Transcriptome Analysis in Air–Liquid Interface Porcine Respiratory Epithelial Cell Cultures Reveals That the Betacoronavirus Porcine Encephalomyelitis Hemagglutinating Virus Induces a Robust Interferon Response to Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Culturing ALI-PRECs
2.2. PHEV In Vitro Culture and Propagation
2.3. PHEV Infection in ALI-PRECs
2.4. PHEV RT-qPCR
2.5. Total RNA Isolation, Reverse Transcription, and Transcriptional Analysis
2.6. Differential Gene Expression Analysis
2.7. Gene Ontology and Network Analysis
2.8. Pathway Analysis
3. Results
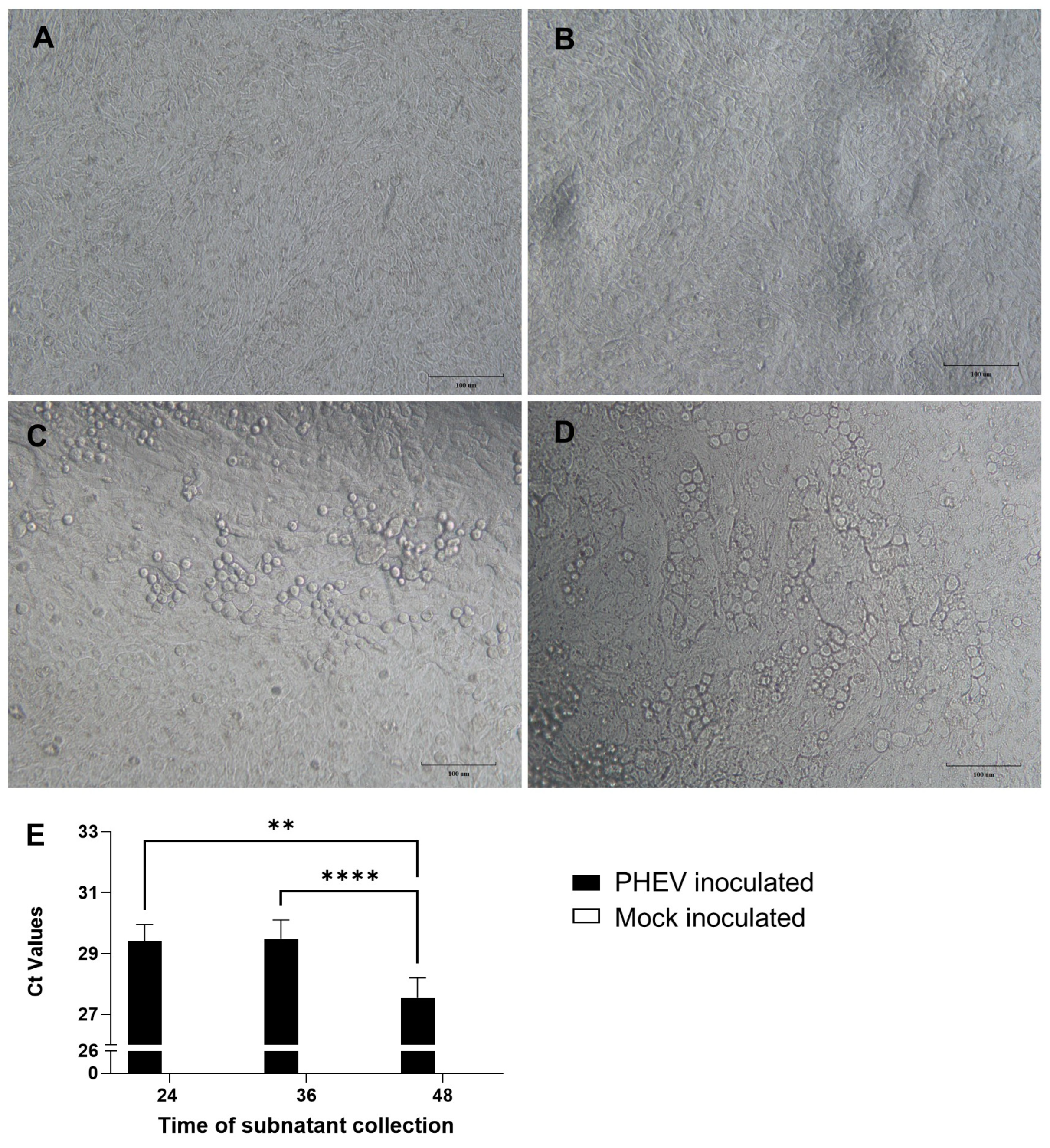
3.1. PHEV Replicates in ALI-PREC Cultures
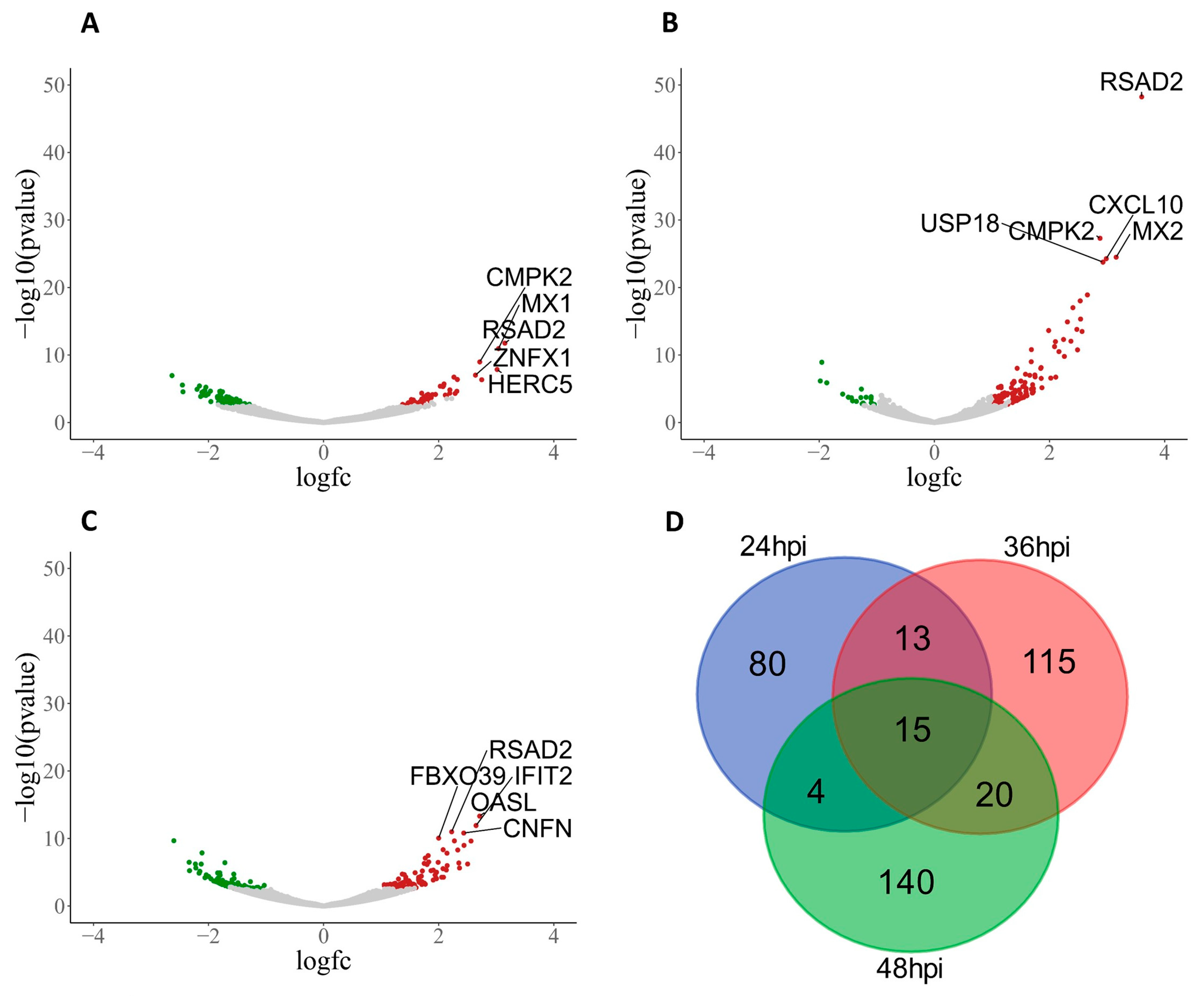
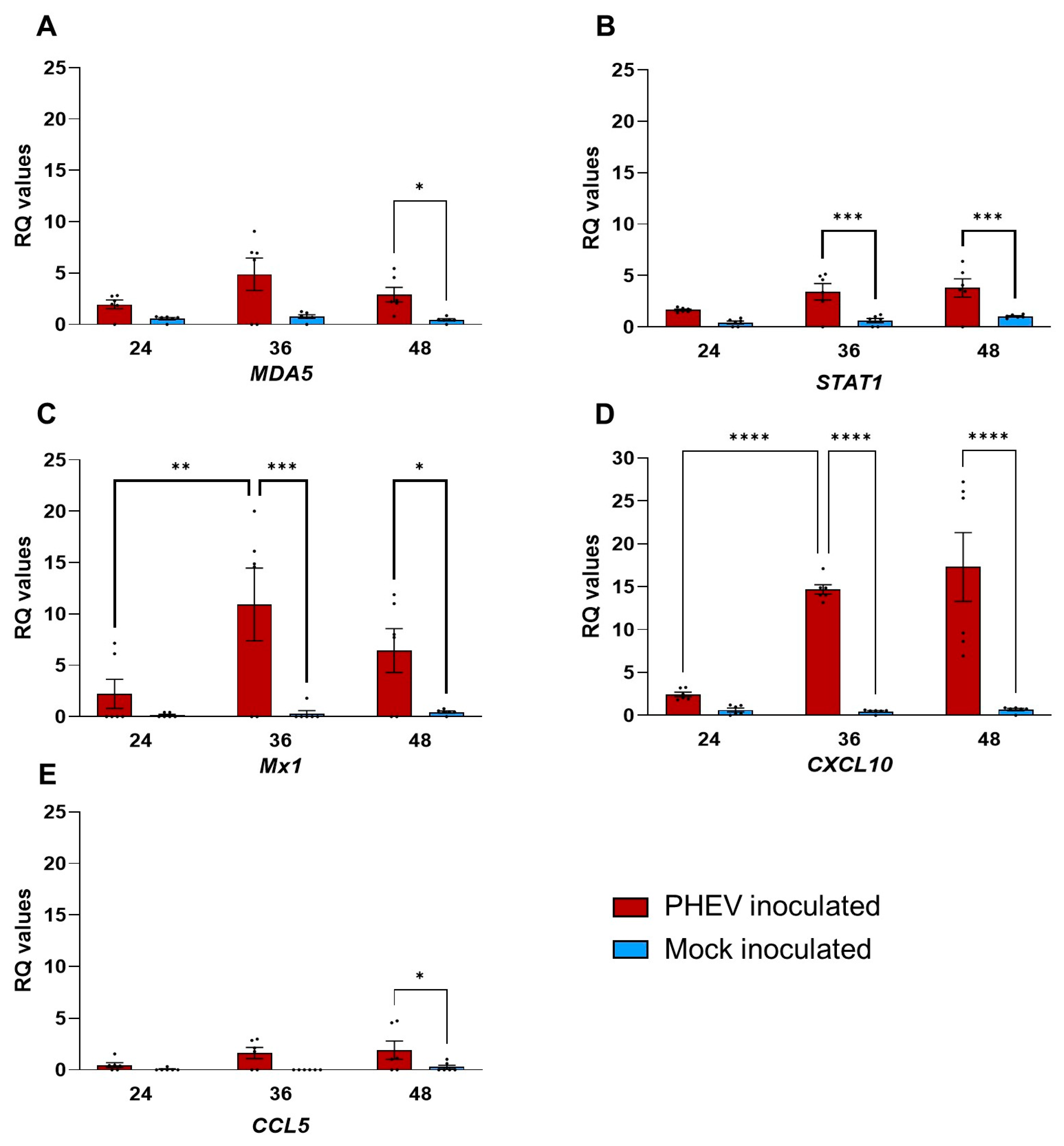
3.2. PHEV-Induced Robust Transcriptional Regulation in ALI-PRECs
3.3. Network Visualization and Analysis of DEGs in ALI-PRECs following PHEV Infection
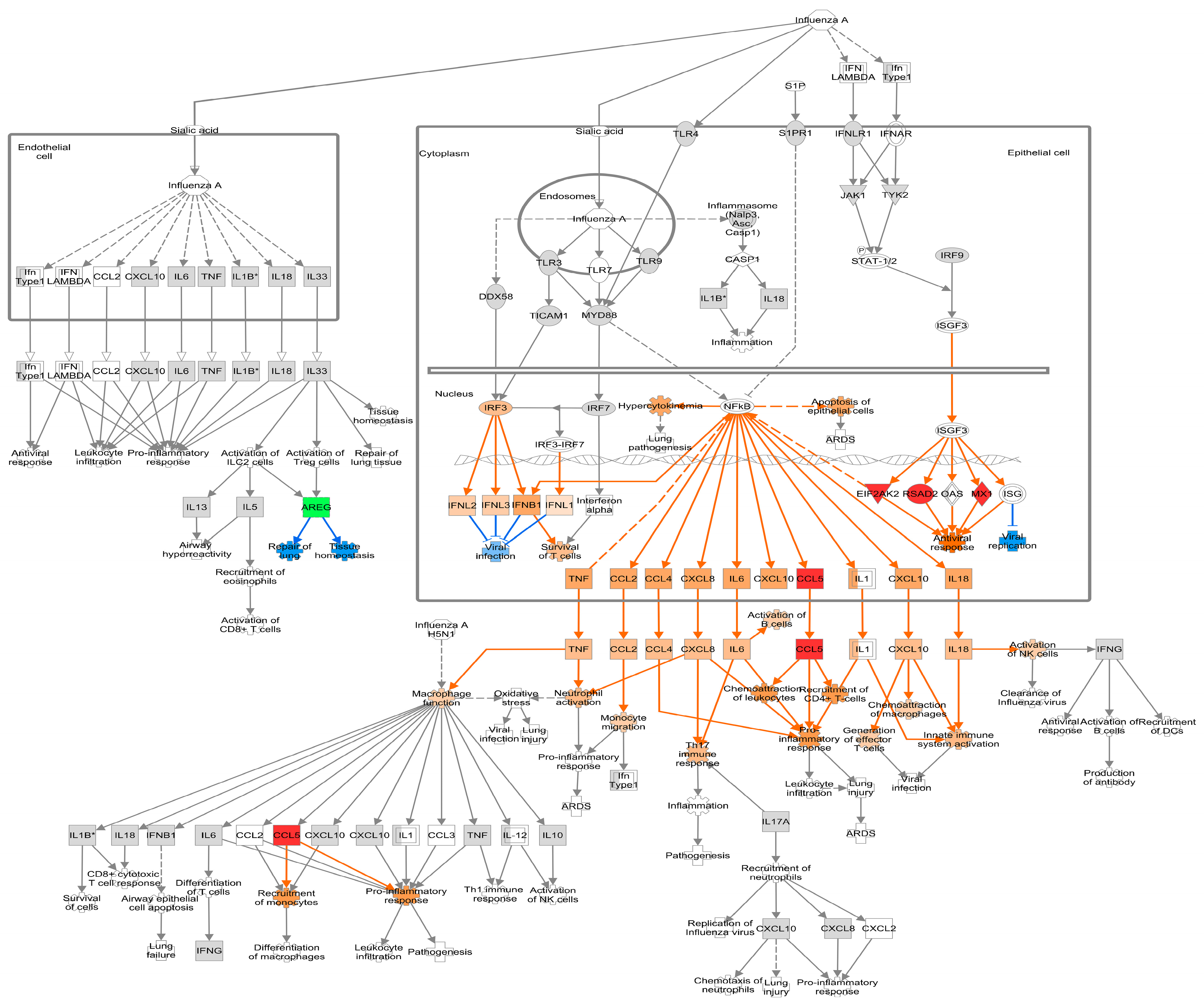
3.4. Pathway Analysis of DEGs Regulated by PHEV in ALI-PRECs
3.4.1. Early Infection
3.4.2. Mid Infection
3.4.3. Late Infection
4. Discussion
4.1. Pattern Recognition Receptors on ALI-PRECs Possibly Initiates a Robust Antiviral Response during PHEV Infection
4.2. Interferon Response in ALI-PRECs and the Role of RSAD2 following PHEV Infection
4.3. Chemokine Response
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mengeling, W.L.; Cutlip, R.C. Experimentally Induced Infection of Newborn Pigs with Hemagglutinating Encephalomyelitis Virus Strain 67N. Am. J. Vet. Res. 1972, 33, 953–956. [Google Scholar]
- Cartwright, S.F.; Lucas, M.; Cavill, J.P.; Gush, A.F.; Blandford, T.B. Vomiting and Wasting Disease of Piglets. Vet. Rec. 1969, 84, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Pensaert, M.B.; Callebaut, P.E. Characteristics of a Coronavirus Causing Vomition and Wasting in Pigs. Arch. Gesamte Virusforsch. 1974, 44, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Dempsey, D.M.; Dutilh, B.E.; Harrach, B.; Harrison, R.L.; Hendrickson, R.C.; Junglen, S.; et al. Changes to Virus Taxonomy and the International Code of Virus Classification and Nomenclature Ratified by the International Committee on Taxonomy of Viruses (2019). Arch. Virol. 2019, 164, 2417–2429. [Google Scholar] [CrossRef] [PubMed]
- Roe, C.K.; Alexander, T.J. A Disease of Nursing Pigs Previously Unreported in Ontario. Can. J. Comp. Med. Vet. Sci. 1958, 22, 305–307. [Google Scholar] [PubMed]
- Greig, A.S.; Mitchell, D.; Corner, A.H.; Bannister, G.L.; Meads, E.B.; Julian, R.J. A Hemagglutinating Virus Producing Encephalomyelitis in Baby Pigs. Can. J. Comp. Med. Vet. Sci. 1962, 26, 49. [Google Scholar] [PubMed]
- Quiroga, M.A.; Cappuccio, J.; Piñeyro, P.; Basso, W.; Moré, G.; Kienast, M.; Schonfeld, S.; Cáncer, J.L.; Arauz, S.; Pintos, M.E.; et al. Hemagglutinating Encephalomyelitis Coronavirus Infection in Pigs, Argentina. Emerg. Infect. Dis. 2008, 14, 484–486. [Google Scholar] [CrossRef] [PubMed]
