Local Power: The Role of Tissue-Resident Immunity in Human Genital Herpes Simplex Virus Reactivation
Abstract
:1. Herpes Simplex Virus 2 Reactivation
2. Tissue Resident Memory (TRM) T Cells
3. TRM during Human HSV Infection
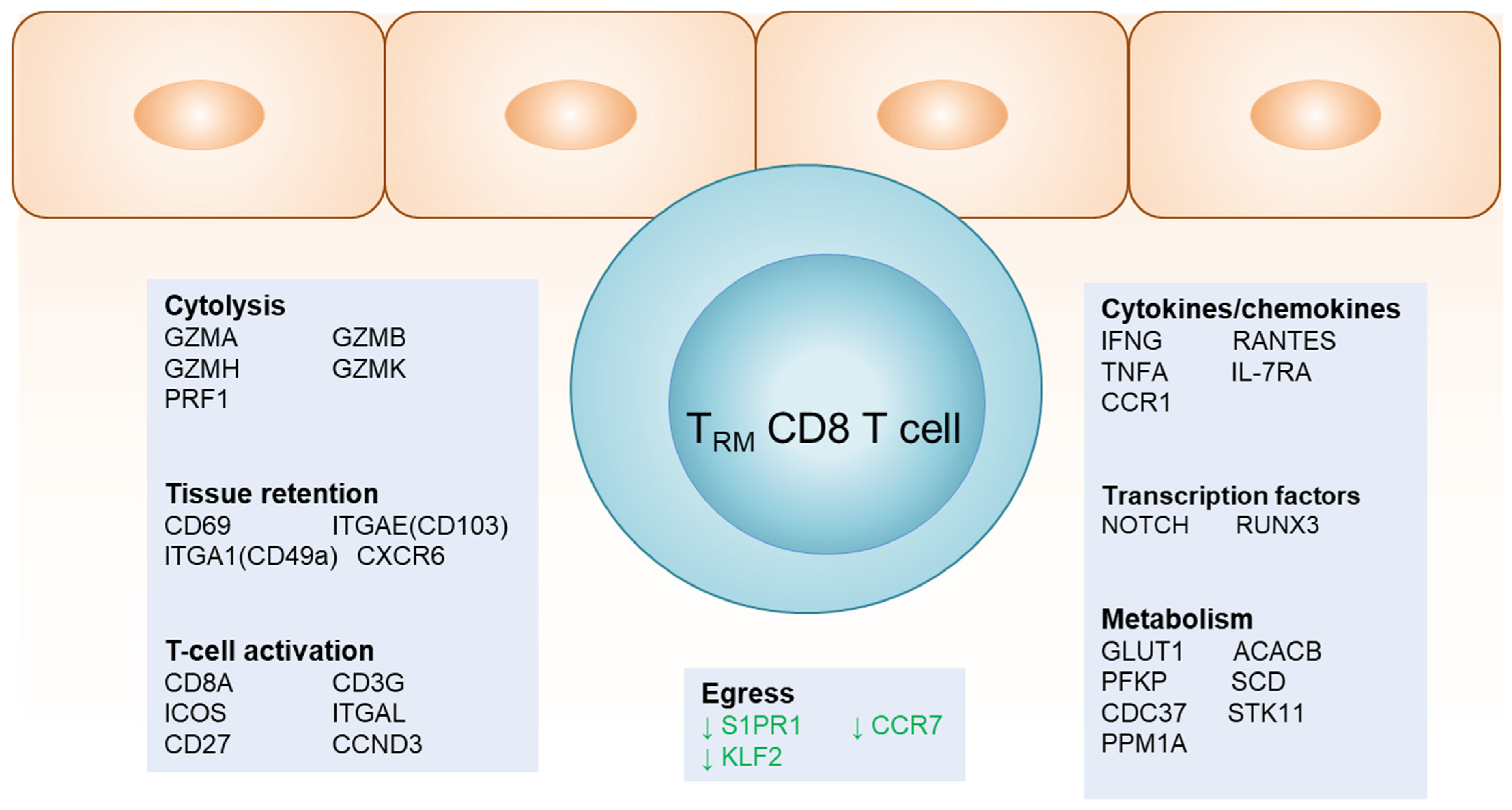
4. Gene Signatures of TRM at the DEJ
5. CD4 T Cells in HSV-2-Affected Tissue
6. Clonality of TRM at the DEJ
7. Impact of TRM on the Epithelia Microenvironment
8. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.E.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PLoS ONE 2015, 10, e0140765. [Google Scholar] [CrossRef]
- Roizman, B.; Knipe, D.; Whitley, R. Herpes Simplex Viruses. In Fields Virology, 6th ed.; Knipe, D.M., Howley, P., Eds.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2021. [Google Scholar]
- Corey, L.; Adams, H.G.; Brown, Z.A.; Holmes, K.K. Genital Herpes Simplex Virus Infections: Clinical Manifestations, Course, and Complications. Ann. Intern. Med. 1983, 98, 958–972. [Google Scholar] [CrossRef]
- Mark, K.E.; Wald, A.; Magaret, A.S.; Selke, S.; Olin, L.; Huang, M.; Corey, L. Rapidly Cleared Episodes of Herpes Simplex Virus Reactivation in Immunocompetent Adults. J. Infect. Dis. 2008, 198, 1141–1149. [Google Scholar] [CrossRef]
- Schiffer, J.T.; Abu-Raddad, L.; Mark, K.E.; Zhu, J.; Selke, S.; Magaret, A.; Wald, A.; Corey, L. Frequent Release of Low Amounts of Herpes Simplex Virus from Neurons: Results of a Mathematical Model. Sci. Transl. Med. 2009, 1, 7ra16. [Google Scholar] [CrossRef] [PubMed]
- Wald, A.; Huang, M.; Carrell, D.; Selke, S.; Corey, L. Polymerase Chain Reaction for Detection of Herpes Simplex Virus (HSV) DNA on Mucosal Surfaces: Comparison with HSV Isolation in Cell Culture. J. Infect. Dis. 2003, 188, 1345–1351. [Google Scholar] [CrossRef]
- Wald, A.; Zeh, J.; Selke, S.; Ashley, R.L.; Corey, L. Virologic Characteristics of Subclinical and Symptomatic Genital Herpes Infections. N. Engl. J. Med. 1995, 333, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, J.T.; Wald, A.; Selke, S.; Corey, L.; Magaret, A. The Kinetics of Mucosal Herpes Simplex Virus–2 Infection in Humans: Evidence for Rapid Viral-Host Interactions. J. Infect. Dis. 2011, 204, 554–561. [Google Scholar] [CrossRef]
- Tronstein, E.; Johnston, C.; Huang, M.-L.; Selke, S.; Magaret, A.; Warren, T.; Corey, L.; Wald, A. Genital Shedding of Herpes Simplex Virus Among Symptomatic and Asymptomatic Persons with HSV-2 Infection. JAMA 2011, 305, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.; Zhu, J.; Jing, L.; Laing, K.J.; McClurkan, C.M.; Klock, A.; Diem, K.; Jin, L.; Stanaway, J.; Tronstein, E.; et al. Virologic and Immunologic Evidence of Multifocal Genital Herpes Simplex Virus 2 Infection. J. Virol. 2014, 88, 4921–4931. [Google Scholar] [CrossRef]
- Schiffer, J.T.; Swan, D.; Al Sallaq, R.; Magaret, A.; Johnston, C.; Mark, K.E.; Selke, S.; Ocbamichael, N.; Kuntz, S.; Zhu, J.; et al. Rapid localized spread and immunologic containment define Herpes simplex virus-2 reactivation in the human genital tract. eLife 2013, 2, e00288. [Google Scholar] [CrossRef]
