Comprehensive Overview of Broadly Neutralizing Antibodies against SARS-CoV-2 Variants
Abstract
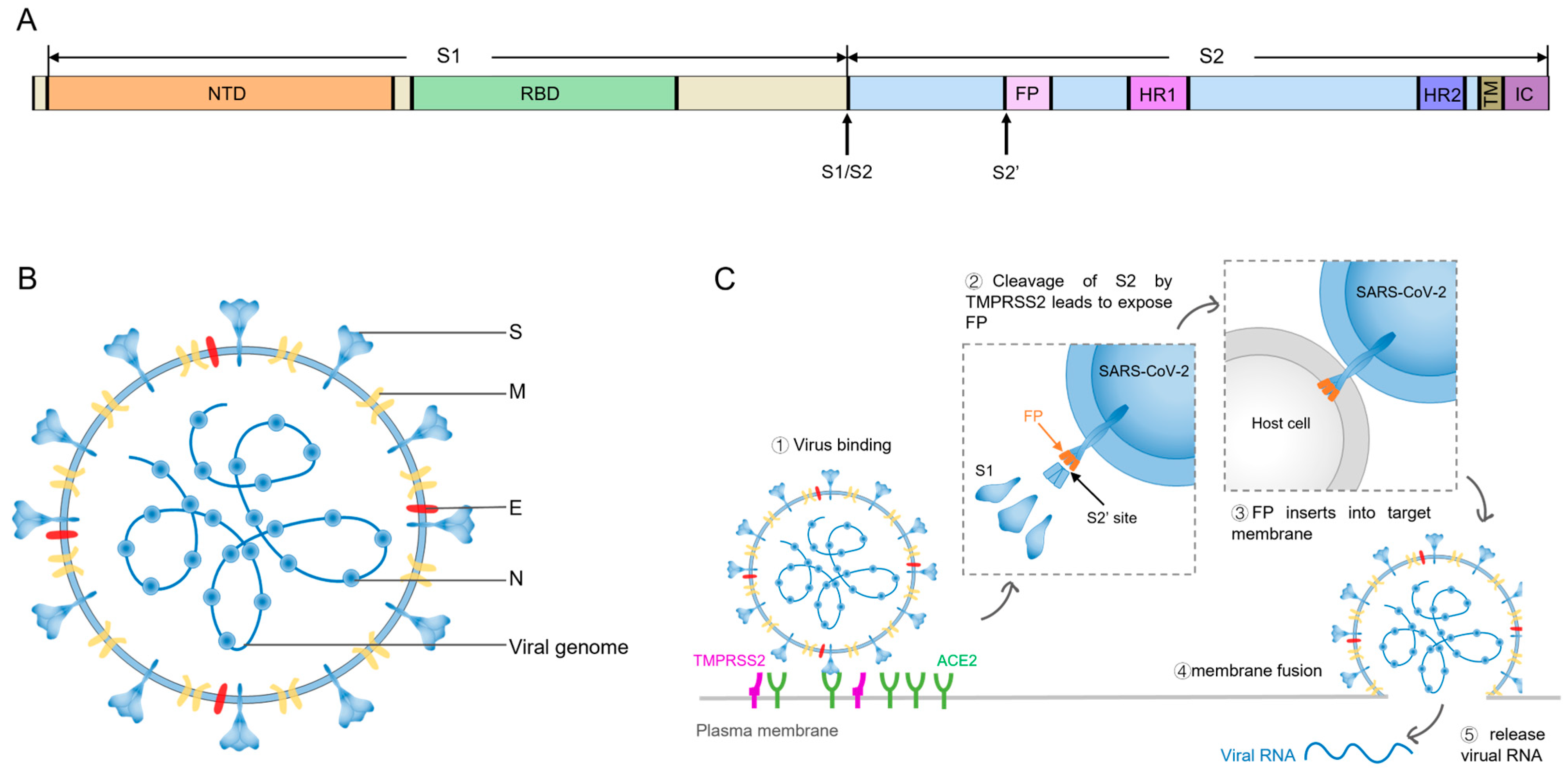
1. Introduction
2. Cumulative Mutations in SARS-CoV-2 Spike
3. Potent bnAbs against SARS-CoV-2
3.1. RBD Antibodies
3.1.1. Class 1 Antibodies
3.1.2. Class 2 Antibodies
3.1.3. Class 3 Antibodies
3.1.4. Class 4 Antibodies
3.1.5. Class 5 Antibodies
3.2. NTD Antibodies
3.3. S2 Antibodies
3.4. Other Antibodies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.Y.; Zhao, R.; Gao, L.J.; Gao, X.F.; Wang, D.P.; Cao, J.M. SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development. Front. Cell Infect. Microbiol. 2020, 10, 587269. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Bai, C.; Zhong, Q.; Gao, G.F. Overview of SARS-CoV-2 genome-encoded proteins. Sci. China Life Sci. 2022, 65, 280–294. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Schoeman, D.; Fielding, B.C. Coronavirus envelope protein: Current knowledge. Virol. J. 2019, 16, 69. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, Q. The Key Site Variation and Immune Challenges in SARS-CoV-2 Evolution. Vaccines 2023, 11, 1472. [Google Scholar] [CrossRef] [PubMed]
- Sturman, L.S.; Holmes, K.V. The molecular biology of coronaviruses. Adv. Virus Res. 1983, 28, 35–112. [Google Scholar]
- Chang, C.K.; Hou, M.H.; Chang, C.F.; Hsiao, C.D.; Huang, T.H. The SARS coronavirus nucleocapsid protein--forms and functions. Antivir. Res. 2014, 103, 39–50. [Google Scholar] [CrossRef]
- Yadav, R.; Chaudhary, J.K.; Jain, N.; Chaudhary, P.K.; Khanra, S.; Dhamija, P.; Sharma, A.; Kumar, A.; Handu, S. Role of Structural and Non-Structural Proteins and Therapeutic Targets of SARS-CoV-2 for COVID-19. Cells 2021, 10, 821. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.; Oton, J.; Qu, K.; Cortese, M.; Zila, V.; McKeane, L.; Nakane, T.; Zivanov, J.; Neufeldt, C.J.; Cerikan, B.; et al. Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature 2020, 588, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed]
- V’Kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Zhou, H. Potent antibodies against immune invasive SARS-CoV-2 Omicron subvariants. Int. J. Biol. Macromol. 2023, 249, 125997. [Google Scholar] [CrossRef]
- Wang, L.; Møhlenberg, M.; Wang, P.; Zhou, H. Immune evasion of neutralizing antibodies by SARS-CoV-2 Omicron. Cytokine Growth Factor. Rev. 2023, 70, 13–25. [Google Scholar] [CrossRef]
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef]
- Viana, R.; Moyo, S.; Amoako, D.G.; Tegally, H.; Scheepers, C.; Althaus, C.L.; Anyaneji, U.J.; Bester, P.A.; Boni, M.F.; Chand, M.; et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 2022, 603, 679–686. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, Y.; Iketani, S.; Nair, M.S.; Li, Z.; Mohri, H.; Wang, M.; Yu, J.; Bowen, A.D.; Chang, J.Y.; et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature 2022, 608, 603–608. [Google Scholar] [CrossRef]
- Challen, R.; Brooks-Pollock, E.; Read, J.M.; Dyson, L.; Tsaneva-Atanasova, K.; Danon, L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: Matched cohort study. Bmj 2021, 372, n579. [Google Scholar] [CrossRef]
- Tegally, H.; Wilkinson, E.; Giovanetti, M.; Iranzadeh, A.; Fonseca, V.; Giandhari, J.; Doolabh, D.; Pillay, S.; San, E.J.; Msomi, N.; et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature 2021, 592, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Iketani, S.; Liu, L.; Guo, Y.; Liu, L.; Chan, J.F.; Huang, Y.; Wang, M.; Luo, Y.; Yu, J.; Chu, H.; et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature 2022, 604, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Saunders, N.; Maes, P.; Guivel-Benhassine, F.; Planchais, C.; Buchrieser, J.; Bolland, W.H.; Porrot, F.; Staropoli, I.; Lemoine, F.; et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022, 602, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Iketani, S.; Li, Z.; Liu, L.; Guo, Y.; Huang, Y.; Bowen, A.D.; Liu, M.; Wang, M.; Yu, J.; et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 2023, 186, 279–286.e8. [Google Scholar] [CrossRef] [PubMed]
- Omotuyi, I.O.; Nash, O.; Ajiboye, O.B.; Iwegbulam, C.G.; Oyinloye, E.B.; Oyedeji, O.A.; Kashim, Z.A.; Okaiyeto, K. Atomistic simulation reveals structural mechanisms underlying D614G spike glycoprotein-enhanced fitness in SARS-COV-2. J. Comput. Chem. 2020, 41, 2158–2161. [Google Scholar] [CrossRef] [PubMed]
- Yurkovetskiy, L.; Wang, X.; Pascal, K.E.; Tomkins-Tinch, C.; Nyalile, T.P.; Wang, Y.; Baum, A.; Diehl, W.E.; Dauphin, A.; Carbone, C.; et al. Structural and Functional Analysis of the D614G SARS-CoV-2 Spike Protein Variant. Cell 2020, 183, 739–751.e8. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Kemp, S.A.; Papa, G.; Datir, R.; Ferreira, I.; Marelli, S.; Harvey, W.T.; Lytras, S.; Mohamed, A.; Gallo, G.; et al. Recurrent emergence of SARS-CoV-2 spike deletion H69/V70 and its role in the Alpha variant B.1.1.7. Cell Rep. 2021, 35, 109292. [Google Scholar] [CrossRef]
- McCallum, M.; De Marco, A.; Lempp, F.A.; Tortorici, M.A.; Pinto, D.; Walls, A.C.; Beltramello, M.; Chen, A.; Liu, Z.; Zatta, F.; et al. N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2. Cell 2021, 184, 2332–2347.e16. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Q.; Wei, P.; Chen, Z.; Aviszus, K.; Yang, J.; Downing, W.; Jiang, C.; Liang, B.; Reynoso, L.; et al. The basis of a more contagious 501Y.V1 variant of SARS-CoV-2. Cell Res. 2021, 31, 720–722. [Google Scholar] [CrossRef]
