Plant-Produced Chimeric Hepatitis E Virus-like Particles as Carriers for Antigen Presentation
Abstract
1. Introduction
2. Results
2.1. Viral Vectors for the Expression of HEV/GFP and HEV/4M2e Hybrid Proteins in Nicotiana benthamiana
2.2. Expression of HEV/GFP and HEV/4M2e in Plants and Protein Purification
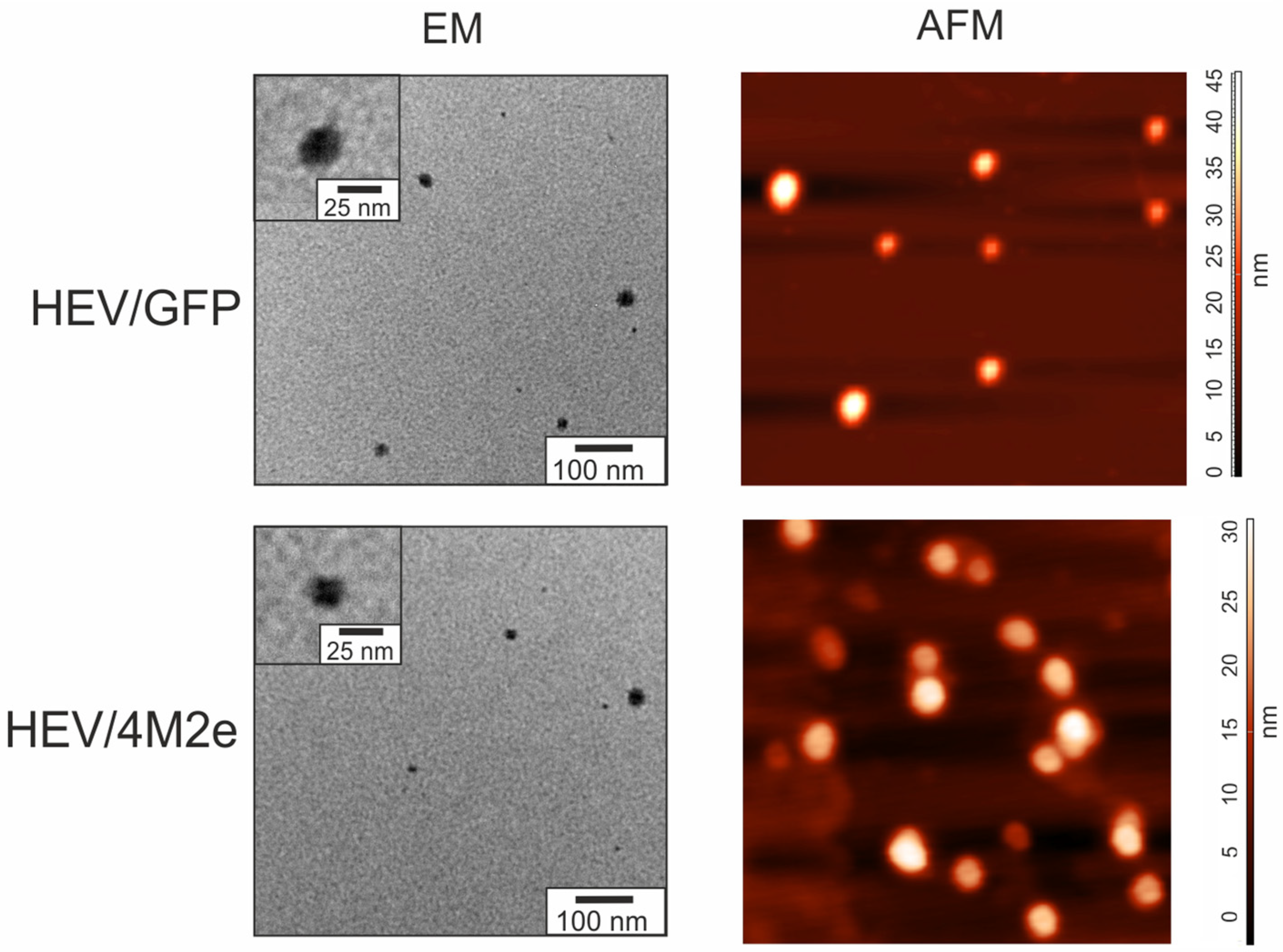
2.3. Plant-Produced HEV/GFP and HEV/4M2e Proteins Formed VLPs
2.4. Inserted Peptides Are Displayed on the Surface of VLPs
3. Discussion
4. Materials and Methods
4.1. Vector Construction
4.2. Agroinfiltration of N. benthamiana Plants
4.3. Protein Isolation and Analysis
4.4. Structural Analysis of Nanoparticles
4.5. ELISA
5. Conclusions
- A recombinant fusion protein comprising truncated HEV capsid with GFP or four tandem copies of M2e peptide inserted at the Tyr485 position can be transiently expressed in plants at a high level using viral vector based on the PVX genome.
