Development of Dry and Liquid Duplex Reagent Mix-Based Polymerase Chain Reaction Assays as Novel Tools for the Rapid and Easy Quantification of Bovine Leukemia Virus (BLV) Proviral Loads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Samples, DNA Extraction, and Plasma Isolation
2.2. Detection of Anti-BLV Antibodies in Plasma Samples
2.3. Determination of the BLV PVL Using Single-CoCoMo Assay
2.4. Development and Optimization of the Liquid Dual-CoCoMo Assay
2.5. Development of the Dry Dual-CoCoMo Assay
2.6. Comparison of the Sensitivity of the Liquid Dual-CoCoMo Assay and Dry Dual-CoCoMo Assay
2.7. Validation of Assay Accuracy for the Clinical Applicability Using Field Samples
2.8. Statistical Analysis
3. Results
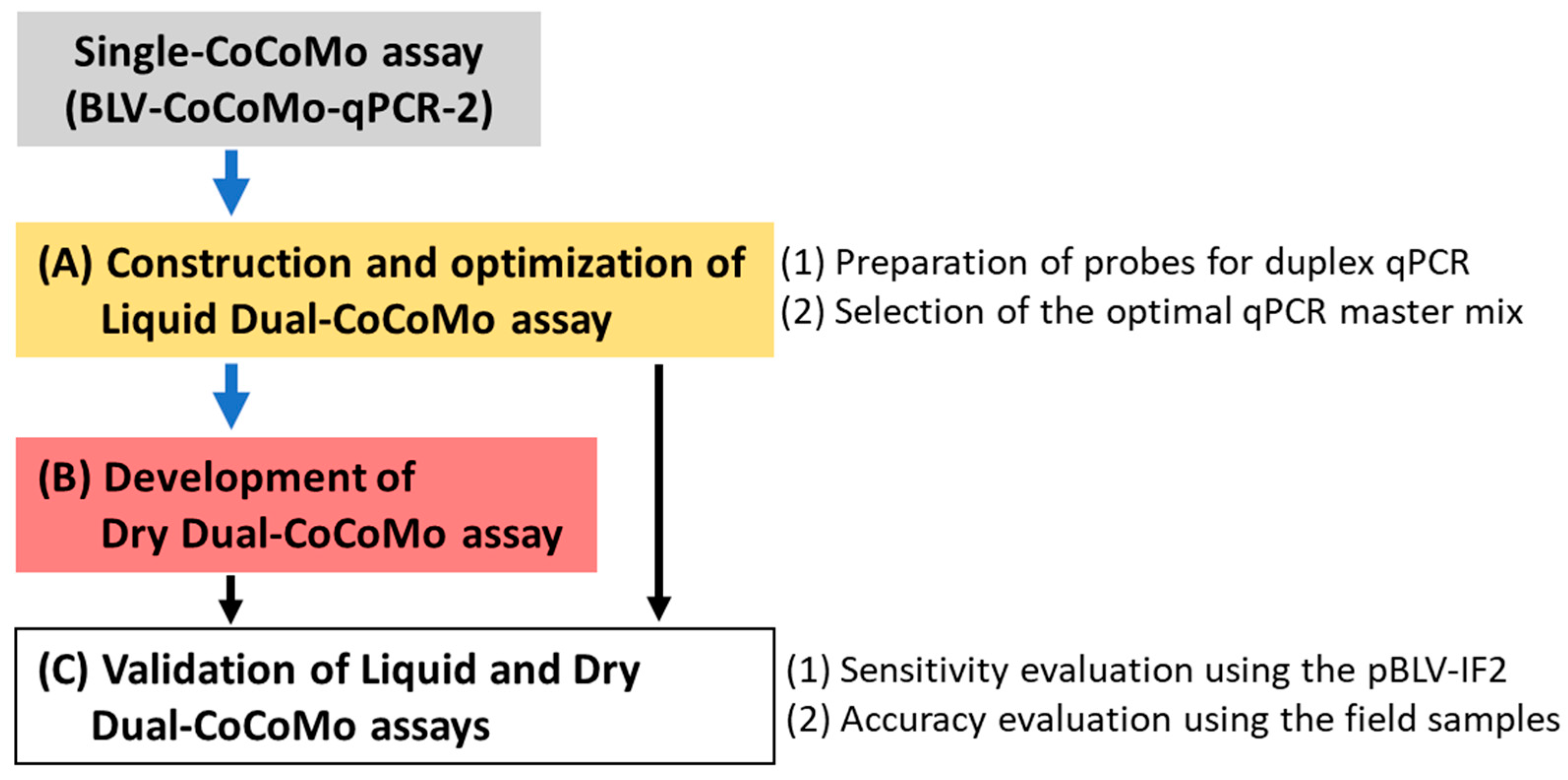
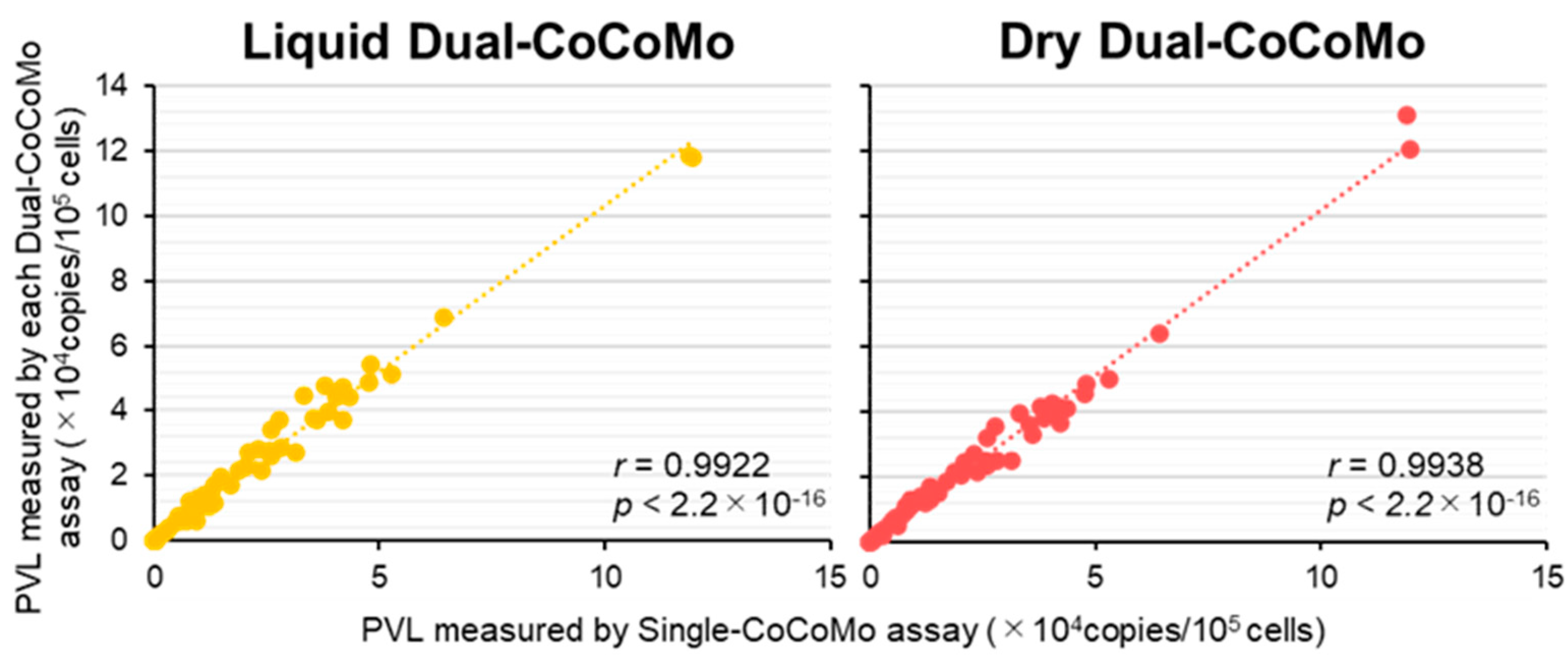
3.1. Construction and Optimization of the Liquid Dual-CoCoMo Assay
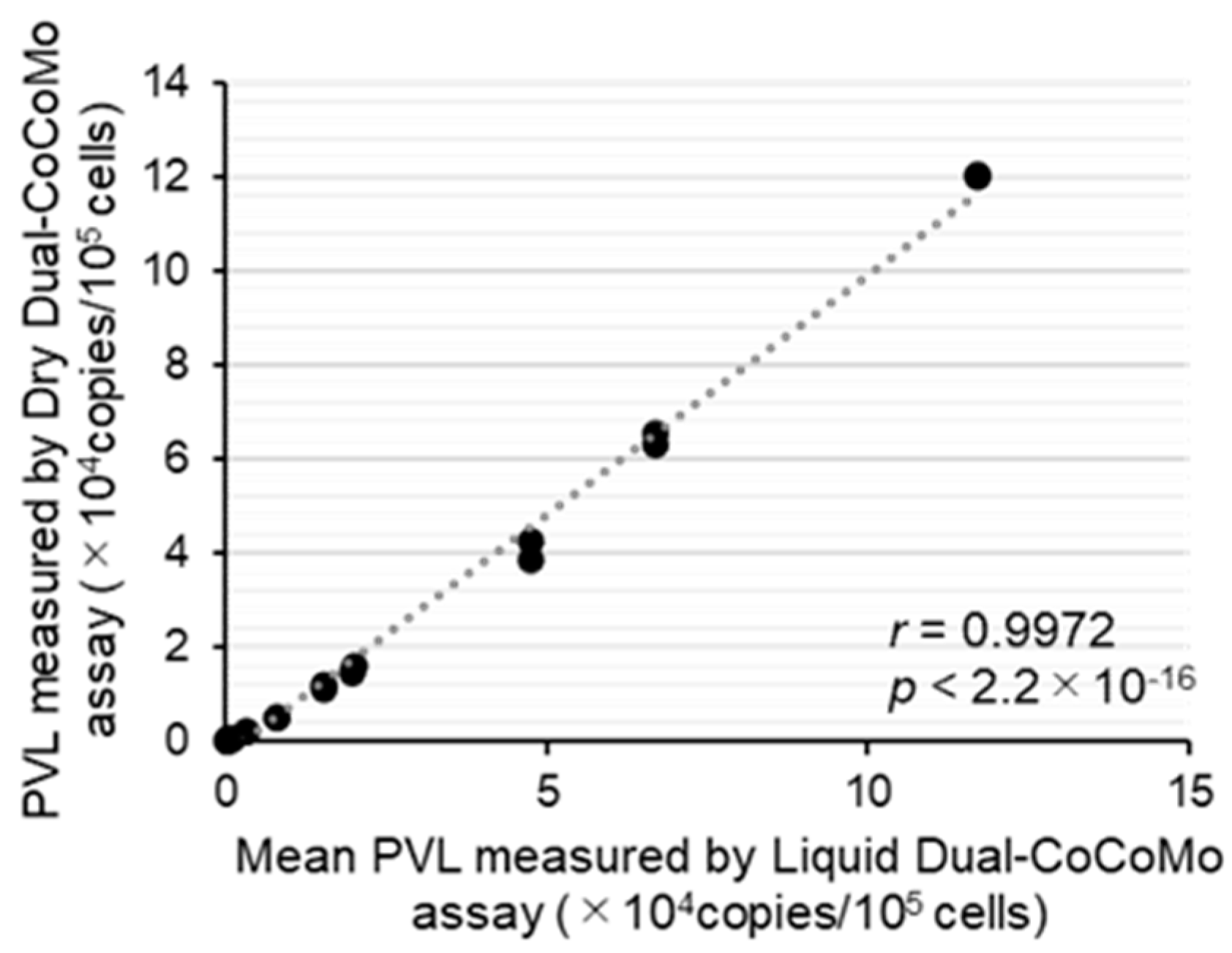
3.2. Development of the Dry Dual-CoCoMo Assay and Correlation Analysis with the Liquid Dual-CoCoMo Assay
3.3. Validation of the Accuracy of the Liquid Dual-CoCoMo Assay and Dry Dual-CoCoMo Assay on BLV Provirus Detection
3.4. Validation of Accuracy Liquid Dual-CoCoMo Assay and Dry Dual-CoCoMo Assay on Field Samples
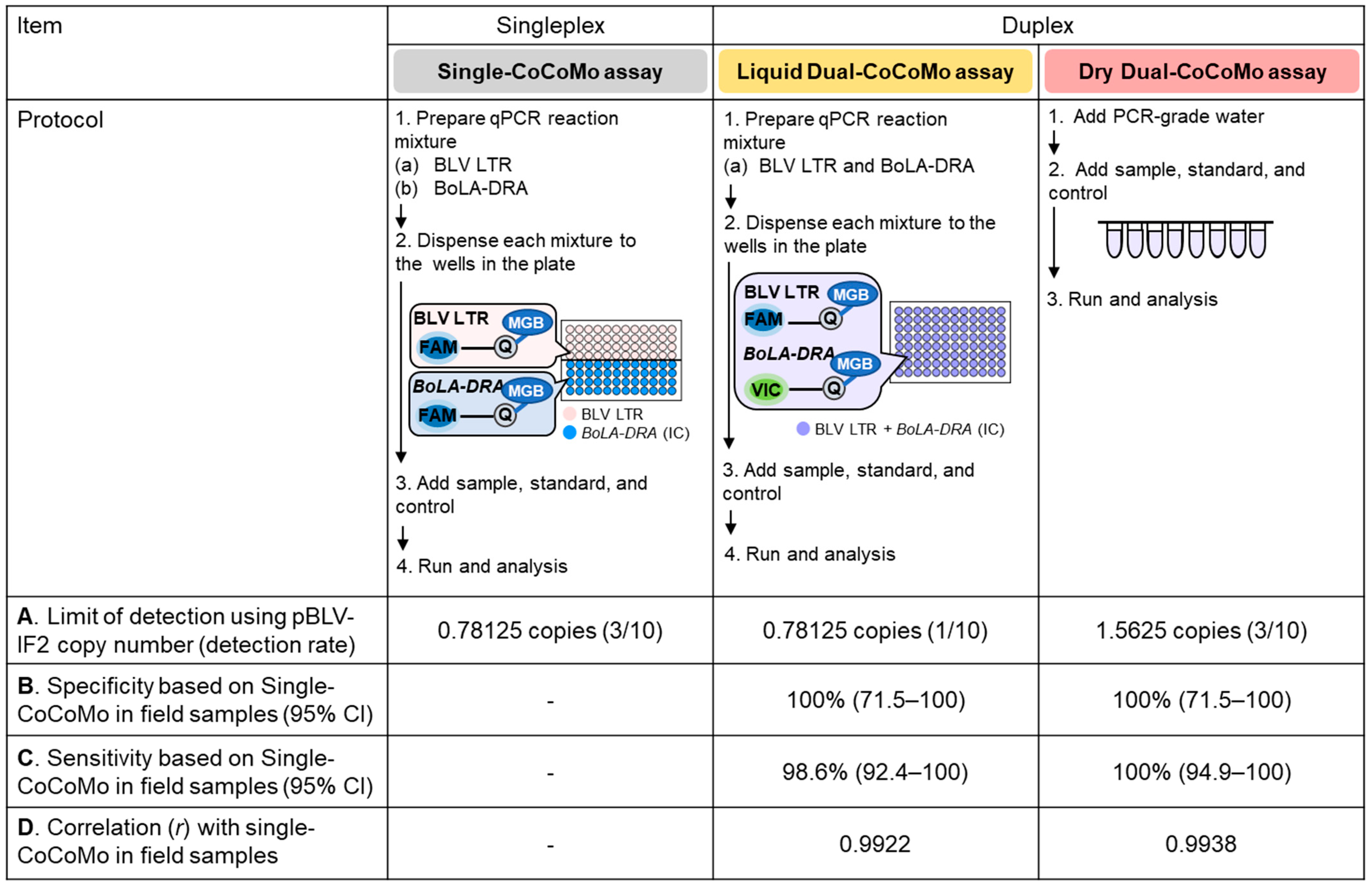
3.5. Comparison of the Characteristic Features of the Single-CoCoMo, Liquid Dual-CoCoMo and Dry Dual-CoCoMo Assays
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aida, Y.; Murakami, H.; Takahashi, M.; Takeshima, S.N. Mechanisms of pathogenesis induced by bovine leukemia virus as a model for human T-cell leukemia virus. Front. Microbiol. 2013, 4, 328. [Google Scholar] [CrossRef] [PubMed]
- EFSA AHAW Panel on Animal Health and Welfare. Scientific opinion on enzootic bovine leukosis. EFSA J. 2015, 13, 4188. [Google Scholar]
- Murakami, K.; Kobayashi, S.; Konishi, M.; Kameyama, K.; Tsutsui, T. Nationwide survey of bovine leukemia virus infection among dairy and beef breeding cattle in Japan from 2009–2011. J. Vet. Med. Sci. 2013, 75, 1123–1126. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.O.; Meas, S.; Park, N.Y.; Kim, Y.H.; Lim, Y.K.; Endoh, D.; Lee, S.I.; Ohashi, K.; Sugimoto, C.; Onuma, M. Seroprevalence of bovine immunodeficiency virus in dairy and beef cattle herds in Korea. J. Vet. Med. Sci. 1999, 61, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wang, X.; Zhou, Y.; Wang, Y.; Zhang, X.; Zheng, Y. Genotyping bovine leukemia virus in dairy cattle of Heilongjiang, northeastern China. BMC Vet. Res. 2019, 15, 179. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fan, W.; Mao, Y.; Yang, Z.; Lu, G.; Zhang, R.; Zhang, H.; Szeto, C.; Wang, C. Bovine leukemia virus infection in cattle of China: Association with reduced milk production and increased somatic cell score. J. Dairy Sci. 2016, 99, 3688–3697. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Kim, E.J.; Ratthanophart, J.; Vitoonpong, R.; Kim, B.H.; Cho, I.S.; Song, J.Y.; Lee, K.K.; Shin, Y.K. Molecular epidemiological and serological studies of bovine leukemia virus (BLV) infection in Thailand cattle. Infect. Genet. Evol. 2016, 41, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Polat, M.; Ohno, A.; Takeshima, S.N.; Kim, J.; Kikuya, M.; Matsumoto, Y.; Mingala, C.N.; Onuma, M.; Aida, Y. Detection and molecular characterization of bovine leukemia virus in Philippine cattle. Arch. Virol. 2015, 160, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Hamada, R.; Metwally, S.; Polat, M.; Borjigin, L.; Ali, A.O.; Abdel-Hady, A.A.A.; Mohamed, A.E.A.; Wada, S.; Aida, Y. Detection and Molecular Characterization of Bovine Leukemia Virus in Egyptian Dairy Cattle. Front. Vet. Sci. 2020, 7, 608. [Google Scholar] [CrossRef] [PubMed]
