First Isolation and Molecular Characterization of Umatilla Virus (Sedoreoviridae, Orbivirus) in Brazil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viral Isolation in Cell Culture
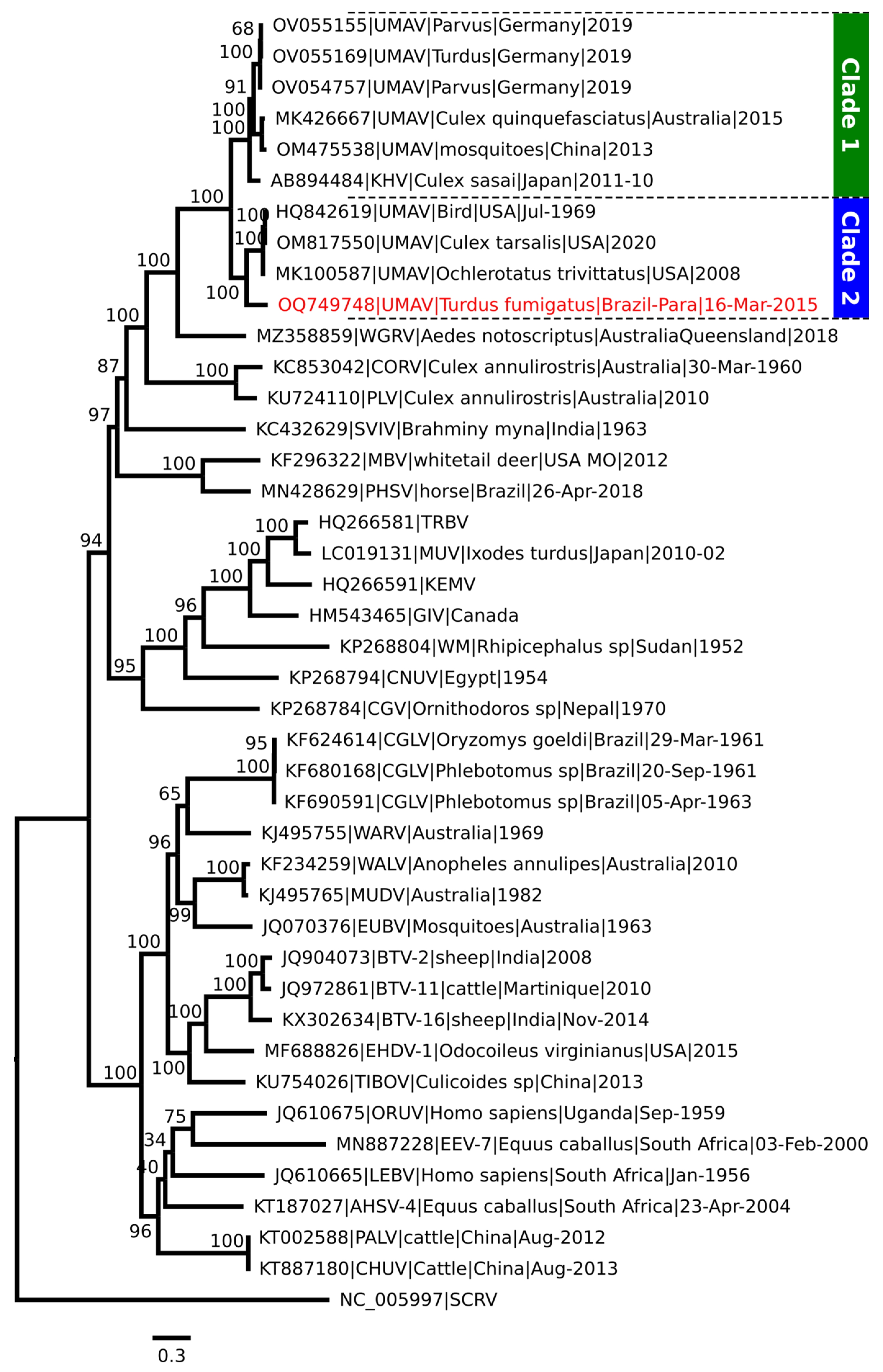
2.2. RNA Extraction, Nucleotide Sequencing, and Phylogenetic Analysis
2.3. Reassortment Analysis
3. Results
3.1. Virus Isolation in Cell Culture and Molecular Analysis
3.2. Reassortment Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matthijnssens, J.; Attoui, H.; Bányai, K.; Brussaard, C.P.D.; Danthi, P.; del Vas, M.; Dermody, T.S.; Duncan, R.; Fāng, Q.; Johne, R.; et al. ICTV Virus Taxonomy Profile: Sedoreoviridae 2022. J. Gen. Virol. 2022, 103, 001782. [Google Scholar] [CrossRef]
- Dilcher, M.; Hasib, L.; Lechner, M.; Wieseke, N.; Middendorf, M.; Marz, M.; Koch, A.; Spiegel, M.; Dobler, G.; Hufert, F.T.; et al. Genetic Characterization of Tribeč Virus and Kemerovo Virus, Two Tick-Transmitted Human-Pathogenic Orbiviruses. Virology 2012, 423, 68–76. [Google Scholar] [CrossRef]
- Zerbini, F.M.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Alfenas-Zerbini, P.; Dempsey, D.M.; Dutilh, B.E.; García, M.L.; Hendrickson, R.C.; et al. Changes to Virus Taxonomy and the ICTV Statutes Ratified by the International Committee on Taxonomy of Viruses (2023). Arch. Virol. 2023, 168, 175. [Google Scholar] [CrossRef]
- Attoui, H.; Jaafar, F.M.; Belhouchet, M.; Aldrovandi, N.; Tao, S.; Chen, B.; Liang, G.; Tesh, R.B.; de Micco, P.; de Lamballerie, X. Yunnan orbivirus, a New Orbivirus Species Isolated from Culex tritaeniorhynchus Mosquitoes in China. J. Gen. Virol. 2005, 86, 3409–3417. [Google Scholar] [CrossRef]
- Wilson, A.J.; Mellor, P.S. Bluetongue in Europe: Past, Present and Future. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2669–2681. [Google Scholar] [CrossRef]
- Belaganahalli, M.N.; Maan, S.; Maan, N.S.; Tesh, R.; Attoui, H.; Mertens, P.P.C. Umatilla Virus Genome Sequencing and Phylogenetic Analysis: Identification of Stretch Lagoon Orbivirus as a New Member of the Umatilla Virus Species. PLoS ONE 2011, 6, e23605. [Google Scholar] [CrossRef]
- MacLachlan, N.J.; Guthrie, A.J. Re-Emergence of Bluetongue, African Horse Sickness, and Other Orbivirus Diseases. Vet. Res. 2010, 41, 35. [Google Scholar] [CrossRef]
- Karabatsos, N. (Ed.) International Catalogue of Arboviruses, Including Certain Other Viruses of Vertebrates, 3rd ed.; Subcommittee on Information Exchange of the American Committee on Arthropod-borne Viruses by the American Society of Tropical Medicine and Hygiene: San Antonio, TX, USA, 1985. [Google Scholar]