- Li, J.; Jia, H.; Tian, M.; Wu, N.; Yang, X.; Qi, J.; Ren, W.; Li, F.; Bian, H. SARS-CoV-2 and Emerging Variants: Unmasking Structure, Function, Infection, and Immune Escape Mechanisms. Front. Cell Infect. Microbiol. 2022, 12, 869832. [Google Scholar] [CrossRef]
- Starr, T.N.; Greaney, A.J.; Hilton, S.K.; Ellis, D.; Crawford, K.H.D.; Dingens, A.S.; Navarro, M.J.; Bowen, J.E.; Tortorici, M.A.; Walls, A.C.; et al. Deep Mutational Scanning of SARS-CoV-2 Receptor Binding Domain Reveals Constraints on Folding and ACE2 Binding. Cell 2020, 182, 1295–1310.e20. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Li, L.; Liu, S.; Wang, Q.; Zhang, D.; Xu, Z.; Han, P.; Li, X.; Peng, Q.; Su, C.; et al. Receptor binding and complex structures of human ACE2 to spike RBD from omicron and delta SARS-CoV-2. Cell 2022, 185, 630–640.e10. [Google Scholar] [CrossRef]
- Khan, A.; Zia, T.; Suleman, M.; Khan, T.; Ali, S.S.; Abbasi, A.A.; Mohammad, A.; Wei, D.Q. Higher infectivity of the SARS-CoV-2 new variants is associated with K417N/T, E484K, and N501Y mutants: An insight from structural data. J. Cell Physiol. 2021, 236, 7045–7057. [Google Scholar] [CrossRef]
- Motozono, C.; Toyoda, M.; Zahradnik, J.; Saito, A.; Nasser, H.; Tan, T.S.; Ngare, I.; Kimura, I.; Uriu, K.; Kosugi, Y.; et al. SARS-CoV-2 spike L452R variant evades cellular immunity and increases infectivity. Cell Host Microbe 2021, 29, 1124–1136.e11. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Garcia-Knight, M.A.; Khalid, M.M.; Servellita, V.; Wang, C.; Morris, M.K.; Sotomayor-González, A.; Glasner, D.R.; Reyes, K.R.; Gliwa, A.S.; et al. Transmission, infectivity, and neutralization of a spike L452R SARS-CoV-2 variant. Cell 2021, 184, 3426–3437.e8. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef]
- West, A.P., Jr.; Wertheim, J.O.; Wang, J.C.; Vasylyeva, T.I.; Havens, J.L.; Chowdhury, M.A.; Gonzalez, E.; Fang, C.E.; Di Lonardo, S.S.; Hughes, S.; et al. Detection and characterization of the SARS-CoV-2 lineage B.1.526 in New York. Nat. Commun. 2021, 12, 4886. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.O.; Jette, C.A.; Abernathy, M.E.; Dam, K.A.; Esswein, S.R.; Gristick, H.B.; Malyutin, A.G.; Sharaf, N.G.; Huey-Tubman, K.E.; Lee, Y.E.; et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature 2020, 588, 682–687. [Google Scholar] [CrossRef]
- Lubinski, B.; Fernandes, M.H.V.; Frazier, L.; Tang, T.; Daniel, S.; Diel, D.G.; Jaimes, J.A.; Whittaker, G.R. Functional evaluation of the P681H mutation on the proteolytic activation of the SARS-CoV-2 variant B.1.1.7 (Alpha) spike. iScience 2022, 25, 103589. [Google Scholar] [CrossRef]
- Saito, A.; Irie, T.; Suzuki, R.; Maemura, T.; Nasser, H.; Uriu, K.; Kosugi, Y.; Shirakawa, K.; Sadamasu, K.; Kimura, I.; et al. Enhanced fusogenicity and pathogenicity of SARS-CoV-2 Delta P681R mutation. Nature 2022, 602, 300–306. [Google Scholar] [CrossRef]
- Shah, M.; Woo, H.G. Omicron: A Heavily Mutated SARS-CoV-2 Variant Exhibits Stronger Binding to ACE2 and Potently Escapes Approved COVID-19 Therapeutic Antibodies. Front. Immunol. 2021, 12, 830527. [Google Scholar] [CrossRef] [PubMed]
- Assadiasl, S.; Fatahi, Y.; Zavvar, M.; Nicknam, M.H. COVID-19: Significance of antibodies. Hum. Antibodies 2020, 28, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Zhang, Y.; Hou, R.; Pan, W.; Liang, H.; Gao, X.; Deng, W.; Huang, X.; Qu, L.; Tang, C.; et al. Deep immunoglobulin repertoire sequencing depicts a comprehensive atlas of spike-specific antibody lineages shared among COVID-19 convalescents. Emerg. Microbes Infect. 2024, 13, 2290841. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Amanat, F.; Thapa, M.; Lei, T.; Ahmed, S.M.S.; Adelsberg, D.C.; Carreño, J.M.; Strohmeier, S.; Schmitz, A.J.; Zafar, S.; Zhou, J.Q.; et al. SARS-CoV-2 mRNA vaccination induces functionally diverse antibodies to NTD, RBD, and S2. Cell 2021, 184, 3936–3948.e10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Schmidt, F.; Weisblum, Y.; Muecksch, F.; Barnes, C.O.; Finkin, S.; Schaefer-Babajew, D.; Cipolla, M.; Gaebler, C.; Lieberman, J.A.; et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature 2021, 592, 616–622. [Google Scholar] [CrossRef]
- Sok, D.; Moldt, B.; Burton, D.R. SnapShot: Broadly neutralizing antibodies. Cell 2013, 155, 728.e1. [Google Scholar] [CrossRef]
- Miller, N.L.; Clark, T.; Raman, R.; Sasisekharan, R. Insights on the mutational landscape of the SARS-CoV-2 Omicron variant receptor-binding domain. Cell Rep. Med. 2022, 3, 100527. [Google Scholar] [CrossRef]
- Tortorici, M.A.; Beltramello, M.; Lempp, F.A.; Pinto, D.; Dang, H.V.; Rosen, L.E.; McCallum, M.; Bowen, J.; Minola, A.; Jaconi, S.; et al. Ultrapotent human antibodies protect against SARS-CoV-2 challenge via multiple mechanisms. Science 2020, 370, 950–957. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, L.; Misasi, J.; Pegu, A.; Zhang, Y.; Harris, D.R.; Olia, A.S.; Talana, C.A.; Yang, E.S.; Chen, M.; et al. Structural basis for potent antibody neutralization of SARS-CoV-2 variants including B.1.1.529. Science 2022, 376, eabn8897. [Google Scholar] [CrossRef]
- de Campos-Mata, L.; Trinité, B.; Modrego, A.; Tejedor Vaquero, S.; Pradenas, E.; Pons-Grífols, A.; Rodrigo Melero, N.; Carlero, D.; Marfil, S.; Santiago, C.; et al. A monoclonal antibody targeting a large surface of the receptor binding motif shows pan-neutralizing SARS-CoV-2 activity. Nat. Commun. 2024, 15, 1051. [Google Scholar] [CrossRef]