- Metwally, S.; Hamada, R.; Ali, A.O.; Mahmoud, H.Y.A.H.; Baker, N.M.; Mohamed, A.E.A.; Wada, S.; Matsumoto, Y.; Aida, Y. Detection and molecular characterization of bovine leukemia virus in beef cattle presented for slaughter in Egypt. J. Vet. Med. Sci. 2020, 82, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Moe, K.K.; Polat, M.; Borjigin, L.; Matsuura, R.; Hein, S.T.; Moe, H.H.; Aida, Y. New evidence of bovine leukemia virus circulating in Myanmar cattle through epidemiological and molecular characterization. PLoS ONE 2020, 15, e0229126. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, V.J.; Bartlett, P.C. Control of Bovine Leukemia Virus in Three US Dairy Herds by Culling ELISA-Positive Cows. Vet. Med. Int. 2019, 2019, 3202184. [Google Scholar] [CrossRef] [PubMed]
- John, E.E.; Keefe, G.; Cameron, M.; Stryhn, H.; McClure, J.T. Development and implementation of a risk assessment and management program for enzootic bovine leukosis in Atlantic Canada. J. Dairy Sci. 2020, 103, 8398–8406. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.L.; Johnson, R.; Wells, S.J. Association between bovine-leukosis virus seroprevalence and herd-level productivity on US dairy farms. Prev. Vet. Med. 2003, 61, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Erskine, R.J.; Bartlett, P.C.; Byrem, T.M.; Render, C.L.; Febvay, C.; Houseman, J.T. Association between bovine leukemia virus, production, and population age in Michigan dairy herds. J. Dairy Sci. 2012, 95, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Nakada, S.; Fujimoto, Y.; Kohara, J.; Makita, K. Economic losses associated with mastitis due to bovine leukemia virus infection. J. Dairy Sci. 2023, 106, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Gillet, N.; Florins, A.; Boxus, M.; Burteau, C.; Nigro, A.; Vandermeers, F.; Balon, H.; Bouzar, A.B.; Defoiche, J.; Burny, A.; et al. Mechanisms of leukemogenesis induced by bovine leukemia virus: Prospects for novel anti-retroviral therapies in human. Retrovirology 2007, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- More, S.; Bøtner, A.; Butterworth, A.; Calistri, P.; Depner, K.; Edwards, S.; Garin-Bastuji, B.; Good, M.; Gortázar Schmidt, C.; Michel, V.; et al. Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): Enzootic bovine leukosis (EBL). EFSA J. 2017, 15, e04956. [Google Scholar] [PubMed]
- Polat, M.; Takeshima, S.N.; Aida, Y. Epidemiology and genetic diversity of bovine leukemia virus. Virol. J. 2017, 14, 209. [Google Scholar] [CrossRef] [PubMed]
- Jimba, M.; Takeshima, S.N.; Murakami, H.; Kohara, J.; Kobayashi, N.; Matsuhashi, T.; Ohmori, T.; Nunoya, T.; Aida, Y. BLV-CoCoMo-qPCR: A useful tool for evaluating bovine leukemia virus infection status. BMC Vet. Res. 2012, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Tajima, S.; Ikawa, Y.; Aida, Y. Complete bovine leukemia virus (BLV) provirus is conserved in BLV-infected cattle throughout the course of B-cell lymphosarcoma development. J. Virol. 1998, 72, 7569–7576. [Google Scholar] [CrossRef] [PubMed]