- Hirano, N.; Nomura, R.; Tawara, T.; Tohyama, K. Neurotropism of Swine Haemagglutinating Encephalomyelitis Virus (Coronavirus) in Mice Depending upon Host Age and Route of Infection. J. Comp. Pathol. 2004, 130, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Yagami, K.; Izumi, Y.; Kajiwara, N.; Sugiyama, F.; Sugiyama, Y. Neurotropism of Mouse-Adapted Haemagglutinating Encephalomyelitis Virus. J. Comp. Pathol. 1993, 109, 21–27. [Google Scholar] [CrossRef]
- Appel, M.; Greig, A.S.; Corner, A.H. Encephalomyelitis of Swine Caused by a Haemagglutinating Virus. IV. Transmission Studies. Res. Vet. Sci. 1965, 6, 482–489. [Google Scholar] [CrossRef]
- Mora-Díaz, J.C.; Temeeyasen, G.; Magtoto, R.; Rauh, R.; Nelson, W.; Carrillo-Ávila, J.A.; Zimmerman, J.; Piñeyro, P.; Giménez-Lirola, L. Infection and Immune Response to Porcine Hemagglutinating Encephalomyelitis Virus in Grower Pigs. Vet. Microbiol. 2021, 253, 108958. [Google Scholar] [CrossRef] [PubMed]
- Meyvisch, C.; Hoorens, J. An Electron Microscopic Study of Experimentally-Induced HEV Encephalitis. Vet. Pathol. 1978, 15, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Mengeling, W.L.; Boothe, A.D.; Ritchie, A.E. Characteristics of a Coronavirus (Strain 67N) of Pigs. Am. J. Vet. Res. 1972, 33, 297–308. [Google Scholar] [PubMed]
- Mora-Díaz, J.C.; Piñeyro, P.E.; Rauh, R.; Nelson, W.; Sankoh, Z.; Gregg, E.; Carrillo-Ávila, J.A.; Shen, H.; Nelli, R.K.; Zimmerman, J.J.; et al. Porcine Hemagglutinating Encephalomyelitis Virus Infection In Vivo and Ex Vivo. J. Virol. 2021, 95, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, L.; Liu, B.; Feng, Y.; Zhou, H.; Zhang, Z.; Wu, Y.; Wang, J.; Gan, Y.; Yuan, T.; et al. Establishment and Comparison of Air-Liquid Interface Culture Systems for Primary and Immortalized Swine Tracheal Epithelial Cells. BMC Cell Biol. 2018, 19, 10. [Google Scholar] [CrossRef]
- Wu, N.H.; Yang, W.; Beineke, A.; Dijkman, R.; Matrosovich, M.; Baumgärtner, W.; Thiel, V.; Valentin-Weigand, P.; Meng, F.; Herrler, G. The Differentiated Airway Epithelium Infected by Influenza Viruses Maintains the Barrier Function despite a Dramatic Loss of Ciliated Cells. Sci. Rep. 2016, 6, 39668. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Wang, Y.; Yuan, W.; Wong, L.B. Ciliogenesis in Cryopreserved Mammalian Tracheal Epithelial Cells Cultured at the Air-Liquid Interface. Cryobiology 2009, 59, 250. [Google Scholar] [CrossRef]
- Hewitt, R.J.; Lloyd, C.M. Regulation of Immune Responses by the Airway Epithelial Cell Landscape. Nat. Rev. Immunol. 2021, 21, 347–362. [Google Scholar] [CrossRef]
- Holtzman, M.J.; Byers, D.E.; Alexander-Brett, J.; Wang, X. The Role of Airway Epithelial Cells and Innate Immune Cells in Chronic Respiratory Disease. Nat. Rev. Immunol. 2014, 14, 686–698. [Google Scholar] [CrossRef]