- ICMBio. Relatório Anual de Rotas e Áreas de Concentração de Aves Migratórias No Brasil; Instituto Chico Mendes de Conservação da Biodiversidade: Brasília, Brazil, 2014. [Google Scholar]
- Martins, F.D.; Castilho, A.F.; Campos, J.; Hatano, F.M.; Rolim, S.G. Fauna da Floresta Nacional de Carajás; Instituto Chico Mendes de Conservação da Biodiversidade: Brasília, Brazil, 2012; 119p. [Google Scholar]
- Barbosa, M.L.; Rocco, I.M.; Felippe, J.M.M.S.; Cruz, A.S. Growth and Maintenance of Aedes albopictus Cell Line, Clone C6/36, in Diferrent Media. Rev. Inst. Adolfo Lutz 1993, 53, 63–70. [Google Scholar] [CrossRef]
- Igarashi, B. Isolation of a Singh’s Aedes Albopictus Cell Clone Sensitive to Dengue and Chikungunya Viruses. J. Gen. Virol. 1978, 40, 531–544. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Peng, Y.; Leung, H.C.M.; Yiu, S.M.; Chin, F.Y.L. IDBA-UD: A de Novo Assembler for Single-Cell and Metagenomic Sequencing Data with Highly Uneven Depth. Bioinformatics 2012, 28, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and Sensitive Protein Alignment Using DIAMOND. Nat. Methods 2014, 12, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Huson, D.H.; Auch, A.F.; Qi, J.; Schuster, S.C. MEGAN Analysis of Metagenomic Data. Genome Res. 2007, 17, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.-Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-Scale Protein Function Classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Abascal, F.; Zardoya, R.; Posada, D. ProtTest: Selection of Best-Fit Models of Protein Evolution. Bioinformatics 2005, 21, 2104–2105. [Google Scholar] [CrossRef] [PubMed]
- Myung, I.J. Tutorial on Maximum Likelihood Estimation. J. Math. Psychol. 2003, 47, 90–100. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Silva, S.P.; Dilcher, M.; Weber, F.; Hufert, F.T.; Weidmann, M.; Cardoso, J.F.; Carvalho, V.L.; Chiang, J.O.; Martins, L.C.; Lima, C.P.S.; et al. Genetic and Biological Characterization of Selected Changuinola Viruses (Reoviridae, Orbivirus) from Brazil. J. Gen. Virol. 2014, 95, 2251–2259. [Google Scholar] [CrossRef]
- Mellor, P.S.; Hamblin, C. African Horse Sickness. Vet. Res. 2004, 35, 445–466. [Google Scholar] [CrossRef]
- Belaganahalli, M.N.; Maan, S.; Maan, N.S.; Nomikou, K.; Pritchard, I.; Lunt, R.; Kirkland, P.D.; Attoui, H.; Brownlie, J.; Mertens, P.P.C. Full Genome Sequencing and Genetic Characterization of Eubenangee Viruses Identify Pata Virus as a Distinct Species within the Genus Orbivirus. PLoS ONE 2012, 7, e31911. [Google Scholar] [CrossRef] [PubMed]