- Kettmann, R.; Deschamps, J.; Cleuter, Y.; Couez, D.; Burny, A.; Marbaix, G. Leukemogenesis by bovine leukemia virus: Proviral DNA integration and lack of RNA expression of viral long terminal repeat and 3′ proximate cellular sequences. Proc. Natl. Acad. Sci. USA 1982, 79, 2465–2469. [Google Scholar] [CrossRef] [PubMed]
- Jimba, M.; Takeshima, S.N.; Matoba, K.; Endoh, D.; Aida, Y. BLV-CoCoMo-qPCR: Quantitation of bovine leukemia virus proviral load using the CoCoMo algorithm. Retrovirology 2010, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Inagaki, Y.; Ohnuki, N.; Sato, R.; Murakami, S.; Imakawa, K. Increasing Bovine leukemia virus (BLV) proviral load is a risk factor for progression of Enzootic bovine leucosis: A prospective study in Japan. Prev. Vet. Med. 2019, 178, 104680. [Google Scholar] [CrossRef] [PubMed]
- Somura, Y.; Sugiyama, E.; Fujikawa, H.; Murakami, K. Comparison of the copy numbers of bovine leukemia virus in the lymph nodes of cattle with enzootic bovine leukosis and cattle with latent infection. Arch. Virol. 2014, 159, 2693–2697. [Google Scholar] [CrossRef] [PubMed]
- Juliarena, M.A.; Barrios, C.N.; Ceriani, M.C.; Esteban, E.N. Hot topic: Bovine leukemia virus (BLV)-infected cows with low proviral load are not a source of infection for BLV-free cattle. J. Dairy Sci. 2016, 99, 4586–4589. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Watanuki, S.; Murakami, H.; Sato, R.; Ishizaki, H.; Aida, Y. Development of a luminescence syncytium induction assay (LuSIA) for easily detecting and quantitatively measuring bovine leukemia virus infection. Arch. Virol. 2018, 163, 1519–1530. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Borjigin, L.; Sato, H.; Takeshima, S.N.; Asaji, S.; Ishizaki, H.; Kawashima, K.; Obuchi, Y.; Sunaga, S.; Ando, A.; et al. Kinetic Study of BLV Infectivity in BLV Susceptible and Resistant Cattle in Japan from 2017 to 2019. Pathogens 2021, 10, 1281. [Google Scholar] [CrossRef] [PubMed]
- Borjigin, L.; Watanuki, S.; Hamada, R.; Bai, L.; Hirose, T.; Sato, H.; Yoneyama, S.; Yasui, A.; Yasuda, S.; Yamanaka, R.; et al. Effectiveness of integrated bovine leukemia virus eradication strategies utilizing cattle carrying resistant and susceptible histocompatibility complex class II DRB3 alleles. J. Dairy Sci. 2023, 106, 9393–9409. [Google Scholar] [CrossRef] [PubMed]
- Mekata, H.; Sekiguchi, S.; Konnai, S.; Kirino, Y.; Horii, Y.; Norimine, J. Horizontal transmission and phylogenetic analysis of bovine leukemia virus in two districts of Miyazaki, Japan. J. Vet. Med. Sci. 2015, 77, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Watanuki, S.; Takeshima, S.N.; Borjigin, L.; Sato, H.; Bai, L.; Murakami, H.; Sato, R.; Ishizaki, H.; Matsumoto, Y.; Aida, Y. Visualizing bovine leukemia virus (BLV)-infected cells and measuring BLV proviral loads in the milk of BLV seropositive dams. Vet. Res. 2019, 50, 102. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Kitamura-Muramatsu, Y.; Saito, S.; Ishizaki, H.; Nakano, M.; Haga, S.; Matoba, K.; Ohno, A.; Murakami, H.; Takeshima, S.N.; et al. Detection of the BLV provirus from nasal secretion and saliva samples using BLV-CoCoMo-qPCR-2: Comparison with blood samples from the same cattle. Virus Res. 2015, 210, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Kohara, J.; Bai, L.; Takeshima, S.N.; Matsumoto, Y.; Hirai, T.; Aida, Y. Correlation between the Biodistribution of Bovine Leukemia Virus in the Organs and the Proviral Load in the Peripheral Blood during Early Stages of Experimentally Infected Cattle. Pathogens 2023, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.W.; Borjigin, L.; Saito, S.; Fukunaga, K.; Saitou, E.; Okazaki, K.; Mizutani, T.; Wada, S.; Takeshima, S.N.; Aida, Y. BoLA-DRB3 Polymorphism is Associated with Differential Susceptibility to Bovine Leukemia Virus-Induced Lymphoma and Proviral Load. Viruses 2020, 12, 352. [Google Scholar] [CrossRef] [PubMed]
