Distribution of SARS-CoV-2 Genomes in Wastewaters and the Associated Potential Infection Risk for Plant Workers in Typical Urban and Peri-Urban Communities of the Buffalo City Region, South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Sample Collection and Processing
2.3. Viral RNA Extraction and Quantification
2.4. Profiling of SARS-CoV-2 Genomes Using Quantitative Real-Time Polymerase Chain Reaction
2.5. Quantitative Microbial Risk Assessment of SARS-CoV-2 Genomes among Wastewater Treatment Plant Operators
- I.
- Hazard Identification: During previous coronavirus outbreaks, the generation of wastewater aerosols and droplets was verified as a crucial mechanism of fecal respiration transmission, and this was also suspected in the ongoing COVID-19 outbreak brought on by SARS-CoV-2 [20]. SARS-CoV-2 incident was identified through an exact number of SAR-CoV-2 viral copies from the positive samples.
- II.
- Exposure Assessment: This describes the characteristic pathways that allow SARS-CoV-2 to spread to people and cause infection. Based on assumptions, the wastewater treatment plant operators were exposed to an aerosolized form of the virus through wind when performing their daily duties such as sampling, manual cleaning, and course screening [21]. These workers were assumed to be present at the wastewater treatment plant site for a period of 6 h a day with different volumetric intakes that were exposed to represent the low-case, moderate-case, and worst-case scenarios. For exposure assessment, the SARS-CoV-2 loads in raw wastewater were used to evaluate the level of risk of infection due to the presence of SARS-CoV-2 in the influents. However, the average viral copies/mL were converted into doses and reference was made to inhalation rates in a study by Dada and Gyawali [21], using 3 different scenarios with a volumetric intake of 2, 10, and 20 mL.
