Metatranscriptomic Sequencing of Sheath Blight-Associated Isolates of Rhizoctonia solani Revealed Multi-Infection by Diverse Groups of RNA Viruses
Abstract
:1. Introduction
2. Materials and Methods
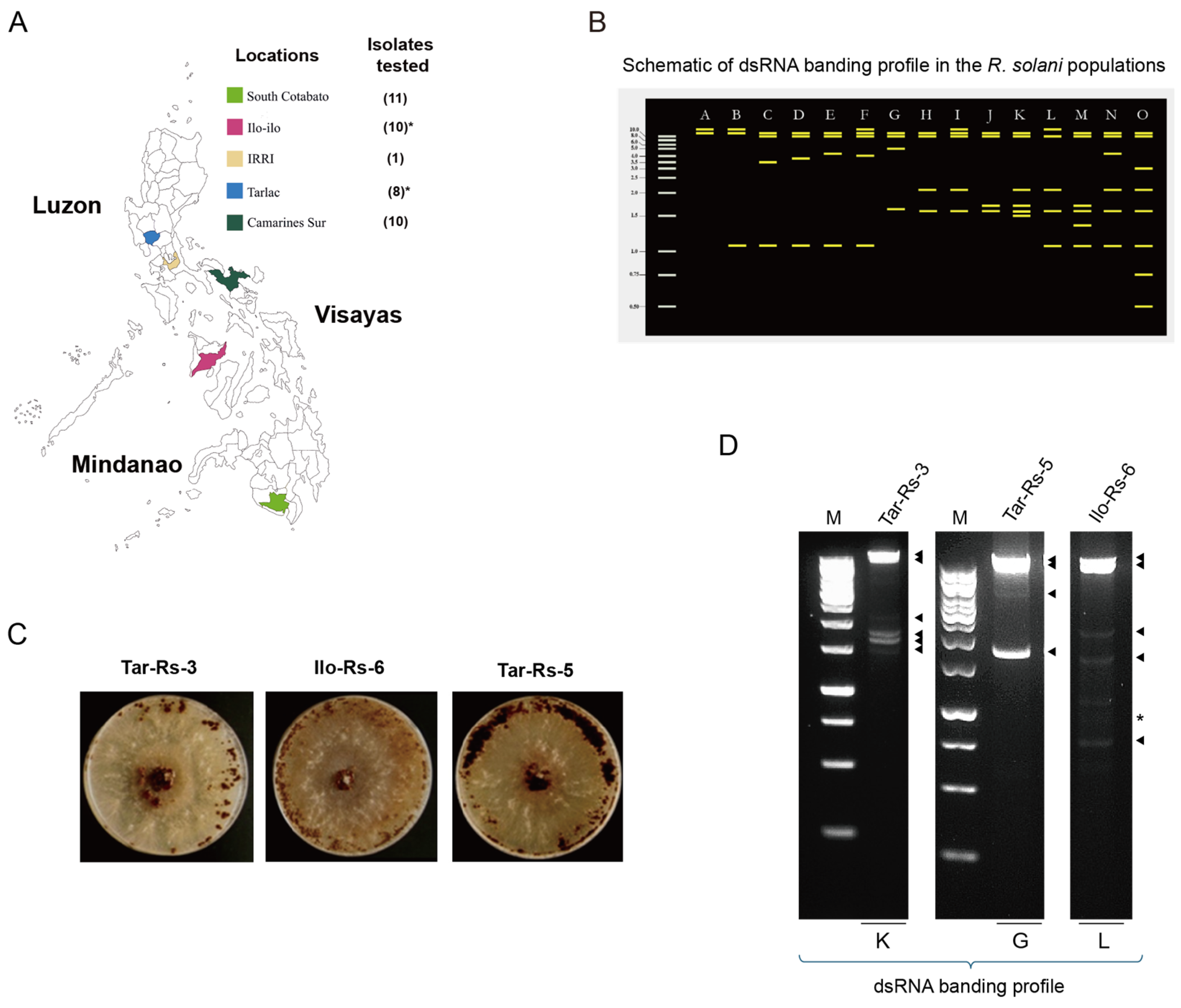
2.1. Collection of Field-Infected Rice Material for Isolation of Rhizoctonia solani
2.2. Phenotype of Rhizoctonia spp. on Artificial Culture Media
2.3. dsRNA Screening among Rhizoctonia solani Collections
2.4. Viral RNA Sequencing and Bioinformatic Analyses
2.5. RT-PCR Validation of Viruses in Fungal Strains