- Nelli, R.K.; Mora-Díaz, J.C.; Giménez-Lirola, L.G. The Betacoronavirus PHEV Replicates and Disrupts the Respiratory Epithelia and Upregulates Key Pattern Recognition Receptor Genes and Downstream Mediators, Including IL-8 and IFN-λ. mSphere 2021, 6, e00820-2. [Google Scholar] [CrossRef]
- Taub, D.D.; Anver, M.; Oppenheim, J.J.; Longo, D.L.; Murphy, W.J. T Lymphocyte Recruitment by Interleukin-8 (IL-8). IL-8-Induced Degranulation of Neutrophils Releases Potent Chemoattractants for Human T Lymphocytes Both In Vitro and In Vivo. J. Clin. Investig. 1996, 97, 1931–1941. [Google Scholar] [CrossRef] [PubMed]
- Pace, E.; Gjomarkaj, M.; Melis, M.; Profita, M.; Spatafora, M.; Vignola, A.M.; Bonsignore, G.; Mody, C.H. Interleukin-8 Induces Lymphocyte Chemotaxis into the Pleural Space. Am. J. Respir. Crit. Care Med. 2012, 159, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. QBase Relative Quantification Framework and Software for Management and Automated Analysis of Real-Time Quantitative PCR Data. Genome Biol. 2008, 8, R19. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-Based Genome Alignment and Genotyping with HISAT2 and HISAT-Genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A Fast Spliced Aligner with Low Memory Requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape Plug-in to Decipher Functionally Grouped Gene Ontology and Pathway Annotation Networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Chen, Y.G.; Hur, S. Cellular Origins of DsRNA, Their Recognition and Consequences. Nat. Rev. Mol. Cell Biol. 2021, 23, 286–301. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, S.; Jia, X.; Ge, Y.; Ling, T.; Nie, M.; Lan, X.; Chen, S.; Xu, A. Mitochondria-Localised ZNFX1 Functions as a DsRNA Sensor to Initiate Antiviral Responses through MAVS. Nat. Cell Biol. 2019, 21, 1346–1356. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Miao, Y.; Qian, L.; Zhang, Y.; Liu, C.; Liu, J.; Zuo, Y.; Feng, Q.; Guo, T.; Zhang, L.; et al. Targeting UBE4A Revives Viperin Protein in Epithelium to Enhance Host Antiviral Defense. Mol. Cell 2020, 77, 734–747.e7. [Google Scholar] [CrossRef] [PubMed]
- Bick, M.J.; Carroll, J.-W.N.; Gao, G.; Goff, S.P.; Rice, C.M.; MacDonald, M.R. Expression of the Zinc-Finger Antiviral Protein Inhibits Alphavirus Replication. J. Virol. 2003, 77, 11555. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, S.; Pontier, D.; Etienne, L. Rapid Evolution of HERC6 and Duplication of a Chimeric HERC5/6 Gene in Rodents and Bats Suggest an Overlooked Role of HERCs in Mammalian Immunity. Front. Immunol. 2020, 11, 3232. [Google Scholar] [CrossRef]
- Dastur, A.; Beaudenon, S.; Kelley, M.; Krug, R.M.; Huibregtse, J.M. Herc5, an Interferon-Induced HECT E3 Enzyme, Is Required for Conjugation of ISG15 in Human Cells. J. Biol. Chem. 2006, 281, 4334–4338. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, M.; Manet, C.; Montagutelli, X. Host Genetic Susceptibility to Viral Infections: The Role of Type I Interferon Induction. Genes. Immun. 2020, 21, 365. [Google Scholar] [CrossRef] [PubMed]