- III.
- Dose Response: Previously conducted QMRAs show that dose-response information for other pathogens is often lacking; hence, that of SARS-CoV-2 does not exist. As such, SARS-CoV-1 was used as a substitute to determine the probability of SARS-CoV-2 concentration to cause an infection with a k constant (4.1 × 102) [23,24]. Dose-response determines the probability of the concentration of the virus to cause an infection using a mathematical model with an exponential model equation that is described as follows:
2.6. Statistical Analysis
3. Results
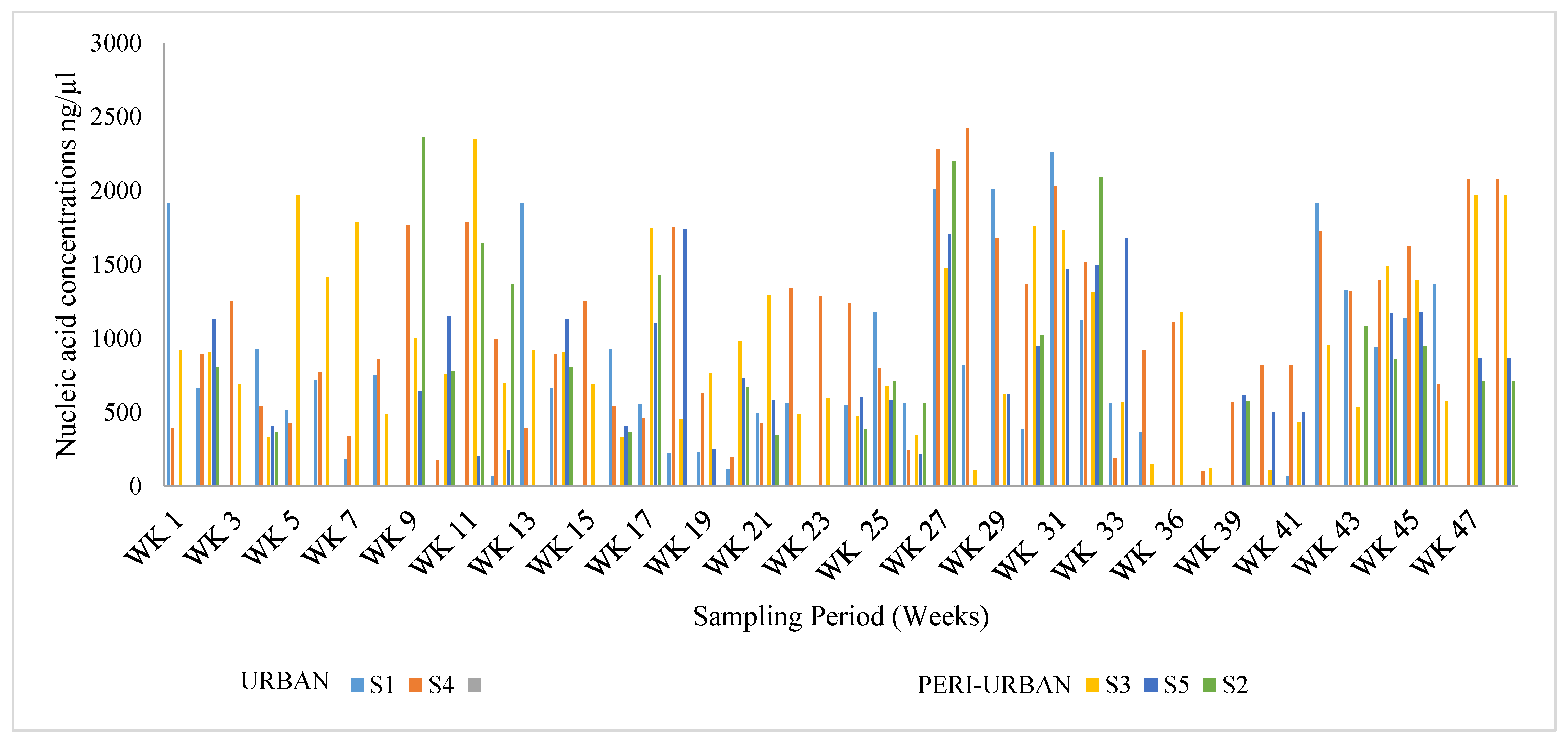
3.1. Viral RNA Extraction and Quantification
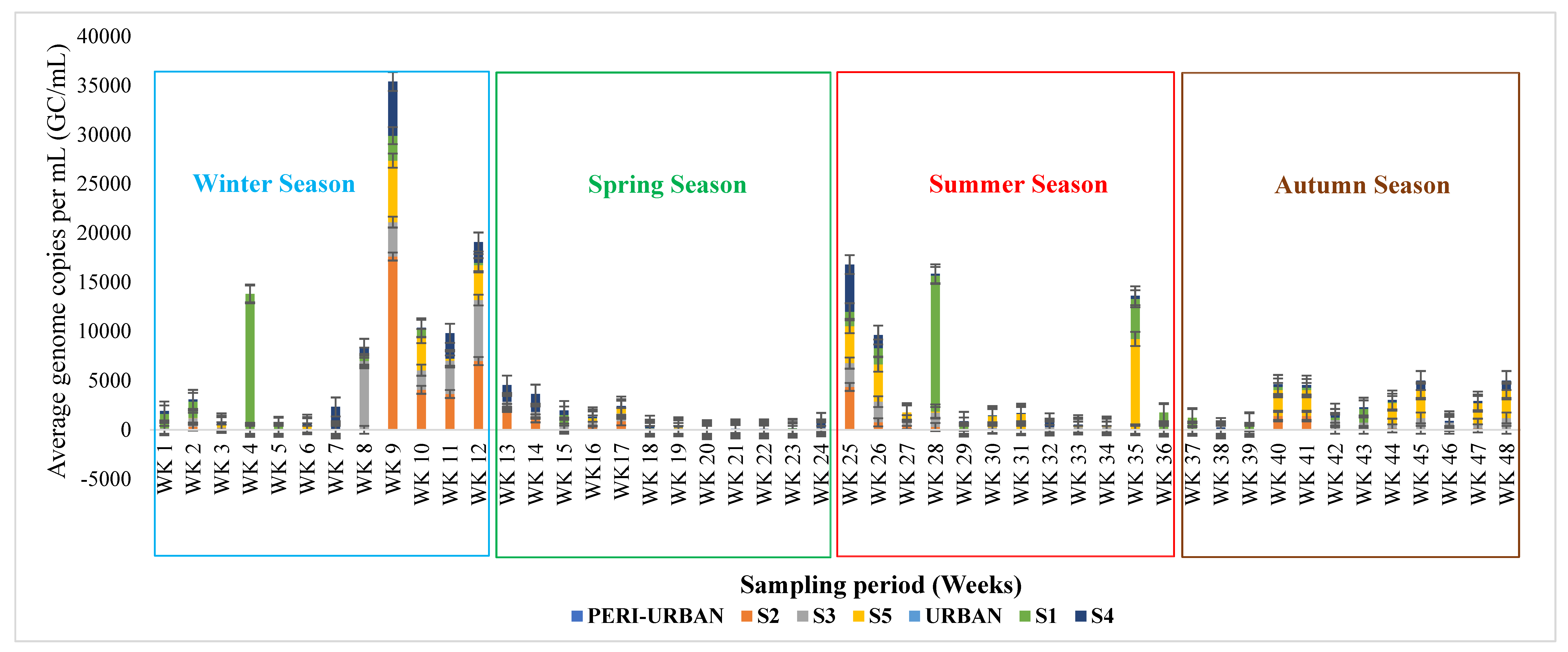
3.2. Profiling of SARS-CoV-2 Genomes Using Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
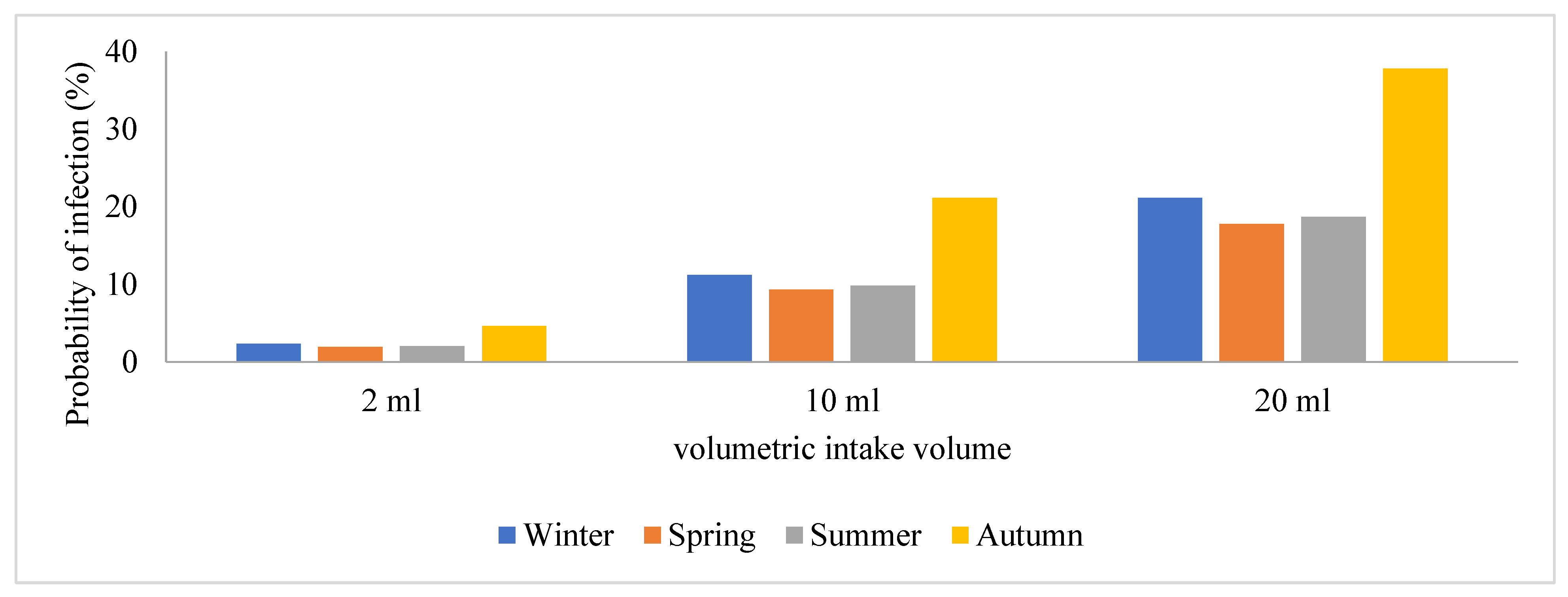
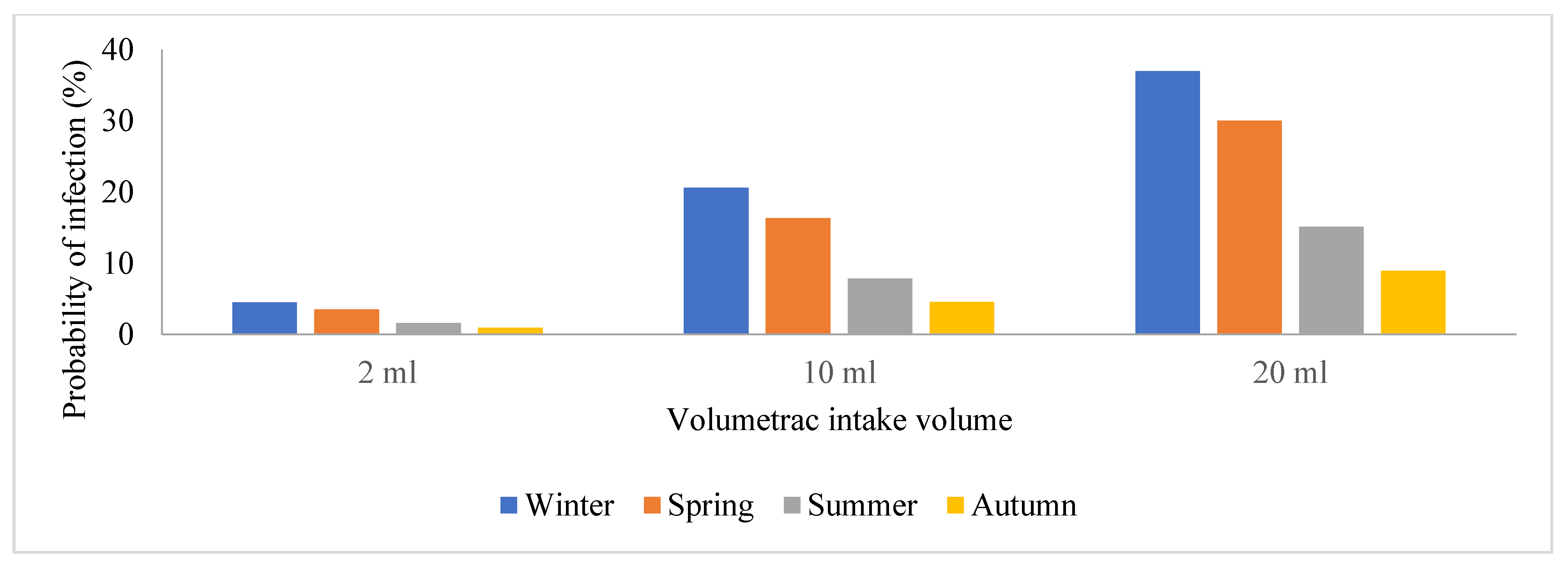
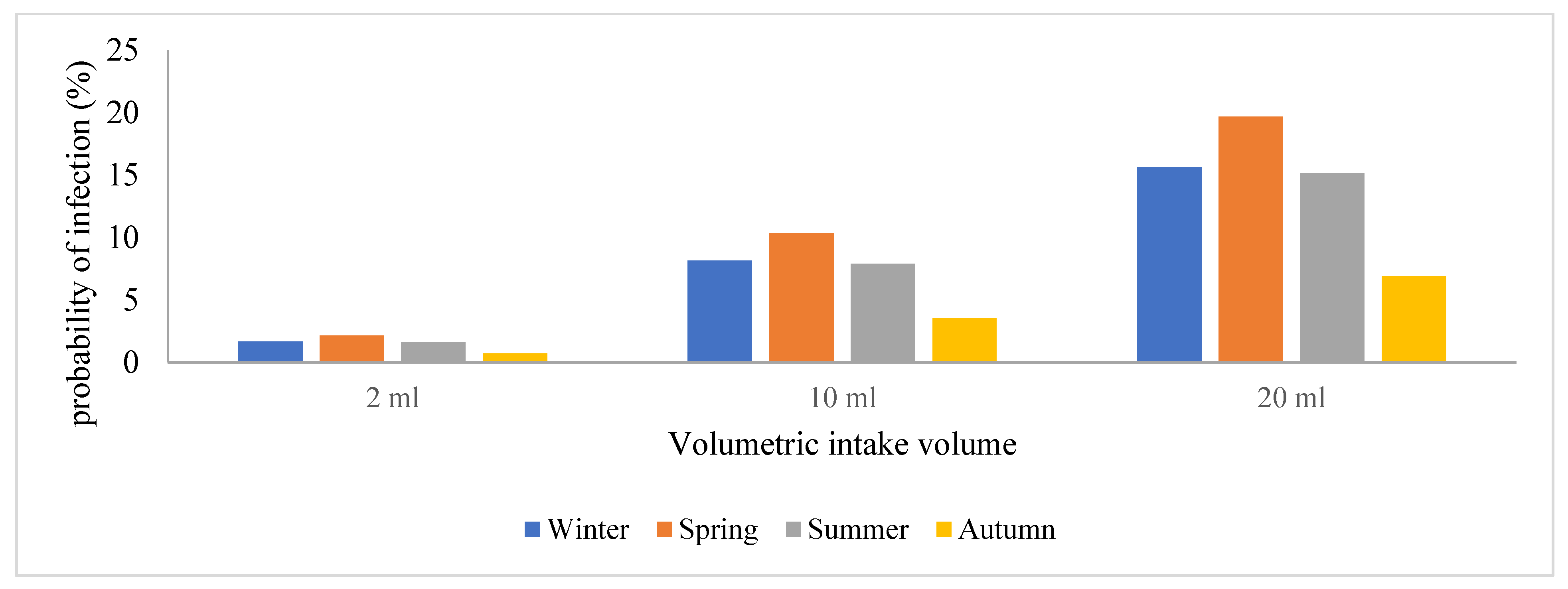
3.3. Quantitative Microbial Risk Assessment of the SARS-CoV-2 Genomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Báez-Santos, Y.M.; St. John, S.E.; Mesecar, A.D. The SARS-coronavirus papain-like protease: Structure, function, and inhibition by designed antiviral compounds. Antivir. Res. 2015, 115, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. 2020, 92, 418–423. [Google Scholar]
- Tegally, H.; Wilkinson, E.; Giovanetti, M.; Iranzadeh, A.; Fonseca, V.; Giandhari, J.; Doolabh, D.; Pillay, S.; San, E.J.; Msomi, N.; et al. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. Medrxiv 2020, 2020, 12. [Google Scholar]
- World Health Organization. Infection Prevention and Control during Health Care When Coronavirus Disease (COVID-19) Is Suspected or Confirmed: Interim Guidance, 12 July 2021 (No. WHO/2019-nCoV/IPC/2021.1). Available online: https://iris.who.int/bitstream/handle/10665/342620/WHO-2019-nCoV-IPC-2021.1-eng.pdf (accessed on 17 April 2024).