3. Results
3.1. The Common Colony Phenotypes and Unique dsRNA Profiles in the Rhizoctonia spp. Collection
3.2. Virus Population in the Three Philippine R. solani Strains
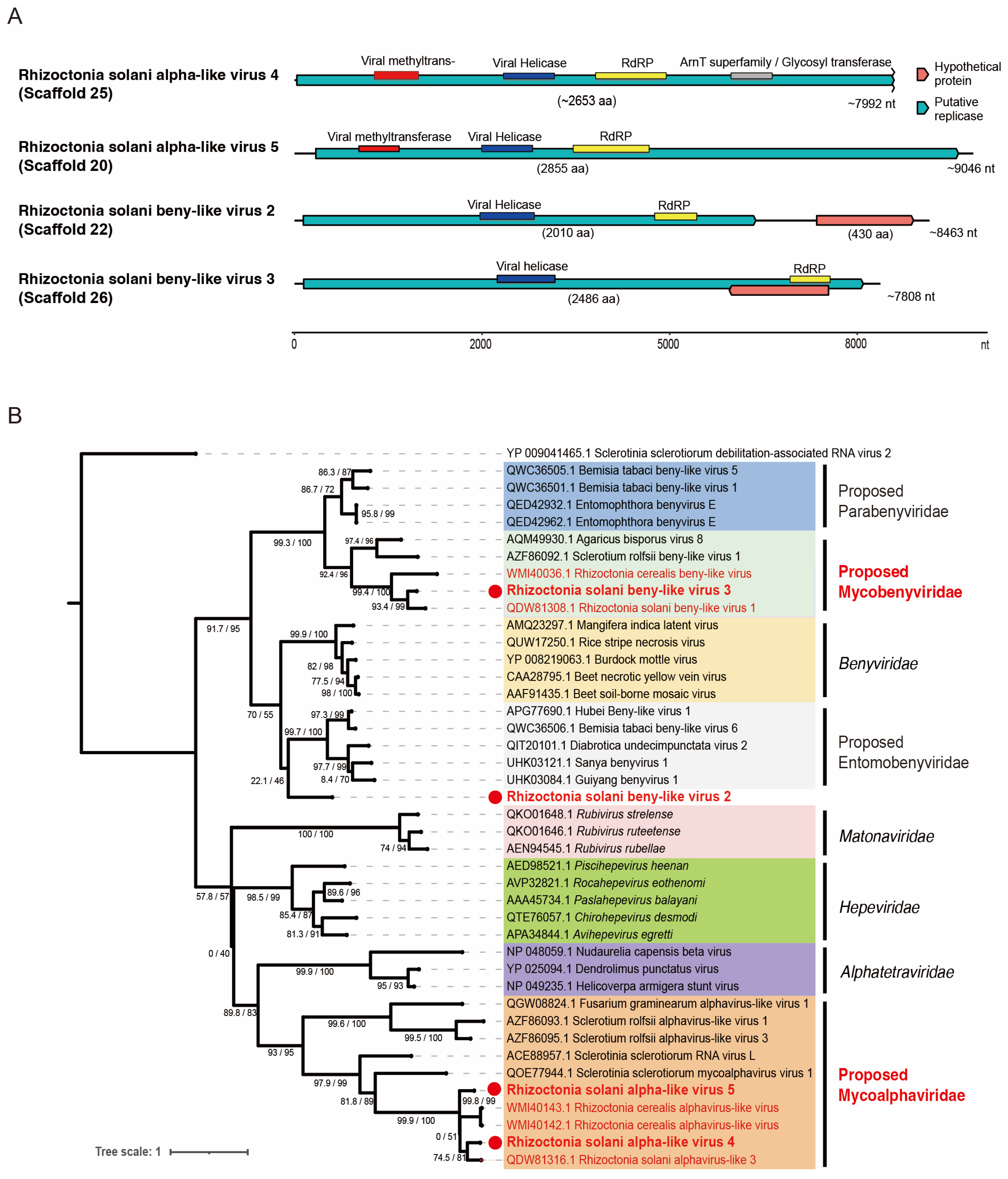
3.3. Viruses of the Order Hepelivirales, Phylum Kitrinoviricota
3.4. Viruses of the Order Martellivirales, Phylum Kitrinoviricota
3.5. Viruses of the Order Ghabrivirales, Phylum Duplornaviricota
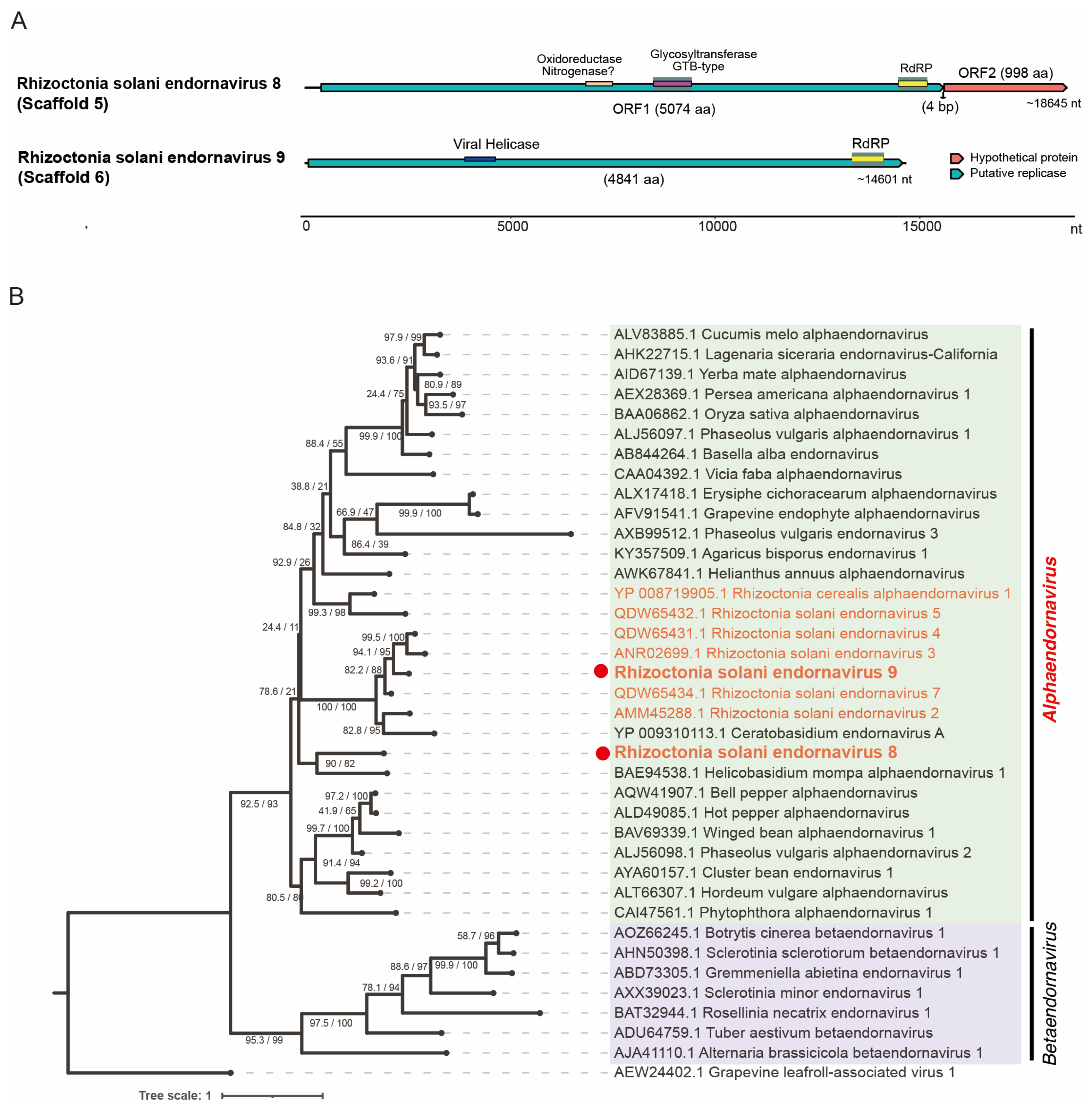
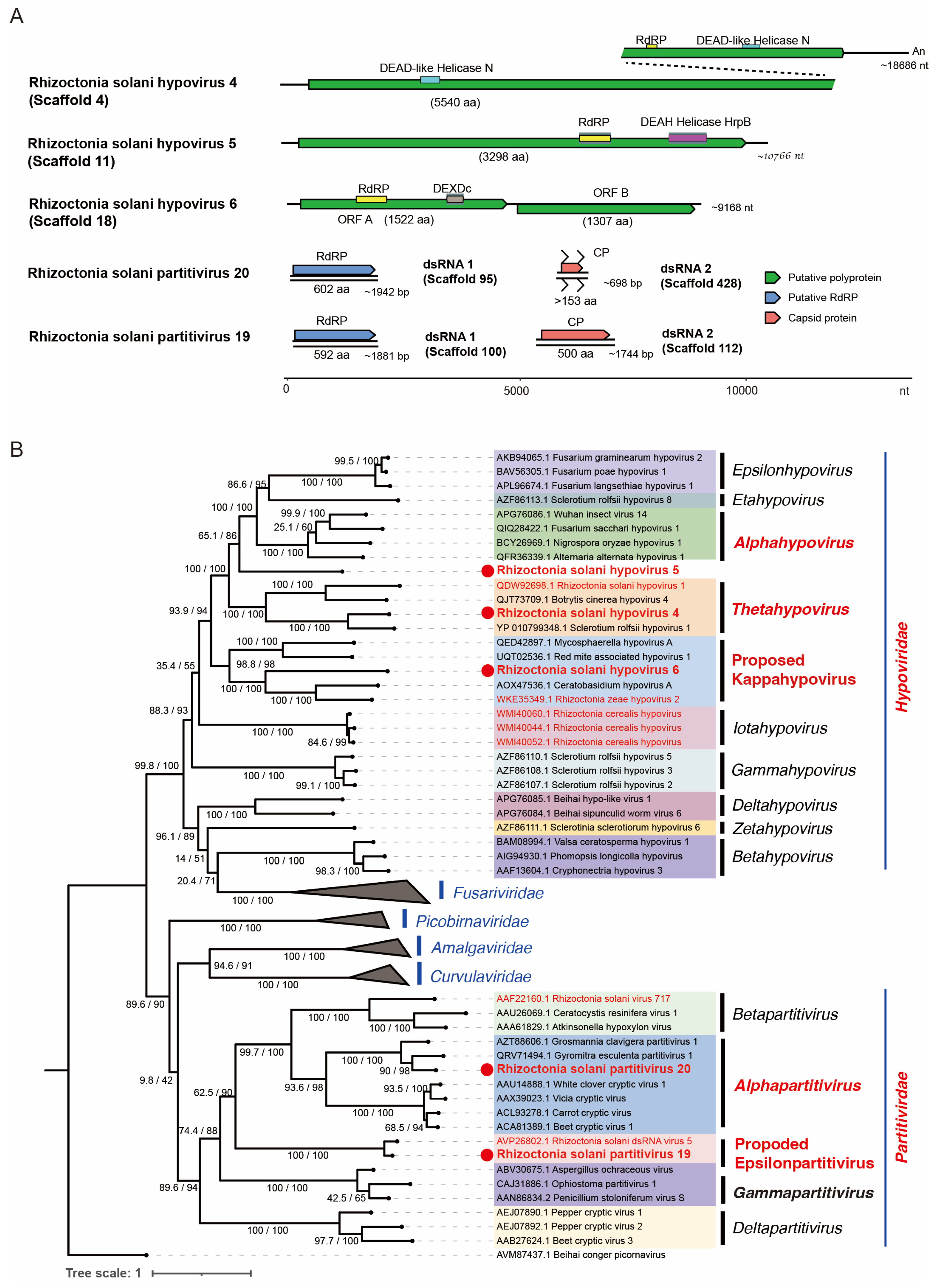
