Development of a Ferritin Protein Nanoparticle Vaccine with PRRSV GP5 Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethics Statement
2.2. Virus, Cells, and Reagents
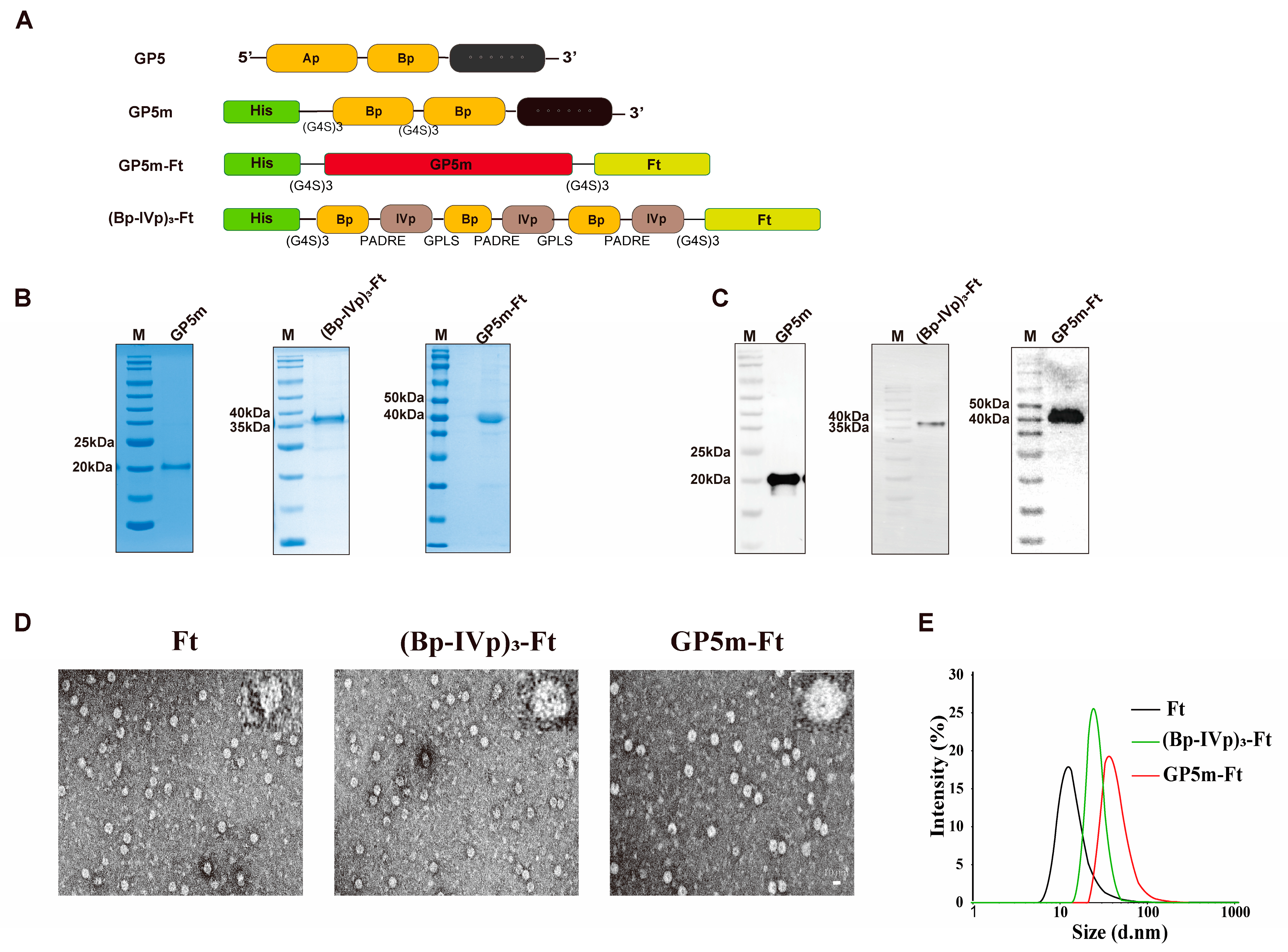
2.3. Expression and Purification of Recombinant Proteins
2.4. Detection of Purified Protein Using Western Blotting
2.5. Dynamic Light Scattering (DLS)
2.6. Transmission Electron Microscope (TEM)
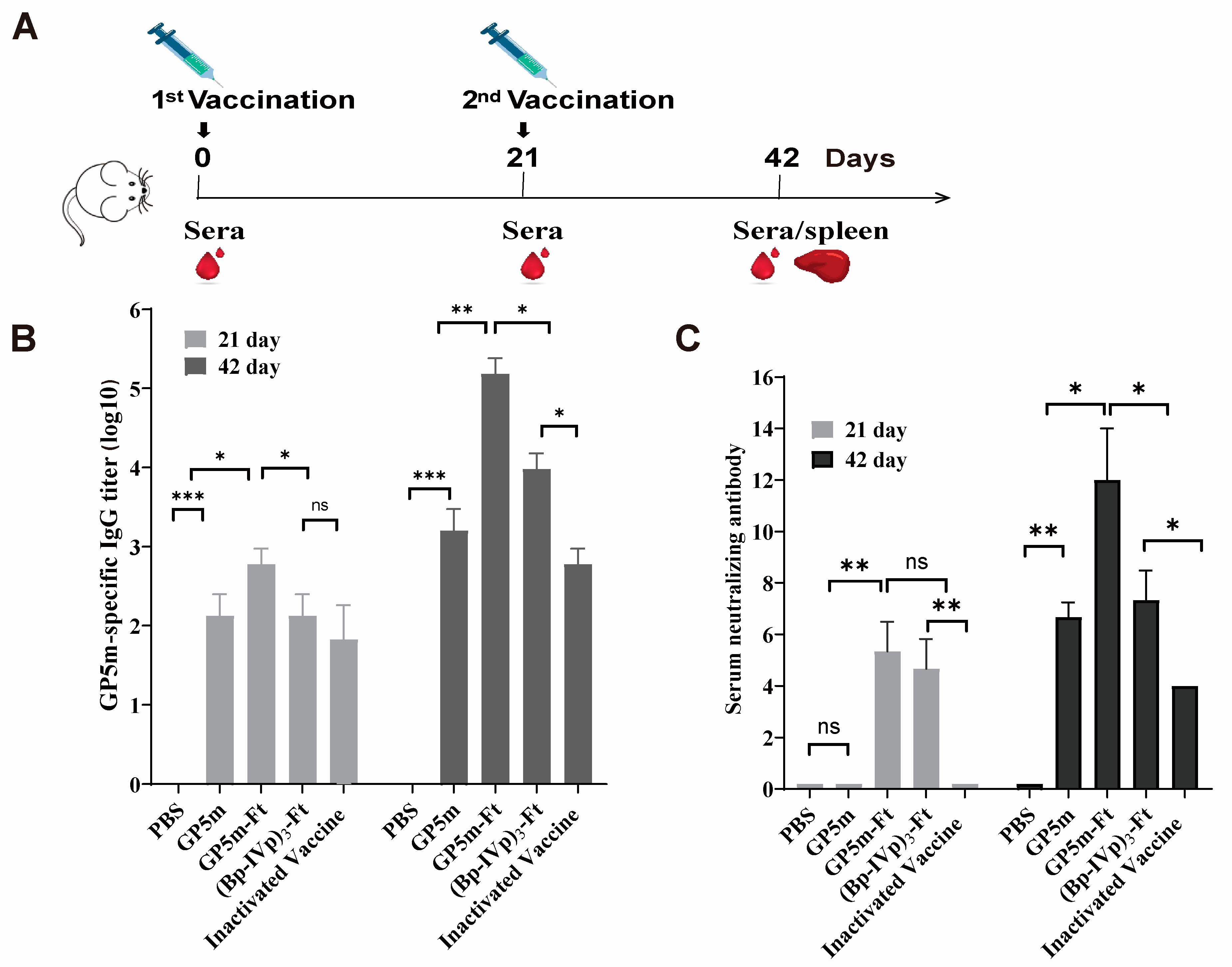
2.7. Vaccination in Mice
2.8. Vaccination and PRRSV Challenge in Piglets
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. PRRSV Serum Neutralization Assays
2.11. Spleen Lymphocyte Proliferation Test in Mice
2.12. ELISpot Analysis of Interferon-Gamma (IFN-γ) in Mice Splenic Lymphocytes
2.13. Testing PBMC Proliferation in Piglets
2.14. Clinical Observation
2.15. Quantitative Analysis of Viral Loads
2.16. Histological Observation
2.17. Statistical Analysis
3. Results
3.1. Design, Purification, and Assembly Validation of Recombinant Nanoparticles
3.2. Humoral Immune Responses of (Bp-IVp)3-Ft and GP5m-Ft in Mice
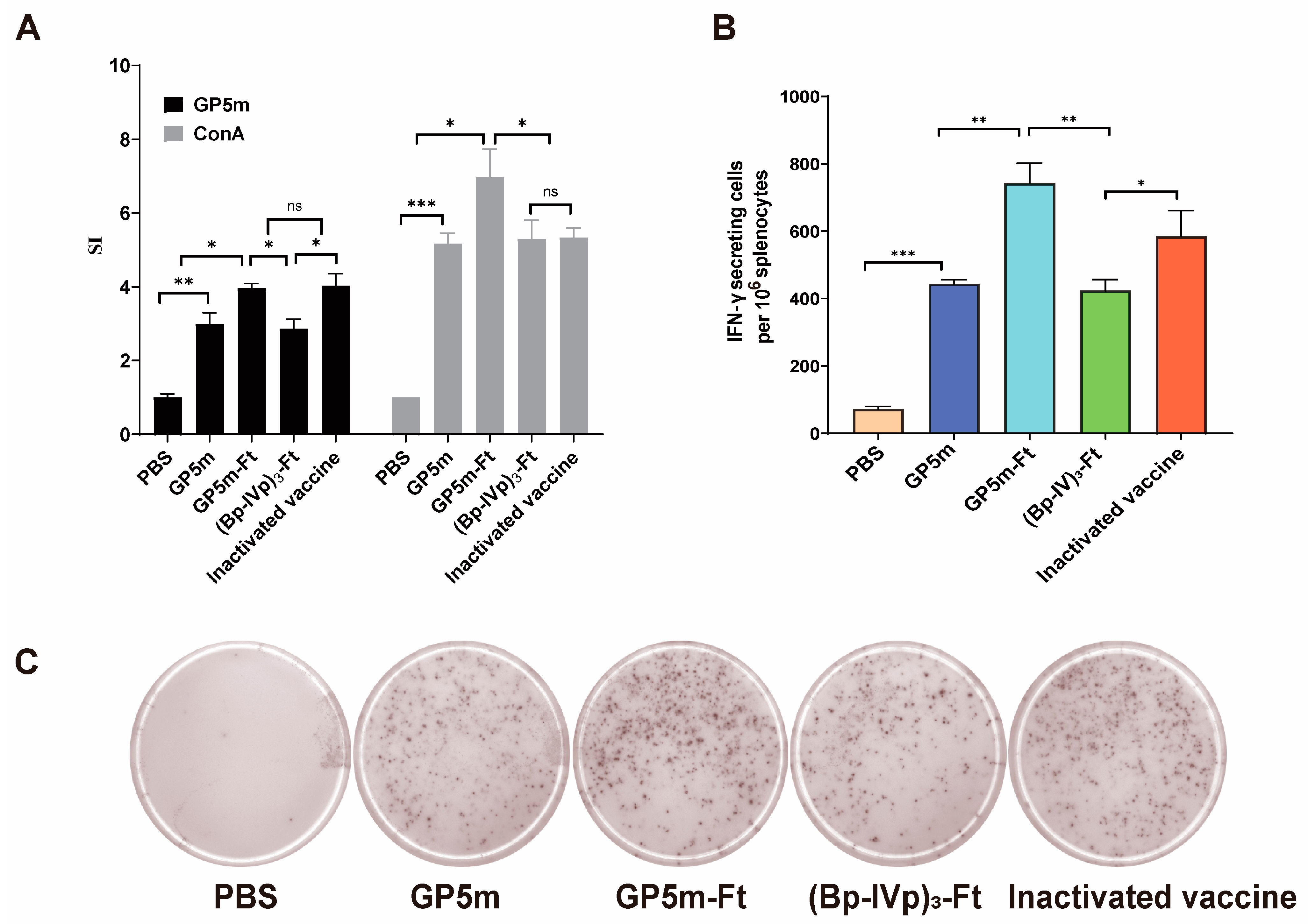
3.3. Cellular Immune Response of (Bp-IVp)3-Ft and GP5m-Ft in Mice
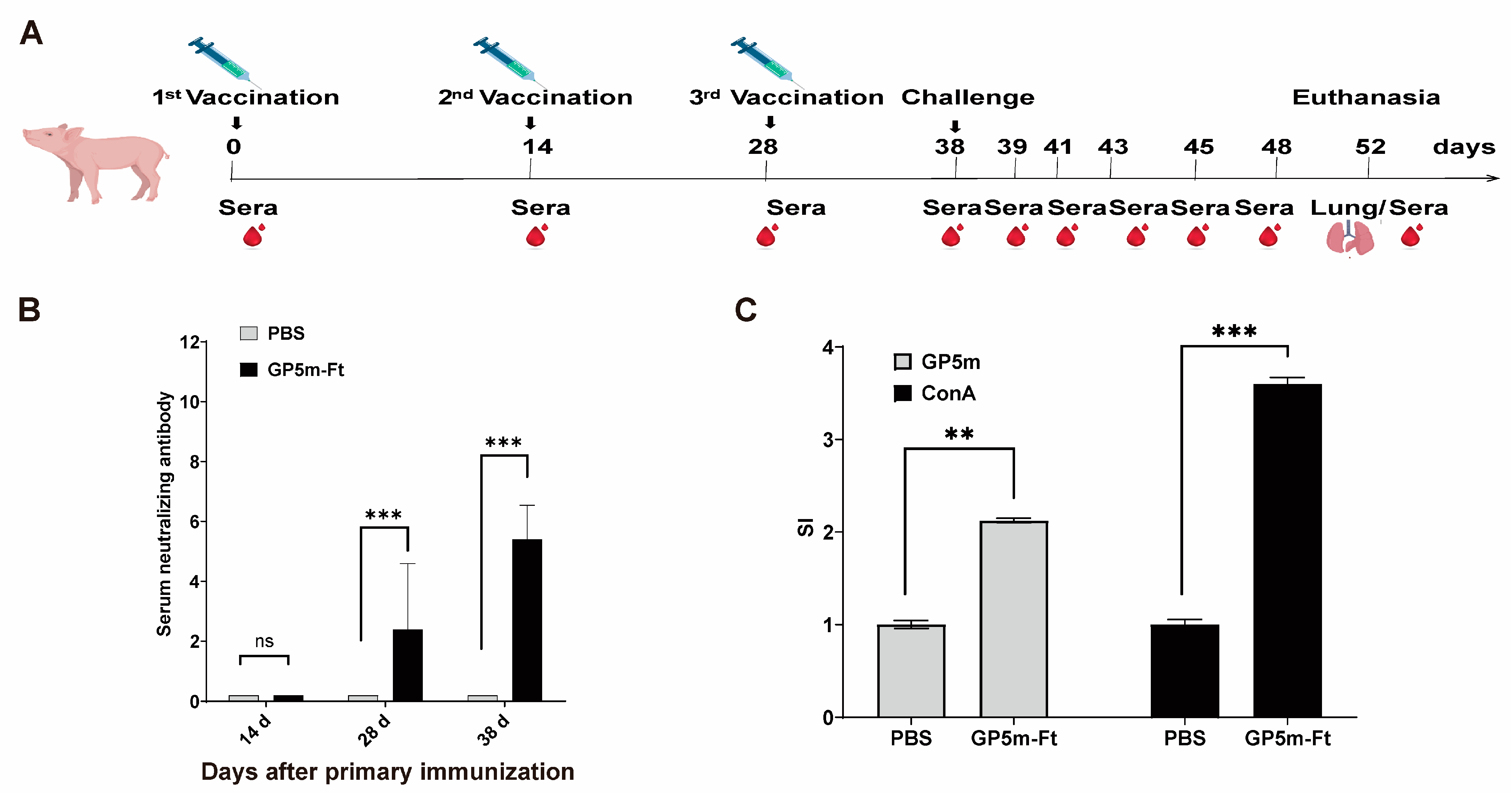
3.4. Evaluation of the Immunogenicity of GP5m-Ft Vaccine in Piglets
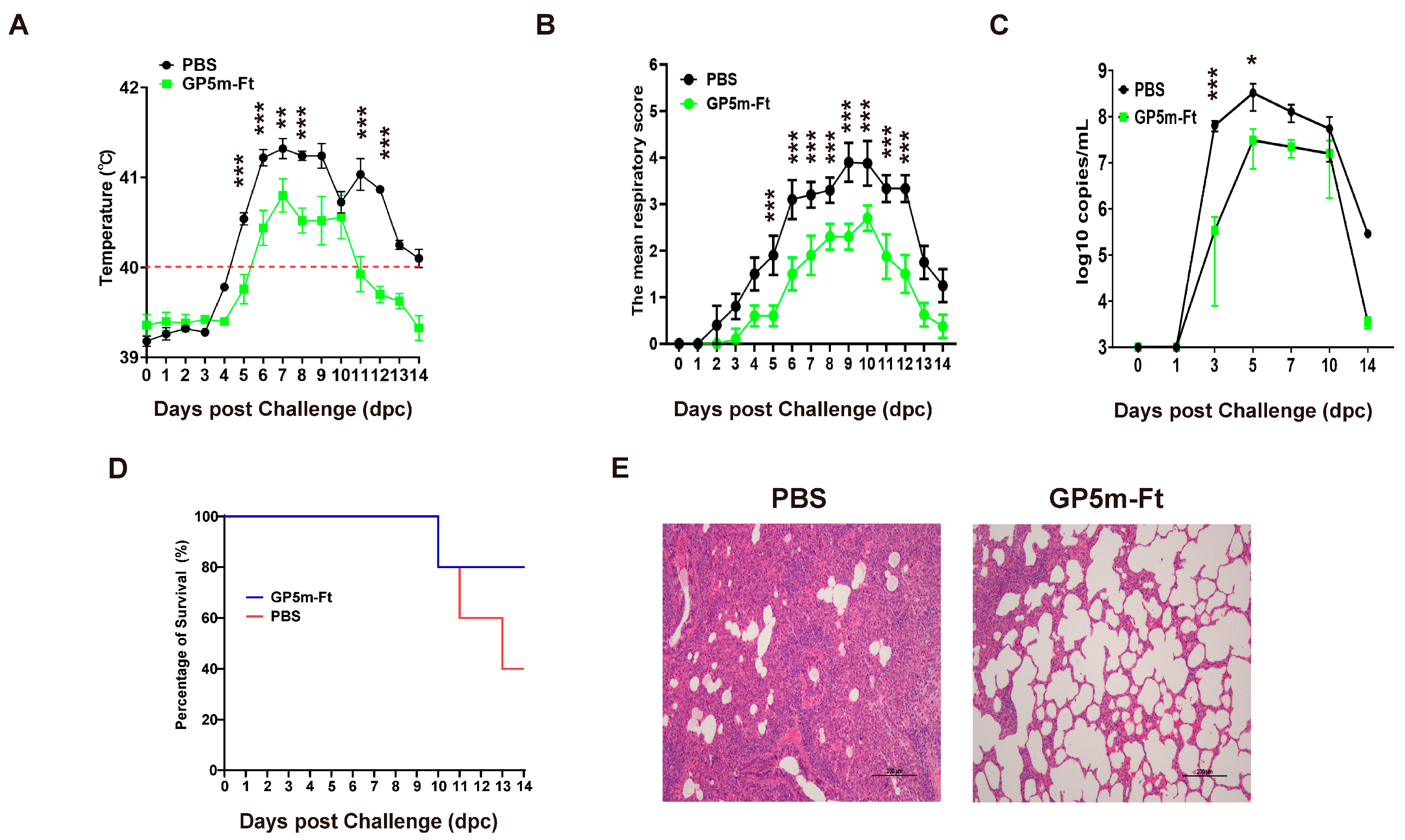
3.5. Immunization with GP5m-Ft Vaccine Provides Effective Protection against PRRSV Challenge in Piglets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Wan, B.; Guo, Z.; Qiao, S.; Li, R.; Xie, S.; Chen, X.; Zhang, G. Genomic analysis of a recombinant NADC30-like porcine reproductive and respiratory syndrome virus in China. Virus Genes 2018, 54, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yang, H. Porcine reproductive and respiratory syndrome in China. Virus Res. 2010, 154, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, X.; Li, R.; Qiao, S.; Zhang, G. The prevalent status and genetic diversity of porcine reproductive and respiratory syndrome virus in China: A molecular epidemiological perspective. Virol. J. 2018, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zheng, Y.; He, Y.; Li, G.; Zhang, H.; Sha, H.; Zhang, Z.; Huang, L.; Zhao, M. Genetic variation and recombination analysis of the GP5 (GP5a) gene of PRRSV-2 strains in China from 1996 to 2022. Front. Microbiol. 