Temporal Changes in Splenic Immune Cell Populations following Infection with a Very Virulent plus MDV in Commercial Meat-Type Chickens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Viruses and Vaccine
2.3. Experimental Design
2.4. Spleen Single-Cell Suspension
2.5. Flow Cytometry-Based Cellular Analysis
2.6. Statistical Analysis
3. Results
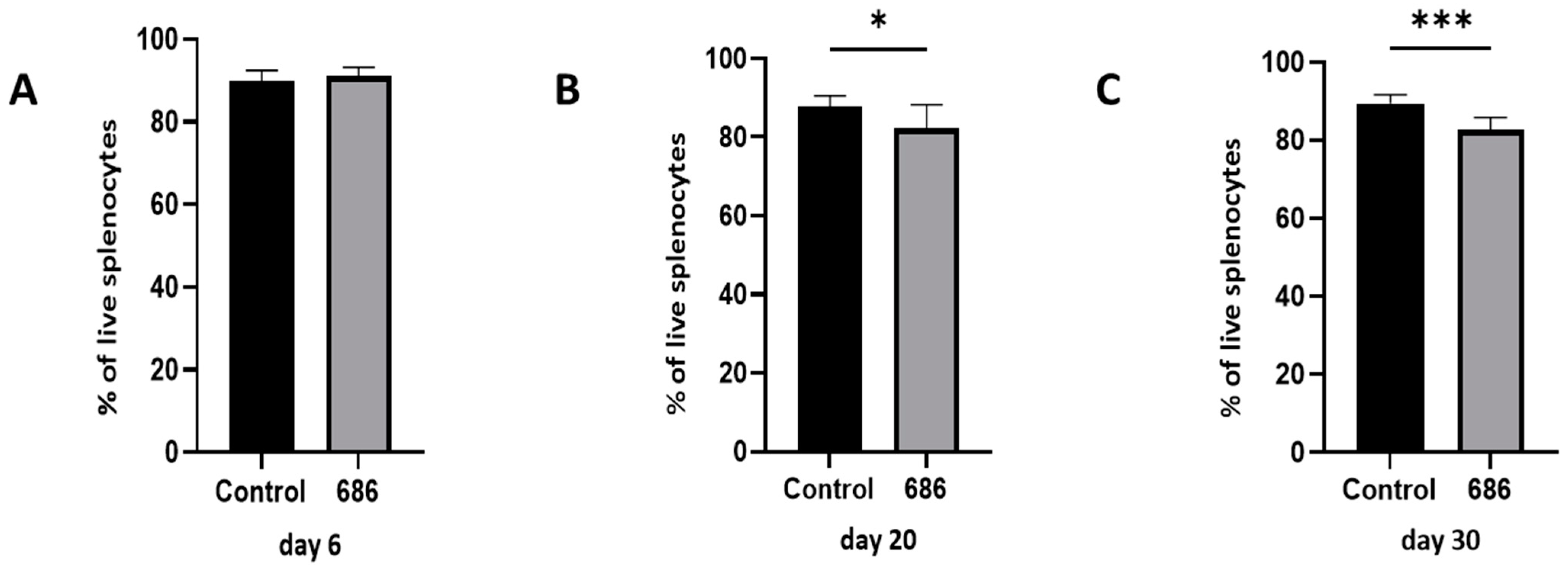
3.1. Effect of the 686 Infection on Live Cells in the Spleen
3.2. Effect of 686 Infection on T Cell Subsets in the Spleen (Figure 2, Figure 3 and Figure 4)
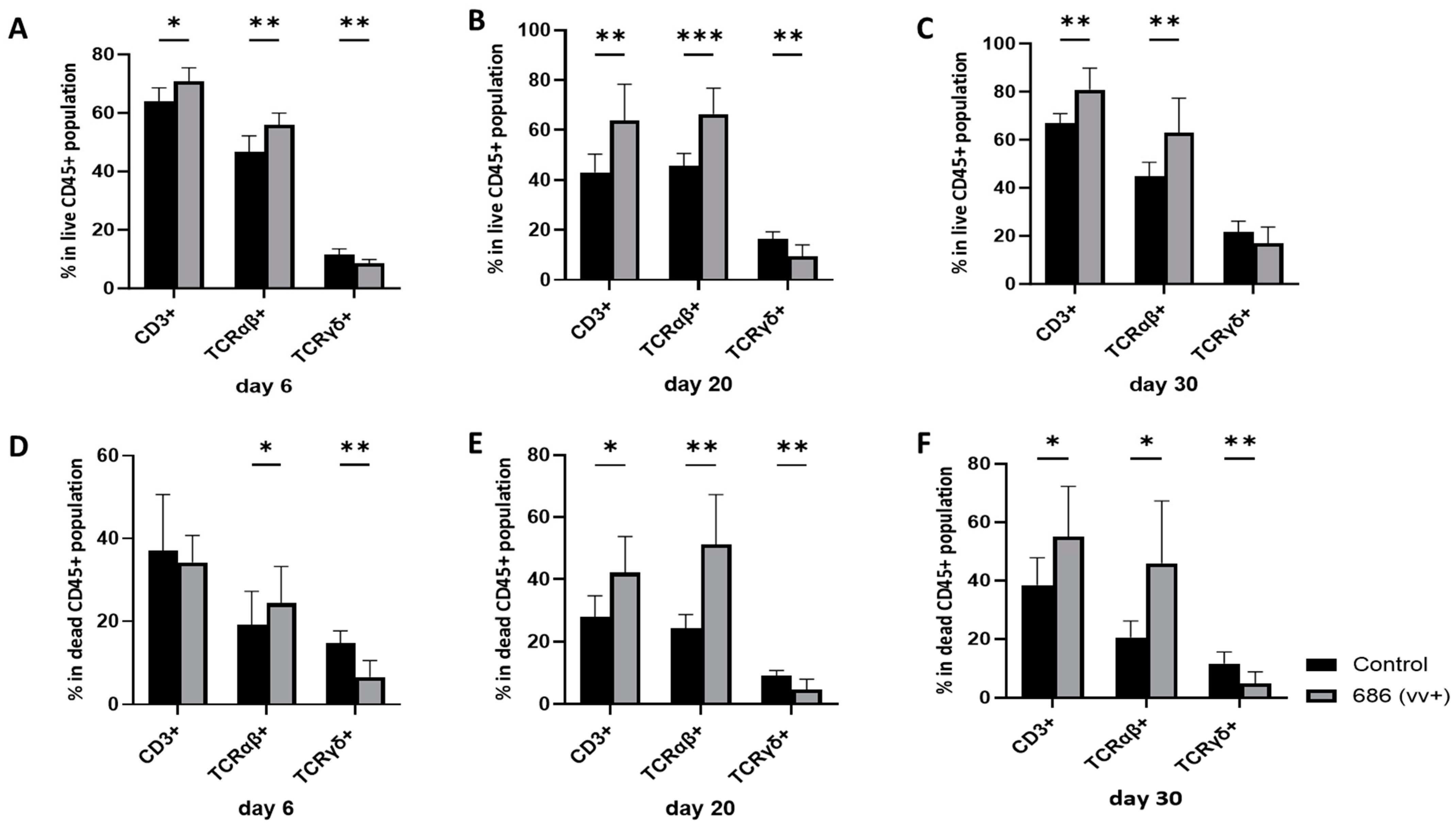


3.3. Effect of 686 Infection on Expression of MHC-I and MHC-II in T Cells in Spleen
3.3.1. MHC-I Expression (Table 1)
| Dpi 3 | Treatment | CD3+ Cells (T Cells) | CD4+ T Cells | CD8β + T Cells | ||||
|---|---|---|---|---|---|---|---|---|
| Live 4 | Dead 4 | Live 4 | Dead 4 | Live 4 | Dead 4 | |||
| Expression of MHC-I 1 (Frequency) | 20 | Control | 23.11 ± 5.59 a | 49.24 ± 5.0 a | 6.066 ± 2.1 a | 3.790 ± 2.1 a | 22.39 ± 4.13 a | 59.41±5.90 a |
| 686 | 35.43 ± 10.8 b | 62.91 ± 11.3b | 19.06 ± 9.6 b | 6.531 ± 2.2 b | 18.90 ± 9.01 a | 48.87±12.4 a | ||
| 30 | Control | 19.99 ± 2.87 a | 37.40 ± 10.8 a | 2.874 ± 0.7 a | 4.774 ± 3.3 a | 22.69 ± 5.68 a | 45.27±6.98 a | |
| 686 | 34.50 ± 9.89 b | 60.53 ± 10.6 b | 15.66 ± 14 b | 7.581 ± 5.0 a | 19.88 ± 9.96 a | 41.08 ± 17.8 a | ||
| Expression of MHC-I (MFI) 2 | 20 | Control | 1840 ± 171.9 a | 3890 ± 895.2 a | 2568 ± 489.2 a | 2205 ± 519.2 a | 2091 ± 179.6 a | 4047 ± 945 a |
| 686 | 2428 ± 408.1b | 3729 ± 1062.6 a | 3212 ± 764.1 a | 3158 ± 904.3 b | 2282 ± 350.4 a | 3590 ± 1204 a | ||
| 30 | Control | 1796 ± 119.4 a | 2812 ± 433.7 a | 2669 ± 144.6 a | 1802 ± 127.3 a | 1918 ± 143.1 a | 2899 ± 414 a | |
| 686 | 2467 ± 528.2 b | 3542 ± 385.9 b | 3474 ± 361.9 b | 2971 ± 481.5 b | 2428 ± 267.1 b | 3422 ± 372 b | ||
3.3.2. MHC-II Expression (Table 2)
| Dpi 3 | Treatment | CD3+ Cells (T Cells) | CD4+ T Cells | CD8β + T Cells | ||||
|---|---|---|---|---|---|---|---|---|
| Live 4 | Dead 4 | Live 4 | Dead 4 | Live 4 | Dead 4 | |||
| Expression of MHC-II 1 (Frequency) | 6 | Control | 31.64 ± 6.83 a | 45.50 ± 6.15 a | 25.27 ± 4.5 a | 1.521 ± 0.6 a | 19.11 ± 7.26 a | 43.67 ± 3.38 a |
| 686 | 26.74 ± 4.47 a | 42.53 ± 2.82 a | 19.57 ± 1.5b | 1.530 ± 0.6 a | 15.78 ± 3.92 a | 47.24 ± 3.22 a | ||
| 20 | Control | 23.79 ± 8.41 a | 47.89 ± 5.00 a | 13.90 ± 5.2 a | 2.627 ± 1.2 a | 25.71 ± 8.16 a | 58.97 ± 5.78 a | |
| 686 | 29.49 ± 5.28 a | 53.83 ± 6.6 a | 19.21 ± 7.4 a | 4.247 ± 1.6 b | 18.54 ± 8.68 a | 49.36 ± 12.5 a | ||
| 30 | Control | 23.70 ± 7.87 a | 32.89 ± 10.9 a | 8.30 ± 3.4 a* | 3.807 ± 1.9 a | 23.04 ± 8.10 a | 51.73 ± 7.71 a | |