3.6. Viruses in the Order Durnavirales, Phylum Pisuviricota
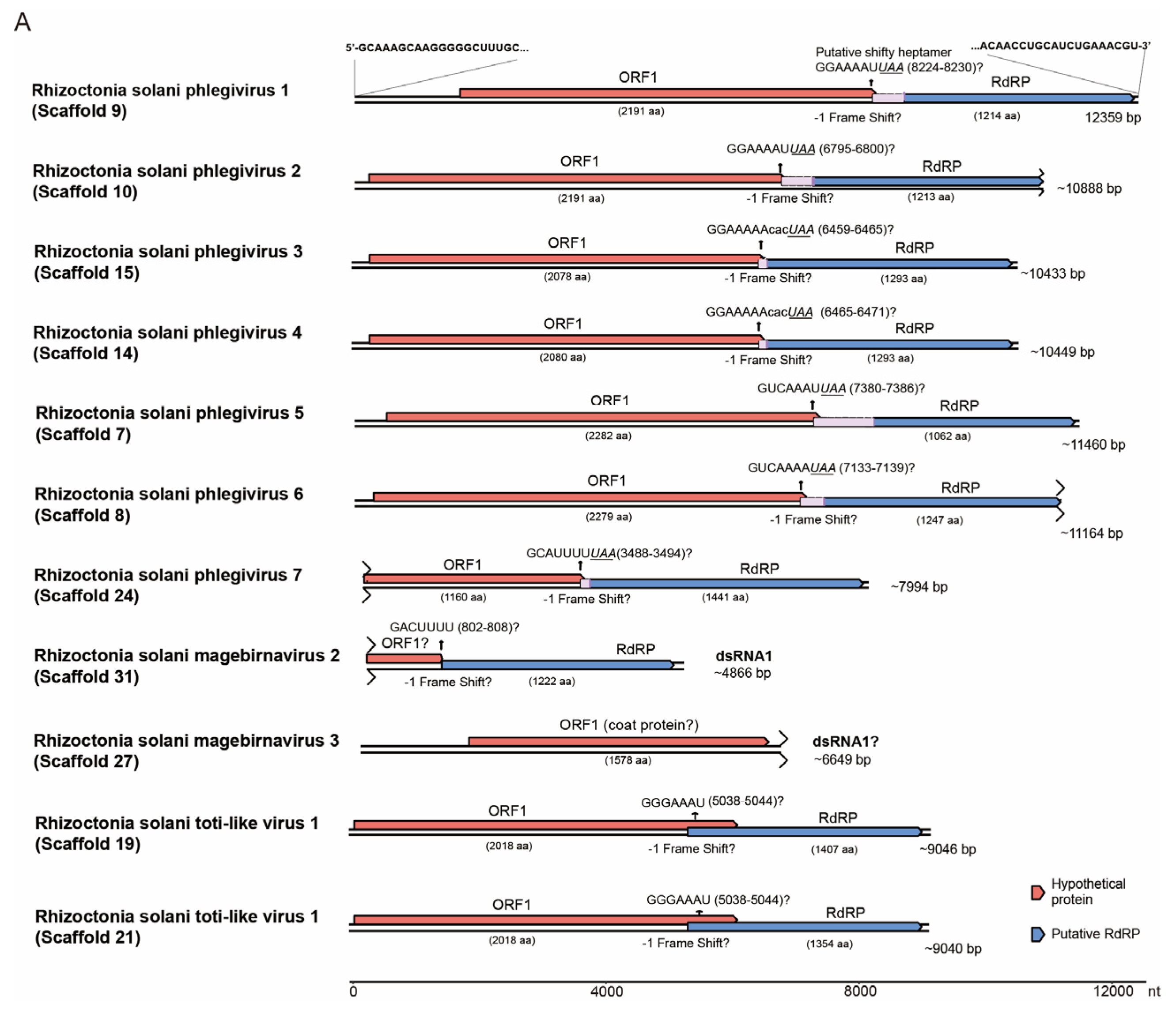
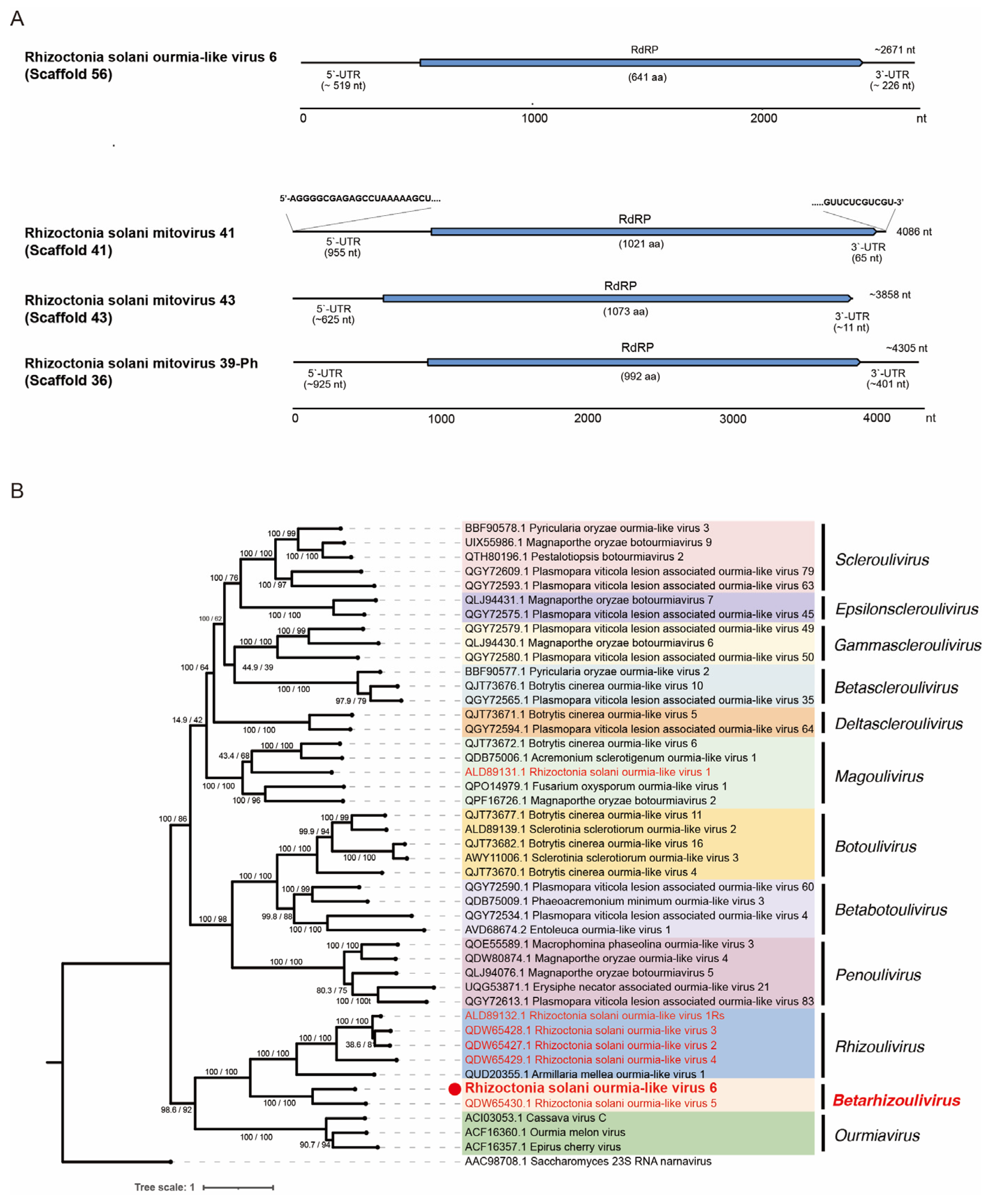
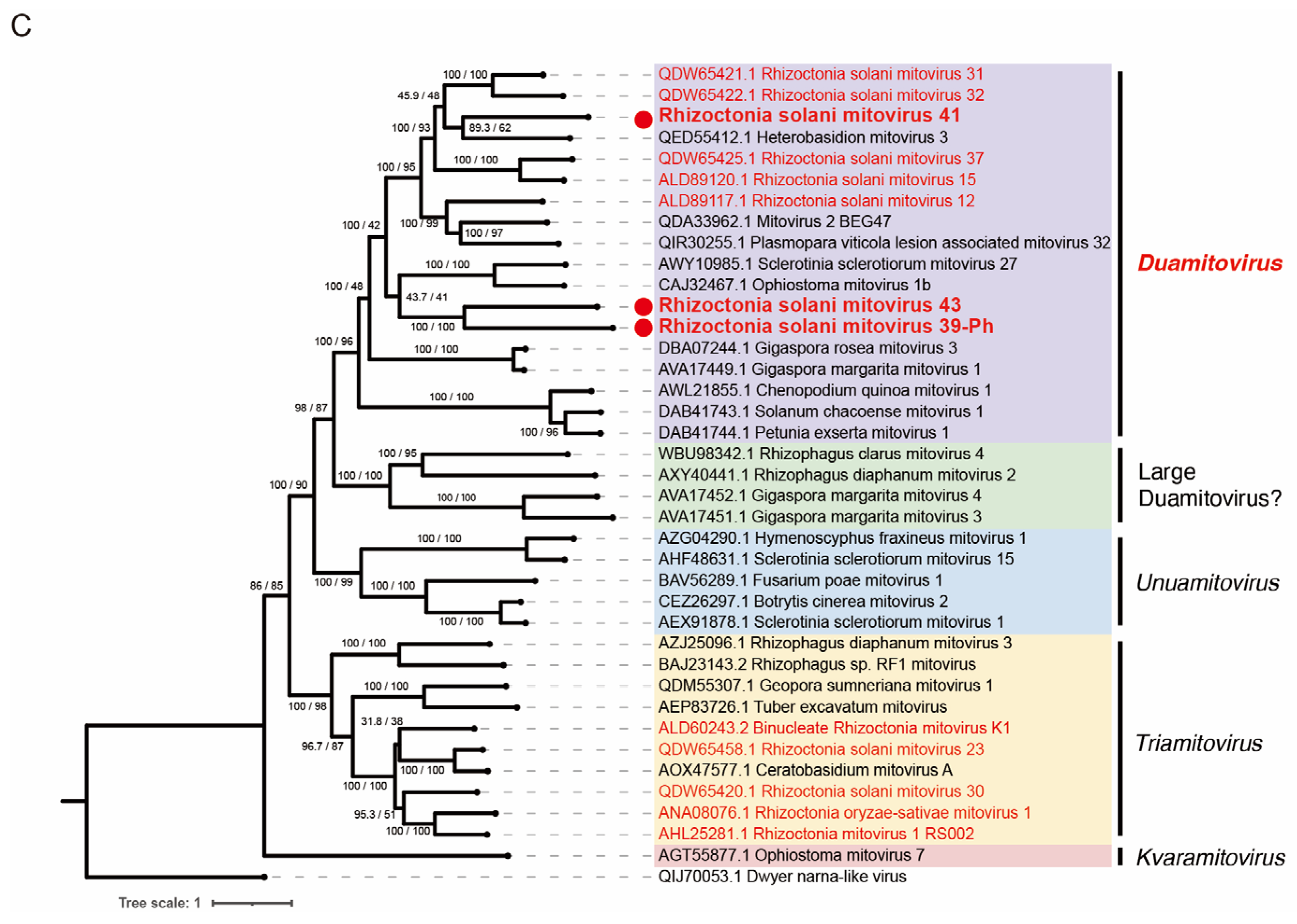
3.7. Viruses in the Families Botourmiaviridae and Mitoviridae within Orders Ourlivirales and Crypavirales, Phylum Lenarviricota
4. Discussion
4.1. First R. solani Virome Report from a Philippine Fungal Population
4.2. High Prevalence Rate of Virus Infection for Rhizoctonia Strains
4.3. Multiple Infections of Single R. solani Strains and Their Stability
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bin Rahman, A.R.; Zhang, J. Trends in rice research: 2030 and beyond. Food Energy Secur. 2023, 12, e390. Available online: https://www.ers.usda.gov/topics/crops/rice/rice-sector-at-a-glance/ (accessed on 1 June 2024). [CrossRef]
- Moore, D.; Robson, G.; Trinci, A. Fungal diseases and loss of world agricultural production. In 21st Century Guidebook to Fungi, 2nd ed.; 2019; Available online: https://www.davidmoore.org.uk/21st_century_guidebook_to_fungi_platinum/ch14_01.htm (accessed on 1 June 2024).