- Zhu, J.; Koelle, D.M.; Cao, J.; Vazquez, J.; Huang, M.L.; Hladik, F.; Wald, A.; Corey, L. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J. Exp. Med. 2007, 204, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Masopust, D.; Vezys, V.; Marzo, A.L.; Lefrançois, L. Preferential Localization of Effector Memory Cells in Nonlymphoid Tissue. Science 2001, 291, 2413–2417. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Lenig, D.; Förster, R.; Lipp, M.; Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999, 401, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Boyman, O.; Hefti, H.P.; Conrad, C.; Nickoloff, B.J.; Suter, M.; Nestle, F.O. Spontaneous Development of Psoriasis in a New Animal Model Shows an Essential Role for Resident T Cells and Tumor Necrosis Factor-α. J. Exp. Med. 2004, 199, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Clark, R.A.; Liu, L.; Wagers, A.J.; Fuhlbrigge, R.C.; Kupper, T.S. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature 2012, 483, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, T.; Wakim, L.M.; Eidsmo, L.; Reading, P.C.; Heath, W.R.; Carbone, F.R. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 2009, 10, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Carbone, F.R. Tissue-Resident Memory T Cells and Fixed Immune Surveillance in Nonlymphoid Organs. J. Immunol. 2015, 195, 17–22. [Google Scholar] [CrossRef]
- Clark, R.A. Resident memory T cells in human health and disease. Sci. Transl. Med. 2015, 7, 269rv1. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.N.; Mackay, L.K. Tissue-resident memory T cells: Local specialists in immune defence. Nat. Rev. Immunol. 2016, 16, 79–89. [Google Scholar] [CrossRef]
- Schenkel, J.M.; Masopust, D. Tissue-Resident Memory T Cells. Immunity 2014, 41, 886–897. [Google Scholar] [CrossRef]
- Bromley, S.K.; Thomas, S.Y.; Luster, A.D. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat. Immunol. 2005, 6, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Flavell, R.A. Cutting edge: Regulation of T cell trafficking and primary immune responses by sphingosine 1-phosphate receptor 1. J. Immunol. 2005, 174, 2485–2488. [Google Scholar] [CrossRef] [PubMed]
- Debes, G.F.; Arnold, C.N.; Young, A.J.; Krautwald, S.; Lipp, M.; Hay, J.B.; Butcher, E.C. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat. Immunol. 2005, 6, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Matloubian, M.; Lo, C.G.; Cinamon, G.; Lesneski, M.J.; Xu, Y.; Brinkmann, V.; Allende, M.L.; Proia, R.L.; Cyster, J.G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004, 427, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Skon, C.N.; Lee, J.-Y.; Anderson, K.G.; Masopust, D.; Hogquist, K.A.; Jameson, S.C. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat. Immunol. 2013, 14, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Cheuk, S.; Schlums, H.; Sérézal, I.G.; Martini, E.; Chiang, S.C.; Marquardt, N.; Gibbs, A.; Detlofsson, E.; Introini, A.; Forkel, M.; et al. CD49a Expression Defines Tissue-Resident CD8+ T Cells Poised for Cytotoxic Function in Human Skin. Immunity 2017, 46, 287–300. [Google Scholar] [CrossRef]