| 686 | 33.91 ± 11.5 a | 49.03 ± 6.61 b | 18.77 ± 15 b* | 4.896 ± 4.2 a | 20.04 ± 5.94 a | 49.78 ± 20.6 a | ||
| Expression of MHC-II (MFI) 2 | 6 | Control | 2151 ± 271 a | 53,336 ± 16,034 a | 1890 ± 161 a | 12,978 ± 2102 a | 2460 ± 548 a | 53,361 ± 18,348 a |
| 686 | 2195 ± 239 a | 22,765 ± 10,118 b | 1907 ± 233 a | 8661 ± 3291 b | 2400 ± 309 a | 19,952 ± 9572 b | ||
| 20 | Control | 2716 ± 347 a | 22,501 ± 8058 a | 1804 ± 229 a | 4179 ± 1098 a | 1946 ± 240 a | 27,155 ± 11,082 a | |
| 686 | 3141 ± 337 b | 8024 ± 4465 b | 2322 ± 366 b | 4279 ± 1324 a | 2087 ± 287 a | 8179 ± 4432 b | ||
| 30 | Control | 1684 ± 296 a | 21,109 ± 5955 a | 1572 ± 228 a | 6899 ± 1344 a | 1673 ± 297 a | 23,445 ± 8857 a | |
| 686 | 2750 ± 1102 b | 18,092 ± 1212 a | 2767 ± 1091b | 7251 ± 855 a | 2214 ± 921 a | 19,933 ± 13,066 a | ||
3.4. Effect of 686 Infection on B Cells and Macrophages in Spleens (Figure 5)
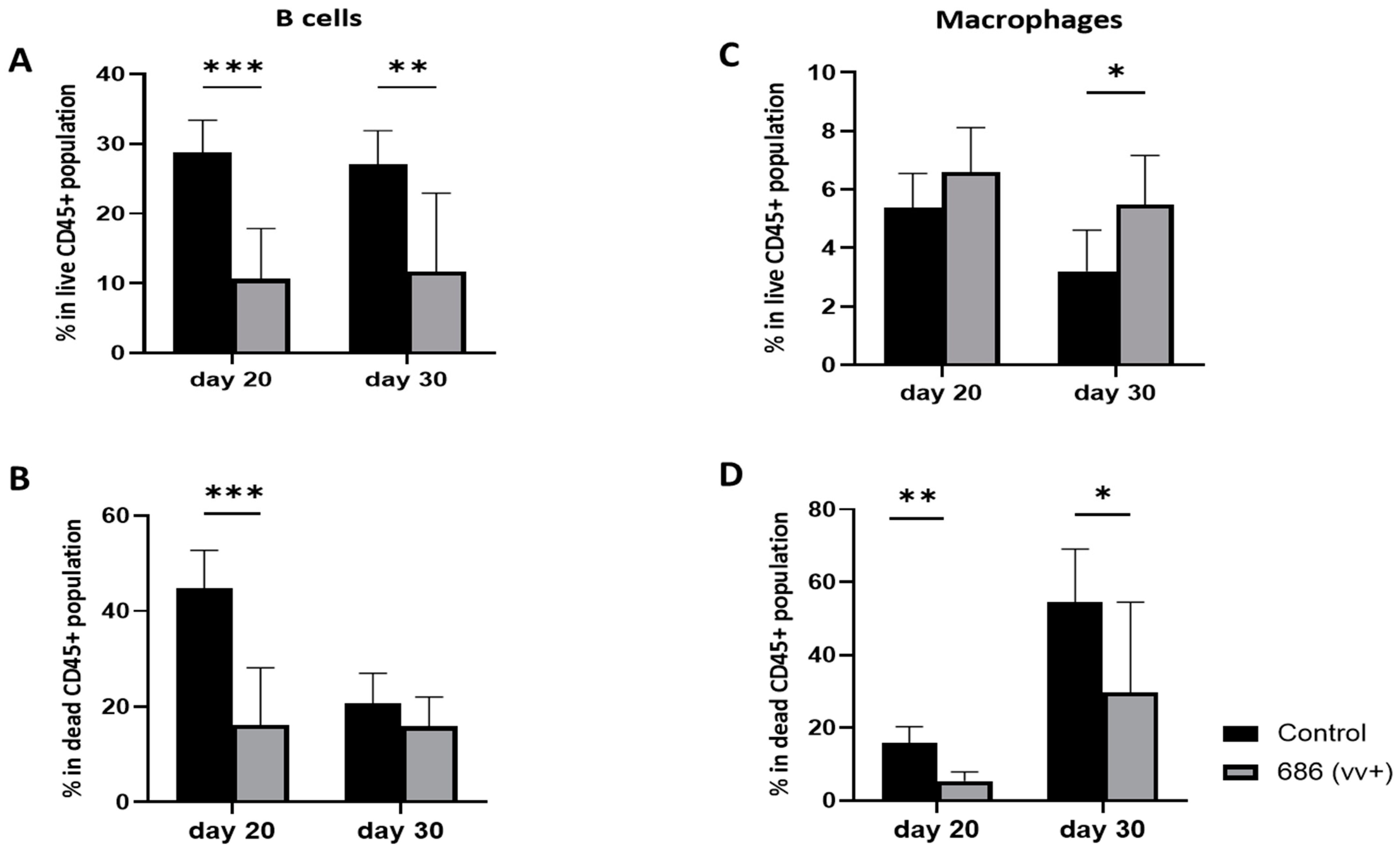
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schat, K.A.; Nair, V.K. Marek’s Disease. In Diseases of Poultry, 12th ed.; Saif, Y.M., Fadly, A.M., Glisson, J.R., McDougald, L.R., Nolan, L.K., Swayne, D.E., Eds.; Blackwell Publishing Professionals: Ames, IA, USA, 2008; pp. 449–514. [Google Scholar]
- Davison, A.J.; Eberle, R.; Ehlers, B.; Hayward, G.S.; McGeoch, D.J.; Minson, A.C.; Pellett, P.E.; Roizman, B.; Studdert, M.J.; Thiry, E. The Order Herpesvirales. Arch. Virol. 2009, 154, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Osterrieder, N.; Kamil, J.P.; Schumacher, D.; Tischer, B.K.; Trapp, S. Marek’s Disease Virus: From Miasma to Model. Nat. Rev. Microbiol. 2006, 4, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Witter, R.L. Increased Virulence of Marek’s Disease Virus Field Isolates. Avian Dis. 1997, 41, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Witter, R.L.; Gimeno, I.M.; Reed, W.M.; Bacon, L.D. An Acute Form of Transient Paralysis Induced by Highly Virulent Strains of Marek’s Disease Virus. Avian Dis. 1999, 43, 704–720. [Google Scholar] [CrossRef] [PubMed]
- Pandiri, A.K.R.; Cortes, A.L.; Lee, L.F.; Gimeno, I.M. Marek’s Disease Virus Infection in the Eye: Chronological Study of the Lesions, Virus Replication, and Vaccine-Induced Protection. Avian Dis. 2008, 52, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Faiz, N.M.; Cortes, A.L.; Guy, J.S.; Fletcher, O.J.; West, M.; Montiel, E.; Gimeno, I.M. Early Infection with Marek’s Disease Virus Can Jeopardize Protection Conferred by Laryngotracheitis Vaccines: A Method to Study MDV-Induced Immunosuppression. Avian Pathol. 