2023, 14, 1238766. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, J.; Li, W.; Xu, H.; Gong, B.; Sun, Q.; Guo, Z.; Li, J.; Xiang, L.; Tang, Y.; et al. Prevalence and genetic evolution of porcine reproductive and respiratory syndrome virus in commercial fattening pig farms in China. Porc. Health Manag. 2024, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Tang, Y.; Liu, T.; Zheng, L.; Wang, T.; Liu, S.; Wang, G.; Cai, X. A natural recombinant PRRSV between HP-PRRSV JXA1-like and NADC30-like strains. Transbound. Emerg. Dis. 2018, 65, 1078–1086. [Google Scholar] [CrossRef]
- Zhou, Z.; Ni, J.; Cao, Z.; Han, X.; Xia, Y.; Zi, Z.; Ning, K.; Liu, Q.; Cai, L.; Qiu, P.; et al. The epidemic status and genetic diversity of 14 highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) isolates from China in 2009. Vet. Microbiol. 2011, 150, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Charoenchanikran, P.; Kedkovid, R.; Sirisereewan, C.; Woonwong, Y.; Arunorat, J.; Sitthichareonchai, P.; Sopipan, N.; Jittimanee, S.; Kesdangsakonwut, S.; Thanawongnuwech, R. Efficacy of Fostera® PRRS modified live virus (MLV) vaccination strategy against a Thai highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) infection. Trop. Anim. Health Prod. 2016, 48, 1351–1359. [Google Scholar] [CrossRef]
- Mair, K.H.; Koinig, H.; Gerner, W.; Höhne, A.; Bretthauer, J.; Kroll, J.J.; Roof, M.B.; Saalmüller, A.; Stadler, K.; Libanova, R. Carbopol improves the early cellular immune responses induced by the modified-life vaccine Ingelvac PRRS® MLV. Vet. Microbiol. 2015, 176, 352–357. [Google Scholar] [CrossRef]
- Oh, T.; Kim, H.; Park, K.H.; Jeong, J.; Yang, S.; Kang, I.; Chae, C. Comparison of four commercial PRRSV MLV vaccines in herds with co-circulation of PRRSV-1 and PRRSV-2. Comp. Immunol. Microbiol. Infect. Dis. 2019, 63, 66–73. [Google Scholar] [CrossRef]