- The plant-produced fusion proteins in vivo formed VLPs displaying GFP and 4M2e.
- HEV coat protein can be used as a carrier for the presentation of large antigens (dozens to hundreds of a.a.).
- Plant-produced HEV particles displaying four copies of M2e peptide could be useful research tools for the development of recombinant vaccines against influenza.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like Particles: Preparation, Immunogenicity and Their Roles as Nanovaccines and Drug Nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, W.; Ma, X.; Sun, X.J.; Fan, J.B.; Wang, Y. Virus-like Particles as Antiviral Vaccine: Mechanism, Design, and Application. Biotechnol. Bioprocess. Eng. 2023, 28, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bhat, T.; Cao, A.; Yin, J. Virus-like Particles: Measures and Biological Functions. Viruses 2022, 14, 383. [Google Scholar] [CrossRef] [PubMed]
- Pushko, P.; Pumpens, P.; Grens, E. Development of Virus-like Particle Technology from Small Highly Symmetric to Large Complex Virus-like Particle Structures. Intervirology 2013, 56, 141–165. [Google Scholar] [CrossRef]
- Lee, Y.T.; Ko, E.J.; Lee, Y.; Kim, K.H.; Kim, M.C.; Lee, Y.N.; Kang, S.M. Intranasal Vaccination with M2e5x Virus-like Particles Induces Humoral and Cellular Immune Responses Conferring Cross-Protection against Heterosubtypic Influenza Viruses. PLoS ONE 2018, 13, e0190868. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zheng, X.; Gai, W.; Wong, G.; Wang, H.; Jin, H.; Feng, N.; Zhao, Y.; Zhang, W.; Li, N.; et al. Novel Chimeric Virus-like Particles Vaccine Displaying MERS-CoV Receptor-Binding Domain Induce Specific Humoral and Cellular Immune Response in Mice. Antivir. Res. 2017, 140, 55–61. [Google Scholar] [CrossRef]
- Marsian, J.; Lomonossoff, G.P. Molecular Pharming-VLPs Made in Plants. Curr. Opin. Biotechnol. 2016, 37, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.F.; Jennings, G.T. Vaccine Delivery: A Matter of Size, Geometry, Kinetics and Molecular Patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, F.; Hemmati-Dinarvand, M.; Karimzade, M.; Rutkowska, D.; Eskandari, M.H.; Khanizadeh, S.; Afsharifar, A. Plant-Derived VLP: A Worthy Platform to Produce Vaccine against SARS-CoV-2. Biotechnol. Lett. 2022, 44, 45–57. [Google Scholar] [CrossRef]
- Hills, R.A.; Howarth, M. Virus-like Particles against Infectious Disease and Cancer: Guidance for the Nano-Architect. Curr. Opin. Biotechnol. 2022, 73, 346–354. [Google Scholar] [CrossRef]
- Roldão, A.; Mellado, M.C.M.; Castilho, L.R.; Carrondo, M.J.T.; Alves, P.M. Virus-like Particles in Vaccine Development. Expert. Rev. Vaccines 2010, 9, 1149–1176. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Zhong, W.; Song, D.; Thornton, S.; Jiang, X.E. Coli-Expressed Recombinant Norovirus Capsid Proteins Maintain Authentic Antigenicity and Receptor Binding Capability. J. Med. Virol. 2004, 74, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Aires, K.A.; Cianciarullo, A.M.; Carneiro, S.M.; Villa, L.L.; Boccardo, E.; Pérez-Martinez, G.; Perez-Arellano, I.; Oliveira, M.L.S.; Ho, P.L. Production of Human Papillomavirus Type 16 L1 Virus-Like Particles by Recombinant Lactobacillus Casei Cells. Appl. Environ. Microbiol. 2006, 72, 745–752. [Google Scholar] [CrossRef][Green Version]