- Charman, M.; McFarlane, S.; Wojtus, J.K.; Sloan, E.; Dewar, R.; Leeming, G.; Al-Saadi, M.; Hunter, L.; Carroll, M.W.; Stewart, J.P.; et al. Constitutive TRIM22 Expression in the Respiratory Tract Confers a Pre-Existing Defence Against Influenza A Virus Infection. Front. Cell. Infect. Microbiol. 2021, 11, 689707. [Google Scholar] [CrossRef]
- Sasidharan Nair, V.; Toor, S.M.; Ali, B.R.; Elkord, E. Dual Inhibition of STAT1 and STAT3 Activation Downregulates Expression of PD-L1 in Human Breast Cancer Cells. Expert Opin. Ther. Targets 2018, 22, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Gailey, C.D.; Wang, E.J.; Brautigan, D.L. Ciliogenesis Associated Kinase 1 (CILK1): Targets and Functions in Various Organ Systems. FEBS Lett. 2019, 593, 2990. [Google Scholar] [CrossRef]
- Schultz, R.; Elenius, V.; Lukkarinen, H.; Saarela, T. Two Novel Mutations in the DNAH11 Gene in Primary Ciliary Dyskinesia (CILD7) with Considerable Variety in the Clinical and Beating Cilia Phenotype. BMC Med. Genet. 2020, 21, 237. [Google Scholar] [CrossRef]
- Hoh, R. The Ciliated Cell Transcriptome; Stanford University: Stanford, CA, USA, 2010. [Google Scholar]
- Fiedler, S.E.; Dudiki, T.; Vijayaraghavan, S.; Carr, D.W. Loss of R2D2 Proteins ROPN1 and ROPN1L Causes Defects in Murine Sperm Motility, Phosphorylation, and Fibrous Sheath Integrity. Biol. Reprod. 2013, 88, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Watt, S.M.; Bühring, H.-J.; Rappold, I.; Chan, J.Y.-H.; Lee-Prudhoe, J.; Jones, T.; Zannettino, A.C.W.; Simmons, P.J.; Doyonnas, R.; Sheer, D.; et al. CD164, a Novel Sialomucin on CD34+ and Erythroid Subsets, Is Located on Human Chromosome 6q21. Blood 1998, 92, 849–866. [Google Scholar] [CrossRef]
- Liu, J.; Knopp, K.A.; Rackaityte, E.; Wang, C.Y.; Laurie, M.T.; Sunshine, S.; Puschnik, A.S.; DeRisi, J.L. Genome-Wide Knockout Screen Identifies Human Sialomucin CD164 as an Essential Entry Factor for Lymphocytic Choriomeningitis Virus. mBio 2022, 13, e00205-22. [Google Scholar] [CrossRef] [PubMed]
- Züst, R.; Cervantes-Barragan, L.; Habjan, M.; Maier, R.; Neuman, B.W.; Ziebuhr, J.; Szretter, K.J.; Baker, S.C.; Barchet, W.; Diamond, M.S.; et al. Ribose 2′-O-Methylation Provides a Molecular Signature for the Distinction of Self and Non-Self MRNA Dependent on the RNA Sensor Mda5. Nat. Immunol. 2011, 12, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fang, P.; Ren, J.; Xia, S.; Zhang, H.; Zhu, X.; Ding, T.; Xiao, S.; Fang, L. Porcine Epidemic Diarrhea Virus Nsp7 Inhibits MDA5 Dephosphorylation to Antagonize Type I Interferon Production. Microbiol. Spectr. 2023, 11, e05017-22. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Fang, L.; Ren, J.; Hong, Y.; Liu, X.; Zhao, Y.; Wang, D.; Peng, G.; Xiao, S. Porcine Deltacoronavirus Accessory Protein NS6 Antagonizes Interferon Beta Production by Interfering with the Binding of RIG-I/MDA5 to Double-Stranded RNA. J. Virol. 2018, 92, 15. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.M.; Santa Maria, F.G.; Lahiri, T.; Friedman, E.; Marié, I.J.; Levy, D.E. PKR Transduces MDA5-Dependent Signals for Type I IFN Induction. PLoS Pathog. 2016, 12, e1005489. [Google Scholar] [CrossRef] [PubMed]