- Roques, E.; Lessard, M.; Archambault, D. The Cholera Toxin B Subunit (CTB) Fused to the Porcine Arterivirus Matrix M and GP5 Envelope Proteins Fails to Enhance the GP5-Specific Antibody Response in Pigs Immunized with Adenovectors. Mol. Biotechnol. 2015, 57, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ni, Y.; Dryman, B.A.; Meng, X.; Zhang, C. Immunogenicity study of plant-made oral subunit vaccine against porcine reproductive and respiratory syndrome virus (PRRSV). Vaccine 2012, 30, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Dang, Z.; Qiu, H.; Fan, X.; Zhou, B.; Cui, B.; Chen, P. Function of PRRSV GP5 envelope protein by using pseudotyped virus. Vet. Microbiol. 2009, 138, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Dokland, T. The structural biology of PRRSV. Virus Res. 2010, 154, 86–97. [Google Scholar] [CrossRef]
- Ostrowski, M.; Galeota, J.A.; Jar, A.M.; Platt, K.B.; Osorio, F.A.; Lopez, O.J. Identification of neutralizing and nonneutralizing epitopes in the porcine reproductive and respiratory syndrome virus GP5 ectodomain. J. Virol. 2002, 76, 4241–4250. [Google Scholar] [CrossRef] [PubMed]
- Piñeyro, P.E.; Kenney, S.P.; Giménez-Lirola, L.G.; Heffron, C.L.; Matzinger, S.R.; Opriessnig, T.; Meng, X. Expression of antigenic epitopes of porcine reproductive and respiratory syndrome virus (PRRSV) in a modified live-attenuated porcine circovirus type 2 (PCV2) vaccine virus (PCV1-2a) as a potential bivalent vaccine against both PCV2 and PRRSV. Virus Res. 2015, 210, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Peng, K.; Tian, J.; Xu, X.; Zhou, E.; Chen, H. Immune response to Fc tagged GP5 glycoproteins of porcine reproductive and respiratory syndrome virus. Viral Immunol. 2014, 27, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Roques, E.; Girard, A.; St-Louis, M.C.; Massie, B.; Gagnon, C.A.; Lessard, M.; Archambault, D. Immunogenic and protective properties of GP5 and M structural proteins of porcine reproductive and respiratory syndrome virus expressed from replicating but nondisseminating adenovectors. Vet. Res. 2013, 44, 17. [Google Scholar] [CrossRef] [PubMed]
- Chia, M.; Hsiao, S.; Chan, H.; Do, Y.; Huang, P.; Chang, H.; Tsai, Y.; Lin, C.; Pang, V.; Jeng, C. The immunogenicity of DNA constructs co-expressing GP5 and M proteins of porcine reproductive and respiratory syndrome virus conjugated by GPGP linker in pigs. Vet. Microbiol. 2010, 146, 189–199. [Google Scholar] [CrossRef]