- Lai, C.C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. COVID-19 Weekly Epidemiological Update 2022: Special Edition: Proposed Working Definitions of SARS-CoV-2 Variants of Interest and Variants of Concern. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 17 April 2024).
- Adedire, O.; John, W.C.; Oladejo, A.O.; Onuwa, G.; Mbah, J.J. An analysis of COVID-19 infections, recoveries and deaths in rivers state, Nigeria. One Health Risk Manag. 2023, 4, 35–42. [Google Scholar] [CrossRef]
- Cahill, N.; Morris, D. Recreational waters–a potential transmission route for SARS-CoV-2 to humans? Sci. Total Environ. 2020, 740, 140122. [Google Scholar] [CrossRef] [PubMed]
- Ebomah, K.E.; Msolo, L.; Okoh, A.I. A Review on SARS-CoV-2 Genome in the Aquatic Environment of Africa: Prevalence, Persistence and the Future Prospects. Water 2022, 14, 2020. [Google Scholar] [CrossRef]
- Landier, J.; Paireau, J.; Rebaudet, S.; Legendre, E.; Lehot, L.; Fontanet, A.; Cauchemez, S.; Gaudart, J. Cold and dry winter conditions are associated with greater SARS-CoV-2 transmission at regional level in western countries during the first epidemic wave. Sci. Rep. 2021, 11, 12756. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.; Li, C. The role of seasonality in the spread of the COVID-19 pandemic. Environ. Res. 2021, 195, 110874. [Google Scholar] [CrossRef]
- Haramoto, E.; Malla, B.; Thakali, O.; Kitajima, M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020, 737, 140405. [Google Scholar] [CrossRef]