- Ohno, A.; Takeshima, S.N.; Matsumoto, Y.; Aida, Y. Risk factors associated with increased bovine leukemia virus proviral load in infected cattle in Japan from 2012 to 2014. Virus Res. 2015, 210, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, S.N.; Kitamura-Muramatsu, Y.; Yuan, Y.; Polat, M.; Saito, S.; Aida, Y. BLV-CoCoMo-qPCR-2: Improvements to the BLV-CoCoMo-qPCR assay for bovine leukemia virus by reducing primer degeneracy and constructing an optimal standard curve. Arch. Virol. 2015, 160, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Chang, L.; Yan, Y.; Wang, L. Development and validation of a duplex real-time PCR for the rapid detection and quantitation of HTLV-1. Virol. J. 2023, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Inabe, K.; Ikuta, K.; Aida, Y. Transmission and propagation in cell culture of virus produced by cells transfected with an infectious molecular clone of bovine leukemia virus. Virology 1998, 245, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, R.; Inabe, K.; Otsuki, H.; Kurokawa, K.; Dohmae, N.; Aida, Y. Three YXXL Sequences of a Bovine Leukemia Virus Transmembrane Protein are Independently Required for Fusion Activity by Controlling Expression on the Cell Membrane. Viruses 2019, 11, 1140. [Google Scholar] [CrossRef] [PubMed]
- Wolffs, P.; Grage, H.; Hagberg, O.; Rådström, P. Impact of DNA polymerases and their buffer systems on quantitative real-time PCR. J. Clin. Microbiol. 2004, 42, 408–411. [Google Scholar] [CrossRef]
- Rahman, M.M.; Badr, Y.; Kamatari, Y.O.; Kitamura, Y.; Shimizu, K.; Okada, A.; Inoshima, Y. Data on proteomic analysis of milk extracellular vesicles from bovine leukemia virus-infected cattle. Data Brief. 2020, 33, 106510. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Ishikawa, H.; Yamauchi, M.; Takashima, S.; Kamatari, Y.O.; Shimizu, K.; Okada, A.; Inoshima, Y. Characterization of mRNA Signature in Milk Small Extracellular Vesicles from Cattle Infected with Bovine Leukemia Virus. Pathogens 2023, 12, 1239. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuchi, A.; Watanuki, S.; Borjigin, L.; Sato, H.; Bai, L.; Matsuura, R.; Kuroda, M.; Murakami, H.; Sato, R.; Asaji, S.; et al. Polymorphism Controls Proviral Load and Infectivity of Bovine Leukemia Virus (BLV) in Milk. Pathogens 2022, 11, 210. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuchi, A.; Bao, A.; Watanuki, S.; Matsuura, R.; Borjigin, L.; Bai, L.; Kuroda, M.; Matsumoto, Y.; Kohara, J.; Aida, Y. Anti-BLV antibodies in whey correlate with bovine leukemia virus disease progression and. Front. Vet. Sci. 2022, 9, 1038101. [Google Scholar] [CrossRef] [PubMed]