- Barfoed, A.M.; Blixenkrone-Møller, M.; Jensen, M.H.; Bøtner, A.; Kamstrup, S. DNA vaccination of pigs with open reading frame 1-7 of PRRS virus. Vaccine 2004, 22, 3628–3641. [Google Scholar] [CrossRef]
- Ma, H.; Li, X.; Li, J.; Zhao, Z.; Zhang, H.; Hao, G.; Chen, H.; Qian, P. Immunization with a recombinant fusion of porcine reproductive and respiratory syndrome virus modified GP5 and ferritin elicits enhanced protective immunity in pigs. Virology 2021, 552, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-C.; Kim, M.-S.; Choi, H.-Y.; Kang, Y.-L.; Choi, I.-Y.; Jung, S.-W.; Jeong, J.-Y.; Kim, M.-C.; Cho, A.Y.; Lee, J.-H.; et al. Porcine Reproductive and Respiratory Syndrome Virus Engineered by Serine Substitution on the 44th Amino Acid of GP5 Resulted in a Potential Vaccine Candidate with the Ability to Produce High Levels of Neutralizing Antibody. Vet. Sci. 2023, 10, 191. [Google Scholar] [CrossRef]
- Duy, D.T.; Kim, H.; Jeong, J.; Park, K.H.; Yang, S.; Oh, T.; Kim, S.; Kang, I.; Chae, C. Comparative evaluation of the efficacy of commercial and prototype PRRS subunit vaccines against an HP-PRRSV challenge. J. Vet. Med. Sci. 2018, 80, 1463–1467. [Google Scholar] [CrossRef]
- Zheng, Q.; Chen, D.; Li, P.; Bi, Z.; Cao, R.; Zhou, B.; Chen, P. Co-expressing GP5 and M proteins under different promoters in recombinant modified vaccinia virus ankara (rMVA)-based vaccine vector enhanced the humoral and cellular immune responses of porcine reproductive and respiratory syndrome virus (PRRSV). Virus Genes 2007, 35, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Song, L.; Zhai, X.; Geng, S.; Pan, Z.; Jiao, X. A porcine reproductive and respiratory syndrome virus (PRRSV) vaccine candidate based on the fusion protein of PRRSV glycoprotein 5 and the Toll-like Receptor-5 agonist Salmonella Typhimurium FljB. BMC Vet. Res. 2015, 11, 121. [Google Scholar] [CrossRef]
