Tanapox Virus and Yaba Monkey Tumor Virus K3 Orthologs Inhibit Primate Protein Kinase R in a Species-Specific Fashion
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Plasmids
2.3. Luciferase-Based Reporter Assays
2.4. Virus and Infections
2.5. PCR of Viral Genomic DNA
2.6. Immunoblot Analyses
2.7. Phylogenetic Analysis
3. Results
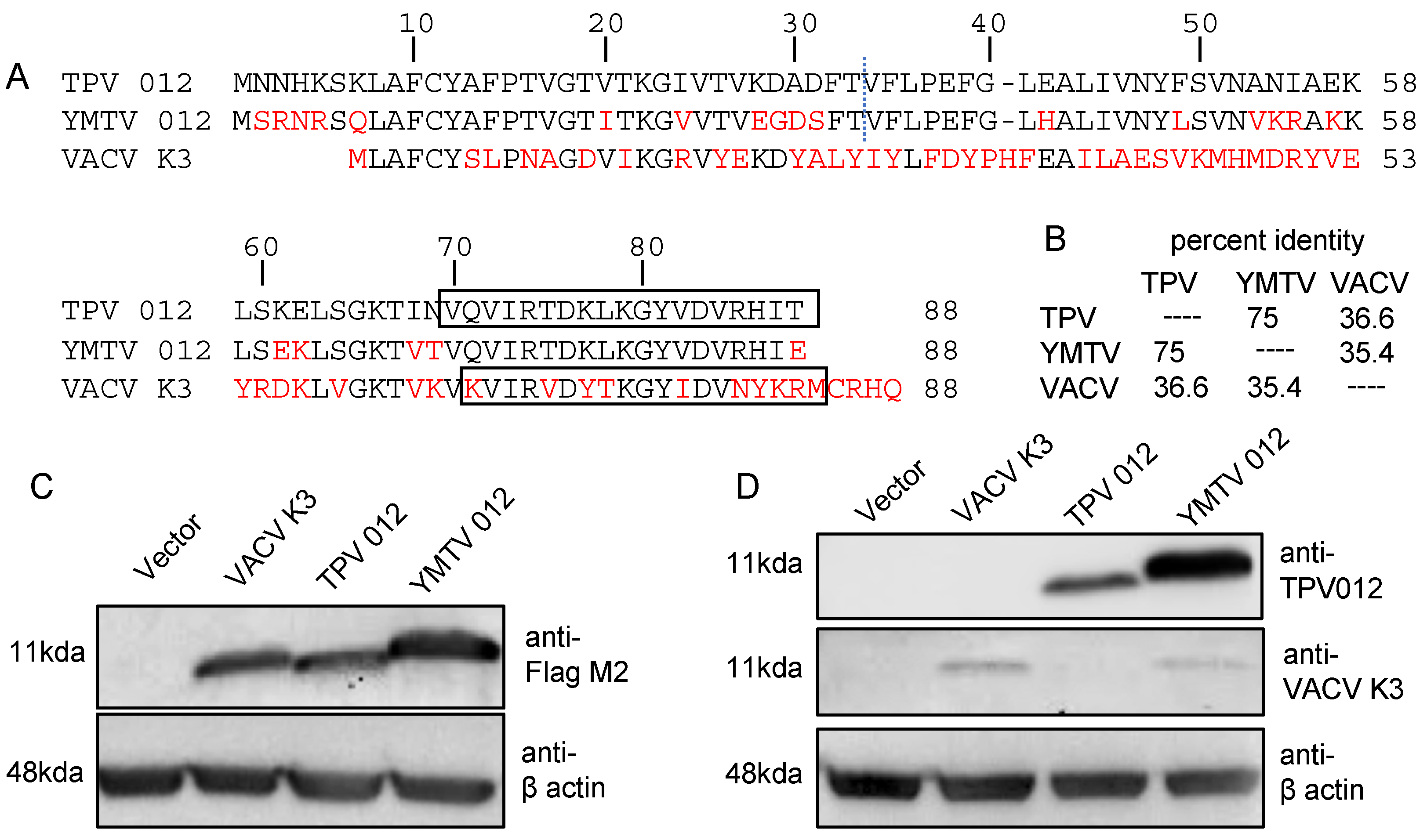
3.1. Species-Specific Inhibition of Primate PKRs by Yatapoxvirus K3 Orthologs
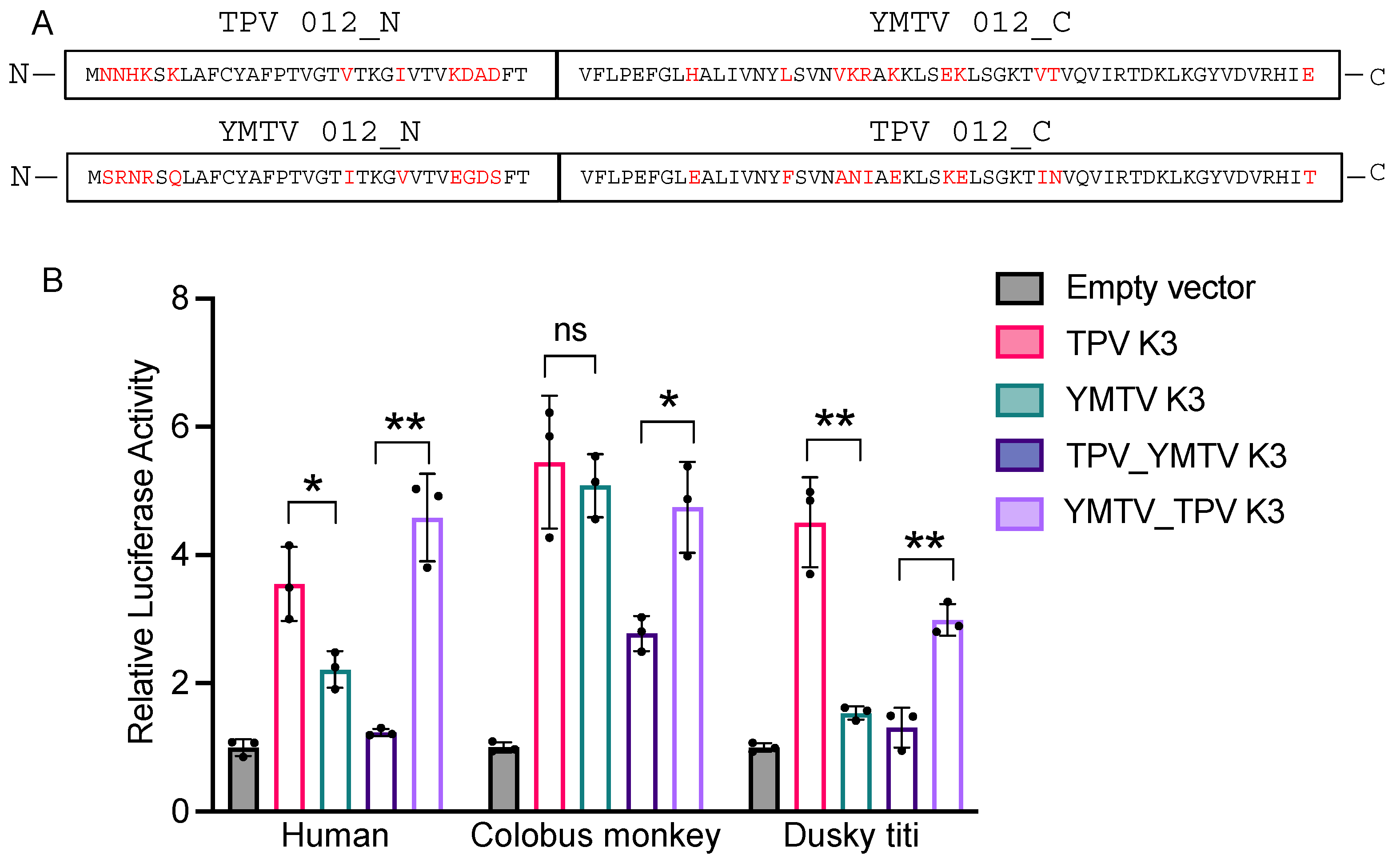
3.2. Differential PKR Inhibition Was Governed by the C-Terminus of Yatapoxvirus K3 Orthologs
3.3. Chimeric Viruses Expressing TPV 012 or YMTV 012 Displayed Cell-Type-Specific Differences in Plaque Formation and Virus Replication
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haller, S.L.; Peng, C.; McFadden, G.; Rothenburg, S. Poxviruses and the evolution of host range and virulence. Infect. Genet. Evol. 2014, 21, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Essani, K.; Smith, G.L. The genome sequence of Yaba-like disease virus, a yatapoxvirus. Virology 2001, 281, 170–192. [Google Scholar] [CrossRef]
- Brunetti, C.R.; Amano, H.; Ueda, Y.; Qin, J.; Miyamura, T.; Suzuki, T.; Li, X.; Barrett, J.W.; McFadden, G. Complete genomic sequence and comparative analysis of the tumorigenic poxvirus Yaba monkey tumor virus. J. Virol. 2003, 77, 13335–13347. [Google Scholar] [CrossRef]
- Downie, A.W.; Taylor-Robinson, C.H.; Caunt, A.E.; Nelson, G.S.; Manson-Bahr, P.E.; Matthews, T.C. Tanapox: A new disease caused by a pox virus. Br. Med. J. 1971, 1, 363–368. [Google Scholar] [CrossRef]
- Jezek, Z.; Arita, I.; Szczeniowski, M.; Paluku, K.M.; Ruti, K.; Nakano, J.H. Human tanapox in Zaire: Clinical and epidemiological observations on cases confirmed by laboratory studies. Bull. World Health Organ. 1985, 63, 1027–1035. [Google Scholar]
- Bearcroft, W.G.; Jamieson, M.F. An outbreak of subcutaneous tumours in rhesus monkeys. Nature 1958, 182, 195–196. [Google Scholar] [CrossRef]
- Knight, J.C.; Novembre, F.J.; Brown, D.R.; Goldsmith, C.S.; Esposito, J.J. Studies on Tanapox virus. Virology 1989, 172, 116–124. [Google Scholar] [CrossRef]