- Tang, Q.Y.; Peng, S.B.; Buresh, R.J.; Zou, Y.B.; Castilla, N.P.; Mew, T.W.; Zhong, X.H. Rice varietal difference in sheath blight development and its association with yield loss at different levels of N fertilization. Field Crop Res. 2007, 102, 219–227. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Elazegui, F.A.; Castilla, N.P.; Teng, P.S. Rice pest constraints in tropical Asia: Quantification of yield losses due to rice pests in a range of production situations. Plant Dis. 2000, 84, 357–369. [Google Scholar] [CrossRef]
- Jia, Y.; Jia, M.H.; Wang, X.; Zhao, H. A Toolbox for Managing Blast and Sheath Blight Diseases of Rice in the United States of America; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Sandoval, R.F.C.; Cumagun, C.J.R. Phenotypic and molecular analyses of Rhizoctonia spp. associated with rice and other hosts. Microorganisms 2019, 7, 88. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Jiang, C.H.; Wang, C.; Chen, L.J.; Li, H.Y.; Xu, Q.; Guo, J.H. An improved strategy for stable biocontrol agents selecting to control rice sheath blight caused by Rhizoctonia solani. Microbiol. Res. 2017, 203, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Carling, D.E.; Baird, R.E.; Gitaitis, R.D.; Brainard, K.A.; Kuninaga, S. Characterization of AG-13, a newly reported anastomosis group of Rhizoctonia solani. Phytopathology 2002, 92, 893–899. [Google Scholar] [CrossRef]
- Cubeta, M.A.; Vilgalys, R. Population biology of the Rhizoctonia solani complex. Phytopathology 1997, 87, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Sneh, B.; Burpee, L.; Ogoshi, A. Identification of Rhizoctonia Species; American Phytopathological Press: St. Paul, MN, USA, 1991. [Google Scholar]
- Stodart, B.J.; Harvey, P.R.; Neate, S.M.; Melanson, D.L.; Scott, E.S. Genetic variation and pathogenicity of anastomosis group 2 isolates of Rhizoctonia solani in Australia. Mycol. Res. 2007, 111, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Kuninaga, S.; Natsuaki, T.; Takeuchi, T.; Yokosawa, R. Sequence variation of the rDNA ITS regions within and between anastomosis groups in Rhizoctonia solani. Curr. Genet. 1997, 32, 237–243. [Google Scholar] [CrossRef]
- Kondo, H.; Botella, L.; Suzuki, N. Mycovirus diversity and evolution revealed/inferred from recent studies. Annu. Rev. Phytopathol. 2022, 60, 307–336. [Google Scholar] [CrossRef]
- Myers, J.M.; Bonds, A.E.; Clemons, R.A.; Thapa, N.A.; Simmons, D.R.; Carter-House, D.; Ortanez, J.; Liu, P.; Miralles-Duran, A.; Desiro, A.; et al. Survey of early-diverging lineages of fungi reveals abundant and diverse mycoviruses. mBio 2020, 11, e02027-20. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pedrajas, M.D.; Canizares, M.C.; Sarmiento-Villamil, J.L.; Jacquat, A.G.; Dambolena, J.S. Mycoviruses in biological control: From basic research to field implementation. Phytopathology 2019, 109, 1828–1839. [Google Scholar] [CrossRef] [PubMed]
- Rigling, D.; Prospero, S. Cryphonectria parasitica, the causal agent of chestnut blight: Invasion history, population biology and disease control. Mol. Plant Pathol. 2018, 19, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Wagemans, J.; Holtappels, D.; Vainio, E.; Rabiey, M.; Marzachi, C.; Herrero, S.; Ravanbakhsh, M.; Tebbe, C.C.; Ogliastro, M.; Ayllon, M.A.; et al. Going viral: Virus-based biological control agents for plant protection. Annu. Rev. Phytopathol. 2022, 60, 21–42. [Google Scholar] [CrossRef] [PubMed]
- Picarelli, M.; Forgia, M.; Rivas, E.B.; Nerva, L.; Chiapello, M.; Turina, M.; Colariccio, A. Extreme diversity of mycoviruses present in isolates of Rhizoctonia solani AG2-2 LP from Zoysia japonica from Brazil. Front. Cell Infect. Microbiol. 2019, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Abdoulaye, A.H.; Hai, D.; Tang, Q.; Jiang, D.; Fu, Y.; Cheng, J.; Lin, Y.; Li, B.; Kotta-Loizou, I.; Xie, J. Two distant helicases in one mycovirus: Evidence of horizontal gene transfer between mycoviruses, coronaviruses and other nidoviruses. Virus Evol. 2021, 7, veab043. [Google Scholar] [CrossRef] [PubMed]
- Andika, I.B.; Wei, S.; Cao, C.; Salaipeth, L.; Kondo, H.; Sun, L. Phytopathogenic fungus hosts a plant virus: A naturally occurring cross-kingdom viral infection. Proc. Natl. Acad. Sci. USA 2017, 114, 12267–12272. [Google Scholar] [CrossRef] [PubMed]
- Abdoulaye, A.H.; Foda, M.F.; Kotta-Loizou, I. Viruses Infecting the plant pathogenic fungus Rhizoctonia solani. Viruses 2019, 11, 1113. [Google Scholar] [CrossRef]
- Li, Y.; Yang, N.; Mu, T.; Wu, X.; Zhao, C. Diversity of mycoviruses present in strains of binucleate Rhizoctonia and multinucleate Rhizoctonia, causal agents for potato stem canker or black scurf. J. Fungi 2023, 9, 214. [Google Scholar] [CrossRef]
- Li, S.; Ma, Z.; Zhang, X.; Cai, Y.; Han, C.; Wu, X. Sixteen novel mycoviruses containing positive single-stranded RNA, double-stranded RNA, and negative single-stranded RNA genomes co-infect a single strain of Rhizoctonia zeae. J. Fungi 2023, 10, 30. [Google Scholar] [CrossRef]
- Zheng, L.; Shu, C.; Zhang, M.; Yang, M.; Zhou, E. Molecular characterization of a novel endornavirus conferring hypovirulence in rice sheath blight fungus Rhizoctonia solani AG-1 IA strain GD-2. Viruses 2019, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhang, M.; Chen, Q.; Zhu, M.; Zhou, E. A novel mycovirus closely related to viruses in the genus Alphapartitivirus confers hypovirulence in the phytopathogenic fungus Rhizoctonia solani. Virology 2014, 456–457, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, S.W.; Ma, Z.H.; Wang, W.J.; Gao, L.H.; Han, C.G.; Yang, A.P.; Wu, X.H. Anastomosis groups and mycovirome of Rhizoctonia isolates causing sugar beet root and crown rot and their sensitivity to flutolanil, thifluzamide, and pencycuron. J. Fungi 2023, 9, 545. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Zhang, X.; Hua, H.; Zhou, T.; Wu, X. Molecular and biological characterization of a novel strain of Alternaria alternata chrysovirus 1 identified from the pathogen Alternaria tenuissima causing watermelon leaf blight. Virus Res. 2020, 280, 197904. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Koley, S.; Mahapatra, S.S. Evaluation of culture media for growth characteristics of Alternaria solani causing early blight of tomato. J. Plant Pathol. Microbiol. 2015, 1, 1–5. [Google Scholar]
- Eusebio-Cope, A.; Suzuki, N. Mycoreovirus genome rearrangements associated with RNA silencing deficiency. Nucleic Acids Res. 2015, 43, 3802–3813. [Google Scholar] [CrossRef] [PubMed]
- Telengech, P.; Hisano, S.; Mugambi, C.; Hyodo, K.; Arjona-Lopez, J.M.; Lopez-Herrera, C.J.; Kanematsu, S.; Kondo, H.; Suzuki, N. Diverse partitiviruses from the phytopathogenic fungus, Rosellinia necatrix. Front. Microbiol. 2020, 11, 1064. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Brister, J.R.; Ako-adjei, D.; Bao, Y.M.; Blinkova, O. NCBI Viral Genomes Resource. Nucleic Acids Res. 2015, 43, D571–D577. [Google Scholar] [CrossRef]
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes de novo assembler. Curr. Protoc. Bioinform. 2020, 70, e102. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bo, Y.; Han, L.; He, J.; Lanczycki, C.J.; Lu, S.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; et al. CDD/SPARCLE: Functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017, 45, D200–D203. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, D. gggenes_CITATION.pdf. R Package Version 0.5.0. 2023. Available online: https://wilkox.org/gggenes/ (accessed on 1 June 2024).