- Shi, R.; Shan, C.; Duan, X.; Chen, Z.; Liu, P.; Song, J.; Song, T.; Bi, X.; Han, C.; Wu, L.; et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature 2020, 584, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Baum, A.; Pascal, K.E.; Russo, V.; Giordano, S.; Wloga, E.; Fulton, B.O.; Yan, Y.; Koon, K.; Patel, K.; et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science 2020, 369, 1010–1014. [Google Scholar] [CrossRef]
- Dong, J.; Zost, S.J.; Greaney, A.J.; Starr, T.N.; Dingens, A.S.; Chen, E.C.; Chen, R.E.; Case, J.B.; Sutton, R.E.; Gilchuk, P.; et al. Genetic and structural basis for SARS-CoV-2 variant neutralization by a two-antibody cocktail. Nat. Microbiol. 2021, 6, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Zost, S.J.; Gilchuk, P.; Case, J.B.; Binshtein, E.; Chen, R.E.; Nkolola, J.P.; Schäfer, A.; Reidy, J.X.; Trivette, A.; Nargi, R.S.; et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature 2020, 584, 443–449. [Google Scholar] [CrossRef]
- Planchais, C.; Fernández, I.; Bruel, T.; de Melo, G.D.; Prot, M.; Beretta, M.; Guardado-Calvo, P.; Dufloo, J.; Molinos-Albert, L.M.; Backovic, M.; et al. Potent human broadly SARS-CoV-2-neutralizing IgA and IgG antibodies effective against Omicron BA.1 and BA.2. J. Exp. Med. 2022, 219, e20220638. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, T.; Zhang, Y.; Yang, E.S.; Schramm, C.A.; Shi, W.; Pegu, A.; Oloniniyi, O.K.; Henry, A.R.; Darko, S.; et al. Ultrapotent antibodies against diverse and highly transmissible SARS-CoV-2 variants. Science 2021, 373, eabh1766. [Google Scholar] [CrossRef]
- Du, W.; Hurdiss, D.L.; Drabek, D.; Mykytyn, A.Z.; Kaiser, F.K.; González-Hernández, M.; Muñoz-Santos, D.; Lamers, M.M.; van Haperen, R.; Li, W.; et al. An ACE2-blocking antibody confers broad neutralization and protection against Omicron and other SARS-CoV-2 variants of concern. Sci. Immunol. 2022, 7, eabp9312. [Google Scholar] [CrossRef]
- Quinti, I.; Mortari, E.P.; Fernandez Salinas, A.; Milito, C.; Carsetti, R. IgA Antibodies and IgA Deficiency in SARS-CoV-2 Infection. Front. Cell Infect. Microbiol. 2021, 11, 655896. [Google Scholar] [CrossRef]
- Starr, T.N.; Czudnochowski, N.; Liu, Z.; Zatta, F.; Park, Y.J.; Addetia, A.; Pinto, D.; Beltramello, M.; Hernandez, P.; Greaney, A.J.; et al. SARS-CoV-2 RBD antibodies that maximize breadth and resistance to escape. Nature 2021, 597, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Qu, P.; Evans, J.P.; Faraone, J.N.; Zheng, Y.M.; Carlin, C.; Anghelina, M.; Stevens, P.; Fernandez, S.; Jones, D.; Lozanski, G.; et al. Enhanced neutralization resistance of SARS-CoV-2 Omicron subvariants BQ.1, BQ.1.1, BA.4.6, BF.7, and BA.2.75.2. Cell Host Microbe 2023, 31, 9–17.e3. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; De Marco, A.; Starr, T.N.; Liu, Z.; Pinto, D.; Walls, A.C.; Zatta, F.; Zepeda, S.K.; Bowen, J.E.; Sprouse, K.R.; et al. Antibody-mediated broad sarbecovirus neutralization through ACE2 molecular mimicry. Science 2022, 375, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Cameroni, E.; Bowen, J.E.; Rosen, L.E.; Saliba, C.; Zepeda, S.K.; Culap, K.; Pinto, D.; VanBlargan, L.A.; De Marco, A.; di Iulio, J.; et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature 2022, 602, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Wang, R.; Ju, B.; Zhang, Q.; Sun, J.; Chen, P.; Zhang, S.; Tian, Y.; Shan, S.; Cheng, L.; et al. Antibody neutralization of SARS-CoV-2 through ACE2 receptor mimicry. Nat. Commun. 2021, 12, 250. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, X.; Wang, Z.; Li, Y.; Wang, C.; Jiang, L.; Zuo, T. Breakthrough infection elicits hypermutated IGHV3-53/3-66 public antibodies with broad and potent neutralizing activity against SARS-CoV-2 variants including the emerging EG.5 lineages. PLoS Pathog. 2023, 19, e1011856. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Ryu, D.K.; Lee, J.; Kim, Y.I.; Seo, J.M.; Kim, Y.G.; Jeong, J.H.; Kim, M.; Kim, J.I.; Kim, P.; et al. A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein. Nat. Commun. 2021, 12, 288. [Google Scholar] [CrossRef]
- Syed, Y.Y. Correction to: Regdanvimab: First Approval. Drugs 2021, 81, 2139. [Google Scholar] [CrossRef]
- Ju, B.; Zhang, Q.; Wang, Z.; Aw, Z.Q.; Chen, P.; Zhou, B.; Wang, R.; Ge, X.; Lv, Q.; Cheng, L.; et al. Infection with wild-type SARS-CoV-2 elicits broadly neutralizing and protective antibodies against omicron subvariants. Nat. Immunol. 2023, 24, 690–699. [Google Scholar] [CrossRef]
- Rouet, R.; Henry, J.Y.; Johansen, M.D.; Sobti, M.; Balachandran, H.; Langley, D.B.; Walker, G.J.; Lenthall, H.; Jackson, J.; Ubiparipovic, S.; et al. Broadly neutralizing SARS-CoV-2 antibodies through epitope-based selection from convalescent patients. Nat. Commun. 2023, 14, 687. [Google Scholar] [CrossRef]