2016, 45, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Calnek, B.W.; Harris, R.W.; Buscaglia, C.; Schat, K.A.; Lucio, B. Relationship between the Immunosuppressive Potential and the Pathotype of Marek’s Disease Virus Isolates. Avian Dis. 1998, 42, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Calnek, B.W. Pathogenesis of Marek’s Disease Virus Infection. In Marek’s Disease; Hirai, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 25–55. ISBN 978-3-642-56863-3. [Google Scholar]
- Schat, K.A. 11—Marek’s Disease Immunosuppression. In Marek’s Disease; Davison, F., Nair, V., Eds.; Academic Press: Oxford, UK, 2004; pp. 142–155. ISBN 15724271. [Google Scholar]
- Faiz, N.M.; Cortes, A.L.; Guy, J.S.; Fletcher, O.J.; Cimino, T.; Gimeno, I.M. Evaluation of Factors Influencing the Development of Late Marek’s Disease Virus-Induced Immunosuppression: Virus Pathotype and Host Sex. Avian Pathol. 2017, 46, 376–385. [Google Scholar] [CrossRef]
- Faiz, N.M.; Cortes, A.L.; Guy, J.S.; Reddy, S.M.; Gimeno, I.M. Differential Attenuation of Marek’s Disease Virus-Induced Tumours and Late-Marek’s Disease Virus-Induced Immunosuppression. J. General. Virol. 2018, 99, 927–936. [Google Scholar] [CrossRef]
- Ball, R.F.; Hill, J.F.; Lyman, J.; Wyatt, A. The Resistance to Marek’s Disease of Chicks from Immunized Breeders. Poult. Sci. 1971, 50, 1084–1090. [Google Scholar] [CrossRef]
- Churchill, A.E.; Payne, L.N.; Chubb, R.C. Immunization against Marek’s Disease Using a Live Attenuated Virus; Academic Press: Cambridge, MA, USA, 1969; Volume 14. [Google Scholar]
- Spencer, J.L.; Robertson, A. Influence of Maternal Antibody on Infection with Virulent or Attenuated Marek’s Disease Herpesvirus. Am. J. Vet. Res. 1972, 33, 393–400. [Google Scholar] [PubMed]
- Calnek, B.W. Effects of Passive Antibody on Early Pathogenesis of Marek’s Disease. Infect. Immun. 1972, 6, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Payne, L.N.; Rennie, M. Pathogenesis of Marek’s Disease in Chicks with and Without Maternal Antibody. JNCI J. Natl. Cancer Inst. 1973, 51, 1559–1573. [Google Scholar] [CrossRef]
- Eidson, C.S.; Fletcher, O.J.; Kleven, S.H.; Anderson, D.P. Detection of Marek’s Disease Antigen in Feather Follicle Epithelium of Chickens Vaccinated Against Marek’s Disease234. JNCI J. Natl. Cancer Inst. 1971, 47, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Eidson, C.S.; Kleven, S.H.; Lacroix, V.M.; Anderson, D.P. Maternal Transfer of Resistance against Development of Marek’s Disease Tumors. Avian Dis. 1972, 16, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, I.M.; Witter, R.L.; Cortes, A.L.; Reed, W.M. Replication Ability of Three Highly Protective Marek’s Disease Vaccines: Implications in Lymphoid Organ Atrophy and Protection. Avian Pathol. 