- Nazarian, S.H.; Barrett, J.W.; Frace, A.M.; Olsen-Rasmussen, M.; Khristova, M.; Shaban, M.; Neering, S.; Li, Y.; Damon, I.K.; Esposito, J.J.; et al. Comparative genetic analysis of genomic DNA sequences of two human isolates of Tanapox virus. Virus Res. 2007, 129, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Sproul, E.E.; Metzgar, R.S.; Grace, J.T., Jr. The pathogenesis of Yaba virus-induced histiocytomas in primates. Cancer Res. 1963, 23, 671–675. [Google Scholar]
- Downie, A.W. Serological evidence of infection with Tana and Yaba pox viruses among several species of monkey. J. Hyg. 1974, 72, 245–250. [Google Scholar] [CrossRef]
- Monroe, B.P.; Nakazawa, Y.J.; Reynolds, M.G.; Carroll, D.S. Estimating the geographic distribution of human Tanapox and potential reservoirs using ecological niche modeling. Int. J. Health Geogr. 2014, 13, 34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ambrus, J.L.; Strandström, H.V. Susceptibility of Old World monkeys to Yaba virus. Nature 1966, 211, 876. [Google Scholar] [CrossRef]
- Grace, J.T., Jr.; Mirand, E.A. Human susceptibility to a simian tumor virus. Ann. N. Y. Acad. Sci. 1963, 108, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Stich, A.; Meyer, H.; Köhler, B.; Fleischer, K. Tanapox: First report in a European traveller and identification by PCR. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D.S. Virus entry: Molecular mechanisms and biomedical applications. Nat. Reviews. Microbiol. 2004, 2, 109–122. [Google Scholar] [CrossRef]
- McFadden, G. Poxvirus tropism. Nat. Reviews. Microbiol. 2005, 3, 201–213. [Google Scholar] [CrossRef]
- Moss, B. Poxvirus cell entry: How many proteins does it take? Viruses 2012, 4, 688–707. [Google Scholar] [CrossRef]
- Jacobs, B.L.; Langland, J.O. When two strands are better than one: The mediators and modulators of the cellular responses to double-stranded RNA. Virology 1996, 219, 339–349. [Google Scholar] [CrossRef]
- Dar, A.C.; Sicheri, F. X-ray crystal structure and functional analysis of vaccinia virus K3L reveals molecular determinants for PKR subversion and substrate recognition. Mol. Cell 2002, 10, 295–305. [Google Scholar] [CrossRef]
- Adomavicius, T.; Guaita, M.; Zhou, Y.; Jennings, M.D.; Latif, Z.; Roseman, A.M.; Pavitt, G.D. The structural basis of translational control by eIF2 phosphorylation. Nat. Commun. 2019, 10, 2136. [Google Scholar] [CrossRef]
- Romano, P.R.; Zhang, F.; Tan, S.L.; Garcia-Barrio, M.T.; Katze, M.G.; Dever, T.E.; Hinnebusch, A.G. Inhibition of double-stranded RNA-dependent protein kinase PKR by vaccinia virus E3: Role of complex formation and the E3 N-terminal domain. Mol. Cell. Biol. 1998, 18, 7304–7316. [Google Scholar] [CrossRef] [PubMed]
