Exploring the Potential of Muridae as Sentinels for Human and Zoonotic Viruses
Abstract
1. Introduction
2. Materials and Methods
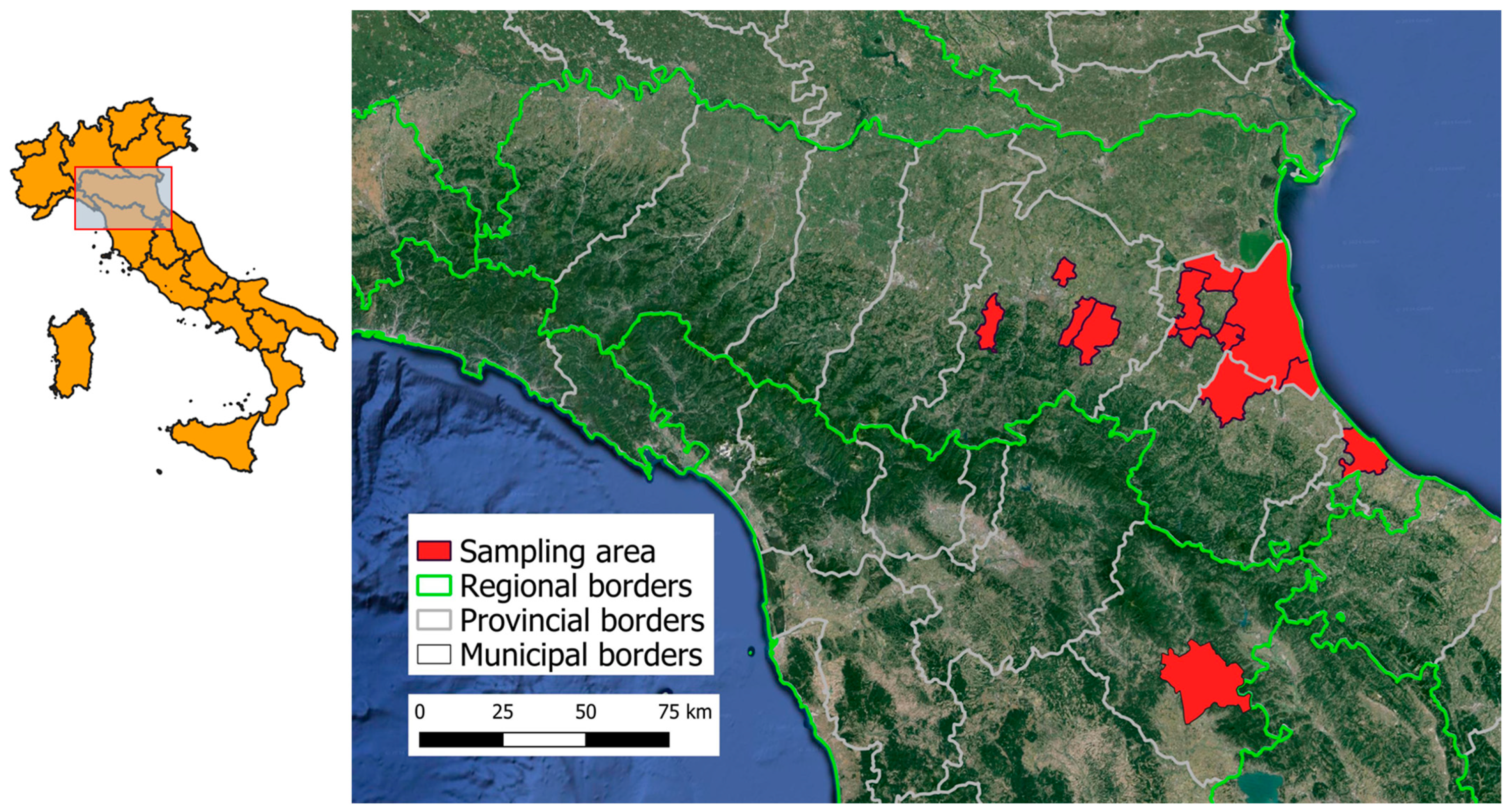
2.1. Study Areas and Sampling
2.2. Preparation of Samples
2.3. Viral Nucleic Acid Detection
2.4. Sanger Sequencing
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Examined Animals
3.2. Virus Detection
3.3. Sanger Sequencing Confirmation of Positive RNA
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef]
- Esposito, M.M.; Turku, S.; Lehrfield, L.; Shoman, A. The Impact of Human Activities on Zoonotic Infection Transmissions. Animals 2023, 13, 1646. [Google Scholar] [CrossRef]
- Liu, B.M.; Mulkey, S.B.; Campos, J.M.; DeBiasi, R.L. Laboratory diagnosis of CNS infections in children due to emerging and re-emerging neurotropic viruses. Pediatr. Res. 2024, 95, 543–550. [Google Scholar] [CrossRef]
- Burgin, C.J.; Colella, J.P.; Kahn, P.L.; Upham, N.S. How many species of mammals are there? J. Mammal. 2018, 99, 1–14. [Google Scholar] [CrossRef]
- Dahmana, H.; Granjon, L.; Diagne, C.; Davoust, B.; Fenollar, F.; Mediannikov, O. Rodents as Hosts of Pathogens and Related Zoonotic Disease Risk. Pathogens 2020, 9, 202. [Google Scholar] [CrossRef]
- Gibb, R.; Redding, D.W.; Chin, K.Q.; Donnelly, C.A.; Blackburn, T.M.; Newbold, T.; Jones, K.E. Zoonotic host diversity increases in human-dominated ecosystems. Nature 2020, 584, 398–402. [Google Scholar] [CrossRef]
- Gravinatti, M.L.; Barbosa, C.M.; Soares, R.M.; Gregori, F. Synanthropic rodents as virus reservoirs and transmitters. Rev. Soc. Bras. Med. Trop. 2020, 53, e20190486. [Google Scholar] [CrossRef]
- Wang, Y.; Lenoch, J.; Kohler, D.; DeLiberto, T.J.; Tang, C.Y.; Li, T.; Tao, Y.J.; Guan, M.; Compton, S.; Zeiss, C.; et al. SARS-CoV-2 Exposure in Norway Rats (Rattus norvegicus) from New York City. mBio 2023, 14, e0362122. [Google Scholar] [CrossRef]
- Santos-Silva, S.; Moraes, D.; Lopez-Lopez, P.; Pauperio, J.; Queiros, J.; Rivero-Juarez, A.; Lux, L.; Ulrich, R.G.; Goncalves, H.M.R.; Van der Poel, W.H.M.; et al. Detection of hepatitis E virus genotype 3 in an Algerian mouse (Mus spretus) in Portugal. Vet. Res. Commun. 2024, 48, 1803–1812. [Google Scholar] [CrossRef]
- Villabruna, N.; Koopmans, M.P.G.; de Graaf, M. Animals as Reservoir for Human Norovirus. Viruses 2019, 11, 478. [Google Scholar] [CrossRef]
- Williams, E.P.; Spruill-Harrell, B.M.; Taylor, M.K.; Lee, J.; Nywening, A.V.; Yang, Z.; Nichols, J.H.; Camp, J.V.; Owen, R.D.; Jonsson, C.B. Common Themes in Zoonotic Spillover and Disease Emergence: Lessons Learned from Bat- and Rodent-Borne RNA Viruses. Viruses 2021, 13, 1509. [Google Scholar] [CrossRef]
- Camp, J.V.; Desvars-Larrive, A.; Nowotny, N.; Walzer, C. Monitoring Urban Zoonotic Virus Activity: Are City Rats a Promising Surveillance Tool for Emerging Viruses? Viruses 2022, 14, 1516. [Google Scholar] [CrossRef]
- Rios-Munoz, L.; Gonzalvez, M.; Caballero-Gomez, J.; Castro-Scholten, S.; Casares-Jimenez, M.; Agullo-Ros, I.; Corona-Mata, D.; Garcia-Bocanegra, I.; Lopez-Lopez, P.; Fajardo, T.; et al. Detection of Rat Hepatitis E Virus in Pigs, Spain, 2023. Emerg. Infect. Dis. 2024, 30, 823–826. [Google Scholar] [CrossRef]
- Rodriguez, C.; Marchand, S.; Sessa, A.; Cappy, P.; Pawlotsky, J.M. Orthohepevirus C hepatitis, an underdiagnosed disease? J. Hepatol. 2023, 79, e39–e41. [Google Scholar] [CrossRef] [PubMed]