- Srivastava, V.; Nand, K.N.; Ahmad, A.; Kumar, R. Yeast-Based Virus-like Particles as an Emerging Platform for Vaccine Development and Delivery. Vaccines 2023, 11, 479. [Google Scholar] [CrossRef] [PubMed]
- Gopal, R.; Schneemann, A. Production and Application of Insect Virus-Based VLPs. Methods Mol. Biol. 2018, 1776, 125–141. [Google Scholar] [CrossRef]
- Rybicki, E.P. Plant Molecular Farming of Virus-like Nanoparticles as Vaccines and Reagents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 2020, 12, e1587. [Google Scholar] [CrossRef]
- Thuenemann, E.C.; Lenzi, P.; Love, A.J.; Taliansky, M.; Bécares, M.; Zuñiga, S.; Enjuanes, L.; Zahmanova, G.G.; Minkov, I.N.; Matic, S.; et al. The use of transient expression systems for the rapid production of virus-like particles in plants. Curr. Pharm. Des. 2013, 19, 5564–5573. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Gomes, A.C.; Vogel, M.; Bachmann, M.F. Interaction of Viral Capsid-Derived Virus-like Particles (VLPs) with the Innate Immune System. Vaccines 2018, 6, 37. [Google Scholar] [CrossRef]
- Shiri, F.; Petersen, K.E.; Romanov, V.; Zou, Q.; Gale, B.K. Characterization and Differential Retention of Q Beta Bacteriophage Virus-like Particles Using Cyclical Electrical Field–Flow Fractionation and Asymmetrical Flow Field–Flow Fractionation. Anal. Bioanal. Chem. 2020, 412, 1563–1572. [Google Scholar] [CrossRef]
- Zahmanova, G.; Takova, K.; Valkova, R.; Toneva, V.; Minkov, I.; Andonov, A.; Lukov, G.L. Plant-Derived Recombinant Vaccines against Zoonotic Viruses. Life 2022, 12, 156. [Google Scholar] [CrossRef]
- Castells-Graells, R.; Lomonossoff, G.P. Plant-Based Production Can Result in Covalent Cross-Linking of Proteins. Plant Biotechnol. J. 2021, 19, 1095–1097. [Google Scholar] [CrossRef]
- Tusé, D.; Tu, T.; McDonald, K.A. Manufacturing Economics of Plant-Made Biologics: Case Studies in Therapeutic and Industrial Enzymes. Biomed. Res. Int. 2014, 2014, 256135. [Google Scholar] [CrossRef] [PubMed]
- Martínez, C.A.; Giulietti, A.M.; Rodríguez Talou, J. Research Advances in Plant-Made Flavivirus Antigens. Biotechnol. Adv. 2012, 30, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Zahmanova, G.G.; Mazalovska, M.; Takova, K.H.; Toneva, V.T.; Minkov, I.N.; Mardanova, E.S.; Ravin, N.V.; Lomonossoff, G.P. Rapid High-Yield Transient Expression of Swine Hepatitis E ORF2 Capsid Proteins in Nicotiana Benthamiana Plants and Production of Chimeric Hepatitis E Virus-Like Particles Bearing the M2e Influenza Epitope. Plants 2020, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; van Eerde, A.; Rimstad, E.; Bock, R.; Branza-Nichita, N.; Yakovlev, I.A.; Clarke, J.L. Plant-Made Vaccines against Viral Diseases in Humans and Farm Animals. Front. Plant Sci. 2023, 14, 1170815. [Google Scholar] [CrossRef] [PubMed]
- Takova, K.; Koynarski, T.; Minkov, G.; Toneva, V.; Mardanova, E.; Ravin, N.; Lukov, G.L.; Zahmanova, G. Development and Optimization of an Enzyme Immunoassay to Detect Serum Antibodies against the Hepatitis E Virus in Pigs, Using Plant-Derived ORF2 Recombinant Protein. Vaccines 2021, 9, 991. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Kato, K.; Li, T.; Takeda, N.; Miyamura, T.; Hammar, L.; Cheng, R.H. Recombinant hepatitis E capsid protein self-assembles into a dual-domain T = 1 particle presenting native virus epitopes. Virology 1999, 265, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Li, T.C.; Yamakawa, Y.; Suzuki, K.; Tatsumi, M.; Razak, M.A.; Uchida, T.; Takeda, N.; Miyamura, T. Expression and self-assembly of empty virus-like particles of hepatitis E virus. J. Virol. 1997, 71, 7207–7213. [Google Scholar] [CrossRef] [PubMed]