- Langland, J.O.; Jacobs, B.L. The role of the PKR-inhibitory genes, E3L and K3L, in determining vaccinia virus host range. Virology 2002, 299, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Langland, J.O.; Jacobs, B.L. Inhibition of PKR by vaccinia virus: Role of the N- and C-terminal domains of E3L. Virology 2004, 324, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.L.; Park, C.; Bruneau, R.C.; Megawati, D.; Zhang, C.; Vipat, S.; Peng, C.; Senkevich, T.G.; Brennan, G.; Tazi, L.; et al. Molecular basis for the host range function of the poxvirus PKR inhibitor E3. bioRxiv 2024. [Google Scholar] [CrossRef]
- Peng, C.; Haller, S.L.; Rahman, M.M.; McFadden, G.; Rothenburg, S. Myxoma virus M156 is a specific inhibitor of rabbit PKR but contains a loss-of-function mutation in Australian virus isolates. Proc. Natl. Acad. Sci. USA 2016, 113, 3855–3860. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Peng, C.; Brennan, G.; Rothenburg, S. Species-specific inhibition of antiviral protein kinase R by capripoxviruses and vaccinia virus. Ann. N.Y. Acad. Sci. 2019, 1438, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Varga, J.; Deschambault, Y. Poxvirus encoded eIF2α homolog, K3 family proteins, is a key determinant of poxvirus host species specificity. Virology 2020, 541, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Peng, C.; Rahman, M.J.; Haller, S.L.; Tazi, L.; Brennan, G.; Rothenburg, S. Orthopoxvirus K3 orthologs show virus- and host-specific inhibition of the antiviral protein kinase PKR. PLoS Pathog. 2021, 17, e1009183. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, K.S.; Esparo, N.M.; Child, S.J.; Geballe, A.P. A Single Amino Acid Dictates Protein Kinase R Susceptibility to Unrelated Viral Antagonists. PLoS Pathog. 2016, 12, e1005966. [Google Scholar] [CrossRef]
- Rahman, M.M.; Liu, J.; Chan, W.M.; Rothenburg, S.; McFadden, G. Myxoma virus protein M029 is a dual function immunomodulator that inhibits PKR and also conscripts RHA/DHX9 to promote expanded host tropism and viral replication. PLoS Pathog. 2013, 9, e1003465. [Google Scholar] [CrossRef]