- Kruger, D.H.; Schonrich, G.; Klempa, B. Human pathogenic hantaviruses and prevention of infection. Hum. Vaccin. 2011, 7, 685–693. [Google Scholar] [CrossRef]
- Vapalahti, O.; Mustonen, J.; Lundkvist, A.; Henttonen, H.; Plyusnin, A.; Vaheri, A. Hantavirus infections in Europe. Lancet Infect. Dis. 2003, 3, 653–661. [Google Scholar] [CrossRef]
- Heyman, P.; Plyusnina, A.; Berny, P.; Cochez, C.; Artois, M.; Zizi, M.; Pirnay, J.P.; Plyusnin, A. Seoul hantavirus in Europe: First demonstration of the virus genome in wild Rattus norvegicus captured in France. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 711–717. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Hantavirus infection. In Annual Epidemiological Report for 2020; ECDC: Stockholm, Sweden, 2023. [Google Scholar]
- Dupinay, T.; Pounder, K.C.; Ayral, F.; Laaberki, M.H.; Marston, D.A.; Lacote, S.; Rey, C.; Barbet, F.; Voller, K.; Nazaret, N.; et al. Detection and genetic characterization of Seoul virus from commensal brown rats in France. Virol. J. 2014, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Jameson, L.J.; Taori, S.K.; Atkinson, B.; Levick, P.; Featherstone, C.A.; van der Burgt, G.; McCarthy, N.; Hart, J.; Osborne, J.C.; Walsh, A.L.; et al. Pet rats as a source of hantavirus in England and Wales, 2013. Eurosurveillance 2013, 18, 20415. [Google Scholar] [CrossRef]
- Plyusnina, A.; Heyman, P.; Baert, K.; Stuyck, J.; Cochez, C.; Plyusnin, A. Genetic characterization of seoul hantavirus originated from norway rats (Rattus norvegicus) captured in Belgium. J. Med. Virol. 2012, 84, 1298–1303. [Google Scholar] [CrossRef]
- Verner-Carlsson, J.; Lohmus, M.; Sundstrom, K.; Strand, T.M.; Verkerk, M.; Reusken, C.; Yoshimatsu, K.; Arikawa, J.; van de Goot, F.; Lundkvist, A. First evidence of Seoul hantavirus in the wild rat population in the Netherlands. Infect. Ecol. Epidemiol. 2015, 5, 27215. [Google Scholar] [CrossRef][Green Version]
- Honig, V.; Kamis, J.; Marsikova, A.; Matejkova, T.; Stopka, P.; Macova, A.; Ruzek, D.; Kvicerova, J. Orthohantaviruses in Reservoir and Atypical Hosts in the Czech Republic: Spillover Infection and Indication of Virus-Specific Tissue Tropism. Microbiol. Spectr. 2022, 10, e0130622. [Google Scholar] [CrossRef]
- Shepherd, J.G.; Blunsum, A.E.; Carmichael, S.; Smollett, K.; Maxwell-Scott, H.; Farmer, E.C.W.; Osborne, J.; MacLean, A.; Ashraf, S.; Shah, R.; et al. Seoul Virus Associated with Pet Rats, Scotland, UK, 2019. Emerg. Infect. Dis. 2021, 27, 2677–2680. [Google Scholar] [CrossRef] [PubMed]
- Reynes, J.M.; Carli, D.; Bour, J.B.; Boudjeltia, S.; Dewilde, A.; Gerbier, G.; Nussbaumer, T.; Jacomo, V.; Rapt, M.P.; Rollin, P.E.; et al. Seoul Virus Infection in Humans, France, 2014–2016. Emerg. Infect. Dis. 2017, 23, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Jameson, L.J.; Newton, A.; Coole, L.; Newman, E.N.; Carroll, M.W.; Beeching, N.J.; Hewson, R.; Christley, R.M. Prevalence of antibodies against hantaviruses in serum and saliva of adults living or working on farms in Yorkshire, United Kingdom. Viruses 2014, 6, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Kallio-Kokko, H.; Laakkonen, J.; Rizzoli, A.; Tagliapietra, V.; Cattadori, I.; Perkins, S.E.; Hudson, P.J.; Cristofolini, A.; Versini, W.; Vapalahti, O.; et al. Hantavirus and arenavirus antibody prevalence in rodents and humans in Trentino, Northern Italy. Epidemiol. Infect. 2006, 134, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Marchino, M.; Sparasci, O.A.; Garcia-Vozmediano, A.; Ceballos, L.; Ru, G.; Robetto, S.; Orusa, R.; MIceli, I.; Baioni, E.; Braghin, S.; et al. First evidence of hantaviruses circulation in rodents host (Mus domesticus) on farms in Piedmont, Northwestern Italy, in a One Health approach. In Proceedings of the Emerging & Re-Emerging Infectious Diseases, MAY 2023; pp. S16–S17. [Google Scholar]