- Avdjieva, I.; Terziyski, I.; Zahmanova, G.; Simeonova, V.; Kulev, O.; Krustev, E.; Krachunov, M.; Nisheva, M.; Vassilev, D. Homology Based Computational Modelling of Hepatitis-E Viral Fusion Capsid Protein. Comptes Rendus L’Academie Bulg. Des Sci. 2019, 72, 358–364. [Google Scholar] [CrossRef]
- Jariyapong, P.; Xing, L.; van Houten, N.E.; Li, T.C.; Weerachatyanukul, W.; Hsieh, B.; Moscoso, C.G.; Chen, C.C.; Niikura, M.; Cheng, H.H.R. Chimeric Hepatitis E Virus-like Particle as a Carrier for Oral-Delivery. Vaccine 2013, 31, 417–424. [Google Scholar] [CrossRef]
- Mardanova, E.S.; Kotlyarov, R.Y.; Stuchinskaya, M.D.; Nikolaeva, L.I.; Zahmanova, G.; Ravin, N.V. High-Yield Production of Chimeric Hepatitis E Virus-Like Particles Bearing the M2e Influenza Epitope and Receptor Binding Domain of SARS-CoV-2 in Plants Using Viral Vectors. Int. J. Mol. Sci. 2022, 23, 15684. [Google Scholar] [CrossRef] [PubMed]
- Sainsbury, F.; Lomonossoff, G.P. Extremely High-Level and Rapid Transient Protein Production in Plants without the Use of Viral Replication. Plant Physiol. 2008, 148, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Marillonnet, S.; Giritch, A.; Gils, M.; Kandzia, R.; Klimyuk, V.; Gleba, Y. In Planta Engineering of Viral RNA Replicons: Efficient Assembly by Recombination of DNA Modules Delivered by Agrobacterium. Proc. Natl. Acad. Sci. USA 2004, 101, 6852–6857. [Google Scholar] [CrossRef] [PubMed]
- Lindbo, J.A. TRBO: A High-Efficiency Tobacco Mosaic Virus RNA-Based Overexpression Vector. Plant Physiol. 2007, 145, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Hoshikawa, K.; Ezura, K.; Okazawa, R.; Fujita, S.; Takaoka, M.; Mason, H.S.; Ezura, H.; Miura, K. Improvement of the Transient Expression System for Production of Recombinant Proteins in Plants. Sci. Rep. 2018, 8, 4755. [Google Scholar] [CrossRef] [PubMed]
- Mardanova, E.S.; Blokhina, E.A.; Tsybalova, L.M.; Peyret, H.; Lomonossoff, G.P.; Ravin, N.V. Efficient Transient Expression of Recombinant Proteins in Plants by the Novel pEff Vector Based on the Genome of Potato Virus X. Front. Plant Sci. 2017, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.Q.; Zhang, M.; Mozdzanowska, K.; Zharikova, D.; Hoff, H.; Wunner, W.; Couch, R.B.; Gerhard, W. Influenza A Virus Infection Engenders a Poor Antibody Response against the Ectodomain of Matrix Protein 2. Virol. J. 2006, 3, 102. [Google Scholar] [CrossRef] [PubMed]
- Kolpe, A.; Schepens, B.; Fiers, W.; Saelens, X. M2-Based Influenza Vaccines: Recent Advances and Clinical Potential. Expert. Rev. Vaccines 2017, 16, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.M.L.; Komarasamy, T.V.; Adnan, N.A.A.B.; Radhakrishnan, A.K.; Balasubramaniam, V.R.M.T. Recent Advances, Approaches and Challenges in the Development of Universal Influenza Vaccines. Influenza Other Respir. Viruses 2024, 18, e13276. [Google Scholar] [CrossRef]