- Alexander, J.; Sidney, J.; Southwood, S.; Ruppert, J.; Oseroff, C.; Maewal, A.; Snoke, K.; Serra, H.M.; Kubo, R.T.; Sette, A.; et al. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity 1994, 1, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.Q.; Alves, P.M.; Roldão, A. Functionalizing Ferritin Nanoparticles for Vaccine Development. Pharmaceutics 2021, 13, 1621. [Google Scholar] [CrossRef]
- Lee, N.K.; Cho, S.; Kim, I.S. Ferritin—A multifaceted protein scaffold for biotherapeutics. Exp. Mol. Med. 2022, 54, 1652–1657. [Google Scholar] [CrossRef]
- Khoshnejad, M.; Parhiz, H.; Shuvaev, V.V.; Dmochowski, I.J.; Muzykantov, V.R. Ferritin-based drug delivery systems: Hybrid nanocarriers for vascular immunotargeting. J. Control Release 2018, 282, 13–24. [Google Scholar] [CrossRef]
- Li, Z.; Cui, K.; Wang, H.; Liu, F.; Huang, K.; Duan, Z.; Wang, F.; Shi, D.; Liu, Q. A milk-based self-assemble rotavirus VP6-ferritin nanoparticle vaccine elicited protection against the viral infection. J. Nanobiotechnol. 2019, 17, 13. [Google Scholar] [CrossRef]
- Li, D.; Song, H.; Li, J.; Liu, X.; Gao, X.; Wu, T.; Zhang, Z.; Li, Y. Expression and Evaluation of a Novel PPRV Nanoparticle Antigen Based on Ferritin Self-Assembling Technology. Pharmaceutics 2022, 14, 1902. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, Y.; Cao, T.; Liu, X.; Liu, X.; Yan, Y.; Shi, Y.; Wang, J.C. Ferritin-based nanomedicine for disease treatment. Med. Rev. 2023, 3, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Chen, L.; Yang, S.; Xia, S.; Xu, Z.; Zhang, T.; Zeng, B.; Yu, T.; Yu, N.; Wang, W.; et al. R848 Adjuvant Laden with Self-Assembled Nanoparticle-Based mRNA Vaccine Elicits Protective Immunity Against H5N1 in Mice. Front. Immunol. 2022, 13, 836274. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Chen, Y.; Li, H.; Fang, K.; Chen, H.; Li, X.; Qian, P. A Self-Assembling Ferritin Nanoplatform for Designing Classical Swine Fever Vaccine: Elicitation of Potent Neutralizing Antibody. Vaccine 2021, 9, 45. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Z.; Tong, X.; Wang, Z.; Xu, S.; Chen, Q.; Zhou, J.; Fang, L.; Wang, D.; Xiao, S. Evolutionary Dynamics of Type 2 Porcine Reproductive and Respiratory Syndrome Virus by Whole-Genome Analysis. Viruses 2021, 13, 2469. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xiao, S.; Wang, Y.; Xu, S.; Jiang, Y.; Chen, H.; Fang, L. Immunogenicity of the highly pathogenic porcine reproductive and respiratory syndrome virus GP5 protein encoded by a synthetic ORF5 gene. Vaccine 2009, 27, 1957–1963. [Google Scholar] [CrossRef]