- Fenwick, C.; Turelli, P.; Duhoo, Y.; Lau, K.; Herate, C.; Marlin, R.; Lamrayah, M.; Campos, J.; Esteves-Leuenberger, L.; Farina, A.; et al. Broadly potent anti-SARS-CoV-2 antibody shares 93% of epitope with ACE2 and provides full protection in monkeys. J. Infect. 2023, 87, 524–537. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Zhuang, X.; Zhang, S.; Chen, Z.; Zou, Y.; Sheng, J.; Li, T.; Tai, W.; Yu, J.; et al. Inactivated vaccine-elicited potent antibodies can broadly neutralize SARS-CoV-2 circulating variants. Nat. Commun. 2023, 14, 2179. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Huang, D.; Lee, C.D.; Wu, N.C.; Jackson, A.M.; Zhu, X.; Liu, H.; Peng, L.; van Gils, M.J.; Sanders, R.W.; et al. Structural and functional ramifications of antigenic drift in recent SARS-CoV-2 variants. Science 2021, 373, 818–823. [Google Scholar] [CrossRef]
- Jones, B.E.; Brown-Augsburger, P.L.; Corbett, K.S.; Westendorf, K.; Davies, J.; Cujec, T.P.; Wiethoff, C.M.; Blackbourne, J.L.; Heinz, B.A.; Foster, D.; et al. The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates. Sci. Transl. Med. 2021, 13, eabf1906. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, F.; Lin, D.; Kong, W.; Cai, X.; Yang, J.; Sun, X.; Cao, P. Rational optimization of a human neutralizing antibody of SARS-CoV-2. Comput. Biol. Med. 2021, 135, 104550. [Google Scholar] [CrossRef]
- Ju, B.; Zhang, Q.; Ge, J.; Wang, R.; Sun, J.; Ge, X.; Yu, J.; Shan, S.; Zhou, B.; Song, S.; et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 2020, 584, 115–119. [Google Scholar] [CrossRef]
- Patrick, C.; Upadhyay, V.; Lucas, A.; Mallela, K.M.G. Biophysical Fitness Landscape of the SARS-CoV-2 Delta Variant Receptor Binding Domain. J. Mol. Biol. 2022, 434, 167622. [Google Scholar] [CrossRef] [PubMed]
- Makdasi, E.; Zvi, A.; Alcalay, R.; Noy-Porat, T.; Peretz, E.; Mechaly, A.; Levy, Y.; Epstein, E.; Chitlaru, T.; Tennenhouse, A.; et al. The neutralization potency of anti-SARS-CoV-2 therapeutic human monoclonal antibodies is retained against viral variants. Cell Rep. 2021, 36, 109679. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y.; Chen, X.; Jiang, Y.; Wang, S.; Huang, Y.; Liu, L.; Li, Y.; Lan, M.; Guo, H.; et al. Structural basis for broad neutralization of human antibody against Omicron sublineages and evasion by XBB variant. J. Virol. 2023, 97, e0113723. [Google Scholar] [CrossRef]
- Yue, C.; Song, W.; Wang, L.; Jian, F.; Chen, X.; Gao, F.; Shen, Z.; Wang, Y.; Wang, X.; Cao, Y. ACE2 binding and antibody evasion in enhanced transmissibility of XBB.1.5. Lancet Infect. Dis. 2023, 23, 278–280. [Google Scholar] [CrossRef]
- Pinto, D.; Park, Y.J.; Beltramello, M.; Walls, A.C.; Tortorici, M.A.; Bianchi, S.; Jaconi, S.; Culap, K.; Zatta, F.; De Marco, A.; et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 2020, 583, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Crespo Casal, M.; Moya, J.; Falci, D.R.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N. Engl. J. Med. 2021, 385, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Magnus, C.L.; Hiergeist, A.; Schuster, P.; Rohrhofer, A.; Medenbach, J.; Gessner, A.; Peterhoff, D.; Schmidt, B. Targeted escape of SARS-CoV-2 in vitro from monoclonal antibody S309, the precursor of sotrovimab. Front. Immunol. 2022, 13, 966236. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhang, J.; Kreutzberger, A.J.B.; Eaton, A.; Edwards, R.J.; Jing, C.; Dai, H.Q.; Sempowski, G.D.; Cronin, K.; Parks, R.; et al. An antibody from single human V(H)-rearranging mouse neutralizes all SARS-CoV-2 variants through BA.5 by inhibiting membrane fusion. Sci. Immunol. 2022, 7, eadd5446. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Sun, P.; Xie, X.; Du, M.; Du, F.; Ye, J.; Kalveram, B.K.; Plante, J.A.; Plante, K.S.; Li, B.; et al. An antibody that neutralizes SARS-CoV-1 and SARS-CoV-2 by binding to a conserved spike epitope outside the receptor binding motif. Sci. Immunol. 2022, 7, eabp9962. [Google Scholar] [CrossRef] [PubMed]
- Westendorf, K.; Žentelis, S.; Wang, L.; Foster, D.; Vaillancourt, P.; Wiggin, M.; Lovett, E.; van der Lee, R.; Hendle, J.; Pustilnik, A.; et al. LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants. Cell Rep. 2022, 39, 110812. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-J.; Pinto, D.; Walls, A.C.; Liu, Z.; De Marco, A.; Benigni, F.; Zatta, F.; Silacci-Fregni, C.; Bassi, J.; Sprouse, K.R.; et al. Imprinted antibody responses against SARS-CoV-2 Omicron sublineages. Science 2022, 378, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Patel, A.; Lai, L.; Chakravarthy, C.; Valanparambil, R.; Reddy, E.S.; Gottimukkala, K.; Davis-Gardner, M.E.; Edara, V.V.; Linderman, S.; et al. Structural insights for neutralization of Omicron variants BA.1, BA.2, BA.4, and BA.5 by a broadly neutralizing SARS-CoV-2 antibody. Sci. Adv. 2022, 8, eadd2032. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Wu, N.C.; Zhu, X.; Lee, C.D.; So, R.T.Y.; Lv, H.; Mok, C.K.P.; Wilson, I.A. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science 2020, 368, 630–633. [Google Scholar] [CrossRef]
- Yuan, M.; Zhu, X.; He, W.T.; Zhou, P.; Kaku, C.I.; Capozzola, T.; Zhu, C.Y.; Yu, X.; Liu, H.; Yu, W.; et al. A broad and potent neutralization epitope in SARS-related coronaviruses. Proc. Natl. Acad. Sci. USA 2022, 119, e2205784119. [Google Scholar] [CrossRef]
- Tortorici, M.A.; Czudnochowski, N.; Starr, T.N.; Marzi, R.; Walls, A.C.; Zatta, F.; Bowen, J.E.; Jaconi, S.; Di Iulio, J.; Wang, Z.; et al. Broad sarbecovirus neutralization by a human monoclonal antibody. Nature 2021, 597, 103–108. [Google Scholar] [CrossRef]
- Huo, J.; Zhao, Y.; Ren, J.; Zhou, D.; Duyvesteyn, H.M.E.; Ginn, H.M.; Carrique, L.; Malinauskas, T.; Ruza, R.R.; Shah, P.N.M.; et al. Neutralization of SARS-CoV-2 by Destruction of the Prefusion Spike. Cell Host Microbe 2020, 28, 497. [Google Scholar] [CrossRef]