- Mardanova, E.S.; Ravin, N.V. Plant-Produced Recombinant Influenza A Vaccines Based on the M2e Peptide. Curr. Pharm. Des. 2018, 24, 1317–1324. [Google Scholar] [CrossRef]
- Neirynck, S.; Deroo, T.; Saelens, X.; Vanlandschoot, P.; Jou, W.M.; Fiers, W. A Universal Influenza A Vaccine Based on the Extracellular Domain of the M2 Protein. Nat. Med. 1999, 5, 1157–1163. [Google Scholar] [CrossRef]
- Feranmi, F. Universal flu vaccine protects against influenza A and B. Lancet Microbe 2022, 3, e902. [Google Scholar] [CrossRef]
- Ravin, N.V.; Blokhina, E.A.; Kuprianov, V.V.; Stepanova, L.A.; Shaldjan, A.A.; Kovaleva, A.A.; Tsybalova, L.M.; Skryabin, K.G. Development of a candidate influenza vaccine based on virus-like particles displaying influenza M2e peptide into the immunodominant loop region of hepatitis B core antigen: Insertion of multiple copies of M2e increases immunogenicity and protective efficiency. Vaccine 2015, 33, 3392–3397. [Google Scholar] [CrossRef]
- Ma, W. Swine Influenza Virus: Current Status and Challenge. Virus Res. 2020, 288, 198118. [Google Scholar] [CrossRef]
- Vincent, A.L.; Perez, D.R.; Rajao, D.; Anderson, T.K.; Abente, E.J.; Walia, R.R.; Lewis, N.S. Influenza A Virus Vaccines for Swine. Vet. Microbiol. 2017, 206, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Cassarino, T.G.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling Protein Tertiary and Quaternary Structure Using Evolutionary Information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef] [PubMed]
- Poria, R.; Kala, D.; Nagraik, R.; Dhir, Y.; Dhir, S.; Singh, B.; Kaushik, N.K.; Noorani, M.S.; Kaushal, A.; Gupta, S. Vaccine Development: Current Trends and Technologies. Life Sci. 2024, 336, 122331. [Google Scholar] [CrossRef] [PubMed]
- Eidenberger, L.; Kogelmann, B.; Steinkellner, H. Plant-Based Biopharmaceutical Engineering. Nat. Rev. Bioeng. 2023, 1, 426–439. [Google Scholar] [CrossRef]
- Rozov, S.M.; Permyakova, N.V.; Deineko, E.V. Main Strategies of Plant Expression System Glycoengineering for Producing Humanized Recombinant Pharmaceutical Proteins. Biochemistry 2018, 83, 215–232. [Google Scholar] [CrossRef]
- Mardanova, E.S.; Takova, K.H.; Toneva, V.T.; Zahmanova, G.G.; Tsybalova, L.M.; Ravin, N.V. A Plant-Based Transient Expression System for the Rapid Production of Highly Immunogenic Hepatitis E Virus-like Particles. Biotechnol. Lett. 2020, 42, 2441–2446. [Google Scholar] [CrossRef]
- Pumpens, P.; Grens, E. HBV Core Particles as a Carrier for B Cell/T Cell Epitopes. Intervirology 2001, 44, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Kratz, P.A.; Böttcher, B.; Nassal, M. Native Display of Complete Foreign Protein Domains on the Surface of Hepatitis B Virus Capsids. Proc. Natl. Acad. Sci. USA 1999, 96, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Skamel, C.; Ploss, M.; Böttcher, B.; Stehle, T.; Wallich, R.; Simon, M.M.; Nassal, M. Hepatitis B Virus Capsid-like Particles Can Display the Complete, Dimeric Outer Surface Protein C and Stimulate Production of Protective Antibody Responses against Borrelia burgdorferi Infection. J. Biol. Chem. 2006, 281, 17474–17481. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.J.; Steele, J.F.C.; Hesketh, E.L.; Walden, M.; Thompson, R.F.; Lomonossoff, G.P.; Ranson, N.A. Combining Transient Expression and Cryo-EM to Obtain High-Resolution Structures of Luteovirid Particles. Structure 2019, 27, 1761–1770.e3. [Google Scholar] [CrossRef] [PubMed]