- Mackay, L.K.; Rahimpour, A.; Ma, J.Z.; Collins, N.; Stock, A.T.; Hafon, M.-L.; Vega-Ramos, J.; Lauzurica, P.; Mueller, S.N.; Stefanovic, T.; et al. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 2013, 14, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Masopust, D.; Vezys, V.; Wherry, E.J.; Barber, D.L.; Ahmed, R. Cutting Edge: Gut Microenvironment Promotes Differentiation of a Unique Memory CD8 T Cell Population. J. Immunol. 2006, 176, 2079–2083. [Google Scholar] [CrossRef]
- Mueller, S.N.; Gebhardt, T.; Carbone, F.R.; Heath, W.R. Memory T Cell Subsets, Migration Patterns, and Tissue Residence. Annu. Rev. Immunol. 2013, 31, 137–161. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Magaret, A.S.; Celum, C.; Wald, A.; Longini, I.M., Jr.; Self, S.G.; Corey, L. Genital Herpes Has Played a More Important Role than Any Other Sexually Transmitted Infection in Driving HIV Prevalence in Africa. PLoS ONE 2008, 3, e2230. [Google Scholar] [CrossRef]
- Bergsbaken, T.; Bevan, M.J. Proinflammatory microenvironments within the intestine regulate the differentiation of tissue-resident CD8+ T cells responding to infection. Nat. Immunol. 2015, 16, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Iijima, N.; Iwasaki, A. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 2014, 346, 93–98. [Google Scholar] [CrossRef]
- Schenkel, J.M.; Fraser, K.A.; Masopust, D. Cutting edge: Resident memory CD8 T cells occupy frontline niches in secondary lymphoid organs. J. Immunol. 2014, 192, 2961–2964. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.V.; Ma, W.; Miron, M.; Granot, T.; Guyer, R.S.; Carpenter, D.J.; Senda, T.; Sun, X.; Ho, S.-H.; Lerner, H.; et al. Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites. Cell Rep. 2017, 20, 2921–2934. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.N.; Zaid, A.; Carbone, F.R. Tissue-Resident T Cells: Dynamic Players in Skin Immunity. Front. Immunol. 2014, 5, 332. [Google Scholar] [CrossRef]
- Schenkel, J.M.; Fraser, K.A.; Beura, L.K.; Pauken, K.E.; Vezys, V.; Masopust, D. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 2014, 346, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Heeg, M.; Goldrath, A.W. Insights into phenotypic and functional CD8+ TRM heterogeneity. Immunol. Rev. 2023, 316, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Zaid, A.; Mackay, L.K.; Rahimpour, A.; Braun, A.; Veldhoen, M.; Carbone, F.R.; Manton, J.H.; Heath, W.R.; Mueller, S.N. Persistence of skin-resident memory T cells within an epidermal niche. Proc. Natl. Acad. Sci. USA 2014, 111, 5307–5312. [Google Scholar] [CrossRef]