- Charrel, R.N.; de Lamballerie, X. Zoonotic aspects of arenavirus infections. Vet. Microbiol. 2010, 140, 213–220. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Director-General Declares the Ongoing Monkeypox Outbreak a Public Health Emergency of International Concern; WHO: Geneva, Switzerland, 2022; Volume 2024. [Google Scholar]
- Upadhayay, S.; Arthur, R.; Soni, D.; Yadav, P.; Navik, U.; Singh, R.; Gurjeet Singh, T.; Kumar, P. Monkeypox infection: The past, present, and future. Int. Immunopharmacol. 2022, 113, 109382. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Lorusso, A. Novel human coronavirus (SARS-CoV-2): A lesson from animal coronaviruses. Vet. Microbiol. 2020, 244, 108693. [Google Scholar] [CrossRef]
- Corman, V.M.; Muth, D.; Niemeyer, D.; Drosten, C. Hosts and Sources of Endemic Human Coronaviruses. Adv. Virus Res. 2018, 100, 163–188. [Google Scholar] [CrossRef]
- Montagutelli, X.; Prot, M.; Levillayer, L.; Salazar, E.B.; Jouvion, G.; Conquet, L.; Beretta, M.; Donati, F.; Albert, M.; Gambaro, F.; et al. Variants with the N501Y mutation extend SARS-CoV-2 host range to mice, with contact transmission. bioRxiv, 2021; preprint. [Google Scholar] [CrossRef]
- Khamrin, P.; Maneekarn, N.; Okitsu, S.; Ushijima, H. Epidemiology of human and animal kobuviruses. Virusdisease 2014, 25, 195–200. [Google Scholar] [CrossRef]
- You, F.F.; Zhang, M.Y.; He, H.; He, W.Q.; Li, Y.Z.; Chen, Q. Kobuviruses carried by Rattus norvegicus in Guangdong, China. BMC Microbiol. 2020, 20, 94. [Google Scholar] [CrossRef] [PubMed]
- Khamrin, P.; Maneekarn, N.; Hidaka, S.; Kishikawa, S.; Ushijima, K.; Okitsu, S.; Ushijima, H. Molecular detection of kobuvirus sequences in stool samples collected from healthy pigs in Japan. Infect. Genet. Evol. 2010, 10, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Diaz Alarcon, R.G.; Liotta, D.J.; Mino, S. Zoonotic RVA: State of the Art and Distribution in the Animal World. Viruses 2022, 14, 2554. [Google Scholar] [CrossRef] [PubMed]
- Tonietti Pde, O.; da Hora, A.S.; Silva, F.D.; Ferrari, K.L.; Brandao, P.E.; Richtzenhain, L.J.; Gregori, F. Simultaneous detection of group a rotavirus in Swine and rat on a pig farm in Brazil. Sci. World J. 2013, 2013, 648406. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lin, X.D.; Huang, K.Y.; Zhang, B.; Shi, M.; Guo, W.P.; Wang, M.R.; Wang, W.; Xing, J.G.; Li, M.H.; et al. Identification of novel and diverse rotaviruses in rodents and insectivores, and evidence of cross-species transmission into humans. Virology 2016, 494, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Lucero, Y.; Matson, D.O.; Ashkenazi, S.; George, S.; O'Ryan, M. Norovirus: Facts and Reflections from Past, Present, and Future. Viruses 2021, 13, 2399. [Google Scholar] [CrossRef] [PubMed]
- Summa, M.; Henttonen, H.; Maunula, L. Human noroviruses in the faeces of wild birds and rodents-new potential transmission routes. Zoonoses Public Health 2018, 65, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Reetz, J.; Johne, R.; Heiberg, A.C.; Petri, S.; Kanig, H.; Ulrich, R.G. The simultaneous occurrence of human norovirus and hepatitis E virus in a Norway rat (Rattus norvegicus). Arch. Virol. 2013, 158, 1575–1578. [Google Scholar] [CrossRef]