- Huang, C.H.; Lee, I.L.; Yeh, I.J.; Liao, J.H.; Ni, C.L.; Wu, S.H.; Chiou, S.H. Upregulation of a non-heme iron-containing ferritin with dual ferroxidase and DNA-binding activities in Helicobacter pylori under acid stress. J. Biochem. 2010, 147, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-H.; Chen, J.; Tong, G.-Z.; Tian, Z.-J.; Zhou, Y.-J.; Li, G.-X.; Li, X.; Peng, J.-M.; An, T.-Q.; Yang, H.-C. A recombinant plasmid co-expressing swine ubiquitin and the GP5 encoding-gene of porcine reproductive and respiratory syndrome virus induces protective immunity in piglets. Vaccine 2008, 26, 1438–1449. [Google Scholar] [CrossRef]
- Liu, D.; Zhou, R.; Zhang, J.; Zhou, L.; Jiang, Q.; Guo, X.; Ge, X.; Yang, H. Recombination analyses between two strains of porcine reproductive and respiratory syndrome virus in vivo. Virus Res. 2011, 155, 473–486. [Google Scholar] [CrossRef]
- Cui, J.; O’Connell, C.M.; Costa, A.; Pan, Y.; Smyth, J.A.; Verardi, P.H.; Burgess, D.J.; Van Kruiningen, H.J.; Garmendia, A.E. A PRRSV GP5-Mosaic vaccine: Protection of pigs from challenge and ex vivo detection of IFNγ responses against several genotype 2 strains. PLoS ONE 2019, 14, e0208801. [Google Scholar]
- Liu, D.; Chen, Y. Epitope screening and vaccine molecule design of PRRSV GP3 and GP5 protein based on immunoinformatics. J. Cell Mol. Med. 2024, 28, e18103. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xiao, S.; Fang, L.; Yu, X.; Song, Y.; Niu, C.; Chen, H. DNA vaccines co-expressing GP5 and M proteins of porcine reproductive and respiratory syndrome virus (PRRSV) display enhanced immunogenicity. Vaccine 2006, 24, 2869–2879. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Yu, Z.; Pang, F.; Xu, X.; Mao, A.; Yuan, W.; He, K.; Li, B. Targeted Delivery of GP5 Antigen of PRRSV to M Cells Enhances the Antigen-Specific Systemic and Mucosal Immune Responses. Front. Cell Infect. Microbiol. 