- Ariotti, S.; Hogenbirk, M.A.; Dijkgraaf, F.E.; Visser, L.L.; Hoekstra, M.E.; Song, J.-Y.; Jacobs, H.; Haanen, J.B.; Schumacher, T.N. Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science 2014, 346, 101–105. [Google Scholar] [CrossRef]
- Khan, T.N.; Mooster, J.L.; Kilgore, A.M.; Osborn, J.F.; Nolz, J.C. Local antigen in nonlymphoid tissue promotes resident memory CD8+ T cell formation during viral infection. J. Exp. Med. 2016, 213, 951–966. [Google Scholar] [CrossRef]
- Mueller, S.N.; Heath, W.R.; McLain, J.D.; Carbone, F.R.; Jones, C.M. Characterization of two TCR transgenic mouse lines specific for herpes simplex virus. Immunol. Cell Biol. 2002, 80, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Mackay, L.K.; Stock, A.T.; Ma, J.Z.; Jones, C.M.; Kent, S.J.; Mueller, S.N.; Heath, W.R.; Carbone, F.R.; Gebhardt, T. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc. Natl. Acad. Sci. USA 2012, 109, 7037–7042. [Google Scholar] [CrossRef]
- Mackay, L.K.; Braun, A.; Macleod, B.L.; Collins, N.; Tebartz, C.; Bedoui, S.; Carbone, F.R.; Gebhardt, T. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J. Immunol. 2015, 194, 2059–2063. [Google Scholar] [CrossRef]
- Mueller, S.N.; Jones, C.M.; Chen, W.; Kawaoka, Y.; Castrucci, M.R.; Heath, W.R.; Carbone, F.R. The Early Expression of Glycoprotein B from Herpes Simplex Virus Can Be Detected by Antigen-Specific CD8+ T Cells. J. Virol. 2003, 77, 2445–2451. [Google Scholar] [CrossRef]
- Shin, H.; Iwasaki, A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 2012, 491, 463–467. [Google Scholar] [CrossRef]
- Gordon, J.; Grafton, G.; Wood, P.M.; Larché, M.; Armitage, R.J. Modelling the human immune response: Can mice be trusted? Curr. Opin. Pharmacol. 2001, 1, 431–435. [Google Scholar] [CrossRef]
- Mestas, J.; Hughes, C.C.W. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef]
- Belshe, R.B.; Leone, P.A.; Bernstein, D.I.; Wald, A.; Levin, M.J.; Stapleton, J.T.; Gorfinkel, I.; Morrow, R.L.A.; Ewell, M.G.; Stokes-Riner, A.; et al. Efficacy Results of a Trial of a Herpes Simplex Vaccine. N. Engl. J. Med. 2012, 366, 34–43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dasgupta, G.; BenMohamed, L. Of mice and not humans: How reliable are animal models for evaluation of herpes CD8+-T cell-epitopes-based immunotherapeutic vaccine candidates? Vaccine 2011, 29, 5824–5836. [Google Scholar] [CrossRef] [PubMed]
- Stanberry, L.R.; Spruance, S.L.; Cunningham, A.L.; Bernstein, D.I.; Mindel, A.; Sacks, S.; Tyring, S.; Aoki, F.Y.; Slaoui, M.; Denis, M.; et al. Glycoprotein-D–Adjuvant Vaccine to Prevent Genital Herpes. N. Engl. J. Med. 2002, 347, 1652–1661. [Google Scholar] [CrossRef] [PubMed]
- Crespi, C.M.; Cumberland, W.G.; Wald, A.; Corey, L.; Blower, S. Longitudinal study of herpes simplex virus type 2 infection using viral dynamic modelling. Sex. Transm. Infect. 2007, 83, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Wald, A.; Corey, L.; Cone, R.; Hobson, A.; Davis, G.; Zeh, J. Frequent genital herpes simplex virus 2 shedding in immunocompetent women. Effect of acyclovir treatment. J. Clin. Investig. 1997, 99, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Wald, A.; Zeh, J.; Selke, S.; Warren, T.; Ryncarz, A.J.; Ashley, R.; Krieger, J.N.; Corey, L. Reactivation of Genital Herpes Simplex Virus Type 2 Infection in Asymptomatic Seropositive Persons. N. Engl. J. Med. 2000, 342, 844–850. [Google Scholar] [CrossRef]