- Fukusho, A.; Ritter, G.D.; Roy, P. Variation in the Bluetongue Virus Neutralization Protein VP2. J. Gen. Virol. 1987, 68 Pt 11, 2967–2973. [Google Scholar] [CrossRef]
- Maan, S.; Maan, N.S.; Samuel, A.R.; Rao, S.; Attoui, H.; Mertens, P.P.C. Analysis and Phylogenetic Comparisons of Full-Length VP2 Genes of the 24 Bluetongue Virus Serotypes. J. Gen. Virol. 2007, 88, 621–630. [Google Scholar] [CrossRef]
- Stamm, D.D. Relationships of Birds and Arboviruses. Auk 1966, 83, 84–97. [Google Scholar] [CrossRef]
- Degallier, N.; da Rosa, A.P.A.T.; da Silva, J.M.C.; Rodrigues, S.G.; da Costa Vasconcelos, P.F.; da Rosa, J.F.S.T.; da Silva, G.P.; da Silva, R.P. Aves Como Hospedeiras de Arbovírus Na Amazônia Brasileira. Bol. do Mus. Para. Emílio Goeldi 1992, 8, 69–111. [Google Scholar]
- Georgopoulou, I.; Tsiouris, V. The Potential Role of Migratory Birds in the Transmission of Zoonoses. Vet. Ital. 2008, 44, 671–677. [Google Scholar]
- Brown, C.R.; O’Brien, V.A. Are Wild Birds Important in the Transport of Arthropod-Borne Viruses? Ornithol. Monogr. 2011, 71, 1–64. [Google Scholar] [CrossRef]
- Weaver, S.C.; Hagenbaugh, A.; Bellew, L.A.; Gousset, L.; Mallampalli, V.; Holland, J.J.; Scott, T.W. Evolution of Alphaviruses in the Eastern Equine Encephalomyelitis Complex. J. Virol. 1994, 68, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Young, D.S.; Kramer, L.D.; Maffei, J.G.; Dusek, R.J.; Backenson, P.B.; Mores, C.N.; Bernard, K.A.; Ebel, G.D. Molecular Epidemiology of Eastern Equine Encephalitis Virus, New York. Emerg. Infect. Dis. 2008, 14, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Cilnis, M.J.; Kang, W.; Weaver, S.C. Genetic Conservation of Highlands J Viruses. Virology 1996, 218, 343–351. [Google Scholar] [CrossRef]
- Maciel-de-Freitas, R.; Neto, R.B.; Gonçalves, J.M.; Codeço, C.T.; Lourenço-de-Oliveira, R. Movement of Dengue Vectors between the Human Modified Environment and an Urban Forest in Rio de Janeiro. J. Med. Entomol. 2006, 43, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Milby, M.M.; Reisen, W.K.; Reeves, W.C. Intercanyon Movement of Marked Culex tarsalis (Diptera: Culicidae)1. J. Med. Entomol. 1983, 20, 193–198. [Google Scholar] [CrossRef]
- Reisen, W.K.; Reeves, W.C. Bionomics and Ecology of Culex tarsalis and Other Potential Mosquito Vector Species. In Epidemiology and Control of Mosquito Borne Arboviruses in California, 1943–1987; California Mosquito & Vector Control Association: Sacramento, CA, USA, 1990; pp. 254–329. [Google Scholar]