- Japhet, M.O.; Famurewa, O.; Adesina, O.A.; Opaleye, O.O.; Wang, B.; Hohne, M.; Bock, C.T.; Mas Marques, A.; Niendorf, S. Viral gastroenteritis among children of 0–5 years in Nigeria: Characterization of the first Nigerian aichivirus, recombinant noroviruses and detection of a zoonotic astrovirus. J. Clin. Virol. 2019, 111, 4–11. [Google Scholar] [CrossRef]
- Neves, E.S.; Mendenhall, I.H.; Borthwick, S.A.; Su, Y.C.F.; Smith, G.J.D. Genetic diversity and expanded host range of astroviruses detected in small mammals in Singapore. One Health 2021, 12, 100218. [Google Scholar] [CrossRef] [PubMed]
- Niendorf, S.; Harms, D.; Hellendahl, K.F.; Heuser, E.; Bottcher, S.; Bock, C.T.; Ulrich, R.G. Presence and Diversity of Different Enteric Viruses in Wild Norway Rats (Rattus norvegicus). Viruses 2021, 13, 992. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; Chin, A.W.; Smith, G.J.; Chan, K.H.; Guan, Y.; Peiris, J.S.; Poon, L.L. Detection of novel astroviruses in urban brown rats and previously known astroviruses in humans. J. Gen. Virol. 2010, 91, 2457–2462. [Google Scholar] [CrossRef] [PubMed]
- De Sabato, L.; Ostanello, F.; De Grossi, L.; Marcario, A.; Franzetti, B.; Monini, M.; Di Bartolo, I. Molecular survey of HEV infection in wild boar population in Italy. Transbound. Emerg. Dis. 2018, 65, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brunink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, M.G.; Anthony, S.J.; Navarrete-Macias, I.; Bestebroer, T.; Munster, V.J.; van Doremalen, N. Updated and Validated Pan-Coronavirus PCR Assay to Detect All Coronavirus Genera. Viruses 2021, 13, 599. [Google Scholar] [CrossRef] [PubMed]
- Klempa, B.; Fichet-Calvet, E.; Lecompte, E.; Auste, B.; Aniskin, V.; Meisel, H.; Denys, C.; Koivogui, L.; ter Meulen, J.; Kruger, D.H. Hantavirus in African wood mouse, Guinea. Emerg. Infect. Dis. 2006, 12, 838–840. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, G.; Mancini, P.; Veneri, C.; Ferraro, G.B.; Lucentini, L.; Iaconelli, M.; Suffredini, E. Detection of Monkeypox Virus DNA in Airport Wastewater, Rome, Italy. Emerg. Infect. Dis. 2023, 29, 193–196. [Google Scholar] [CrossRef]
- da Silva, A.K.; Le Saux, J.C.; Parnaudeau, S.; Pommepuy, M.; Elimelech, M.; Le Guyader, F.S. Evaluation of removal of noroviruses during wastewater treatment, using real-time reverse transcription-PCR: Different behaviors of genogroups I and II. Appl. Env. Microbiol. 2007, 73, 7891–7897. [Google Scholar] [CrossRef]
- Svraka, S.; Duizer, E.; Vennema, H.; de Bruin, E.; van der Veer, B.; Dorresteijn, B.; Koopmans, M. Etiological role of viruses in outbreaks of acute gastroenteritis in The Netherlands from 1994 through 2005. J. Clin. Microbiol. 2007, 45, 1389–1394. [Google Scholar] [CrossRef]