- Koelle, D.M.; Corey, L. Recent Progress in Herpes Simplex Virus Immunobiology and Vaccine Research. Clin. Microbiol. Rev. 2003, 16, 96–113. [Google Scholar] [CrossRef] [PubMed]
- Posavad, C.M.; Koelle, D.M.; Shaughnessy, M.F.; Corey, L. Severe genital herpes infections in HIV-infected individuals with impaired herpes simplex virus-specific CD8+cytotoxic T lymphocyte responses. Proc. Natl. Acad. Sci. USA 1997, 94, 10289–10294. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Zhu, J.; Phasouk, K.; Koelle, D.M.; Wald, A.; Corey, L. An Effector Phenotype of CD8+ T Cells at the Junction Epithelium during Clinical Quiescence of Herpes Simplex Virus 2 Infection. J. Virol. 2012, 86, 10587–10596. [Google Scholar] [CrossRef]
- Zhu, J.; Hladik, F.; Woodward, A.; Klock, A.; Peng, T.; Johnston, C.; Remington, M.; Magaret, A.; Koelle, D.M.; Wald, A.; et al. Persistence of HIV-1 receptor–positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat. Med. 2009, 15, 886–892. [Google Scholar] [CrossRef]
- Zhu, J.; Peng, T.; Johnston, C.; Phasouk, K.; Kask, A.S.; Klock, A.; Jin, L.; Diem, K.; Koelle, D.M.; Wald, A.; et al. Immune surveillance by CD8αα+ skin-resident T cells in human herpes virus infection. Nature 2013, 497, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Unger, P.-P.A.; Oja, A.E.; Khemai-Mehraban, T.; Ouwendijk, W.J.D.; Hombrink, P.; Verjans, G.M.G.M. T-cells in human trigeminal ganglia express canonical tissue-resident memory T-cell markers. J. Neuroinflamm. 2022, 19, 249. [Google Scholar] [CrossRef]
- Verjans, G.M.G.M.; Hintzen, R.Q.; van Dun, J.M.; Poot, A.; Milikan, J.C.; Laman, J.D.; Langerak, A.W.; Kinchington, P.R.; Osterhaus, A.D.M.E. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc. Natl. Acad. Sci. USA 2007, 104, 3496–3501. [Google Scholar] [CrossRef]
- Palmer, C.S.; Ostrowski, M.; Balderson, B.; Christian, N.; Crowe, S.M. Glucose Metabolism Regulates T Cell Activation, Differentiation, and Functions. Front. Immunol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Byersdorfer, C.A. The role of Fatty Acid oxidation in the metabolic reprograming of activated T-cells. Front. Immunol. 2014, 5, 641. [Google Scholar] [CrossRef]
- MacIver, N.J.; Jacobs, S.R.; Wieman, H.L.; Wofford, J.A.; Coloff, J.L.; Rathmell, J.C. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J. Leukoc. Biol. 2008, 84, 949–957. [Google Scholar] [CrossRef]
- Pan, Y.; Tian, T.; Park, C.O.; Lofftus, S.Y.; Mei, S.; Liu, X.; Luo, C.; O’malley, J.T.; Gehad, A.; Teague, J.E.; et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 2017, 543, 252–256. [Google Scholar] [CrossRef]