- Gal-Ben-Ari, S.; Barrera, I.; Ehrlich, M.; Rosenblum, K. PKR: A Kinase to Remember. Front. Mol. Neurosci. 2019, 11, 480. [Google Scholar] [CrossRef] [PubMed]
- Sadler, A.J.; Latchoumanin, O.; Hawkes, D.; Mak, J.; Williams, B.R.G. An Antiviral Response Directed by PKR Phosphorylation of the RNA Helicase A. PLoS Pathog. 2009, 5, e1000311. [Google Scholar] [CrossRef]
- García, M.A.; Gil, J.; Ventoso, I.; Guerra, S.; Domingo, E.; Rivas, C.; Esteban, M. Impact of Protein Kinase PKR in Cell Biology: From Antiviral to Antiproliferative Action. Microbiol. Mol. Biol. Rev. 2006, 70, 1032. [Google Scholar] [CrossRef]
- Xing, J.; Zhang, A.; Du, Y.; Fang, M.; Minze, L.J.; Liu, Y.J.; Li, X.C.; Zhang, Z. Identification of Poly(ADP-Ribose) Polymerase 9 (PARP9) as a Noncanonical Sensor for RNA Virus in Dendritic Cells. Nat. Commun. 2021, 12, 2681. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, D.; Roswit, W.T.; Jin, X.; Patel, A.C.; Patel, D.A.; Agapov, E.; Wang, Z.; Tidwell, R.M.; Atkinson, J.J.; et al. PARP9-DTX3L Ubiquitin Ligase Targets Host Histone H2BJ and Viral 3C Protease to Enhance Interferon Signaling and Control Viral Infection. Nat. Immunol. 2015, 16, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Feng, Y.; Sun, H.; Yang, S.; Sun, T.; Guo, X.; Ji, F.; Wu, B.; Zhou, D. Scavenger Receptor MARCO Contributes to Macrophage Phagocytosis and Clearance of Tumor Cells. Exp. Cell Res. 2021, 408, 112862. [Google Scholar] [CrossRef]
- Barkal, A.A.; Brewer, R.E.; Markovic, M.; Kowarsky, M.; Barkal, S.A.; Zaro, B.W.; Krishnan, V.; Hatakeyama, J.; Dorigo, O.; Barkal, L.J.; et al. CD24 Signalling through Macrophage Siglec-10 Is a Target for Cancer Immunotherapy. Nature 2019, 572, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.R.; Maute, R.L.; Dulken, B.W.; Hutter, G.; George, B.M.; McCracken, M.N.; Gupta, R.; Tsai, J.M.; Sinha, R.; Corey, D.; et al. PD-1 Expression by Tumour-Associated Macrophages Inhibits Phagocytosis and Tumour Immunity. Nature 2017, 545, 495–499. [Google Scholar] [CrossRef]
- Shi, B.; Chu, J.; Huang, T.; Wang, X.; Li, Q.; Gao, Q.; Xia, Q.; Luo, S. The Scavenger Receptor MARCO Expressed by Tumor-Associated Macrophages Are Highly Associated With Poor Pancreatic Cancer Prognosis. Front. Oncol. 2021, 11, 4518. [Google Scholar] [CrossRef]
- Schulz, D.; Severin, Y.; Zanotelli, V.R.T.; Bodenmiller, B. In-Depth Characterization of Monocyte-Derived Macrophages Using a Mass Cytometry-Based Phagocytosis Assay. Sci. Rep. 2019, 9, 1925. [Google Scholar] [CrossRef]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Cheng, L.; Li, J.; Wang, X.; Wang, F.; et al. Single-Cell Landscape of Bronchoalveolar Immune Cells in Patients with COVID-19. Nat. Med. 2020, 26, 842–844. [Google Scholar] [CrossRef]