- Elde, N.C.; Child, S.J.; Geballe, A.P.; Malik, H.S. Protein kinase R reveals an evolutionary model for defeating viral mimicry. Nature 2009, 457, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.G.; Young, L.; Chuang, R.Y.; Venter, J.C.; Hutchison, C.A., 3rd; Smith, H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 2009, 6, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Vipat, S.; Brennan, G.; Park, C.; Haller, S.L.; Rothenburg, S. Rapid, Seamless Generation of Recombinant Poxviruses using Host Range and Visual Selection. J. Vis. Exp. JoVE 2020, 159, e61049. [Google Scholar] [CrossRef]
- Rothenburg, S.; Seo, E.J.; Gibbs, J.S.; Dever, T.E.; Dittmar, K. Rapid evolution of protein kinase PKR alters sensitivity to viral inhibitors. Nat. Struct. Mol. Biol. 2009, 16, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Beattie, E.; Tartaglia, J.; Paoletti, E. Vaccinia virus-encoded eIF-2 alpha homolog abrogates the antiviral effect of interferon. Virology 1991, 183, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Esposito, J.; Condit, R.; Obijeski, J. The preparation of orthopoxvirus DNA. J. Virol. Methods 1981, 2, 175–179. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Higgins, D.G. Clustal Omega for making accurate alignments of many protein sequences. Protein Sci. 2018, 27, 135–145. [Google Scholar] [CrossRef]
- Nejepinska, J.; Malik, R.; Wagner, S.; Svoboda, P. Reporters transiently transfected into mammalian cells are highly sensitive to translational repression induced by dsRNA expression. PLoS ONE 2014, 9, e87517. [Google Scholar] [CrossRef]
- Kawagishi-Kobayashi, M.; Silverman, J.B.; Ung, T.L.; Dever, T.E. Regulation of the protein kinase PKR by the vaccinia virus pseudosubstrate inhibitor K3L is dependent on residues conserved between the K3L protein and the PKR substrate eIF2alpha. Mol. Cell. Biol. 1997, 17, 4146–4158. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.V.; Chang, H.W.; Jacobs, B.L.; Kaufman, R.J. The E3L and K3L vaccinia virus gene products stimulate translation through inhibition of the double-stranded RNA-dependent protein kinase by different mechanisms. J. Virol. 1993, 67, 1688–1692. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Peng, C.; Zhang, C.; Stoian, A.M.M.; Tazi, L.; Brennan, G.; Rothenburg, S. Maladaptation after a virus host switch leads to increased activation of the pro-inflammatory NF-κB pathway. Proc. Natl. Acad. Sci. USA 2022, 119, e2115354119. [Google Scholar] [CrossRef] [PubMed]