- Kageyama, T.; Kojima, S.; Shinohara, M.; Uchida, K.; Fukushi, S.; Hoshino, F.B.; Takeda, N.; Katayama, K. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J. Clin. Microbiol. 2003, 41, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Loisy, F.; Atmar, R.L.; Guillon, P.; Le Cann, P.; Pommepuy, M.; Le Guyader, F.S. Real-time RT-PCR for norovirus screening in shellfish. J. Virol. Methods 2005, 123, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jothikumar, N.; Kang, G.; Hill, V.R. Broadly reactive TaqMan assay for real-time RT-PCR detection of rotavirus in clinical and environmental samples. J. Virol. Methods 2009, 155, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lin, X.D.; Wang, W.; Shi, M.; Guo, W.P.; Zhang, X.H.; Xing, J.G.; He, J.R.; Wang, K.; Li, M.H.; et al. Isolation and characterization of a novel arenavirus harbored by Rodents and Shrews in Zhejiang province, China. Virology 2015, 476, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Reuter, G.; Egyed, L. Bovine kobuvirus in europe. Emerg. Infect. Dis. 2009, 15, 822–823. [Google Scholar] [CrossRef] [PubMed]
- Gallimore, C.I.; Taylor, C.; Gennery, A.R.; Cant, A.J.; Galloway, A.; Lewis, D.; Gray, J.J. Use of a heminested reverse transcriptase PCR assay for detection of astrovirus in environmental swabs from an outbreak of gastroenteritis in a pediatric primary immunodeficiency unit. J. Clin. Microbiol. 2005, 43, 3890–3894. [Google Scholar] [CrossRef] [PubMed]
- Noel, J.S.; Lee, T.W.; Kurtz, J.B.; Glass, R.I.; Monroe, S.S. Typing of human astroviruses from clinical isolates by enzyme immunoassay and nucleotide sequencing. J. Clin. Microbiol. 1995, 33, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Finkbeiner, S.R.; Le, B.M.; Holtz, L.R.; Storch, G.A.; Wang, D. Detection of newly described astrovirus MLB1 in stool samples from children. Emerg. Infect. Dis. 2009, 15, 441–444. [Google Scholar] [CrossRef]
- Larsson, A. AliView: A fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef]
- To, K.K.W.; Chan, W.M.; Li, K.S.M.; Lam, C.S.F.; Chen, Z.; Tse, H.; Lau, S.K.P.; Woo, P.C.Y.; Yuen, K.Y. High prevalence of four novel astrovirus genotype species identified from rodents in China. J. Gen. Virol. 2017, 98, 1004–1015. [Google Scholar] [CrossRef]
- Lupusoru, G.; Andronesi, A.G.; Lupusoru, M.; Ailincai, I.; Sfeatcu, R.; Vacaroiu, I.; Banu, M.; Achim, C.; Ismail, G. Hantavirus infections in the South-Eastern European countries: A study of two cases and literature review. Exp. Ther. Med. 2023, 26, 430. [Google Scholar] [CrossRef] [PubMed]
- Ricco, M.; Peruzzi, S.; Ranzieri, S.; Balzarini, F.; Valente, M.; Marchesi, F.; Bragazzi, N.L. Hantavirus infections in Italy: Not reported doesn't mean inexistent. Acta Biomed. 2021, 92, e2021324. [Google Scholar] [CrossRef]
- Reuter, G.; Boros, A.; Takats, K.; Matics, R.; Pankovics, P. A novel mammarenavirus (family Arenaviridae) in hedgehogs (Erinaceus roumanicus) in Europe. Arch. Virol. 2023, 168, 174. [Google Scholar] [CrossRef] [PubMed]
- Vilibic-Cavlek, T.; Savic, V.; Ferenc, T.; Mrzljak, A.; Barbic, L.; Bogdanic, M.; Stevanovic, V.; Tabain, I.; Ferencak, I.; Zidovec-Lepej, S. Lymphocytic Choriomeningitis-Emerging Trends of a Neglected Virus: A Narrative Review. Trop. Med. Infect. Dis. 2021, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Tagliapietra, V.; Rosa, R.; Hauffe, H.C.; Laakkonen, J.; Voutilainen, L.; Vapalahti, O.; Vaheri, A.; Henttonen, H.; Rizzoli, A. Spatial and temporal dynamics of lymphocytic choriomeningitis virus in wild rodents, northern Italy. Emerg. Infect. Dis. 2009, 15, 1019–1025. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lanave, G.; Dowgier, G.; Decaro, N.; Albanese, F.; Brogi, E.; Parisi, A.; Losurdo, M.; Lavazza, A.; Martella, V.; Buonavoglia, C.; et al. Novel Orthopoxvirus and Lethal Disease in Cat, Italy. Emerg. Infect. Dis. 2018, 24, 1665–1673. [Google Scholar] [CrossRef]