- Cole, D.C.; Chung, Y.; Gagnidze, K.; Hajdarovic, K.H.; Rayon-Estrada, V.; Harjanto, D.; Bigio, B.; Gal-Toth, J.; Milner, T.A.; McEwen, B.S.; et al. Loss of APOBEC1 RNA-Editing Function in Microglia Exacerbates Age-Related CNS Pathophysiology. Proc. Natl. Acad. Sci. USA 2017, 114, 13272–13277. [Google Scholar] [CrossRef]
- Kim, K.; Calabrese, P.; Wang, S.; Qin, C.; Rao, Y.; Feng, P.; Chen, X.S. The Roles of APOBEC-Mediated RNA Editing in SARS-CoV-2 Mutations, Replication and Fitness. Sci. Rep. 2022, 12, 14972. [Google Scholar] [CrossRef]
- Mourier, T.; Sadykov, M.; Carr, M.J.; Gonzalez, G.; Hall, W.W.; Pain, A. Host-Directed Editing of the SARS-CoV-2 Genome. Biochem. Biophys. Res. Commun. 2021, 538, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Perelygin, A.A.; Zharkikh, A.A.; Scherbik, S.V.; Brinton, M.A. The Mammalian 2′-5′ Oligoadenylate Synthetase Gene Family: Evidence for Concerted Evolution of Paralogous Oas1 Genes in Rodentia and Artiodactyla. J. Mol. Evol. 2006, 63, 562–576. [Google Scholar] [CrossRef]
- Melchjorsen, J.; Kristiansen, H.; Christiansen, R.; Rintahaka, J.; Matikainen, S.; Paludan, S.R.; Hartmann, R. Differential Regulation of the OASL and OAS1 Genes in Response to Viral Infections. J. Interf. Cytokine Res. 2009, 29, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Choi, U.Y.; Kang, J.S.; Hwang, Y.S.; Kim, Y.J. Oligoadenylate Synthase-like (OASL) Proteins: Dual Functions and Associations with Diseases. Exp. Mol. Med. 2015, 47, e144. [Google Scholar] [CrossRef]
- Drappier, M.; Michiels, T. Inhibition of the OAS/RNase L Pathway by Viruses. Curr. Opin. Virol. 2015, 15, 19. [Google Scholar] [CrossRef]
- Lee, D.; Le Pen, J.; Yatim, A.; Dong, B.; Aquino, Y.; Ogishi, M.; Pescarmona, R.; Talouarn, E.; Rinchai, D.; Zhang, P.; et al. Inborn Errors of OAS-RNase L in SARS-CoV-2-Related Multisystem Inflammatory Syndrome in Children. Science (1979) 2023, 379, eabo3627. [Google Scholar] [CrossRef] [PubMed]
- Ciancanelli, M.J.; Abel, L.; Zhang, S.Y.; Casanova, J.L. Host Genetics of Severe Influenza: From Mouse Mx1 to Human IRF7. Curr. Opin. Immunol. 2016, 38, 109–120. [Google Scholar] [CrossRef]
- Andolfo, I.; Russo, R.; Lasorsa, V.A.; Cantalupo, S.; Rosato, B.E.; Bonfiglio, F.; Frisso, G.; Abete, P.; Cassese, G.M.; Servillo, G.; et al. Common Variants at 21q22.3 Locus Influence MX1 and TMPRSS2 Gene Expression and Susceptibility to Severe COVID-19. iScience 2021, 24, 102322. [Google Scholar] [CrossRef]
- Diamond, M.S.; Farzan, M. The Broad-Spectrum Antiviral Functions of IFIT and IFITM Proteins. Nat. Rev. Immunol. 2012, 13, 46–57. [Google Scholar] [CrossRef]
- Perng, Y.C.; Lenschow, D.J. ISG15 in Antiviral Immunity and Beyond. Nat. Rev. Microbiol. 2018, 16, 423–439. [Google Scholar] [CrossRef]