2018, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Ma, H.; Cao, L.; Jing, J.; Xiao, P.; Sun, W.; Xie, C.; Wen, S.; Li, Y.; Tian, M.; et al. Immunogenicity of recombinant vaccinia virus vaccines co-expressing GP3/GP5 of European PRRSV and Cap protein of PCV2 in pigs. Appl. Microbiol. Biotechnol. 2018, 102, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Jiang, P.; Wang, X.; Li, Y.; Du, Y.; Wang, X. Enhanced immune responses of mice inoculated recombinant adenoviruses expressing GP5 by fusion with GP3 and/or GP4 of PRRS virus. Virus Res. 2008, 136, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Morales-Hernández, S.; Ugidos-Damboriena, N.; López-Sagaseta, J. Self-Assembling Protein Nanoparticles in the Design of Vaccines: 2022 Update. Vaccine 2022, 10, 1447. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-I.; Kim, D.; Yu, K.-M.; Seo, H.D.; Lee, S.-A.; Casel, M.A.B.; Jang, S.-G.; Kim, S.; Jung, W.; Lai, C.-J.; et al. Development of Spike Receptor-Binding Domain Nanoparticles as a Vaccine Candidate against SARS-CoV-2 Infection in Ferrets. mBio 2021, 12, e00230-21. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, I.S.; Joyce, M.G.; Chen, R.E.; Leung, K.; McKee, K.; Druz, A.; Van Galen, J.G.; Kanekiyo, M.; Tsybovsky, Y.; Yang, E.S.; et al. Two-Component Ferritin Nanoparticles for Multimerization of Diverse Trimeric Antigens. ACS Infect. Dis. 2018, 4, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.A.; Yang, Z.; Gnanapragasam, P.N.P.; Ou, S.; Dam, K.-M.A.; Wang, H.; Bjorkman, P.J. Construction, characterization, and immunization of nanoparticles that display a diverse array of influenza HA trimers. PLoS ONE 2021, 16, e0247963. [Google Scholar] [CrossRef]
- Wang, B.; Li, S.; Qiao, Y.; Fu, Y.; Nie, J.; Jiang, S.; Yao, X.; Pan, Y.; Zhao, L.; Wu, C.; et al. Self-assembling ferritin nanoparticles coupled with linear sequences from canine distemper virus haemagglutinin protein elicit robust immune responses. J. Nanobiotechnol. 2022, 20, 32. [Google Scholar] [CrossRef]