- Gierynska, M.; Szulc-Dabrowska, L.; Struzik, J.; Gregorczyk-Zboroch, K.P.; Mielcarska, M.B.; Toka, F.N.; Schollenberger, A.; Biernacka, Z. Orthopoxvirus Zoonoses-Do We Still Remember and Are Ready to Fight? Pathogens 2023, 12, 363. [Google Scholar] [CrossRef]
- Girón-Guzmán, I.; Díaz-Reolid, A.; Truchado, P.; Carcereny, A.; Garcia-Pedemonte, D.; Hernaez, B.; Bosch, A.; Pintó, R.M.; Guix, S.; Allende, A.; et al. Wastewater based epidemiology beyond SARS-CoV-2: Spanish wastewater reveals the current spread of Monkeypox virus. medRxiv 2022, preprint. [Google Scholar] [CrossRef]
- de Jonge, E.F.; Peterse, C.M.; Koelewijn, J.M.; van der Drift, A.R.; van der Beek, R.; Nagelkerke, E.; Lodder, W.J. The detection of monkeypox virus DNA in wastewater samples in the Netherlands. Sci. Total Env. 2022, 852, 158265. [Google Scholar] [CrossRef]
- Colombo, V.C.; Sluydts, V.; Marien, J.; Vanden Broecke, B.; Van Houtte, N.; Leirs, W.; Jacobs, L.; Iserbyt, A.; Hubert, M.; Heyndrickx, L.; et al. SARS-CoV-2 surveillance in Norway rats (Rattus norvegicus) from Antwerp sewer system, Belgium. Transbound. Emerg. Dis. 2022, 69, 3016–3021. [Google Scholar] [CrossRef]
- Fisher, A.M.; Airey, G.; Liu, Y.; Gemmell, M.; Thomas, J.; Bentley, E.G.; Whitehead, M.A.; Paxton, W.A.; Pollakis, G.; Paterson, S.; et al. The ecology of viruses in urban rodents with a focus on SARS-CoV-2. Emerg. Microbes Infect. 2023, 12, 2217940. [Google Scholar] [CrossRef] [PubMed]
- Trogu, T.; Canziani, S.; Tolini, C.; Carrera, M.; Sozzi, E.; Lelli, D.; Lavazza, A.; Mandola, M.L.; Robetto, S.; Marchino, M.; et al. Virological investigation in synanthropic rodents in North Italy. Int. J. Infect. Dis. 2023, 130, S85. [Google Scholar] [CrossRef]
- Funk, C.J.; Manzer, R.; Miura, T.A.; Groshong, S.D.; Ito, Y.; Travanty, E.A.; Leete, J.; Holmes, K.V.; Mason, R.J. Rat respiratory coronavirus infection: Replication in airway and alveolar epithelial cells and the innate immune response. J. Gen. Virol. 2009, 90, 2956–2964. [Google Scholar] [CrossRef] [PubMed]
- Bergallo, M.; Galliano, I.; Dapra, V.; Rassu, M.; Montanari, P.; Tovo, P.A. Molecular Detection of Human Astrovirus in Children With Gastroenteritis, Northern Italy. Pediatr. Infect. Dis. J. 2018, 37, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, B.; Ianiro, G.; Iaccarino, D.; D'Apice, F.; Ferraro, A.; Race, M.; Spasiano, D.; Esposito, E.; Monini, M.; Serra, F.; et al. A potential risk assessment tool to monitor pathogens circulation in coastal waters. Env. Res. 2021, 200, 111748. [Google Scholar] [CrossRef]
- Pankovics, P.; Boros, A.; Laszlo, Z.; Szekeres, S.; Foldvari, G.; Altan, E.; Delwart, E.; Reuter, G. Genome characterization, prevalence and tissue distribution of astrovirus, hepevirus and norovirus among wild and laboratory rats (Rattus norvegicus) and mice (Mus musculus) in Hungary. Infect. Genet. Evol. 2021, 93, 104942. [Google Scholar] [CrossRef]
- Kuczera, K.; Orlowska, A.; Smreczak, M.; Frant, M.; Trebas, P.; Rola, J. Prevalence of Astroviruses in Different Animal Species in Poland. Viruses 2024, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Doro, R.; Farkas, S.L.; Martella, V.; Banyai, K. Zoonotic transmission of rotavirus: Surveillance and control. Expert. Rev. Anti Infect. Ther. 2015, 13, 1337–1350. [Google Scholar] [CrossRef]