- Inenaga, T.; Fukuoka, K.; Sumida, M.; Aiba, S.; Nishikaku, K.; Matsuno, Y.; Kobayashi, T.; Imakawa, K. Low proviral load in the Kumamoto strain of Japanese Brown cattle infected with the bovine leukemia virus. BMC Vet. Res. 2023, 19, 185. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Song, J.K.; Shin, S.; Kim, H. Comparison of Multiplex Real-Time PCR and PCR-Reverse Blot Hybridization Assays for the Direct and Rapid Detection of Porcine Circovirus Type 2 Genotypes. Front. Vet. Sci. 2020, 7, 200. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhai, Z.; Huang, K.; Zhang, N.; Yuan, Y.; Shang, Y.; Luo, Y. A novel universal primer-multiplex-PCR method with sequencing gel electrophoresis analysis. PLoS ONE 2012, 7, e22900. [Google Scholar] [CrossRef] [PubMed]
- Rola-Łuszczak, M.; Finnegan, C.; Olech, M.; Choudhury, B.; Kuźmak, J. Development of an improved real time PCR for the detection of bovine leukaemia provirus nucleic acid and its use in the clarification of inconclusive serological test results. J. Virol. Methods 2013, 189, 258–264. [Google Scholar] [CrossRef]
- Lew, A.E.; Bock, R.E.; Molloy, J.B.; Minchin, C.M.; Robinson, S.J.; Steer, P. Sensitive and specific detection of proviral bovine leukemia virus by 5’ Taq nuclease PCR using a 3’ minor groove binder fluorogenic probe. J. Virol. Methods 2004, 115, 167–175. [Google Scholar] [CrossRef]
- Yoneyama, S.; Kobayashi, S.; Matsunaga, T.; Tonosaki, K.; Leng, D.; Sakai, Y.; Yamada, S.; Kimura, A.; Ichijo, T.; Hikono, H.; et al. Comparative Evaluation of Three Commercial Quantitative Real-Time PCRs Used in Japan for Bovine Leukemia Virus. Viruses 2022, 14, 1182. [Google Scholar] [CrossRef]
- Elnifro, E.M.; Ashshi, A.M.; Cooper, R.J.; Klapper, P.E. Multiplex PCR: Optimization and application in diagnostic virology. Clin. Microbiol. Rev. 2000, 13, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Depner, K.; Schirrmeier, H.; Beer, M. A universal heterologous internal control system for duplex real-time RT-PCR assays used in a detection system for pestiviruses. J. Virol. Methods 2006, 136, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, S.; Iguchi, Y.; Shibata, N.; Teramura, I.; Kitagawa, T.; Yamanaka, H. Real-time multiplex PCR for simultaneous detection of multiple species from environmental DNA: An application on two Japanese medaka species. Sci. Rep. 2018, 8, 9138. [Google Scholar] [CrossRef] [PubMed]
- Chua, A.L.; Elina, H.T.; Lim, B.H.; Yean, C.Y.; Ravichandran, M.; Lalitha, P. Development of a dry reagent-based triplex PCR for the detection of toxigenic and non-toxigenic Vibrio cholerae. J. Med. Microbiol. 2011, 60, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Seise, B.; Pollok, S.; Seyboldt, C.; Weber, K. Dry-reagent-based PCR as a novel tool for the rapid detection of Clostridium spp. J. Med. Microbiol. 2013, 62, 1588–1591. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, S.; Ramlal, S.; Kingston, J.; Batra, H.V. Thermostabilization of indigenous multiplex polymerase chain reaction reagents for detection of enterotoxigenic Staphylococcus aureus. J. Microbiol. Immunol. Infect. 2018, 51, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Kamau, E.; Alemayehu, S.; Feghali, K.C.; Juma, D.W.; Blackstone, G.M.; Marion, W.R.; Obare, P.; Ogutu, B.; Ockenhouse, C.F. Sample-ready multiplex qPCR assay for detection of malaria. Malar. J. 2014, 13, 158. [Google Scholar] [CrossRef] [PubMed]