- Dzimianski, J.V.; Scholte, F.E.M.; Bergeron, É.; Pegan, S.D. ISG15: It’s Complicated. J. Mol. Biol. 2019, 431, 4203–4216. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, K.J.; Hahn, C.S.; Kim, K.I.; Yan, M.; Rosario, D.; Li, L.; De La Torre, J.C.; Zhang, D.E. Role of ISG15 Protease UBP43 (USP18) in Innate Immunity to Viral Infection. Nat. Med. 2004, 10, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, N.R.; Heikel, G.; Michlewski, G. TRIM25 and Its Emerging RNA-Binding Roles in Antiviral Defense. Wiley Interdiscip. Rev. RNA 2020, 11, e1588. [Google Scholar] [CrossRef]
- Hu, M.M.; Shu, H.B. Multifaceted Roles of TRIM38 in Innate Immune and Inflammatory Responses. Cell. Mol. Immunol. 2017, 14, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Errett, J.S.; Suthar, M.S.; McMillan, A.; Diamond, M.S.; Gale, M. The Essential, Nonredundant Roles of RIG-I and MDA5 in Detecting and Controlling West Nile Virus Infection. J. Virol. 2013, 87, 11416–11425. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Serrano, E.E.; Gizzi, A.S.; Arnold, J.J.; Grove, T.L.; Almo, S.C.; Cameron, C.E. Viperin Reveals Its True Function. Annu. Rev. Virol. 2020, 7, 421–446. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Lv, J.; Wang, W.; Guo, R.; Zhong, C.; Antia, A.; Zeng, Q.; Li, J.; Liu, Q.; Zhou, J.; et al. CMPK2 Is a Host Restriction Factor That Inhibits Infection of Multiple Coronaviruses in a Cell-Intrinsic Manner. PLoS Biol. 2023, 21, e3002039. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Yaneva, R.; Cresswell, P. Viperin: A Multifunctional, Interferon-Inducible Protein That Regulates Virus Replication. Cell Host Microbe 2011, 10, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Heydari, H.; Golmohammadi, R.; Mirnejad, R.; Tebyanian, H.; Fasihi-Ramandi, M.; Moosazadeh Moghaddam, M. Antiviral Peptides against Coronaviridae Family: A Review. Peptides 2021, 139, 170526. [Google Scholar] [CrossRef]
- Wu, J.; Chi, H.; Fu, Y.; Cao, A.; Shi, J.; Zhu, M.; Zhang, L.; Hua, D.; Huang, J. The Antiviral Protein Viperin Interacts with the Viral N Protein to Inhibit Proliferation of Porcine Epidemic Diarrhea Virus. Arch. Virol. 2020, 165, 2279. [Google Scholar] [CrossRef]
- Sun, M.; Yu, Z.; Luo, M.; Li, B.; Pan, Z.; Ma, J.; Yao, H. Screening Host Antiviral Proteins under the Enhanced Immune Responses Induced by a Variant Strain of Porcine Epidemic Diarrhea Virus. Microbiol. Spectr. 2022, 10, e0066122. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Huang, X.; Shan, Y.; Xu, J.; Gao, Q.; Xu, X.; Zhang, C.; Shi, F.; Yue, M.; He, F.; et al. Transcriptome Analysis Revealed Inhibition of Lipid Metabolism in 2-D Porcine Enteroids by Infection with Porcine Epidemic Diarrhea Virus. Vet. Microbiol. 2022, 273, 109525. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Liu, X.; Hu, D.; Luo, Y.; Zhang, G.; Liu, P. Swine Enteric Coronaviruses (PEDV, TGEV, and PDCoV) Induce Divergent Interferon-Stimulated Gene Responses and Antigen Presentation in Porcine Intestinal Enteroids. Front. Immunol. 2022, 12, 5946. [Google Scholar] [CrossRef]