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Neang, S.; Bincader, S.; Rangsuwan, S.; Keawmanee, P.; Rin, S.; Salaipeth, L.; Das, S.; Kondo, H.; Suzuki, N.; Sato, I.; et al. Omnipresence of partitiviruses in rice aggregate sheath spot symptom-associated fungal Isolates from paddies in Thailand. Viruses 2021, 13, 2269. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Dolja, V.V.; Krupovic, M.; Varsani, A.; Wolf, Y.I.; Yutin, N.; Zerbini, F.M.; Kuhn, J.H. Global organization and proposed megataxonomy of the virus world. Microbiol. Mol. Biol. Rev. 2020, 84, e00061-19. [Google Scholar] [CrossRef] [PubMed]
- Gilmer, D.; Ratti, C.; Ictv Report, C. ICTV Virus Taxonomy Profile: Benyviridae. J. Gen. Virol. 2017, 98, 1571–1572. [Google Scholar] [CrossRef]
- Valverde, R.A.; Khalifa, M.E.; Okada, R.; Fukuhara, T.; Sabanadzovic, S.; Ictv Report, C. ICTV virus taxonomy profile: Endornaviridae. J. Gen. Virol. 2019, 100, 1204–1205. [Google Scholar] [CrossRef]
- Ong, J.W.L.; Li, H.; Sivasithamparam, K.; Dixon, K.W.; Jones, M.G.K.; Wylie, S.J. Novel Endorna-like viruses, including three with two open reading frames, challenge the membership criteria and taxonomy of the Endornaviridae. Virology 2016, 499, 203–211. [Google Scholar] [CrossRef]
- Sato, Y.; Caston, J.; Hillman, B.I.; Kim, D.-H.; Kondo, H.; Nibert, M.L.; Lanza, D.; Sabanadzovic, S.; Stenger, D.; Wu, M.; et al. 015F.A.v2.Ghabrivirales_reorg.docx. ICTV 2023. Available online: https://ictv.global/filebrowser/download/15196 (accessed on 1 June 2024).
- Zhong, J.; Chen, C.Y.; Gao, B.D. Genome sequence of a novel mycovirus of Rhizoctonia solani, a plant pathogenic fungus. Virus Genes 2015, 51, 167–170. [Google Scholar] [CrossRef]
- Kozlakidis, Z.; Hacker, C.V.; Bradley, D.; Jamal, A.; Phoon, X.; Webber, J.; Brasier, C.M.; Buck, K.W.; Coutts, R.H. Molecular characterisation of two novel double-stranded RNA elements from Phlebiopsis gigantea. Virus Genes 2009, 39, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.M.; Jamal, A.; Phoon, X.; Korhonen, K.; Coutts, R.H. Incidence of Phlebiopsis gigantea large virus-1 in a collection of Phlebiopsis gigantea isolates. Arch. Virol. 2011, 156, 2091–2094. [Google Scholar] [CrossRef]
- Petrzik, K.; Sarkisova, T.; Stary, J.; Koloniuk, I.; Hrabakova, L.; Kubesova, O. Molecular characterization of a new monopartite dsRNA mycovirus from mycorrhizal Thelephora terrestris (Ehrh.) and its detection in soil oribatid mites (Acari: Oribatida). Virology 2016, 489, 12–19. [Google Scholar] [CrossRef]
- Dinman, J.D.; Icho, T.; Wickner, R.B. A -1 ribosomal frameshift in a double-stranded RNA virus of yeast forms a gag-pol fusion protein. Proc. Natl. Acad. Sci. USA 1991, 88, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Salaipeth, L.; Lin, Y.H.; Sasaki, A.; Kanematsu, S.; Suzuki, N. A novel bipartite double-stranded RNA mycovirus from the white root rot fungus Rosellinia necatrix: Molecular and biological characterization, taxonomic considerations, and potential for biological control. J. Virol. 2009, 83, 12801–12812. [Google Scholar] [CrossRef]
- Sato, Y.; Miyazaki, N.; Kanematsu, S.; Xie, J.; Ghabrial, S.A.; Hillman, B.I.; Suzuki, N.; Ictv Report, C. ICTV Virus Taxonomy Profile: Megabirnaviridae. J. Gen. Virol. 2019, 100, 1269–1270. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Zhao, Y.; Zhou, T.; Wu, X.; Zhao, C. Six novel mycoviruses containing positive single-stranded RNA and double-stranded RNA genomes co-infect a single strain of the Rhizoctonia solani AG-3 PT. Viruses 2022, 14, 813. [Google Scholar] [CrossRef]
- Zhang, M.; He, Z.; Huang, X.; Shu, C.; Zhou, E. Genome organizations and functional analyses of a novel gammapartitivirus from Rhizoctonia solani AG-1 IA strain D122. Viruses 2021, 13, 2254. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, H.; Zhang, M.; Cao, X.; Zhou, E. The complete genomic sequence of a novel mycovirus from Rhizoctonia solani AG-1 IA strain B275. Arch. Virol. 2013, 158, 1609–1612. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Z.; Zhu, H.J.; Gao, B.D.; Zhou, Q.; Zhong, J. Diverse, novel mycoviruses from the virome of a hypovirulent Sclerotium rolfsii strain. Front. Plant Sci. 2018, 9, 1738. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Velasco, L.; Ayllon, M.A.; Suzuki, N.; Lee-Marzano, S.Y.; Sun, L.; Sabanadzovic, S.; Turina, M. ICTV Virus Taxonomy Profile: Hypoviridae 2023. J. Gen. Virol. 2023, 104, 001848. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.H.; Sun, L.; Chiba, S.; Araki, H.; Suzuki, N. Coupled termination/reinitiation for translation of the downstream open reading frame B of the prototypic hypovirus CHV1-EP713. Nucleic Acids Res. 2009, 37, 3645–3659. [Google Scholar] [CrossRef] [PubMed]
- Vainio, E.J.; Chiba, S.; Ghabrial, S.A.; Maiss, E.; Roossinck, M.; Sabanadzovic, S.; Suzuki, N.; Xie, J.; Nibert, M.; Ictv Report, C. ICTV Virus Taxonomy Profile: Partitiviridae. J. Gen. Virol. 2018, 99, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Nerva, L.; Silvestri, A.; Ciuffo, M.; Palmano, S.; Varese, G.C.; Turina, M. Transmission of Penicillium aurantiogriseum partiti-like virus 1 to a new fungal host (Cryphonectria parasitica) confers higher resistance to salinity and reveals adaptive genomic changes. Environ. Microbiol. 2017, 19, 4480–4492. [Google Scholar] [CrossRef] [PubMed]