2011, 40, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.R.; Auten, K.; Heidari, M.; Buscaglia, C. Correlation between Marek’s Disease Virus Pathotype and Replication. Avian Dis. 2014, 58, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.F.M.F.; Wong, C.W.; Walkden-Brown, S.W.; Colditz, I.G.; Arzey, K.E.; Groves, P.J. Immunosuppressive Effects of Marek’s Disease Virus (MDV) and Herpesvirus of Turkeys (HVT) in Broiler Chickens and the Protective Effect of HVT Vaccination against MDV Challenge. Avian Pathol. 2002, 31, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Walkden-Brown, S.; Islam, A.; Islam, A.; Burgess, S.; Groves, P.; Cooke, J. Pathotyping of Australian Isolates of Marek’s Disease Virus in Commercial Broiler Chickens Vaccinated with Herpesvirus of Turkeys (HVT) or Bivalent (HVT/SB1) Vaccine and Association with Viral Load in the Spleen and Feather Dander. Aust. Vet. J. 2013, 91, 341–350. [Google Scholar] [CrossRef]
- Gimeno, I.M.; Cortes, A.L.; Faiz, N.; Villalobos, T.; Badillo, H.; Barbosa, T. Efficacy of Various HVT Vaccines (Conventional and Recombinant) Against Marek’s Disease in Broiler Chickens: Effect of Dose and Age of Vaccination. Avian Dis. 2016, 60, 662–668. [Google Scholar] [CrossRef]
- Gimeno, I.M.; Cortes, A.L.; Reddy, S.M.; López de Juan Abad, B.; Käser, T.; Limsatanun, A. Highly Virulent Marek’s Disease Virus Strains Affect T Lymphocyte Function and Viability of Splenocytes in Commercial Meat-Type Chickens. Avian Pathol. 2019, 48, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Witter, R.L.; Kreager, K.S. Serotype 1 Viruses Modified by Backpassage or Insertional Mutagenesis: Approaching the Threshold of Vaccine Efficacy in Marek’s Disease. Avian Dis. 2004, 48, 768–782. [Google Scholar] [CrossRef]
- Witter, R.L.; Nazerian, K.; Purchase, H.G.; Burgoyne, G.H. Isolation from Turkeys of a Cell-Associated Herpesvirus Antigenically Related to Marek’s Disease Virus. Am. J. Vet. Res. 1970, 31, 525–538. [Google Scholar] [PubMed]
- Gimeno, I.M.; Cortes, A.L.; Silva, R.F. Load of Challenge Marek’s Disease Virus DNA in Blood as a Criterion for Early Diagnosis of Marek’s Disease Tumors. Avian Dis. 2008, 52, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, I.M.; Witter, R.L.; Reed, W.M. Four Distinct Neurologic Syndromes in Marek’s Disease: Effect of Viral Strain and Pathotype. Avian Dis. 1999, 43, 721–737. [Google Scholar] [CrossRef] [PubMed]
- Shek, W.R.; Calnek, B.W.; Schat, K.A.; Chen, C.H. Characterization of Marek’s Disease Virus-Infected Lymphocytes: Discrimination between Cytolytically and Latently Infected Cells. J. Natl. Cancer Inst. 1983, 70, 485–491. [Google Scholar] [PubMed]