- Medema, G.; Heijnen, L.; Elsinga, G.; Italiaander, R.; Brouwer, A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported Covid-19 prevalence in the early stage of the epidemic in The Netherlands. Environ. Sci. Technol. Lett. 2020, 7, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, A.; Ganesan, H.; Raja, S.; Govindasamy, V.; Arunachalam, M.; Narayanasamy, A. Novel wastewater surveillance strategy for early detection of coronavirus disease 2019 hotspots. J. Environ. Health 2020, 17, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Carducci, A.; Donzelli, G.; Cioni, L.; Verani, M. Quantitative Microbial Risk Assessment in Occupational Settings Applied to the Airborne Human Adenovirus Infection. Int. J. Environ. Res. Public Health 2016, 13, 733–743. [Google Scholar] [CrossRef]
- Kavese, K.; Mbali, A.; Anyikwa, I. The Gig Economy, Digital Labour Platforms, and Independent Employment in the Eastern Cape; Eastern Cape Socio-Economic Consultative Council: East London, South Africa, 2022. [Google Scholar]
- Department for Environment and Rural Affairs. National Policy Statement for Wastewater: A Framework Document for Planning Decisions on Nationally Significant Wastewater Infrastructure; The Stationery Office: London, UK, 2012. [Google Scholar]
- Walsh, K.A.; Jordan, K.; Clyne, B.; Rohde, D.; Drummond, L.; Byrne, P.; Ahern, S.; Carty, P.G.; O’Brien, K.K.; O’Murchu, E.; et al. SARS-CoV-2 detection, viral load, and infectivity over the course of an infection. J. Infect. 2020, 81, 357–371. [Google Scholar] [CrossRef]
- Qongwe, V.V.; Ebomah, K.E.; Msolo, L.; Nontongana, N.; Okoh, A.I. Using Quantitative Microbial Risk Assessment (QMRA) of SARS-CoV-2 to understand possible exposure to health risks in selected wastewater treatment plants located in the Eastern region of South Africa. Microb. Risk Anal. 2024, 26, 100293. [Google Scholar] [CrossRef]
- Sherchan, S.P.; Shahin, S.; Ward, L.M.; Tandukar, S.; Aw, T.G.; Schmitz, B.; Ahmed, W.; Kitajima, M. First detection of SARS-CoV-2 RNA in wastewater in North America: A study in Louisiana, USA. Sci. Total Environ. 2020, 743, 140621. [Google Scholar] [CrossRef] [PubMed]