- Reisen, W.K.; Fang, Y.; Lothrop, H.D.; Martinez, V.M.; Wilson, J.; O’connor, P.; Carney, R.; Cahoon-Young, B.; Shafii, M.; Brault, A.C. Overwintering of West Nile Virus in Southern California. J. Med. Entomol. 2006, 43, 344–355. [Google Scholar] [CrossRef]
- Jourdain, E.; Gauthier-Clerc, M.; Bicout, D.; Sabatier, P. Bird Migration Routes and Risk for Pathogen Dispersion into Western Mediterranean Wetlands. Emerg. Infect. Dis. 2007, 13, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Stutchbury, B.J.M.; Tarof, S.A.; Done, T.; Gow, E.; Kramer, P.M.; Tautin, J.; Fox, J.W.; Afanasyev, V. Tracking Long-Distance Songbird Migration by Using Geolocators. Science 2009, 323, 896. [Google Scholar] [CrossRef] [PubMed]
- ICMBio. Relatório de Rotas e Áreas de Concentração de Aves Migratórias No Brasil; Instituto Chico Mendes de Conservação da Biodiversidade: Brasília, Brazil, 2019. [Google Scholar]
- Owen, J.; Moore, F.; Panella, N.; Edwards, E.; Bru, R.; Hughes, M.; Komar, N. Migrating Birds as Dispersal Vehicles for West Nile Virus. Ecohealth 2006, 3, 79–85. [Google Scholar] [CrossRef]
- Agarwal, A.; Parida, M.; Dash, P.K. Impact of Transmission Cycles and Vector Competence on Global Expansion and Emergence of Arboviruses. Rev. Med. Virol. 2017, 27, e1941. [Google Scholar] [CrossRef]
- Tangudu, C.S.; Charles, J.; Hurt, S.L.; Dunphy, B.M.; Smith, R.C.; Bartholomay, L.C.; Blitvich, B.J. Skunk River Virus, a Novel Orbivirus Isolated from Aedes trivittatus in the United States. J. Gen. Virol. 2019, 100, 295–300. [Google Scholar] [CrossRef]
- Oliveira, C.F. Relatório de Gestão do Exercício 2017; Instituto Evandro Chagas: Rio de Janeiro, Brazil, 2018. [Google Scholar]

| Virus | VP1 | VP2 | VP3 | VP4 | VP5 | VP6 | VP7 | NS1 | NS2 | NS3 |
|---|---|---|---|---|---|---|---|---|---|---|
| nt/aa | nt/aa | nt/aa | nt/aa | nt/aa | nt/aa | nt/aa | nt/aa | nt/aa | nt/aa | |
| BTV | 51.6/47.5 | 26.6/9.8 | 47.0/35.8 | 50.1/45.0 | 44.3/32.3 | 34.5/21.5 | 38.3/24.4 | 33.0/19.4 | 30.5/16.3 | 29.9/14.8 |
| CORV | 56.8/58.0 | 29.1/15.0 | 52.8/47.8 | 53.2/50.1 | 52.2/46.9 | 39.0/23.2 | 45.2/41.5 | 41.9/30.8 | 40.6/30.7 | 34.2/20.0 |
| KHV | 74.9/89.0 | 60.6/58.0 | 79.5/95.1 | 71.0/77.9 | 73.4/82.8 | 73.6/64.6 | 81.0/91.7 | 76.0/87.1 | 70.6/77.1 | 67.0/66.5 |
| MOBV | 55.8/51.4 | 29.9/11.8 | 51.0/43.5 | 50.0/42.1 | 48.8/37.8 | 38.9/25.7 | 46.4/32.3 | 41.5/26.7 | 37.3/23.3 | 28.3/19.4 |
| PLV | 51.2/47.0 | 26.0/7.5 | 47.0/35.6 | 50.4/43.9 | 43.2/29.9 | 32.0/18.1 | 40.3/25.3 | 34.5/20.3 | 32.3/17.8 | 27.9/11.8 |