- Heenemann, K.; Lapp, S.; Teifke, J.P.; Fichtner, D.; Mettenleiter, T.C.; Vahlenkamp, T.W. Development of a Bovine leukemia virus polymerase gene-based real-time polymerase chain reaction and comparison with an envelope gene-based assay. J. Vet. Diagn. Investig. 2012, 24, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Gillet, N.A.; Hamaidia, M.; de Brogniez, A.; Gutiérrez, G.; Renotte, N.; Reichert, M.; Trono, K.; Willems, L. Bovine Leukemia Virus Small Noncoding RNAs Are Functional Elements That Regulate Replication and Contribute to Oncogenesis In Vivo. PLoS Pathog. 2016, 12, e1005588. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.I.; Alvarez, I.; Trono, K.G.; Jaworski, J.P. Quantification of bovine leukemia virus proviral DNA using a low-cost real-time polymerase chain reaction. J. Dairy Sci. 2018, 101, 6366–6374. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Notsu, K.; Matsuura, Y.; Mitoma, S.; El Daous, H.; Norimine, J.; Sekiguchi, S. Development of droplet digital PCR for quantification of bovine leukemia virus proviral load using unpurified genomic DNA. J. Virol. Methods 2023, 315, 114706. [Google Scholar] [CrossRef] [PubMed]
- De Brun, M.L.; Cosme, B.; Petersen, M.; Alvarez, I.; Folgueras-Flatschart, A.; Flatschart, R.; Panei, C.J.; Puentes, R. Development of a droplet digital PCR assay for quantification of the proviral load of bovine leukemia virus. J. Vet. Diagn. Investig. 2022, 34, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, J.P.; Pluta, A.; Rola-Łuszczak, M.; McGowan, S.L.; Finnegan, C.; Heenemann, K.; Carignano, H.A.; Alvarez, I.; Murakami, K.; Willems, L.; et al. Interlaboratory Comparison of Six Real-Time PCR Assays for Detection of Bovine Leukemia Virus Proviral DNA. J. Clin. Microbiol. 2018, 56, e00304-18. [Google Scholar] [CrossRef] [PubMed]



| Sample No. | Single-CoCoMo a | Dual-CoCoMo b | ||||
|---|---|---|---|---|---|---|
| TBIRD c | GeneAce d | |||||
| Mean PVL e | SD f | Mean PVL | SD | Mean PVL | SD | |
| B1 | 112 | 18 | 44 | 21 | 51 | 8 |
| B2 | 435 | 38 | 175 | 24 | 318 | 29 |
| B3 | 937 | 85 | 520 | 45 | 894 | 76 |
| B4 | 3027 | 147 | 2079 | 224 | 3155 | 224 |
| B5 | 5902 | 300 | 6699 | 465 | 7730 | 301 |
| B6 | 12,046 | 521 | 13,950 | 588 | 15,175 | 678 |
| B7 | 14,883 | 365 | 18,699 | 1369 | 19,680 | 329 |
| B8 | 40,870 | 1941 | 44,153 | 3765 | 47,304 | 1369 |
| B9 | 63,016 | 2118 | 61,627 | 3799 | 66,705 | 2516 |
| B10 | 119,317 | 2804 | 104,926 | 5260 | 116,959 | 4202 |
| Method | qPCR Master Mix | Sample No. | Proviral Load (Copies/105 Cells) | Intra-Assay CV f (%) | Inter-Assay CV g (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Exp. 1 Mean (SD e) | Exp. 2 Mean (SD) | Exp. 3 Mean (SD) | Exp. 1 | Exp. 2 | Exp. 3 | Exp. 1~3 | |||
| Single-CoCoMo a | TBIRD c | B1 | 129 (7) | 93 (6) | 114 (16) | 5.7 | 6.9 | 13.6 | 16.1 |
| B2 | 420 (15) | 425 (47) | 461 (44) | 3.6 | 11.0 | 9.6 | 5.1 | ||
| B3 | 910 (16) | 944 (160) | 957 (41) | 1.8 | 16.9 | 4.2 | 2.6 | ||
| B4 | 2997 (164) | 3039 (63) | 3044 (231) | 5.5 | 2.1 | 7.6 | 0.9 | ||
| B5 | 6015 (318) | 5764 (219) | 5927 (404) | 5.3 | 3.8 | 6.8 | 2.2 | ||
| B6 | 12,030 (585) | 11,895 (224) | 12,213 (786) | 4.9 | 1.9 | 6.4 | 1.3 | ||
| B7 | 15,019 (423) | 14,929 (412) | 14,702 (321) | 2.8 | 2.8 | 2.2 | 1.1 | ||
| B8 | 41,771 (1971) | 41,638 (959) | 39,201 (1997) | 4.7 | 2.3 | 5.1 | 3.5 | ||
| B9 | 64,189 (1713) | 62,639 (3337) | 62,219 (806) | 2.7 | 5.3 | 1.3 | 1.6 | ||
| B10 | 119,641 (2699) | 121,469 (1137) | 116,841 (2562) | 2.3 | 0.9 | 2.2 | 2.0 | ||
| Dual-CoCoMo b | TBIRD | B1 | 33 (10) | 30 (9) | 68 (13) | 29.3 | 29.9 | 19.6 | 48.4 |
| B2 | 182 (19) | 189 (13) | 155 (30) | 10.5 | 6.8 | 19.5 | 10.2 | ||
| B3 | 528 (57) | 517 (56) | 514 (41) | 10.8 | 10.9 | 8.0 | 1.4 | ||