- Sankhala, R.S.; Dussupt, V.; Chen, W.-H.; Bai, H.; Martinez, E.J.; Jensen, J.L.; Rees, P.A.; Hajduczki, A.; Chang, W.C.; Choe, M.; et al. Antibody targeting of conserved sites of vulnerability on the SARS-CoV-2 spike receptor-binding domain. Structure 2024, 32, 131–147.e7. [Google Scholar] [CrossRef]
- Liu, H.; Wu, N.C.; Yuan, M.; Bangaru, S.; Torres, J.L.; Caniels, T.G.; van Schooten, J.; Zhu, X.; Lee, C.D.; Brouwer, P.J.M.; et al. Cross-Neutralization of a SARS-CoV-2 Antibody to a Functionally Conserved Site Is Mediated by Avidity. Immunity 2020, 53, 1272–1280.e5. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Edwards, R.J.; Manne, K.; Martinez, D.R.; Schäfer, A.; Alam, S.M.; Wiehe, K.; Lu, X.; Parks, R.; Sutherland, L.L.; et al. In vitro and in vivo functions of SARS-CoV-2 infection-enhancing and neutralizing antibodies. Cell 2021, 184, 4203–4219.e32. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Iketani, S.; Guo, Y.; Reddem, E.R.; Casner, R.G.; Nair, M.S.; Yu, J.; Chan, J.F.; Wang, M.; Cerutti, G.; et al. An antibody class with a common CDRH3 motif broadly neutralizes sarbecoviruses. Sci. Transl. Med. 2022, 14, eabn6859. [Google Scholar] [CrossRef]
- Jensen, J.L.; Sankhala, R.S.; Dussupt, V.; Bai, H.; Hajduczki, A.; Lal, K.G.; Chang, W.C.; Martinez, E.J.; Peterson, C.E.; Golub, E.S.; et al. Targeting the Spike Receptor Binding Domain Class V Cryptic Epitope by an Antibody with Pan-Sarbecovirus Activity. J. Virol. 2023, 97, e0159622. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, H.; Zhang, Y.; Yuan, L.; Wang, Y.; Zhang, T.; Wang, S.; Zhang, J.; Yu, H.; Xiong, H.; et al. Three SARS-CoV-2 antibodies provide broad and synergistic neutralization against variants of concern, including Omicron. Cell Rep. 2022, 39, 110862. [Google Scholar] [CrossRef]
- Bullen, G.; Galson, J.D.; Hall, G.; Villar, P.; Moreels, L.; Ledsgaard, L.; Mattiuzzo, G.; Bentley, E.M.; Masters, E.W.; Tang, D.; et al. Cross-Reactive SARS-CoV-2 Neutralizing Antibodies From Deep Mining of Early Patient Responses. Front. Immunol. 2021, 12, 678570. [Google Scholar] [CrossRef]
- Dussupt, V.; Sankhala, R.S.; Mendez-Rivera, L.; Townsley, S.M.; Schmidt, F.; Wieczorek, L.; Lal, K.G.; Donofrio, G.C.; Tran, U.; Jackson, N.D.; et al. Low-dose in vivo protection and neutralization across SARS-CoV-2 variants by monoclonal antibody combinations. Nat. Immunol. 2021, 22, 1503–1514. [Google Scholar] [CrossRef]
- Li, T.; Cai, H.; Zhao, Y.; Li, Y.; Lai, Y.; Yao, H.; Liu, L.D.; Sun, Z.; van Vlissingen, M.F.; Kuiken, T.; et al. Uncovering a conserved vulnerability site in SARS-CoV-2 by a human antibody. EMBO Mol. Med. 2021, 13, e14544. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xue, W.; Zheng, Q.; Song, S.; Yang, C.; Xiong, H.; Zhang, S.; Hong, M.; Zhang, Y.; Yu, H.; et al. Cross-neutralizing antibodies bind a SARS-CoV-2 cryptic site and resist circulating variants. Nat. Commun. 2021, 12, 5652. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, L.; Park, Y.J.; Tortorici, M.A.; Czudnochowski, N.; Walls, A.C.; Beltramello, M.; Silacci-Fregni, C.; Pinto, D.; Rosen, L.E.; Bowen, J.E.; et al. Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell 2020, 183, 1024–1042.e21. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Yan, R.; Zhang, J.; Zhang, G.; Zhang, Y.; Hao, M.; Zhang, Z.; Fan, P.; Dong, Y.; Yang, Y.; et al. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science 2020, 369, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, G.; Guo, Y.; Zhou, T.; Gorman, J.; Lee, M.; Rapp, M.; Reddem, E.R.; Yu, J.; Bahna, F.; Bimela, J.; et al. Potent SARS-CoV-2 neutralizing antibodies directed against spike N-terminal domain target a single supersite. Cell Host Microbe 2021, 29, 819–833.e7. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.F.N.; Mesquita, F.P.; Amaral, J.L.; Landim, P.G.C.; Lima, K.R.P.; Costa, M.B.; Farias, I.R.; Belém, M.O.; Pinto, Y.O.; Moreira, H.H.T.; et al. The spike glycoprotein of SARS-CoV-2: A review of how mutations of spike glycoproteins have driven the emergence of variants with high transmissibility and immune escape. Int. J. Biol. Macromol. 2022, 208, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Lok, S.M. An NTD supersite of attack. Cell Host Microbe 2021, 29, 744–746. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, P.; Nair, M.S.; Yu, J.; Rapp, M.; Wang, Q.; Luo, Y.; Chan, J.F.; Sahi, V.; Figueroa, A.; et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature 2020, 584, 450–456. [Google Scholar] [CrossRef]
- Cerutti, G.; Guo, Y.; Wang, P.; Nair, M.S.; Wang, M.; Huang, Y.; Yu, J.; Liu, L.; Katsamba, P.S.; Bahna, F.; et al. Neutralizing antibody 5-7 defines a distinct site of vulnerability in SARS-CoV-2 spike N-terminal domain. Cell Rep. 2021, 37, 109928. [Google Scholar] [CrossRef]
- Li, D.; Sempowski, G.D.; Saunders, K.O.; Acharya, P.; Haynes, B.F. SARS-CoV-2 Neutralizing Antibodies for COVID-19 Prevention and Treatment. Annu. Rev. Med. 2022, 73, 1–16. [Google Scholar] [CrossRef]
- Olukitibi, T.A.; Ao, Z.; Warner, B.; Unat, R.; Kobasa, D.; Yao, X. Significance of Conserved Regions in Coronavirus Spike Protein for Developing a Novel Vaccine against SARS-CoV-2 Infection. Vaccines 2023, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Shrock, E.; Fujimura, E.; Kula, T.; Timms, R.T.; Lee, I.H.; Leng, Y.; Robinson, M.L.; Sie, B.M.; Li, M.Z.; Chen, Y.; et al. Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science 2020, 370, eabd4250. [Google Scholar] [CrossRef] [PubMed]