- Calnek, B.W.; Higgins, D.A.; Fabricant, J. Rous Sarcoma Regression in Chickens Resistant or Susceptible to Marek’s Disease. Avian Dis. 1975, 19, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Schat, K.A.; Chen, C.-L.H.; Shek, W.R.; Calnek, B.W. Surface Antigens on Marek’s Disease Lymphoblastoid Tumor Cell Lines23. JNCI J. Natl. Cancer Inst. 1982, 69, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Powell, P.C.; Mustill, B.M.; Rennie, M. The Role of Histocompatibility Antigens in Cell-mediated Cytotoxicity against Marek’s Disease Tumour-derived Lymphoblastoid Cell Lines. Avian Pathol. 1983, 12, 461–468. [Google Scholar] [CrossRef]
- Morimura, T.; Hattori, M.; Ohashi, K.; Sugimoto, C.; Onuma, M. Immunomodulation of Peripheral T Cells in Chickens Infected with Marek’s Disease Virus: Involvement in Immunosuppression. J. Gen. Virol. 1995, 76, 2979–2985. [Google Scholar] [CrossRef]
- Morimura, T.; Ohashi, K.; Kon, Y.; Hattori, M.; Sugimoto, C.; Onuma, M. Apoptosis and CD8-down-Regulation in the Thymus of Chickens Infected with Marek’s Disease Virus Brief Report; Springer: Berlin, Germany, 1996; Volume 141. [Google Scholar]
- Smith, A.L.; Göbel, T.W. Avian T Cells: Antigen Recognition and Lineages. In Avian Immunology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 91–102. [Google Scholar] [CrossRef]
- Pieper, J.; Methner, U.; Berndt, A. Heterogeneity of Avian Γδ T Cells. Vet. Immunol. Immunopathol. 2008, 124, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Laursen, A.M.S.; Kulkarni, R.R.; Taha-Abdelaziz, K.; Plattner, B.L.; Read, L.R.; Sharif, S. Characterizaton of Gamma Delta T Cells in Marek’s Disease Virus (Gallid Herpesvirus 2) Infection of Chickens. Virology 2018, 522, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Fenzl, L.; Göbel, T.W.; Neulen, M.L. Γδ T Cells Represent a Major Spontaneously Cytotoxic Cell Population in the Chicken. Dev. Comp. Immunol. 2017, 73, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Quere, P.; Coopert, M.D.; Thorbecket, G.J. Characterization of Suppressor T Cells for Antibody Production by Chicken Spleen Cells I. Antigen-Induced Suppressor Cells Are CT8+, TcRl+ (Yb) T Cells. Immunology 1990, 71, 517. [Google Scholar] [PubMed]
- Berndt, A.; Pieper, J.; Methner, U. Circulating Γδ T Cells in Response to Salmonellaenterica Serovar Enteritidis Exposure in Chickens. Infect. Immun. 2006, 74, 3967–3978. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Zhang, F.; Yang, Y.; Shang, S. The Evaluation of Cellular Immunity to Avian Viral Diseases: Methods, Applications, and Challenges. Front. Microbiol. 2021, 12, 794514. [Google Scholar] [CrossRef] [PubMed]