- Ianiro, G.; Di Bartolo, I.; De Sabato, L.; Pampiglione, G.; Ruggeri, F.M.; Ostanello, F. Detection of uncommon G3P[3] rotavirus A (RVA) strain in rat possessing a human RVA-like VP6 and a novel NSP2 genotype. Infect. Genet. Evol. 2017, 53, 206–211. [Google Scholar] [CrossRef]
- Sachsenroder, J.; Braun, A.; Machnowska, P.; Ng, T.F.F.; Deng, X.; Guenther, S.; Bernstein, S.; Ulrich, R.G.; Delwart, E.; Johne, R. Metagenomic identification of novel enteric viruses in urban wild rats and genome characterization of a group A rotavirus. J. Gen. Virol. 2014, 95, 2734–2747. [Google Scholar] [CrossRef]
- Firth, C.; Bhat, M.; Firth, M.A.; Williams, S.H.; Frye, M.J.; Simmonds, P.; Conte, J.M.; Ng, J.; Garcia, J.; Bhuva, N.P.; et al. Detection of zoonotic pathogens and characterization of novel viruses carried by commensal Rattus norvegicus in New York City. mBio 2014, 5, e01933-14. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.; Headley, S.A.; Diniz, J.A.; Pereira, A.H.; Lorenzetti, E.; Alfieri, A.A.; Alfieri, A.F. Extra-intestinal detection of canine kobuvirus in a puppy from Southern Brazil. Arch. Virol. 2017, 162, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Boros, A.; Orlovacz, K.; Pankovics, P.; Szekeres, S.; Foldvari, G.; Fahsbender, E.; Delwart, E.; Reuter, G. Diverse picornaviruses are prevalent among free-living and laboratory rats (Rattus norvegicus) in Hungary and can cause disseminated infections. Infect. Genet. Evol. 2019, 75, 103988. [Google Scholar] [CrossRef] [PubMed]
- Alfano, F.; Lucibelli, M.G.; Serra, F.; Levante, M.; Rea, S.; Gallo, A.; Petrucci, F.; Pucciarelli, A.; Picazio, G.; Monini, M.; et al. Identification of Aichivirus in a Pet Rat (Rattus norvegicus) in Italy. Animals 2024, 14, 1765. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; He, W.; Fu, J.; Li, Y.; He, H.; Chen, Q. Epidemiological Evidence for Fecal-Oral Transmission of Murine Kobuvirus. Front. Public Health 2022, 10, 865605. [Google Scholar] [CrossRef] [PubMed]
- Boswell, C.A.; Mundo, E.E.; Ulufatu, S.; Bumbaca, D.; Cahaya, H.S.; Majidy, N.; Van Hoy, M.; Schweiger, M.G.; Fielder, P.J.; Prabhu, S.; et al. Comparative Physiology of Mice and Rats: Radiometric Measurement of Vascular Parameters in Rodent Tissues. Mol. Pharm. 2014, 11, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.; Murray, K.A.; Zambrana-Torrelio, C.; Morse, S.S.; Rondinini, C.; Di Marco, M.; Breit, N.; Olival, K.J.; Daszak, P. Global hotspots and correlates of emerging zoonotic diseases. Nat. Commun. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
| Virus Family | Target Virus/Taxon | Assay | Primers/Probes (5′-3′ Sequence) | References |
|---|---|---|---|---|
| Coronaviridae | SARS-CoV-2 | RT-qPCR | RdRp_SARSr-F: GTGARATGGTCATGTGTGGCGG RdRp_SARSr-P2: FAM-CAGGTGGAACCTCATCAGGAGATGC-BBQ RdRp_SARSr-R: CARATGTTAAASACACTATTAGCATA | [49] |
| Sarbecoviruses | RT-qPCR | E_Sarbeco_F: ACAGGTACGTTAATAGTTAATAGCGT E_Sarbeco_P1: FAM-ACACTAGCCATCCTTACTGCGCTTCG-BBQ E_Sarbeco_R: ATATTGCAGCAGTACGCACACA | [49] | |
| Coronavirus | RT-PCR | Pan_CoV_F-1: GGTGGGAYTAYCCHAARTGYGA Pan_CoV_R-1: CCRTCATCAGAHARWATCAT Pan_CoV_R-2: CCRTCATCACTHARWATCAT | [50] | |
| Semi-nested PCR | Pan_CoV_F-2: GAYTAYCCHAARTGTGAYAGA Pan_CoV_F-3: GAYTAYCCHAARTGTGAYMGH Pan_CoV_R-1: CCRTCATCAGAHARWATCAT Pan_CoV_R-2: CCRTCATCACTHARWATCAT | [50] | ||
| Hantaviridae | Hantaviruses | RT-PCR, nested | HAN-L-F1: ATGTAYGTBAGTGCWGATGC HAN-L-R1: AACCADTCWGTYCCRTCATC HAN-L-F2: TGCWGATGCHACIAARTGGTC HAN-L-R2: GCRTCRTCWGARTGRTGDGCAA | [51] |