- Ladner, J.T.; Henson, S.N.; Boyle, A.S.; Engelbrektson, A.L.; Fink, Z.W.; Rahee, F.; D’Ambrozio, J.; Schaecher, K.E.; Stone, M.; Dong, W.; et al. Epitope-resolved profiling of the SARS-CoV-2 antibody response identifies cross-reactivity with endemic human coronaviruses. Cell Rep. Med. 2021, 2, 100189. [Google Scholar] [CrossRef]
- Crowley, A.R.; Natarajan, H.; Hederman, A.P.; Bobak, C.A.; Weiner, J.A.; Wieland-Alter, W.; Lee, J.; Bloch, E.M.; Tobian, A.A.R.; Redd, A.D.; et al. Boosting of cross-reactive antibodies to endemic coronaviruses by SARS-CoV-2 infection but not vaccination with stabilized spike. eLife 2022, 11, e75228. [Google Scholar] [CrossRef]
- Pannus, P.; Neven, K.Y.; De Craeye, S.; Heyndrickx, L.; Vande Kerckhove, S.; Georges, D.; Michiels, J.; Francotte, A.; Van Den Bulcke, M.; Zrein, M.; et al. Poor Antibody Response to BioNTech/Pfizer Coronavirus Disease 2019 Vaccination in Severe Acute Respiratory Syndrome Coronavirus 2-Naive Residents of Nursing Homes. Clin. Infect. Dis. 2022, 75, e695–e704. [Google Scholar] [CrossRef] [PubMed]
- Piepenbrink, M.S.; Park, J.G.; Deshpande, A.; Loos, A.; Ye, C.; Basu, M.; Sarkar, S.; Khalil, A.M.; Chauvin, D.; Woo, J.; et al. Potent universal beta-coronavirus therapeutic activity mediated by direct respiratory administration of a Spike S2 domain-specific human neutralizing monoclonal antibody. PLoS Pathog. 2022, 18, e1010691. [Google Scholar] [CrossRef]
- Li, C.J.; Chang, S.C. SARS-CoV-2 spike S2-specific neutralizing antibodies. Emerg. Microbes Infect. 2023, 12, 2220582. [Google Scholar] [CrossRef]
- Pinto, D.; Sauer, M.M.; Czudnochowski, N.; Low, J.S.; Tortorici, M.A.; Housley, M.P.; Noack, J.; Walls, A.C.; Bowen, J.E.; Guarino, B.; et al. Broad betacoronavirus neutralization by a stem helix-specific human antibody. Science 2021, 373, 1109–1116. [Google Scholar] [CrossRef]
- Shi, W.; Wang, L.; Zhou, T.; Sastry, M.; Yang, E.S.; Zhang, Y.; Chen, M.; Chen, X.; Choe, M.; Creanga, A.; et al. Vaccine-elicited murine antibody WS6 neutralizes diverse beta-coronaviruses by recognizing a helical stem supersite of vulnerability. Structure 2022, 30, 1233–1244.e7. [Google Scholar] [CrossRef]
- Zhou, P.; Yuan, M.; Song, G.; Beutler, N.; Shaabani, N.; Huang, D.; He, W.T.; Zhu, X.; Callaghan, S.; Yong, P.; et al. A human antibody reveals a conserved site on beta-coronavirus spike proteins and confers protection against SARS-CoV-2 infection. Sci. Transl. Med. 2022, 14, eabi9215. [Google Scholar] [CrossRef] [PubMed]
- Hurlburt, N.K.; Homad, L.J.; Sinha, I.; Jennewein, M.F.; MacCamy, A.J.; Wan, Y.H.; Boonyaratanakornkit, J.; Sholukh, A.M.; Jackson, A.M.; Zhou, P.; et al. Structural definition of a pan-sarbecovirus neutralizing epitope on the spike S2 subunit. Commun. Biol. 2022, 5, 342. [Google Scholar] [CrossRef] [PubMed]
- Jennewein, M.F.; MacCamy, A.J.; Akins, N.R.; Feng, J.; Homad, L.J.; Hurlburt, N.K.; Seydoux, E.; Wan, Y.H.; Stuart, A.B.; Edara, V.V.; et al. Isolation and characterization of cross-neutralizing coronavirus antibodies from COVID-19+ subjects. Cell Rep. 2021, 36, 109353. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Chao, T.L.; Chang, T.Y.; Hsiao, C.C.; Lu, D.C.; Chiang, Y.W.; Lai, G.C.; Tsai, Y.M.; Fang, J.T.; Ieong, S.; et al. Neutralizing Monoclonal Antibodies Inhibit SARS-CoV-2 Infection through Blocking Membrane Fusion. Microbiol. Spectr. 2022, 10, e0181421. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yi, C.; Zhu, Y.; Ding, L.; Xia, S.; Chen, X.; Liu, M.; Gu, C.; Lu, X.; Fu, Y.; et al. Neutralization mechanism of a human antibody with pan-coronavirus reactivity including SARS-CoV-2. Nat. Microbiol. 2022, 7, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Low, J.S.; Jerak, J.; Tortorici, M.A.; McCallum, M.; Pinto, D.; Cassotta, A.; Foglierini, M.; Mele, F.; Abdelnabi, R.; Weynand, B.; et al. ACE2-binding exposes the SARS-CoV-2 fusion peptide to broadly neutralizing coronavirus antibodies. Science 2022, 377, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Dacon, C.; Tucker, C.; Peng, L.; Lee, C.-C.D.; Lin, T.-H.; Yuan, M.; Cong, Y.; Wang, L.; Purser, L.; Williams, J.K.; et al. Broadly neutralizing antibodies target the coronavirus fusion peptide. Science 2022, 377, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.L.; Chiang, C.Y.; Lai, S.C.; Yu, C.Y.; Huang, Y.L.; Liao, H.C.; Liao, C.L.; Chen, H.W.; Liu, S.J. Monoclonal antibody targeting the conserved region of the SARS-CoV-2 spike protein to overcome viral variants. JCI Insight 2022, 7, e157597. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jiang, S.; Ying, T. Single-Domain Antibodies As Therapeutics against Human Viral Diseases. Front. Immunol. 2017, 8, 1802. [Google Scholar] [CrossRef]
- Wu, Y.; Li, C.; Xia, S.; Tian, X.; Kong, Y.; Wang, Z.; Gu, C.; Zhang, R.; Tu, C.; Xie, Y.; et al. Identification of Human Single-Domain Antibodies against SARS-CoV-2. Cell Host Microbe 2020, 27, 891–898.e5. [Google Scholar] [CrossRef]