- Pleshakova, T.O.; Bukharina, N.S.; Archakov, A.I.; Ivanov, Y.D. Atomic Force Microscopy for Protein Detection and Their Physicoсhemical Characterization. Int. J. Mol. Sci. 2018, 19, 1142. [Google Scholar] [CrossRef] [PubMed]
- Lampinen, V.; Heinimäki, S.; Laitinen, O.H.; Pesu, M.; Hankaniemi, M.M.; Blazevic, V.; Hytönen, V.P. Modular Vaccine Platform Based on the Norovirus-like Particle. J. Nanobiotechnol. 2021, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, N.; Chuan, Y.P.; Lua, L.H.L.; Middelberg, A.P.J. Modular Engineering of a Microbially-Produced Viral Capsomere Vaccine for Influenza. Chem. Eng. Sci. 2013, 103, 12–20. [Google Scholar] [CrossRef]
- Zykova, A.A.; Blokhina, E.A.; Stepanova, L.A.; Shuklina, M.A.; Tsybalova, L.M.; Kuprianov, V.V.; Ravin, N.V. Nanoparticles based on artificial self-assembling peptide and displaying M2e peptide and stalk HA epitopes of influenza A virus induce potent humoral and T-cell responses and protect against the viral infection. Nanomedicine 2022, 39, 102463. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, S.M.; Zhao, Z.-S.; Lo, C.-Y.; Misplon, J.A.; Liu, T.; Ye, Z.; Hogan, R.J.; Wu, Z.; Benton, K.A.; Tumpey, T.M.; et al. Matrix Protein 2 Vaccination and Protection against Influenza Viruses, Including Subtype H5N1. Emerg. Infect. Dis. 2007, 13, 426–435. [Google Scholar] [CrossRef]
- Kotlyarov, R.Y.; Kuprianov, V.V.; Migunov, A.I.; Stepanova, L.A.; Tsybalova, L.M.; Kiselev, O.I.; Ravin, N.V.; Skryabin, K.G. Development of Recombinant Vaccine against A(H1N1) 2009 Influenza Based on Virus-like Nanoparticles Carrying the Extracellular Domain of M2 Protein. Acta Naturae 2010, 2, 71–76. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]




| Particles | HEV/GFP | HEV/4M2e |
|---|---|---|
| Electron microscopy | ||
| Diameter (nm) 1 | 24 ± 8 | 21 ± 6 |
| PDI | 0.11 | 0.08 |
| Atomic force microscopy | ||
| Height (nm) 1 | 37 ± 14 | 19 ± 6 |
| PDI 2 | 0.14 | 0.10 |
| Height/diameter ratio 1 | 0.12 ± 0.02 | 0.21 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mardanova, E.S.; Vasyagin, E.A.; Kotova, K.G.; Zahmanova, G.G.; Ravin, N.V. Plant-Produced Chimeric Hepatitis E Virus-like Particles as Carriers for Antigen Presentation. Viruses 2024, 16, 1093. https://doi.org/10.3390/v16071093
Mardanova ES, Vasyagin EA, Kotova KG, Zahmanova GG, Ravin NV. Plant-Produced Chimeric Hepatitis E Virus-like Particles as Carriers for Antigen Presentation. Viruses. 2024; 16(7):1093. https://doi.org/10.3390/v16071093
Chicago/Turabian StyleMardanova, Eugenia S., Egor A. Vasyagin, Kira G. Kotova, Gergana G. Zahmanova, and Nikolai V. Ravin. 2024. "Plant-Produced Chimeric Hepatitis E Virus-like Particles as Carriers for Antigen Presentation" Viruses 16, no. 7: 1093. https://doi.org/10.3390/v16071093
APA StyleMardanova, E. S., Vasyagin, E. A., Kotova, K. G., Zahmanova, G. G., & Ravin, N. V. (2024). Plant-Produced Chimeric Hepatitis E Virus-like Particles as Carriers for Antigen Presentation. Viruses, 16(7), 1093. https://doi.org/10.3390/v16071093