- Sliepen, K.; Ozorowski, G.; Burger, J.A.; van Montfort, T.; Stunnenberg, M.; LaBranche, C.; Montefiori, D.C.; Moore, J.P.; Ward, A.B.; Sanders, R.W. Presenting native-like HIV-1 envelope trimers on ferritin nanoparticles improves their immunogenicity. Retrovirology 2015, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Guo, Y.; Li, M.; Cao, C.; Wang, J.; Gao, M. Recombinant ferritin nanoparticles can induce dendritic cell maturation through TLR4/NF-kappaB pathway. Biotechnol. Lett. 2020, 42, 2489–2500. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, B.; Zhu, Y.; Tan, W.; Zhu, M. Ferritin nanoparticle-based SARS-CoV-2 RBD vaccine induces a persistent antibody response and long-term memory in mice. Cell Mol. Immunol. 2021, 18, 749–751. [Google Scholar] [CrossRef] [PubMed]
- Burakova, Y.; Madera, R.; McVey, S.; Schlup, J.R.; Shi, J. Adjuvants for Animal Vaccines. Viral Immunol. 2018, 31, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Dewangan, H.K. Nanoparticles as Adjuvants in Vaccine Delivery. Crit. Rev. Ther. Drug Carrier Syst. 2020, 37, 183–204. [Google Scholar] [CrossRef]
- D’Amico, C.; Fontana, F.; Cheng, R.; Santos, H.A. Development of vaccine formulations: Past, present, and future. Drug Deliv Transl. Res. 2021, 11, 353–372. [Google Scholar] [CrossRef]
| Primer and Probe | Sequence (5′-3′) |
|---|---|
| PRRSV N Forward | TTCCCTCTAGCGACTGAAGA |
| PRRSV N Reverse | AGTTCCAGCACCCTGATTG |
| Probe 6 FAM | GCAATTGTGTCTGTCGTCGATCCAGA-BHQ1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, X.; Ma, J.; Zhou, Y.; Xiao, S.; Xiao, X.; Fang, L. Development of a Ferritin Protein Nanoparticle Vaccine with PRRSV GP5 Protein. Viruses 2024, 16, 991. https://doi.org/10.3390/v16060991
Chang X, Ma J, Zhou Y, Xiao S, Xiao X, Fang L. Development of a Ferritin Protein Nanoparticle Vaccine with PRRSV GP5 Protein. Viruses. 2024; 16(6):991. https://doi.org/10.3390/v16060991
Chicago/Turabian StyleChang, Xinjian, Jun Ma, Yanrong Zhou, Shaobo Xiao, Xun Xiao, and Liurong Fang. 2024. "Development of a Ferritin Protein Nanoparticle Vaccine with PRRSV GP5 Protein" Viruses 16, no. 6: 991. https://doi.org/10.3390/v16060991
APA StyleChang, X., Ma, J., Zhou, Y., Xiao, S., Xiao, X., & Fang, L. (2024). Development of a Ferritin Protein Nanoparticle Vaccine with PRRSV GP5 Protein. Viruses, 16(6), 991. https://doi.org/10.3390/v16060991