- Dada, A.C.; Gyawali, P. Quantitative microbial risk assessment (QMRA) of occupational exposure to SARS-CoV-2 in wastewater treatment plants. Sci. Total Environ. 2021, 763, 142989. [Google Scholar] [CrossRef] [PubMed]
- Zaneti, R.N.; Girardi, V.; Spilki, F.R.; Mena, K.; Westphalen, A.P.C.; da Costa Colares, E.R.; Pozzebon, A.G.; Etchepare, R.G. Quantitative microbial risk assessment of SARS-CoV-2 for workers in wastewater treatment plants. Sci. Total Environ. 2021, 754, 142163. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Impact of COVID-19 on Health and Care Workers: A Closer Look at Deaths; No. WHO/HWF/WorkingPaper/2021.1; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Arslan, M.; Xu, B.; GamalEl-Din, M. Transmission of SARS-CoV-2 via faecal-oral and aerosols-borne routes: Environmental dynamics and implications for wastewater management in underprivileged societies. Sci. Total Environ. 2020, 743, 140709. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, J.; Xiao, A.; Gu, X.; Lee, W.L.; Armas, F.; Kauffman, K.; Hanage, W.; Matus, M.; Ghaeli, N.; et al. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. mSystems 2020, 5, 10-1128. [Google Scholar] [CrossRef]
- Wurtzer, S.; Marechal, V.; Mouchel, J.M.; Moulin, L. Time course quantitative detection of SARS-CoV-2 in Parisian wastewaters correlates with COVID-19 confirmed cases. medRxiv 2020. [Google Scholar]
- Galloway, S.E. Emergence of SARS-CoV-2 b. 1.1. 7 lineages—United States, December 29, 2020–January 12, 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J.L.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [CrossRef] [PubMed]