- Koelle, D.M.; Dong, L.; Jing, L.; Laing, K.J.; Zhu, J.; Jin, L.; Selke, S.; Wald, A.; Varon, D.; Huang, M.-L.; et al. HSV-2-Specific Human Female Reproductive Tract Tissue Resident Memory T Cells Recognize Diverse HSV Antigens. Front. Immunol. 2022, 13, 867962. [Google Scholar] [CrossRef]
- Donaghy, H.; Bosnjak, L.; Harman, A.N.; Marsden, V.; Tyring, S.K.; Meng, T.-C.; Cunningham, A.L. Role for Plasmacytoid Dendritic Cells in the Immune Control of Recurrent Human Herpes Simplex Virus Infection. J. Virol. 2009, 83, 1952–1961. [Google Scholar] [CrossRef]
- Peng, T.; Zhu, J.; Klock, A.; Phasouk, K.; Huang, M.-L.; Koelle, D.M.; Wald, A.; Corey, L. Evasion of the Mucosal Innate Immune System by Herpes Simplex Virus Type 2. J. Virol. 2009, 83, 12559–12568. [Google Scholar] [CrossRef]
- Wakim, L.M.; Waithman, J.; van Rooijen, N.; Heath, W.R.; Carbone, F.R. Dendritic Cell-Induced Memory T Cell Activation in Nonlymphoid Tissues. Science 2008, 319, 198–202. [Google Scholar] [CrossRef]
- Moss, N.J.; Magaret, A.; Laing, K.J.; Kask, A.S.; Wang, M.; Mark, K.E.; Schiffer, J.T.; Wald, A.; Koelle, D.M. Peripheral Blood CD4 T-Cell and Plasmacytoid Dendritic Cell (pDC) Reactivity to Herpes Simplex Virus 2 and pDC Number Do Not Correlate with the Clinical or Virologic Severity of Recurrent Genital Herpes. J. Virol. 2012, 86, 9952–9963. [Google Scholar] [CrossRef]
- Granelli-Piperno, A.; Shimeliovich, I.; Pack, M.; Trumpfheller, C.; Steinman, R.M. HIV-1 selectively infects a subset of nonmaturing BDCA1-positive dendritic cells in human blood. J. Immunol. 2006, 176, 991–998. [Google Scholar] [CrossRef]
- Pope, M.; Betjes, M.; Romani, N.; Hirmand, H.; Cameron, P.; Hoffman, L.; Gezelter, S.; Schuler, G.; Steinman, R. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell 1994, 78, 389–398. [Google Scholar] [CrossRef]
- Celum, C.; Wald, A.; Hughes, J.; Sanchez, J.; Reid, S.; Delany-Moretlwe, S.; Cowan, F.; Casapia, M.; Ortiz, A.; Fuchs, J.; et al. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: A randomised, double-blind, placebo-controlled trial. Lancet 2008, 371, 2109–2119. [Google Scholar] [CrossRef]
- Freeman, E.E.; Weiss, H.A.; Glynn, J.R.; Cross, P.L.; Whitworth, J.A.; Hayes, R.J. Herpes simplex virus 2 infection increases HIV acquisition in men and women: Systematic review and meta-analysis of longitudinal studies. Aids 2006, 20, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Watson-Jones, D.; Weiss, H.A.; Rusizoka, M.; Changalucha, J.; Baisley, K.; Mugeye, K.; Tanton, C.; Ross, D.; Everett, D.; Clayton, T.; et al. Effect of Herpes Simplex Suppression on Incidence of HIV among Women in Tanzania. N. Engl. J. Med. 2008, 358, 1560–1571. [Google Scholar] [CrossRef]
- Wald, A.; Carrell, D.; Remington, M.; Kexel, E.; Zeh, J.; Corey, L. Two-Day Regimen of Acyclovir for Treatment of Recurrent Genital Herpes Simplex Virus Type 2 Infection. Clin. Infect. Dis. 2002, 34, 944–948. [Google Scholar] [CrossRef]