| B4 | 2119 (325) | 1923 (113) | 2196 (153) | 15.4 | 5.9 | 7.0 | 6.8 | ||
| B5 | 6131 (22) | 7008 (277) | 6958 (242) | 0.4 | 4.0 | 3.5 | 7.4 | ||
| B6 | 13,827 (238) | 13,381 (251) | 14,641 (198) | 1.7 | 1.9 | 1.4 | 4.6 | ||
| B7 | 17,967 (1237) | 18,099 (902) | 20,031 (1072) | 6.9 | 5.0 | 5.4 | 6.2 | ||
| B8 | 41,453 (604) | 41,897 (259) | 49,109 (924) | 1.5 | 0.6 | 1.9 | 9.7 | ||
| B9 | 60,183 (1327) | 58,469 (1537) | 66,229 (1933) | 2.2 | 2.6 | 2.9 | 6.6 | ||
| B10 | 101,333 (2817) | 102,531 (1014) | 110,915 (4468) | 2.8 | 1.0 | 4.0 | 5.0 | ||
| GeneAce d | B1 | 57 (10) | 47 (4) | 49 (7) | 18.1 | 9.1 | 14.2 | 10.4 | |
| B2 | 306 (27) | 323 (30) | 324 (36) | 8.8 | 9.3 | 11.1 | 3.2 | ||
| B3 | 912 (26) | 903 (140) | 867 (31) | 2.8 | 15.5 | 3.6 | 2.7 | ||
| B4 | 3178 (279) | 3240 (158) | 3048 (263) | 8.8 | 4.9 | 8.6 | 3.1 | ||
| B5 | 7768 (170) | 7800 (305) | 7621 (462) | 2.2 | 3.9 | 6.1 | 1.2 | ||
| B6 | 15,025 (338) | 15,776 (224) | 14,725 (891) | 2.3 | 1.4 | 6.0 | 3.6 | ||
| B7 | 19,689 (346) | 19,911 (110) | 19,441 (366) | 1.8 | 0.6 | 1.9 | 1.2 | ||
| B8 | 47,481 (823) | 48,245 (1476) | 46,185 (1176) | 1.7 | 3.1 | 2.5 | 2.2 | ||
| B9 | 69,026 (655) | 66,257 (2987) | 64,833 (1524) | 0.9 | 4.5 | 2.3 | 3.2 | ||
| B10 | 117,881 (3506) | 119,300 (5001) | 113,695 (2800) | 3.0 | 4.2 | 2.5 | 2.5 | ||
| pBLV-IF 2 a (Copy Number) | BLV Provirus Detection Frequency (%) | |||||
|---|---|---|---|---|---|---|
| Single-CoCoMo b | Liquid Dual-CoCoMo c | Dry Dual-CoCoMo | ||||
| 100 | 3/3 d | (100) | 3/3 | (100) | 3/3 | (100) |
| 50 | 3/3 | (100) | 3/3 | (100) | 3/3 | (100) |
| 25 | 3/3 | (100) | 3/3 | (100) | 3/3 | (100) |
| 12.5 | 3/3 | (100) | 3/3 | (100) | 3/3 | (100) |
| 6.25 | 3/3 | (100) | 3/3 | (100) | 2/3 | (67) |
| 3.125 | 8/10 | (80) | 7/10 | (70) | 7/10 | (70) |
| 1.5625 | 4/10 | (40) | 3/10 | (30) | 3/10 | (30) |
| 0.78125 | 3/10 | (30) | 1/10 | (10) | 0/10 | (0) |
| 0 | 0/3 | (0) | 0/3 | (0) | 0/3 | (0) |
| Sample | ELISA a | Proviral Load b (Copies/105 Cells) | Sample | ELISA | Proviral Load (Copies/105 Cells) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Single-CoCoMo | Liquid Dual-CoCoMo | Dry Dual-CoCoMo | Single-CoCoMo | Liquid Dual-CoCoMo | Dry Dual-CoCoMo | ||||
| BLV-uninfected cattle | BLV-infected cattle without lymphoma | ||||||||
| J2 | - | 0 | 0 | 0 | S42 | + | 8130 | 12,271 | 11,065 |
| J5 | - | 0 | 0 | 0 | S56 | + | 8132 | 12,168 | 10,102 |
| J9 | - | 0 | 0 | 0 | H63 | + | 9168 | 8393 | 12,971 |
| H10 | - | 0 | 0 | 0 | H82 | + | 9342 | 6379 | 11,372 |
| J16 | - | 0 | 0 | 0 | S24 | + | 9810 | 13,238 | 12,318 |
| J24 | - | 0 | 0 | 0 | S54 | + | 11,242 | 14,063 | 13,927 |
| J29 | - | 0 | 0 | 0 | H69 | + | 11,844 | 10,444 | 12,204 |
| H28 | - | 0 | 0 | 0 | J39k | + | 12,183 | 10,536 | 11,956 |
| A1 | - | 0 | 0 | 0 | J23k | + | 12,702 | 14,566 | 14,549 |
| A2 | - | 0 | 0 | 0 | H57 | + | 13,435 | 16,964 | 17,127 |
| A3 | - | 0 | 0 | 0 | C77 | + | 13,441 | 11,529 | 12,966 |
| BLV-infected cattle without lymphoma | J84 | + | 15,019 | 19,689 | 15,142 | ||||
| S23 | + | 13 | 0 | 5 | J42 | + | 17,012 | 17,014 | 18,212 |
| S7 | + | 24 | 2 | 4 | H4 | + | 18,874 | 21,916 | 21,665 |
| S34 | + | 26 | 4 | 13 | H75 | + | 20,212 | 22,598 | 20,631 |
| J8 | + | 32 | 3 | 15 | S39 | + | 21,042 | 27,235 | 24,285 |
| S10 | + | 33 | 11 | 34 | S43 | + | 23,270 | 28,199 | 26,686 |
| Bs1 | + | 37 | 1 | 9 | J41k | + | 23,927 | 21,800 | 21,335 |