- Meijerink, N.; Van Haarlem, D.A.; Velkers, F.C.; Stegeman, A.J.; Rutten, V.P.M.G.; Jansen, C.A. Analysis of Chicken Intestinal Natural Killer Cells, a Major IEL Subset during Embryonic and Early Life. Dev. Comp. Immunol. 2021, 114, 103857. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama-Kato, A.; Shojadoost, B.; Boodhoo, N.; Raj, S.; Alizadeh, M.; Fazel, F.; Fletcher, C.; Zheng, J.; Gupta, B.; Abdul-Careem, M.F.; et al. Activated Chicken Gamma Delta T Cells Are Involved in Protective Immunity against Marek’s Disease. Viruses 2023, 15, 285. [Google Scholar] [CrossRef]
- Burgess, S.C.; Basaran, B.H.; Davison, T.F. Resistance to Marek’s Disease Herpesvirus-Induced Lymphoma Is Multiphasic and Dependent on Host Genotype. Vet. Pathol. 2001, 38, 129–142. [Google Scholar] [CrossRef]
- Burgess, S.C.; Davison, T.F. Identification of the Neoplastically Transformed Cells in Marek’s Disease Herpesvirus-Induced Lymphomas: Recognition by the Monoclonal Antibody AV37. J. Virol. 2002, 76, 7276–7292. [Google Scholar] [CrossRef]
- Nazerian, K.; Sharma, J.M. Brief Communication: Detection of T-Cell Surface Antigens in a Marek’s Disease Lymphoblastoid Cell Line. JNCI J. Natl. Cancer Inst. 1975, 54, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Schat, K.A.; Chen, C.L.; Calnek, B.W.; Char, D. Transformation of T-Lymphocyte Subsets by Marek’s Disease Herpesvirus. J. Virol. 1991, 65, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Baigent, S.J.; Davison, F. Marek’s Disease Virus: Biology and Life Cycle. In Marek’s Disease; Davison, F., Nair, V., Eds.; Academic Press: Oxford, UK, 2004; pp. 62–77, i–ii. [Google Scholar] [CrossRef]
- Sarson, A.J.; Parvizi, P.; Lepp, D.; Quinton, M.; Sharif, S. Transcriptional Analysis of Host Responses to Marek’s Disease Virus Infection in Genetically Resistant and Susceptible Chickens. Anim. Genet. 2008, 39, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, L.G.; Chisari, F. V Noncytolytic Control of Viral Infections by the Innate and Adaptive Immuneresponse. Annu. Rev. Immunol. 2001, 19, 65–91. [Google Scholar] [CrossRef] [PubMed]
- Boodhoo, N.; Gurung, A.; Sharif, S.; Behboudi, S. Marek’s Disease in Chickens: A Review with Focus on Immunology. Vet. Res. 2016, 47, 119. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Schat, K.A. Inhibitory effects of nitric oxide and gamma interferon on in vitro and in vivo replication of Marek’s disease virus. J. Virol. 2000, 74, 3605–3612. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Careem, M.F.; Haq, K.; Shanmuganathan, S.; Read, L.R.; Schat, K.A.; Heidari, M.; Sharif, S. Induction of Innate Host Responses in the Lungs of Chickens Following Infection with a Very Virulent Strain of Marek’s Disease Virus. Virology 2009, 393, 250–257. [Google Scholar] [CrossRef]