| Poxviridae | Monkeypox virus | PCR, nested | G2R-1st cycle F: ATAGCACCACATGCACCATC G2R-1st cycle R: AAAGGTATCCGAACCACACG MPVX G F mod: GGAAAGTGTAAAGACAACGAATACAG MPVX G R mod: GCTATCACATAATCTGAAAGCGTA | [52] |
| Caliciviridae | Norovirus GI | RT-qPCR | QNIF4: CGCTGGATGCGNTTCCAT NV1LCR: CCTTAGACGCCATCATCATTTAC NVGG1p: FAM- TGGACAGGAGAYCGCRATCT-BHQ1 | [53,54] |
| Norovirus GII | RT-qPCR | QNIF2: ATGTTCAGRTGGATGAGRTTCTCWGA COG2R: TCGACGCCATCTTCATTCACA QNIFS: FAM- AGC ACG TGG GAG GGC GAT CG -BHQ1 | [55,56] | |
| Reoviridae | Group A rotavirus | RT-qPCR | JVKF: CAGTGGTTGATGCTCAAGATGGA JVKR: TCATTGTAATCATATTGAATACCCA JVKP: FAM-ACAACTGCAGCTTCAAAAGAAGWGT-BHQ | [57] |
| Arenaviridae | Mammarenavirus | RT-PCR, nested | Arena-F1: AYNGGNACNCCRTTNGC Arena-R1: TCHTAYAARGARCARGTDGGDGG Arena-F2: GGNACYTCHTCHCCCCANAC Arena-R2: AGYAARTGGGGNCCNAYKATG | [58] |
| Picornaviridae | Kobuvirus | RT-PCR | UNIV-kobu-F: TGGAYTACAAG(/R)TGTTTTGATGC UNIV-kobu-R: ATGTTGTTRATGATGGTGTTGA | [59] |
| Astroviridae | Mamastrovirus | RT-PCR, nested | Mon269: CAACTCAGGAAACAGGGTGT Mon270: TCAGATGCATTGTCATTGGT Mon269N GACCAAAACCTGCAATATGTCA | [60,61] |
| RT-PCR | SF0073: ATTGGACTCGATTTGATGG SF0076: CTGGCTTAACCCACATTCC | [62] |
| Specie | Sex | Age Class (%) | Weght (g) | Samples | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adult | Subadult | Min | Max | Median | Fecal | Lung | Liver | Total | ||
| Mus musculus (House mice) (n = 13; F = 2, M = 11) | F | 2 (100) | 0 (0.0) | 17 | 20.4 | 18.7 | 2 | 2 | 2 | 6 |
| M | 10 (90.9) | 1 (9.1) | 7 | 70 | 22.1 | 6 | 9 | 4 | 19 | |
| Rattus norvegicus (Brown rats) (n = 70; F = 24, M = 46) | F | 18 (75.0) | 6 (25.0) | 45 | 390 | 195.2 | 22 | 23 | 20 | 65 |
| M | 22 (47.8) | 24 (52.2) | 28.4 | 470 | 156.5 | 41 | 36 | 34 | 111 | |
| Rattus rattus (Black rats) (n = 45; F = 17, M = 28) | F | 12 (70.6) | 5 (29.4) | 29 | 195 | 97.6 | 16 | 15 | 14 | 45 |
| M | 15 (53.6) | 13 (46.4) | 18 | 165 | 87.6 | 24 | 18 | 11 | 53 | |
| Total (n = 128) | 79 (61.7) | 49 (38.3) | - | - | - | 111 | 103 | 85 | 299 | |
| Specie * | Sample Type | No. of Samples Tested | Detected Viruses (No. of Positive Samples) | |
|---|---|---|---|---|
| Rattus norvegicus (Brown rats) | Fecal/rectal swab | 63 | MukV | (6) |
| AstV | (1) | |||
| Liver | 54 | - | (0) | |
| Lung | 59 | MukV | (1) | |
| Rattus rattus (Black rats) | Fecal/rectal swab | 40 | MukV | (7) |
| Liver | 25 | - | (0) | |
| Lung | 33 | - | (0) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Bartolo, I.; De Sabato, L.; Ianiro, G.; Vaccari, G.; Dini, F.M.; Ostanello, F.; Monini, M. Exploring the Potential of Muridae as Sentinels for Human and Zoonotic Viruses. Viruses 2024, 16, 1041. https://doi.org/10.3390/v16071041
Di Bartolo I, De Sabato L, Ianiro G, Vaccari G, Dini FM, Ostanello F, Monini M. Exploring the Potential of Muridae as Sentinels for Human and Zoonotic Viruses. Viruses. 2024; 16(7):1041. https://doi.org/10.3390/v16071041
Chicago/Turabian StyleDi Bartolo, Ilaria, Luca De Sabato, Giovanni Ianiro, Gabriele Vaccari, Filippo Maria Dini, Fabio Ostanello, and Marina Monini. 2024. "Exploring the Potential of Muridae as Sentinels for Human and Zoonotic Viruses" Viruses 16, no. 7: 1041. https://doi.org/10.3390/v16071041
APA StyleDi Bartolo, I., De Sabato, L., Ianiro, G., Vaccari, G., Dini, F. M., Ostanello, F., & Monini, M. (2024). Exploring the Potential of Muridae as Sentinels for Human and Zoonotic Viruses. Viruses, 16(7), 1041. https://doi.org/10.3390/v16071041