- Ayllon, M.A.; Turina, M.; Xie, J.; Nerva, L.; Marzano, S.L.; Donaire, L.; Jiang, D.; Consortium, I.R. ICTV Virus Taxonomy Profile: Botourmiaviridae. J. Gen. Virol. 2020, 101, 454–455. [Google Scholar] [CrossRef] [PubMed]
- Macino, G.; Coruzzi, G.; Nobrega, F.G.; Li, M.; Tzagoloff, A. Use of the UGA terminator as a tryptophan codon in yeast mitochondria. Proc. Natl. Acad. Sci. USA 1979, 76, 3784–3785. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Falloon, R.E.; Stewart, A.; Pitman, A.R. Novel mitoviruses in Rhizoctonia solani AG-3PT infecting potato. Fungal Biol. 2016, 120, 338–350. [Google Scholar] [CrossRef]
- Nibert, M.L. Mitovirus UGA(Trp) codon usage parallels that of host mitochondria. Virology 2017, 507, 96–100. [Google Scholar] [CrossRef]
- Ezawa, T.; Silvestri, A.; Maruyama, H.; Tawaraya, K.; Suzuki, M.; Duan, Y.; Turina, M.; Lanfranco, L. Structurally distinct mitoviruses: Are they an ancestral lineage of the Mitoviridae exclusive to arbuscular mycorrhizal fungi (Glomeromycotina)? mBio 2023, 14, e0024023. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Mazumdar, P.; Harikrishna, J.A.; Babu, S. Sheath blight of rice: A review and identification of priorities for future research. Planta 2019, 250, 1387–1407. [Google Scholar] [CrossRef] [PubMed]
- Marzano, S.Y.L.; Nelson, B.D.; Ajayi-Oyetunde, O.; Bradley, C.A.; Hughes, T.J.; Hartman, G.L.; Eastburn, D.M.; Domier, L.L. Identification of diverse mycoviruses through metatranscriptomics characterization of the viromes of five major fungal plant pathogens. J. Virol. 2016, 90, 6846–6863. [Google Scholar] [CrossRef]
- Bartholomaus, A.; Wibberg, D.; Winkler, A.; Puhler, A.; Schluter, A.; Varrelmann, M. Deep Sequencing Analysis Reveals the Mycoviral Diversity of the Virome of an Avirulent Isolate of Rhizoctonia solani AG-2-2 IV. PLoS ONE 2016, 11, e0165965. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, C.; Zhang, M.L.; Mei, Y.; Zhou, E.X. Diversity of dsRNA viruses infecting rice sheath blight fungus Rhizoctonia solani AG-1 IA. Rice Sci. 2018, 25, 57–60. [Google Scholar]
- Domingo, D.D.; Bawingan, P.A.; Bharathan, S.; Bharathan, N. Molecular screening and characterization of dsRNA from wild-type and mutant strains of Rhizoctonia solani Kuhn Isolates. Philipp. J. Sci. 2014, 143, 61–72. [Google Scholar]
- Zanzinger, D.H.; Bandy, B.P.; Tavantzis, S.M. High frequency of finding double-stranded RNA in naturally occurring isolates of Rhizoctonia solani. J. Gen. Virol. 1984, 65, 1601–1605. [Google Scholar] [CrossRef]
- Bharathan, N.; Saso, H.; Gudipati, L.; Bharathan, S.; Whited, K. Double-stranded RNA: Distribution and analysis among isolates of Rhizoctonia solani AG-2 to -13. Plant Pathol. 2005, 54, 196–203. [Google Scholar] [CrossRef]
- Li, W.; Sun, H.; Cao, S.; Zhang, A.; Zhang, H.; Shu, Y.; Chen, H. Extreme diversity of mycoviruses present in single strains of Rhizoctonia cerealis, the pathogen of wheat sharp eyespot. Microbiol. Spectr. 2023, 11, e0052223. [Google Scholar] [CrossRef]
- Li, D.Y.; Li, S.; Wei, S.H.; Sun, W.X. Strategies to manage rice sheath blight: Lessons from interactions between rice and Rhizoctonia solani. Rice 2021, 14, 21. [Google Scholar] [CrossRef]
- Senapati, M.; Tiwari, A.; Sharma, N.; Chandra, P.; Bashyal, B.M.; Ellur, R.K.; Bhowmick, P.K.; Bollinedi, H.; Vinod, K.K.; Singh, A.K.; et al. Rhizoctonia solani Kuhn pathophysiology: Status and prospects of sheath blight disease management in rice. Front. Plant Sci. 2022, 13, 881116. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Chen, W.R.; Niu, Y.F.; Xu, P.; Zhang, L.F.; Yu, S.H.; Yang, G.H.; Mo, X.H. Complete nucleotide sequence of a novel alphapartitivirus from Rhizoctonia solani AG-4 HG III isolate SM03. Arch. Virol. 2022, 167, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Bian, R.; Andika, I.B.; Pang, T.; Lian, Z.; Wei, S.; Niu, E.; Wu, Y.; Kondo, H.; Liu, X.; Sun, L. Facilitative and synergistic interactions between fungal and plant viruses. Proc. Natl. Acad. Sci. USA 2020, 117, 3779–3788. [Google Scholar] [CrossRef] [PubMed]
- Andika, I.B.; Tian, M.; Bian, R.; Cao, X.; Luo, M.; Kondo, H.; Sun, L. Cros-kingdom interactions between plant and fungal viruses. Annu. Rev. Virol. 2023, 10, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Bryner, S.F.; Rigling, D. Hypovirus virulence and vegetative incompatibility in populations of the chestnut blight fungus. Phytopathology 2012, 102, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.H.; Lakshman, D.K.; Tavantzis, S.M. Association of distinct double-stranded RNAs with enhanced or diminished virulence in Rhizoctonia solani infecting potato. Mol. Plant Microbe Interact. 1997, 10, 1002–1009. [Google Scholar] [CrossRef]
- Hillman, B.I.; Aulia, A.; Suzuki, N. Viruses of plant-interacting fungi. Adv. Virus Res. 2018, 100, 99–116. [Google Scholar]