- Vieira Gomes, A.M.; Souza Carmo, T.; Silva Carvalho, L.; Mendonça Bahia, F.; Parachin, N.S. Comparison of Yeasts as Hosts for Recombinant Protein Production. Microorganisms 2018, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.C.; Dever, T.E.; Sicheri, F. Higher-order substrate recognition of eIF2alpha by the RNA-dependent protein kinase PKR. Cell 2005, 122, 887–900. [Google Scholar] [CrossRef]
- Hand, E.S.; Haller, S.L.; Peng, C.; Rothenburg, S.; Hersperger, A.R. Ectopic expression of vaccinia virus E3 and K3 cannot rescue ectromelia virus replication in rabbit RK13 cells. PLoS ONE 2015, 10, e0119189. [Google Scholar] [CrossRef] [PubMed]
- Brennan, G.; Kitzman, J.O.; Rothenburg, S.; Shendure, J.; Geballe, A.P. Adaptive gene amplification as an intermediate step in the expansion of virus host range. PLoS Pathog. 2014, 10, e1004002. [Google Scholar] [CrossRef] [PubMed]
- Brennan, G.; Kitzman, J.O.; Shendure, J.; Geballe, A.P. Experimental Evolution Identifies Vaccinia Virus Mutations in A24R and A35R That Antagonize the Protein Kinase R Pathway and Accompany Collapse of an Extragenic Gene Amplification. J. Virol. 2015, 89, 9986–9997. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Smith, C.; Geballe, A.P.; Rothenburg, S.; Kitzman, J.O.; Brennan, G. Gene amplification acts as a molecular foothold to facilitate cross-species adaptation and evasion of multiple antiviral pathways. Virus Evol. 2022, 8, veac105. [Google Scholar] [CrossRef]
- Rothenburg, S.; Brennan, G. Species-Specific Host-Virus Interactions: Implications for Viral Host Range and Virulence. Trends Microbiol. 2020, 28, 46–56. [Google Scholar] [CrossRef]
- Bratke, K.A.; McLysaght, A.; Rothenburg, S. A survey of host range genes in poxvirus genomes. Infect. Genet. Evol. 2013, 14, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Myskiw, C.; Arsenio, J.; Hammett, C.; van Bruggen, R.; Deschambault, Y.; Beausoleil, N.; Babiuk, S.; Cao, J. Comparative analysis of poxvirus orthologues of the vaccinia virus E3 protein: Modulation of protein kinase R activity, cytokine responses, and virus pathogenicity. J. Virol. 2011, 85, 12280–12291. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Chao, J.; Xiang, Y. Identification from diverse mammalian poxviruses of host-range regulatory genes functioning equivalently to vaccinia virus C7L. Virology 2008, 372, 372–383. [Google Scholar] [CrossRef] [PubMed]