- Erez-Garcia, F.; Martin-Vicente, M.; Rojas-Garcia, R.L.; Castilla-Garcia, L.; Munoz-Gomez, M.J.; Hervas Fernandez, I.; Gonzalez Ventosa, V.; Vidal-Alcantara, E.J.; Cuadros-Gonzalez, J.; Bermejo-Martin, J.F.; et al. High SARS-CoV-2 Viral Load and Low CCL5 Expression Levels in the Upper Respiratory Tract Are Associated With COVID-19 Severity. J. Infect. Dis. 2022, 225, 977. [Google Scholar] [CrossRef] [PubMed]
- Tyner, J.W.; Uchida, O.; Kajiwara, N.; Kim, E.Y.; Patel, A.C.; O’Sullivan, M.P.; Walter, M.J.; Schwendener, R.A.; Cook, D.N.; Danoff, T.M.; et al. CCL5-CCR5 Interaction Provides Antiapoptotic Signals for Macrophage Survival during Viral Infection. Nat. Med. 2005, 11, 1180. [Google Scholar] [CrossRef]
- Culley, F.J.; Pennycook, A.M.J.; Tregoning, J.S.; Dodd, J.S.; Walzl, G.; Wells, T.N.; Hussell, T.; Openshaw, P.J.M. Role of CCL5 (RANTES) in Viral Lung Disease. J. Virol. 2006, 80, 8151–8157. [Google Scholar] [CrossRef] [PubMed]
- Elemam, N.M.; Talaat, I.M.; Maghazachi, A.A. CXCL10 Chemokine: A Critical Player in RNA and DNA Viral Infections. Viruses 2022, 14, 2445. [Google Scholar] [CrossRef]
- Mahalingam, S.; Farber, J.M.; Karupiah, G. The Interferon-Inducible Chemokines MuMig and Crg-2 Exhibit Antiviral Activity In Vivo. J. Virol. 1999, 73, 1479–1491. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davila, K.M.S.; Nelli, R.K.; Mora-Díaz, J.C.; Sang, Y.; Miller, L.C.; Giménez-Lirola, L.G. Transcriptome Analysis in Air–Liquid Interface Porcine Respiratory Epithelial Cell Cultures Reveals That the Betacoronavirus Porcine Encephalomyelitis Hemagglutinating Virus Induces a Robust Interferon Response to Infection. Viruses 2024, 16, 939. https://doi.org/10.3390/v16060939
Davila KMS, Nelli RK, Mora-Díaz JC, Sang Y, Miller LC, Giménez-Lirola LG. Transcriptome Analysis in Air–Liquid Interface Porcine Respiratory Epithelial Cell Cultures Reveals That the Betacoronavirus Porcine Encephalomyelitis Hemagglutinating Virus Induces a Robust Interferon Response to Infection. Viruses. 2024; 16(6):939. https://doi.org/10.3390/v16060939
Chicago/Turabian StyleDavila, Kaitlyn M. Sarlo, Rahul K. Nelli, Juan C. Mora-Díaz, Yongming Sang, Laura C. Miller, and Luis G. Giménez-Lirola. 2024. "Transcriptome Analysis in Air–Liquid Interface Porcine Respiratory Epithelial Cell Cultures Reveals That the Betacoronavirus Porcine Encephalomyelitis Hemagglutinating Virus Induces a Robust Interferon Response to Infection" Viruses 16, no. 6: 939. https://doi.org/10.3390/v16060939
APA StyleDavila, K. M. S., Nelli, R. K., Mora-Díaz, J. C., Sang, Y., Miller, L. C., & Giménez-Lirola, L. G. (2024). Transcriptome Analysis in Air–Liquid Interface Porcine Respiratory Epithelial Cell Cultures Reveals That the Betacoronavirus Porcine Encephalomyelitis Hemagglutinating Virus Induces a Robust Interferon Response to Infection. Viruses, 16(6), 939. https://doi.org/10.3390/v16060939