- Van Heeke, G.; Allosery, K.; De Brabandere, V.; De Smedt, T.; Detalle, L.; de Fougerolles, A. Nanobodies® Nanobody is a registered trademark of Ablynx NV. as inhaled biotherapeutics for lung diseases. Pharmacol. Ther. 2017, 169, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ren, Y.; Aw, Z.Q.; Chen, B.; Yang, Z.; Lei, Y.; Cheng, L.; Liang, Q.; Hong, J.; Yang, Y.; et al. Broadly neutralizing and protective nanobodies against SARS-CoV-2 Omicron subvariants BA.1, BA.2, and BA.4/5 and diverse sarbecoviruses. Nat. Commun. 2022, 13, 7957. [Google Scholar] [CrossRef] [PubMed]
- Buffington, J.; Duan, Z.; Kwon, H.J.; Hong, J.; Li, D.; Feng, M.; Xie, H.; Ho, M. Identification of nurse shark V(NAR) single-domain antibodies targeting the spike S2 subunit of SARS-CoV-2. Faseb J. 2023, 37, e22973. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cheng, L.; Fu, M.; Huang, B.; Zhu, L.; Xu, S.; Shi, H.; Zhang, D.; Yuan, H.; Nawaz, W.; et al. A potent bispecific nanobody protects hACE2 mice against SARS-CoV-2 infection via intranasal administration. Cell Rep. 2021, 37, 109869. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Jian, F.; Wang, J.; Yu, Y.; Song, W.; Yisimayi, A.; Wang, J.; An, R.; Chen, X.; Zhang, N.; et al. Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution. Nature 2023, 614, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.; Seow, J.; Huettner, I.; Khan, H.; Kouphou, N.; Acors, S.; Winstone, H.; Pickering, S.; Galao, R.P.; Dupont, L.; et al. Neutralization potency of monoclonal antibodies recognizing dominant and subdominant epitopes on SARS-CoV-2 Spike is impacted by the B.1.1.7 variant. Immunity 2021, 54, 1276–1289.e6. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Yang, Y.; Zhang, X. Neutralizing antibodies for the prevention and treatment of COVID-19. Cell Mol. Immunol. 2021, 18, 2293–2306. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Focosi, D. SARS-CoV-2 variants resistant to monoclonal antibodies in immunocompromised patients constitute a public health concern. J. Clin. Investig. 2023, 133, e168603. [Google Scholar] [CrossRef]
- Levin, M.J.; Ustianowski, A.P.; De Wit, S.; Launay, O.; Avila, M.; Seegobin, S.D.; Templeton, A.; Yuan, Y.; Ambery, P.; Arends, R.H.; et al. LB5. PROVENT: Phase 3 Study of Efficacy and Safety of AZD7442 (Tixagevimab/Cilgavimab) for Pre-exposure Prophylaxis of COVID-19 in Adults. Open Forum Infect. Dis. 2021, 8, S810. [Google Scholar] [CrossRef]

| Site of the Mutations | Mutations | Mutations Shared with VOCs | Impact of the Mutation |
|---|---|---|---|
| NTD | Δ69-70 | Alpha, Omicron | decreases neutralization potency |
| T95I | Omicron | increases transmission, associated with immune escape | |
| RBD | G339D | Omicron | increases transmission by enhancing interaction between S protein and ACE2 |
| S371L | Omicron | increases immune escape | |
| K417N/T | Beta, Gamma, Omicron | reduces affinity between the RBD and ACE2, associated with immune escape | |
| N440K | Omicron | increases binding between the RBD and ACE2 | |
| L452R | Delta | increases affinity between the RBD and ACE2, promotes immune escape | |
| T478K | Delta, Omicron | enhances the ACE2 interaction, increases immune escape | |
| E484A/K | Beta, Gamma, Omicron | E484K enhances binding between the RBD and ACE2, significantly increases immune escape E484A decreases affinity between the RBD and ACE2, stimulates severe immune escape | |
| Q493R | Omicron | contributes to immune escape | |
| N501Y | Alpha, Beta, Gamma, Omicron | increases transmission by enhancing interaction between S protein and ACE2 | |
| S1/S2 cleavage site | D614G | Alpha, Beta, Gamma, Delta, Omicron | increases transmission |
| P681H/R | Delta, Omicron | facilitates S cleavage, endows moderate immune escape ability |
| Target | Epitope | NAbs | Origin | SARS-CoV-2 VOCs and Omicron Sub-Lineages | Emergency Use Authorization |
|---|---|---|---|---|---|
| RBD | Class 1 | S2E12 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2 | |
| A23-58.1 | Human | Alpha, Beta, Gamma, Delta, BA.1 | |||
| B1-182.1 | Human | Alpha, Beta, Gamma, Delta, BA.1 | |||
| Cv2.1169 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2 | |||
| 87G7 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2 | |||
| 17T2 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.4/5, BQ.1.1, XBB.1.5, XBB.1.16, BA.2.86 | |||
| CB6 | Human | Alpha, Delta | Etesevimab * | ||
| REGN10933 | Human | Alpha, Delta | Casirivimab * | ||
| AZD8895 | Human | Alpha, Beta, Gamma, Delta, BA.1 | Tixagevimab * | ||
| S2K146 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.1.1, BA.2, BA.3, BA.2.12.1, BA.4/5, BQ.1, BQ.1.1 | |||
| GAR05 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.5 | |||
| P2C-1F11 | Human | Alpha, Beta, Gamma, Delta, BA.2, BA.2.75, BA.4/5, BF.7 | |||
| CT-P59 | Human | Alpha, Beta, Gamma, Delta, Omicron | Regdanvimab | ||
| P4J15 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.4/5, BA.2.75.2, BQ.1, BQ.1.1, XBB.1, XBB.1.5, CH.1.1, XBB.1.16, XBB.1.16.1, XBB.2.3, EG.1, EG.5.1 | |||
| P2-1B1 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.2.12.1, BA.2.75, BA.3, BA.4/5 | |||
| P5-1C8 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.2.12.1, BA.2.75, BA.3, BA.4/5 | |||
| P5S-2B10 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.2.12.1, BA.2.75, BA.3, BA.4/5 | |||
| P5S-2B6 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.2.12.1, BA.2.75, BA.3, BA.4/5 | |||
| P5-1H1 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.2.12.1, BA.2.75, BA.3, BA.4/5 | |||
| 10-5B | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.2.12.1, BA.3 | |||