- Maldonado, L.; Teague, J.E.; Morrow, M.P.; Jotova, I.; Wu, T.C.; Wang, C.; Desmarais, C.; Boyer, J.D.; Tycko, B.; Robins, H.S.; et al. Intramuscular Therapeutic Vaccination Targeting HPV16 Induces T Cell Responses That Localize in Mucosal Lesions. Sci. Transl. Med. 2014, 6, 221ra13. [Google Scholar] [CrossRef] [PubMed]
- Robins, H.S.; Campregher, P.V.; Srivastava, S.K.; Wacher, A.; Turtle, C.J.; Kahsai, O.; Riddell, S.R.; Warren, E.H.; Carlson, C.S. Comprehensive assessment of T-cell receptor β-chain diversity in αβ T cells. Blood 2009, 114, 4099–4107. [Google Scholar] [CrossRef]
- Ford, E.S.; Li, A.; Laing, K.J.; Dong, L.; Diem, K.; Jing, L.; Basu, K.; Ott, M.; Tartaglia, J.; Gurunathan, S.; et al. Expansion of the HSV-2-specific T cell repertoire in skin after immunotherapeutic HSV-2 vaccine. medRxiv, 2024; prepint. [Google Scholar] [CrossRef]
- Peng, T.; Phasouk, K.; Sodroski, C.N.; Sun, S.; Hwangbo, Y.; Layton, E.D.; Jin, L.; Klock, A.; Diem, K.; Magaret, A.S.; et al. Tissue-Resident-Memory CD8+ T Cells Bridge Innate Immune Responses in Neighboring Epithelial Cells to Control Human Genital Herpes. Front. Immunol. 2021, 12, 735643. [Google Scholar] [CrossRef]
- Leib, D.A. Counteraction of interferon-induced antiviral responses by herpes simplex viruses. Curr. Top. Microbiol. Immunol. 2002, 269, 171–185. [Google Scholar] [CrossRef]
- Casrouge, A.; Zhang, S.-Y.; Eidenschenk, C.; Jouanguy, E.; Puel, A.; Yang, K.; Alcais, A.; Picard, C.; Mahfoufi, N.; Nicolas, N.; et al. Herpes Simplex Virus Encephalitis in Human UNC-93B Deficiency. Science 2006, 314, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, S.; Jouanguy, E.; Al-Hajjar, S.; Fieschi, C.; Al-Mohsen, I.Z.; Al-Jumaah, S.; Yang, K.; Chapgier, A.; Eidenschenk, C.; Eid, P.; et al. Impaired response to interferon-α/β and lethal viral disease in human STAT1 deficiency. Nat. Genet. 2003, 33, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, P.; Swan, D.A.; Duke, E.R.; Corey, L.; Zhu, J.; Davé, V.A.; Spuhler, L.R.; Lund, J.M.; Prlic, M.; Schiffer, J.T. Tissue-resident T cell derived cytokines eliminate herpes simplex virus-2-infected cells. J. Clin. Investig. 2020, 130, 2903–2919. [Google Scholar] [CrossRef]
- Reddi, T.S.; Merkl, P.E.; Lim, S.-Y.; Letvin, N.L.; Knipe, D.M. Tripartite Motif 22 (TRIM22) protein restricts herpes simplex virus 1 by epigenetic silencing of viral immediate-early genes. PLoS Pathog. 2021, 17, e1009281. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Miner, M.D. Local Power: The Role of Tissue-Resident Immunity in Human Genital Herpes Simplex Virus Reactivation. Viruses 2024, 16, 1019. https://doi.org/10.3390/v16071019
Zhu J, Miner MD. Local Power: The Role of Tissue-Resident Immunity in Human Genital Herpes Simplex Virus Reactivation. Viruses. 2024; 16(7):1019. https://doi.org/10.3390/v16071019
Chicago/Turabian StyleZhu, Jia, and Maurine D. Miner. 2024. "Local Power: The Role of Tissue-Resident Immunity in Human Genital Herpes Simplex Virus Reactivation" Viruses 16, no. 7: 1019. https://doi.org/10.3390/v16071019