- Sato, Y.; Hisano, S.; Lopez-Herrera, C.J.; Kondo, H.; Suzuki, N. Three-layered complex interactions among capsidless (+)ssRNA yadokariviruses, dsRNA viruses, and a fungus. mBio 2022, 13, e0168522. [Google Scholar] [CrossRef]


| Virus Abbreviation a | Segments | Scaffold No. b | Length | Read | Accession No. | BLASTx Top Hit Virus i | Identity (%) |
|---|---|---|---|---|---|---|---|
| (nt or bp) | Counts | ||||||
| RsALV4 | 25 | 7992 | 1200 | MW596323 | Rhizoctonia cerealis alphavirus-like virus | 47.06 | |
| RsALV5 | 20 | 9046 | 5722 | PP262119 | Rhizoctonia cerealis alphavirus-like virus | 46.45 | |
| RsBLV2 | 22 | 8463 | 31,965 | MW574435 | Bemisia tabaci beny-like virus 6 | 34.64 | |
| RsBLV3 | 26 | 7808 | 707 | MW574436 | Rhizoctonia solani beny-like virus 1 | 46.53 | |
| RsBLV4 | 269 | 1026 | 73 | MW596324 | Rhizoctonia solani beny-like virus 1 | 69.66 | |
| RsEV8 | 5 | 18,645 | 2183 | MW574437 | Rhizoctonia solani endornavirus 1 | 44.57 | |
| RsEV9 | 6 | 14,601 | 3299 | MW574438 | Rhizoctonia solani endornavirus 7 | 38.6 | |
| RsEV1 c | 2 | 19,876 | 2652 | MW596327 | Rhizoctonia solani endornavirus 1 | 90.79 | |
| 3 | 19,860 | 3552 | MW596329 | Rhizoctonia solani endornavirus 1 | 82 | ||
| 1 | 20,088 | 5629 | MW596328 | Rhizoctonia solani endornavirus 1 | 82.14 | ||
| RsPhV3 d | 15 | 10,433 | 2637 | MW574447 | Rhizoctonia solani dsRNA virus 7 | 45.46 | |
| RsPhV1 e | 9 | 12,389 | 1913 | MW596343 | Rhizoctonia solani dsRNA virus 7 | 60.43 | |
| RsPhV2 e | 10 | 10,888 | 2040 | MW596348 | Rhizoctonia solani dsRNA virus 7 | 60.09 | |
| RsPhV5 f | 7 | 11,460 | 2911 | MW596349 | Rhizoctonia solani dsRNA virus 7 | 58.88 | |
| RsPhV6 f | 8 | 11,164 | 2386 | MW596350 | Rhizoctonia solani dsRNA virus 7 | 59.1 | |
| RsPhV7 g | 24 | 7994 | 787 | MW574448 | Phlebiopsis gigantea large virus 1 | 30.49 | |
| RsPhV8 g | 40 | 4180 | 16,318 | MW574449 | Lentinula edodes mycovirus HKB | 37.28 | |
| RsPhV4 d | 14 | 10,449 | 1987 | MW596344 | Rhizoctonia zeae RNA virus 1 | 42.52 | |
| RsMBV2 | dsRNA1 | 31 | 4866 | 676 | MW560186 | Pterostylis megabirnavirus-like RdRP | 70.26 |
| RsMBV3 g | dsRNA1? | 27 | 6649 | 3867 | MW596351 | Rhizoctonia zeae megabirnavirus 1 CP | 26.28 |
| RsTLV1 | 19 | 9046 | 32,346 | MW574450 | XiangYun toti-like virus 7 | 30.82 | |
| 21 | 9040 | 22,486 | MW574451 | XiangYun toti-like virus 7 | 31.03 | ||
| RsRV c | 16 | 10,190 | 11,311 | MW596346 | Rhizoctonia solani RNA virus | 85.19 | |
| 13 | 10,453 | 8801 | MW596347 | Rhizoctonia solani RNA virus | 85.41 | ||
| 12 | 10,467 | 7239 | PP334487 | Rhizoctonia solani RNA virus | 85.98 | ||
| RsYnV1g | 377 | 785 | 46 | PP334490 | Rhizoctonia zeae yadonushi virus 2 | 53.01 | |
| RsHV4 | 4 | 18,697 | 86,912 | MW574439 | Sclerotium rolfsii hypovirus 1 | 59.07 | |
| RsHV5 | 11 | 10,766 | 1538 | MW574440 | Nigrospora oryzae hypovirus 1 | 33.16 | |
| RsHV6 | 18 | 9168 | 10,872 | MW574441 | Rhizoctonia zeae hypovirus 2 | 33.16 | |
| 38 | 4235 | 904 | MW574442 | Rhizoctonia zeae hypovirus 2 | 27.61 | ||
| 34 | 4489 | 4949 | MW574443 | Rhizoctonia zeae hypovirus 2 | 27 | ||
| RsPV20 | dsRNA1 | 95 | 1942 | 2586 | MW596340 | Grosmannia clavigera partitivirus 1 RdRP | 66.83 |
| dsRNA2 | 428 | 698 | 50 | PP334488 | Grosmannia clavigera partitivirus 1 CP | 41.49 | |
| RsPV19 | dsRNA1 | 100 | 1881 | 286 | MW596341 | Rhizoctonia solani dsRNA virus 5 | 82.6 |
| dsRNA2 | 112 | 1744 | 242 | PP262120 | Rhizoctonia solani dsRNA virus 5 | 70.53 | |
| RsPV-SM03 c | dsRNA1 | 92 | 1976 | 8112 | MW596342 | Rhizoctonia solani partitivirus SM03 | 98.96 |
| dsRNA2 | 102 | 1852 | 1624 | PP334489 | Rhizoctonia solani partitivirus SM03 | 96.72 | |
| RsRV1 c | 66 | 2321 | 534 | MW596338 | Rhizoctonia solani dsRNA virus 1 | 98.39 | |
| RsMV39-Ph h | 36 | 4305 | 1,374,127 | MW574444 | Rhizoctonia solani mitovirus 15 | 59.17 | |
| RsMV41 | 41 | 4086 | 2,530,917 | MW596335 | Binucleate Rhizoctonia mitovirus 8 | 62.14 | |
| RsMV43 | 43 | 3858 | 4,698,115 | MW596337 | Binucleate Rhizoctonia mitovirus 16 | 72.66 | |
| RsOLV6 | 56 | 2671 | 306 | MW574434 | Rhizoctonia solani ormia-like virus 5 | 42.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urzo, M.L.R.; Guinto, T.D.; Eusebio-Cope, A.; Budot, B.O.; Yanoria, M.J.T.; Jonson, G.B.; Arakawa, M.; Kondo, H.; Suzuki, N. Metatranscriptomic Sequencing of Sheath Blight-Associated Isolates of Rhizoctonia solani Revealed Multi-Infection by Diverse Groups of RNA Viruses. Viruses 2024, 16, 1152. https://doi.org/10.3390/v16071152
Urzo MLR, Guinto TD, Eusebio-Cope A, Budot BO, Yanoria MJT, Jonson GB, Arakawa M, Kondo H, Suzuki N. Metatranscriptomic Sequencing of Sheath Blight-Associated Isolates of Rhizoctonia solani Revealed Multi-Infection by Diverse Groups of RNA Viruses. Viruses. 2024; 16(7):1152. https://doi.org/10.3390/v16071152
Chicago/Turabian StyleUrzo, Michael Louie R., Timothy D. Guinto, Ana Eusebio-Cope, Bernard O. Budot, Mary Jeanie T. Yanoria, Gilda B. Jonson, Masao Arakawa, Hideki Kondo, and Nobuhiro Suzuki. 2024. "Metatranscriptomic Sequencing of Sheath Blight-Associated Isolates of Rhizoctonia solani Revealed Multi-Infection by Diverse Groups of RNA Viruses" Viruses 16, no. 7: 1152. https://doi.org/10.3390/v16071152