- COVID, W. , 19. Sentinel Hospital Surveillance Update, Week 53. 2 January 2021; National Institute for Communicable Diseases: Johannesburg, South Africa, 2021. [Google Scholar]
- Castiglioni, S.; Schiarea, S.; Pellegrinelli, L.; Primache, V.; Galli, C.; Bubba, L.; Mancinelli, F.; Marinelli, M.; Cereda, D.; Ammoni, E.; et al. SARS-CoV-2 RNA in urban wastewater samples to monitor the COVID-19 pandemic in Lombardy, Italy. Sci. Total Environ. 2022, 806, 150816. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Angel, N.; Edson, J.; Bibby, K.; Bivins, A.; O’Brien, J.W.; Choi, P.M.; Kitajima, M.; Simpson, S.L.; Li, J.; et al. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: A proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020, 728, 138764. [Google Scholar] [CrossRef] [PubMed]
- Miura, F.; Kitajima, M.; Omori, R. Duration of SARS-CoV-2 viral shedding in faeces as a parameter for wastewater-based epidemiology: Re-analysis of patient data using a shedding dynamics model. Sci. Total Environ. 2021, 769, 144549. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.A.; Morden, E.; Rousseau, P.; Arendse, J.; Bam, J.L.; Boloko, L.; Cloete, K.; Cohen, C.; Chetty, N.; Dane, P.; et al. Outcomes of laboratory-confirmed SARS-CoV-2 infection during resurgence driven by Omicron lineages BA. 4 and BA. 5 compared with previous waves in the Western Cape Province, South Africa. Int. J. Infect. Dis. 2023, 127, 63–68. [Google Scholar] [CrossRef]
- Han, H.; Luo, Q.; Mo, F.; Long, L.; Zheng, W. SARS-CoV-2 RNA more readily detected in induced sputum than in throat swabs of convalescent COVID-19 patients. Lancet Infect. Dis. 2020, 20, 655–656. [Google Scholar] [CrossRef]




| Site | Capacity of the Plant (Megaliters/Day) | Technology Use of Wastewater Treatment Plants | Area (Coverage) | Population Density | Population | Catchment Area |
|---|---|---|---|---|---|---|