| KXD01 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.3, BA.4/5, BA.2.75, BF.7, BQ.1, XBB, XBB.1, XBB.1.5, XBB.1.16, EG.5, EG.5.1, FL.1.5, FL.1.5.1 | |||
| KXD02 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.3, BA.4/5, BA.2.75, BF.7, BQ.1, XBB, XBB.1, XBB.1.5, XBB.1.16, EG.5, EG.5.1, FL.1.5, FL.1.5.1 | |||
| KXD03 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.3, BA.4/5, BA.2.75, BF.7, BQ.1, XBB, XBB.1, XBB.1.5, XBB.1.16, EG.5, EG.5.1, FL.1.5, FL.1.5.1, HK.3 | |||
| KXD04 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.3, BA.4/5, BF.7, BQ.1, XBB.1.5, XBB.1.16 | |||
| KXD05 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.3, BA.4/5, BA.2.75, BF.7, BQ.1, XBB.1.5 | |||
| KXD06 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.3, BA.4/5, BA.2.75, BF.7, BQ.1, XBB.1.5, XBB.1.16 | |||
| Class 2 | LY-CoV555 | Human | Alpha | Bamlanivimab * | |
| P2B-2F6 | Human | Alpha | |||
| MD65 | Human | Alpha, Beta | |||
| P5S-2A9 | Human | Alpha, Beta, Gamma, Delta, BA.2, BA.2.12.1, BA.2.75, BA.3, and BA.4/5 | |||
| Class 3 | S309 | Human | Alpha, Beta, Gamma, Delta, BA.1.1, BA.2, BA.2.12.1, BA.2.75, BA.4/5, XBB | Sotrovimab * | |
| SP1-77 | Humanised Mouse | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.3, BA.4/5, and BA.2.12.1 | |||
| SW186 | Mouse | Alpha, Beta, Gamma, Delta, BA.1 | |||
| LY-CoV1404 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.2.12.1, BA.2.75, BA.4/5, BF.7 | Bebtelovimab * | ||
| AZD1061 | Human | Alpha, Beta, Gamma, Delta | Cilgavimab * | ||
| S2X324 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.3, BA.4, BA.5, BA.2.12.1, BA.2.75 | |||
| P2S-2E9 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.2.12.1, BA.2.75, BA.3, BA.4/5 | |||
| REGN10987 | Human | Alpha, Beta, Gamma, Delta | Imdevimab * | ||
| 1G11 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.1.1, BA.2, BA.2.12.1, BA.4/5, BF.7 | |||
| 002-S21F2 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.2.12.1, BA.4/5 | |||
| 6-2C | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2.12.1, BA.2.75, BA.3, BA.4/5, BA.4.6, BF.7 | |||
| 3-2A2-4 | Alpaca | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.4/5 | |||
| Class 4 | COVA1-16 | Human | Alpha, Beta, Gamma, Delta | ||
| ADG-20 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.1.1 | |||
| S2X259 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.1.1, BA.3 | |||
| DH1047 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.1.1 | |||
| 10-40 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.1.1, BA.2, BA.2.12.1, and BA.4/5 | |||
| Nb70 | Alpaca | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.4/5 | |||
| 1-2C7 | Alpaca | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.4/5 | |||
| Class 5 | S2H97 | Human | Alpha, Beta, Gamma, Delta, BA.1 | ||
| XMA09 | Human | Alpha, Beta, Gamma, Delta, Omicron | |||
| WRAIR-2057 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BQ.1.1, XBB.1.5 | |||
| WRAIR-2063 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BQ.1.1, XBB.1.5 | |||
| FD20 | Human | Alpha, Beta, Gamma, Delta | |||
| 7D6 | Mouse | Alpha, Beta, Gamma | |||
| 6D6 | Mouse | Alpha, Beta, Gamma | |||
| S2 | stem helix | 1249A8 | Human | Alpha, Beta, Gamma, Delta, Omicron | |
| CV3-25 | Human | Alpha, Beta, Gamma, Delta, Omicron | |||
| CC40.8 | Human | Alpha, Beta, Gamma, Delta | |||
| S2P6 | Human | Alpha, Beta, Gamma, Delta | |||
| WS6 | Mouse | Alpha, Beta, Gamma, Delta, Omicron | |||
| S2-4D | Mouse | Alpha, Beta, Gamma, Delta, Omicron | |||
| S2-5D | Mouse | Alpha, Beta, Gamma, Delta, Omicron | |||
| S2-8D | Mouse | Alpha, Beta, Gamma, Delta, Omicron | |||
| S2-4A | Mouse | Alpha, Beta, Gamma, Delta, Omicron | |||
| S2A9 | Shark | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.4/5 | |||
| fusion peptide | 76E1 | Human | Alpha, Beta, Gamma, Delta, Omicron | ||
| VN01H1 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2 | |||
| C77G12 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2 | |||
| COV44-62 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.4/5 | |||
| COV44-79 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.2, BA.4/5 | |||
| heptad repeat | hMab5.17 | Humanised from Immunised Mouse | Alpha, Beta, Gamma, Delta | ||
| NTD | 4-18 | Human | Alpha, Beta, Gamma, Delta | ||
| 5-7 | Human | Alpha, Beta, Gamma, Delta, BA.1, BA.1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, L.; Li, T.; Xue, W.; Zhang, S.; Wang, H.; Liu, H.; Gu, Y.; Xia, N.; Li, S. Comprehensive Overview of Broadly Neutralizing Antibodies against SARS-CoV-2 Variants. Viruses 2024, 16, 900. https://doi.org/10.3390/v16060900
Cui L, Li T, Xue W, Zhang S, Wang H, Liu H, Gu Y, Xia N, Li S. Comprehensive Overview of Broadly Neutralizing Antibodies against SARS-CoV-2 Variants. Viruses. 2024; 16(6):900. https://doi.org/10.3390/v16060900
Chicago/Turabian StyleCui, Lingyan, Tingting Li, Wenhui Xue, Sibo Zhang, Hong Wang, Hongjing Liu, Ying Gu, Ningshao Xia, and Shaowei Li. 2024. "Comprehensive Overview of Broadly Neutralizing Antibodies against SARS-CoV-2 Variants" Viruses 16, no. 6: 900. https://doi.org/10.3390/v16060900
APA StyleCui, L., Li, T., Xue, W., Zhang, S., Wang, H., Liu, H., Gu, Y., Xia, N., & Li, S. (2024). Comprehensive Overview of Broadly Neutralizing Antibodies against SARS-CoV-2 Variants. Viruses, 16(6), 900. https://doi.org/10.3390/v16060900