- Feng, Z.-Q.; Lian, T.; Huang, Y.; Zhu, Q.; Liu, Y.-P. Expression Pattern of Genes of RLR-Mediated Antiviral Pathway in Different-Breed Chicken Response to Marek’s Disease Virus Infection. Biomed. Res. Int. 2013, 2013, 419256. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.A.; Miller, L. Comparison of Macrophage Function in Several Commercial Broiler Genetic Lines. Poult. Sci. 1991, 70, 2094–2101. [Google Scholar] [CrossRef]
- Chakraborty, P.; Vervelde, L.; Dalziel, R.G.; Wasson, P.S.; Nair, V.; Dutia, B.M.; Kaiser, P. Marek’s Disease Virus Infection of Phagocytes: A de Novo in Vitro Infection Model. J. General. Virol. 2017, 98, 1080–1088. [Google Scholar] [CrossRef]
- Lee, L.F.; Sharma, J.M.; Nazerian, K.; Witter, R.L. Suppression of Mitogen-Induced Proliferation of Normal Spleen Cells by Macrophages from Chickens Inoculated with Marek’s Disease Virus. J. Immunol. 1978, 120, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Ostuni, R.; Kratochvill, F.; Murray, P.J.; Natoli, G. Macrophages and Cancer: From Mechanisms to Therapeutic Implications. Trends Immunol. 2015, 36, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Schuren, A.B.C.; Costa, A.I.; Wiertz, E.J.H.J. Recent Advances in Viral Evasion of the MHC Class I Processing Pathway. Curr. Opin. Immunol. 2016, 40, 43–50. [Google Scholar] [CrossRef]
- Hunt, H.D.; Lupiani, B.; Miller, M.M.; Gimeno, I.; Lee, L.F.; Parcells, M.S. Marek’s Disease Virus Down-Regulates Surface Expression of MHC (B Complex) Class I (BF) Glycoproteins during Active but Not Latent Infection of Chicken Cells. Virology 2001, 282, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.M.; Davidson, I.; Burgess, S.C.; Heller, E.D. Major Histocompatibility Complex Class I Is Downregulated in Marek’s Disease Virus Infected Chicken Embryo Fibroblasts and Corrected by Chicken Interferon. Comp. Immunol. Microbiol. Infect. Dis. 2003, 26, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Jarosinski, K.W.; Hunt, H.D.; Osterrieder, N. Down-Regulation of MHC Class I by the Marek’s Disease Virus (MDV) UL49.5 Gene Product Mildly Affects Virulence in a Haplotype-Specific Fashion. Virology 2010, 405, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Hunt, H.D.; Parcells, M.S.; van Santen, V.; Ewald, S.J. Two Class I Genes of the Chicken MHC Have Different Functions: BF1 Is Recognized by NK Cells While BF2 Is Recognized by CTLs. Immunogenetics 2018, 70, 599–611. [Google Scholar] [CrossRef]