| SVIV | 55.8/53.9 | 28.5/12.3 | 51.2/448 | 48.7/44.4 | 47.9/34.6 | 33.9/24.8 | 48.4/36.1 | 35.8/22.0 | 38.5/27.7 | 27.7/18.2 |
| UMAV | 80.6/94.7 | 61.5/60.0 | 83.8/97.7 | 77.0/85.0 | 76.2/89.4 | 82.8/76.7 | 86.6/98.9 | 80.9/93.6 | 81.4/94.6 | 83.1/92.0 |
| YUOV | 54.8/50.3 | 29.1/11.7 | 49.5/40.7 | 48.0/42.8 | 48.2/35.7 | 34.5/23.4 | 45.4/30.4 | 38.9/22.8 | 35.6/24.1 | 29.4/17.0 |
| Segment * | Protein | Mapping Reads | Coverage | Genome Size (nt) | 5′ UTR Length | ORF Size (nt) | Situation ORF | Protein (aa) | Weight (KDa) | 3′ UTR Length | NCBI ID |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | RNA Pol Orbiviruses (VP1) | 577 | 21 | 3920 | 11 | 3900 | Complete | 1299 | 147,807 | 9 | OQ749748 |
| 2 | Inner layer core VP3 Orbiviruses (T2) | 451 | 23 | 2728 | 18 | 2710 | Incomplete | 902 | 103,146 | NA | OQ749749 |
| 3 | Capsid VP2 Orbiviruses (VP2) | 827 | 50 | 2311 | 17 | 2294 | Incomplete | 764 | 89,838 | NA | OQ749750 |
| 4 | Orbiviruses VP4 (Cap) | 1899 | 134 | 1979 | 4 | 1947 | Complete | 648 | 75,829 | 28 | OQ749751 |
| 5 | Orbiviruses NS1 (TuP) | 621 | 47 | 1771 | 13 | 1743 | Complete | 580 | 66,995 | 15 | OQ749752 |
| 6 | Capsid VP5 Orbiviruses | 486 | 43 | 1641 | 27 | 1590 | Complete | 529 | 5884 | 24 | OQ749753 |
| 7 | BTV NS2 | 486 | 53 | 1318 | 60 | 1224 | Complete | 407 | 46,073 | 34 | OQ749754 |
| 8 | Orbiviruses VP7 capsid (T13) | 237 | 31 | 1074 | 8 | 1056 | Complete | 351 | 38,953 | 10 | OQ749755 |
| 9 | Orbiviruses VP6 (Hel) | 394 | 50 | 999 | 24 | 975 | Incomplete | 324 | 34,632 | NA | OQ749756 |
| 10 | Orbiviruses NS3 | 93 | 15 | 843 | 12 | 795 | Complete | 264 | 29,362 | 36 | OQ749757 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, L.; Silva, S.; Cruz, A.C.; da Silva, E.; Wanzeller, A.L.; Carvalho, V.; Chiang, J.; Martins, L. First Isolation and Molecular Characterization of Umatilla Virus (Sedoreoviridae, Orbivirus) in Brazil. Viruses 2024, 16, 1050. https://doi.org/10.3390/v16071050
Barros L, Silva S, Cruz AC, da Silva E, Wanzeller AL, Carvalho V, Chiang J, Martins L. First Isolation and Molecular Characterization of Umatilla Virus (Sedoreoviridae, Orbivirus) in Brazil. Viruses. 2024; 16(7):1050. https://doi.org/10.3390/v16071050
Chicago/Turabian StyleBarros, Landeson, Sandro Silva, Ana Cecília Cruz, Eliana da Silva, Ana Lúcia Wanzeller, Valéria Carvalho, Jannifer Chiang, and Lívia Martins. 2024. "First Isolation and Molecular Characterization of Umatilla Virus (Sedoreoviridae, Orbivirus) in Brazil" Viruses 16, no. 7: 1050. https://doi.org/10.3390/v16071050