| Common Name | Scientific Name | GenBank Accession |
|---|---|---|
| Human | Homo sapiens | NM_002759 |
| Gorilla | Gorilla gorilla | EU733258.1 |
| Bonobo | Pan paniscus | EU733255.1 |
| Chimpanzee | Pan troglodytes | EU733256.1 |
| Orangutan | Pongo pygmaeus pgmaeus | EU733259.1 |
| White-cheeked gibbon | Nomascus leucogenys | EU733257.1 |
| White-bearded gibbon | Hylobates albibarbis | EU733270.1 |
| Siamang | Symphalangus syndactylus | EU733271.1 |
| Colobus monkey | Colobus guereza | EU733267.1 |
| François’ leaf monkey | Trachypithecus francoisi | EU733268.1 * |
| Baboon | Papio anubis | XM_009184002.4 * |
| Sooty mangabey | Cercocebus torquatus atys | EU733262.1 |
| Rhesus macaque | Macaca mulatta | EU733261.1 |
| Talapoin monkey | Miopithecus talapoin talapoin | EU733269.1 |
| African green monkey | Clorocebus aethiops | EU733254.1 |
| Patas monkey | Erythrocebus patas | EU733260.1 |
| Black-handed spider monkey | Ateles geoffroyi | EU733263.1 |
| Woolly monkey | Lagothrix lagotricha | EU733266.1 |
| Tamarin | Saguinus labiatus | EU733264.1 |
| Dusky titi | Callicebus moloch | EU733265.1 |
| Owl monkey | Aotus trivirgatus | FJ374685.1 |
| Rabbit | Oryctolagus cuniculus | NM_001082213.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Megawati, D.; Stroup, J.N.; Park, C.; Clarkson, T.; Tazi, L.; Brennan, G.; Rothenburg, S. Tanapox Virus and Yaba Monkey Tumor Virus K3 Orthologs Inhibit Primate Protein Kinase R in a Species-Specific Fashion. Viruses 2024, 16, 1095. https://doi.org/10.3390/v16071095
Megawati D, Stroup JN, Park C, Clarkson T, Tazi L, Brennan G, Rothenburg S. Tanapox Virus and Yaba Monkey Tumor Virus K3 Orthologs Inhibit Primate Protein Kinase R in a Species-Specific Fashion. Viruses. 2024; 16(7):1095. https://doi.org/10.3390/v16071095
Chicago/Turabian StyleMegawati, Dewi, Jeannine N. Stroup, Chorong Park, Taylor Clarkson, Loubna Tazi, Greg Brennan, and Stefan Rothenburg. 2024. "Tanapox Virus and Yaba Monkey Tumor Virus K3 Orthologs Inhibit Primate Protein Kinase R in a Species-Specific Fashion" Viruses 16, no. 7: 1095. https://doi.org/10.3390/v16071095
APA StyleMegawati, D., Stroup, J. N., Park, C., Clarkson, T., Tazi, L., Brennan, G., & Rothenburg, S. (2024). Tanapox Virus and Yaba Monkey Tumor Virus K3 Orthologs Inhibit Primate Protein Kinase R in a Species-Specific Fashion. Viruses, 16(7), 1095. https://doi.org/10.3390/v16071095