- Dalgaard, T.; Boving, M.K.; Handberg, K.; Jensen, K.H.; Norup, L.R.; Juul-Madsen, H.R. Brief Report MHC Expression on Spleen Lymphocyte Subsets in Genetically Resistant and Susceptible Chickens Infected with Marek’s Disease Virus. Viral Immunol. 2009, 22, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Calnek, B.W.; Schat, K.A.; Ross, L.J.N.; Chen, C.H. Further Characterization of Marek’s Disease Virus-infected Lymphocytes. II. In Vitro Infection. Int. J. Cancer 1984, 33, 399–406. [Google Scholar] [CrossRef]
- Niikura, M.; Kim, T.; Hunt, H.D.; Burnside, J.; Morgan, R.W.; Dodgson, J.B.; Cheng, H.H. Marek’s Disease Virus up-Regulates Major Histocompatibility Complex Class II Cell Surface Expression in Infected Cells. Virology 2007, 359, 212–219. [Google Scholar] [CrossRef]
- Yu, C.; Liu, Q.; Qin, A.; Hu, X.; Xu, W.; Qian, K.; Shao, H.; Jin, W. Expression Kinetics of Chicken Β2-Microglobulin and Class i MHC in Vitro and in Vivo during Marek’s Disease Viral Infections. Vet. Res. Commun. 2013, 37, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Davison, F.; Kaiser, P. Immunity to Marek’s Disease. In Marek’s Disease; Academic Press: Cambridge, MA, USA, 2004; pp. 126–141. [Google Scholar] [CrossRef]
- Katneni, U.K. Innate Patterning of the Immune Response to Marek’s Disease Virus (MDV) During Pathogenesis and Vaccination; University of Delaware: Newark, DE, USA, 2015. [Google Scholar]
- Thanthrige-Don, N.; Read, L.R.; Faizal Abdul-Careem, M.; Mohammadi, H.; Mallick, A.I.; Sharif, S. Marek’s Disease Virus Influences the Expression of Genes Associated with IFN-g-Inducible MHC Class II Expression. Viral Immunol. 2010, 23, 227–232. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaled, N.; Kulkarni, R.R.; Käser, T.; Gimeno, I.M. Temporal Changes in Splenic Immune Cell Populations following Infection with a Very Virulent plus MDV in Commercial Meat-Type Chickens. Viruses 2024, 16, 1092. https://doi.org/10.3390/v16071092
Khaled N, Kulkarni RR, Käser T, Gimeno IM. Temporal Changes in Splenic Immune Cell Populations following Infection with a Very Virulent plus MDV in Commercial Meat-Type Chickens. Viruses. 2024; 16(7):1092. https://doi.org/10.3390/v16071092
Chicago/Turabian StyleKhaled, Nagwa, Raveendra R. Kulkarni, Tobias Käser, and Isabel M. Gimeno. 2024. "Temporal Changes in Splenic Immune Cell Populations following Infection with a Very Virulent plus MDV in Commercial Meat-Type Chickens" Viruses 16, no. 7: 1092. https://doi.org/10.3390/v16071092
APA StyleKhaled, N., Kulkarni, R. R., Käser, T., & Gimeno, I. M. (2024). Temporal Changes in Splenic Immune Cell Populations following Infection with a Very Virulent plus MDV in Commercial Meat-Type Chickens. Viruses, 16(7), 1092. https://doi.org/10.3390/v16071092