| S1 | 0.4 | Stabilization pond | 2.130 km2 | 230.0/km2 | 69,900 | Mcantsi river |
| S2 | 2 | Stabilization pond | 8.082 km2 | 1400/km2 | 11,192 | Yellowwoods river |
| S3 | 7.5 | Bio-filters | 4.646 km2 | 3900/km2 | 18,189 | Buffalo river |
| S4 | 7 | Activated sludge model | 65.52 km2 | 520/km2 | 34,019 | Buffalo river |
| S5 | 8 | Activated sludge model | 17.289 km2 | 1300/km2 | 21,783 | Mdizeni stream |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngqwala, B.; Msolo, L.; Ebomah, K.E.; Nontongana, N.; Okoh, A.I. Distribution of SARS-CoV-2 Genomes in Wastewaters and the Associated Potential Infection Risk for Plant Workers in Typical Urban and Peri-Urban Communities of the Buffalo City Region, South Africa. Viruses 2024, 16, 871. https://doi.org/10.3390/v16060871
Ngqwala B, Msolo L, Ebomah KE, Nontongana N, Okoh AI. Distribution of SARS-CoV-2 Genomes in Wastewaters and the Associated Potential Infection Risk for Plant Workers in Typical Urban and Peri-Urban Communities of the Buffalo City Region, South Africa. Viruses. 2024; 16(6):871. https://doi.org/10.3390/v16060871
Chicago/Turabian StyleNgqwala, Balisa, Luyanda Msolo, Kingsley Ehi Ebomah, Nolonwabo Nontongana, and Anthony Ifeanyi Okoh. 2024. "Distribution of SARS-CoV-2 Genomes in Wastewaters and the Associated Potential Infection Risk for Plant Workers in Typical Urban and Peri-Urban Communities of the Buffalo City Region, South Africa" Viruses 16, no. 6: 871. https://doi.org/10.3390/v16060871
APA StyleNgqwala, B., Msolo, L., Ebomah, K. E., Nontongana, N., & Okoh, A. I. (2024). Distribution of SARS-CoV-2 Genomes in Wastewaters and the Associated Potential Infection Risk for Plant Workers in Typical Urban and Peri-Urban Communities of the Buffalo City Region, South Africa. Viruses, 16(6), 871. https://doi.org/10.3390/v16060871