| S40 | - | 38 | 3 | 14 | J17k | + | 25,710 | 27,802 | 24,704 |
| S37 | + | 88 | 24 | 66 | S50 | + | 26,127 | 34,154 | 31,848 |
| S14 | + | 96 | 35 | 90 | sS1 | + | 26,131 | 26,053 | 23,207 |
| S35 | + | 110 | 53 | 103 | S52 | + | 27,660 | 37,228 | 35,544 |
| C78 | + | 129 | 57 | 15 | sS3 | + | 28,132 | 28,830 | 25,113 |
| C95 | + | 276 | 69 | 170 | C72 | + | 31,463 | 27,133 | 25,000 |
| S11 | + | 386 | 274 | 462 | H85 | + | 33,257 | 44,667 | 39,258 |
| H100 | + | 420 | 306 | 207 | H103 | + | 35,373 | 37,492 | 36,080 |
| S53 | + | 567 | 517 | 839 | H3 | + | 35,952 | 37,429 | 33,172 |
| H65 | + | 910 | 912 | 564 | S13 | + | 37,760 | 47,798 | 41,548 |
| S44 | + | 1657 | 2062 | 2092 | J37k | + | 38,719 | 39,600 | 37,791 |
| S36 | + | 1891 | 2159 | 2140 | J22k | + | 40,527 | 44,114 | 42,501 |
| S21 | + | 2081 | 2657 | 2822 | H102 | + | 41,553 | 46,496 | 37,861 |
| C60 | + | 2997 | 3178 | 1914 | H80 | + | 41,771 | 47,481 | 40,279 |
| S51 | + | 3116 | 4172 | 3982 | J21 | + | 42,014 | 45,401 | 41,479 |
| S49 | + | 4866 | 5767 | 5854 | sS2 | + | 43,500 | 44,218 | 41,095 |
| S28 | + | 4956 | 6022 | 6314 | J18k | + | 47,530 | 48,853 | 45,261 |
| S55 | + | 5402 | 6457 | 5822 | H107 | + | 47,893 | 54,363 | 48,606 |
| S12 | + | 5565 | 7740 | 7038 | J7k | + | 52,898 | 51,397 | 50,210 |
| H106 | + | 5722 | 6075 | 7377 | H87 | + | 64,189 | 69,026 | 64,248 |
| H88 | + | 6015 | 7768 | 4893 | H51 | + | 119,641 | 117,881 | 120,377 |
| J58 | + | 6849 | 7672 | 7945 | BLV-infected cattle with lymphoma | ||||
| C94 | + | 7309 | 5943 | 8482 | E3 | + | 42,080 | 37,035 | 36,511 |
| H81 | + | 7882 | 6493 | 9152 | E57 | + | 118,919 | 118,257 | 131,025 |
| (a) | Single-CoCoMo | Total | ||
| Positive | Negative | |||
| Liquid Dual-CoCoMo | Positive | 70 | 0 | 70 |
| Negative | 1 | 11 | 12 | |
| Total | 71 | 11 | 82 | |
| Sensitivity (%) | Specificity (%) | |||
| (95% CI) | (95% CI) | |||
| 98.6 | 100 | |||
| (92.4–100) | (71.5–100) | |||
| (b) | Single-CoCoMo | Total | ||
| Positive | Negative | |||
| Dry Dual-CoCoMo | Positive | 71 | 0 | 71 |
| Negative | 0 | 11 | 11 | |
| Total | 71 | 11 | 82 | |
| Sensitivity (%) | Specificity (%) | |||
| (95% CI) | (95% CI) | |||
| 100 | 100 | |||
| (94.9–100) | (71.5–100) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanuki, S.; Shoji, K.; Izawa, M.; Okami, M.; Ye, Y.; Bao, A.; Liu, Y.; Saitou, E.; Sugiyama, K.; Endo, M.; et al. Development of Dry and Liquid Duplex Reagent Mix-Based Polymerase Chain Reaction Assays as Novel Tools for the Rapid and Easy Quantification of Bovine Leukemia Virus (BLV) Proviral Loads. Viruses 2024, 16, 1016. https://doi.org/10.3390/v16071016
Watanuki S, Shoji K, Izawa M, Okami M, Ye Y, Bao A, Liu Y, Saitou E, Sugiyama K, Endo M, et al. Development of Dry and Liquid Duplex Reagent Mix-Based Polymerase Chain Reaction Assays as Novel Tools for the Rapid and Easy Quantification of Bovine Leukemia Virus (BLV) Proviral Loads. Viruses. 2024; 16(7):1016. https://doi.org/10.3390/v16071016
Chicago/Turabian StyleWatanuki, Sonoko, Kazuyuki Shoji, Masaki Izawa, Mitsuaki Okami, Yingbao Ye, Aronggaowa Bao, Yulin Liu, Etsuko Saitou, Kimikazu Sugiyama, Michiru Endo, and et al. 2024. "Development of Dry and Liquid Duplex Reagent Mix-Based Polymerase Chain Reaction Assays as Novel Tools for the Rapid and Easy Quantification of Bovine Leukemia Virus (BLV) Proviral Loads" Viruses 16, no. 7: 1016. https://doi.org/10.3390/v16071016







