Elucidating the Immune Response to SARS-CoV-2: Natural Infection versus Covaxin/Covishield Vaccination in a South Indian Population
Abstract
1. Introduction
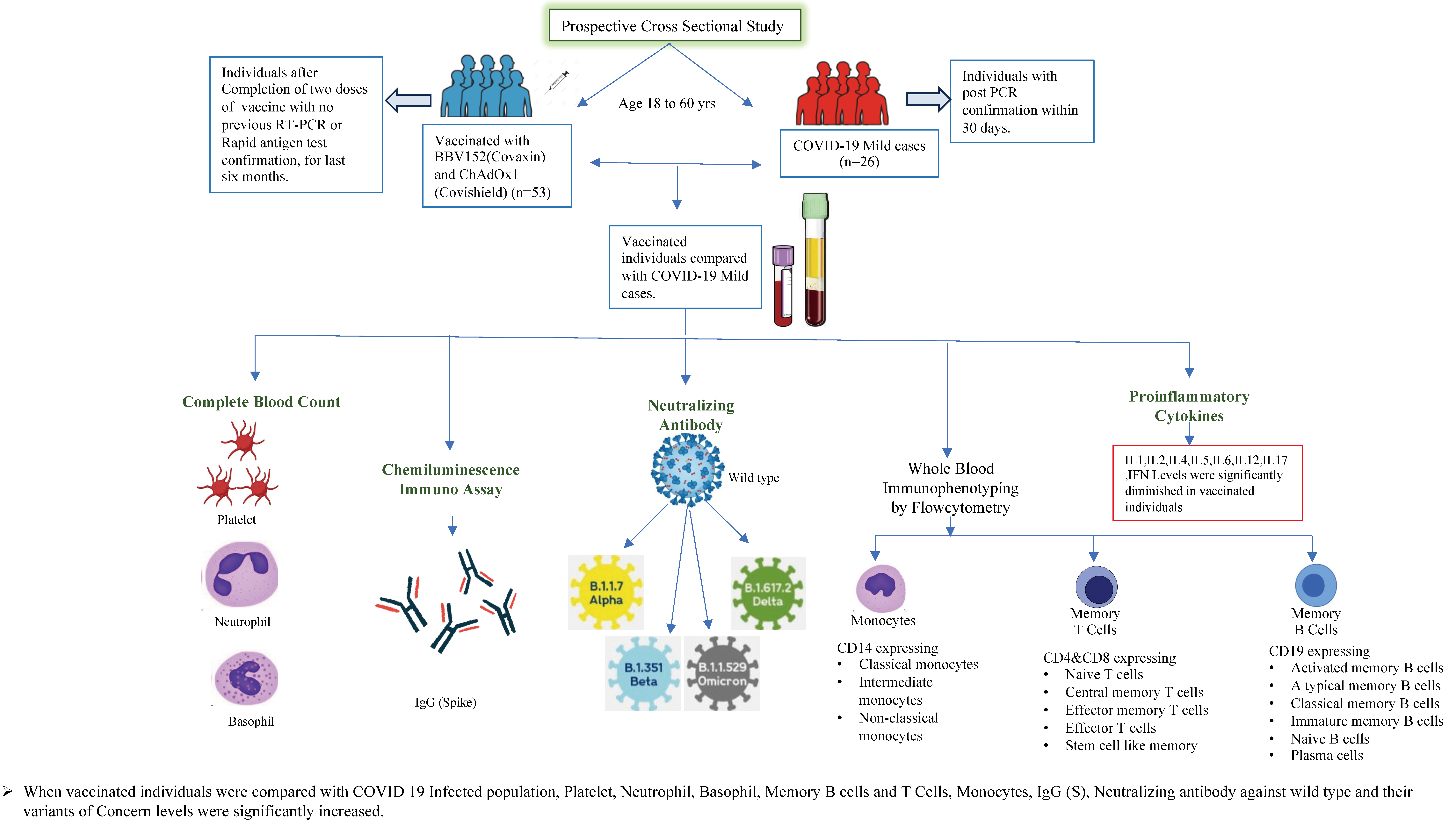
2. Materials and Methods
2.1. Study Procedure
2.2. Ex Vivo Immunophenotyping
2.3. Multiplex ELISA
2.4. Antibody Assays
3. Statistical Analysis
4. Results
4.1. COVID-19 Vaccinated and Infected Cohort
4.2. Alterations of Hematological Parameters in COVID-19 Vaccinated versus Naturally Infected Individuals
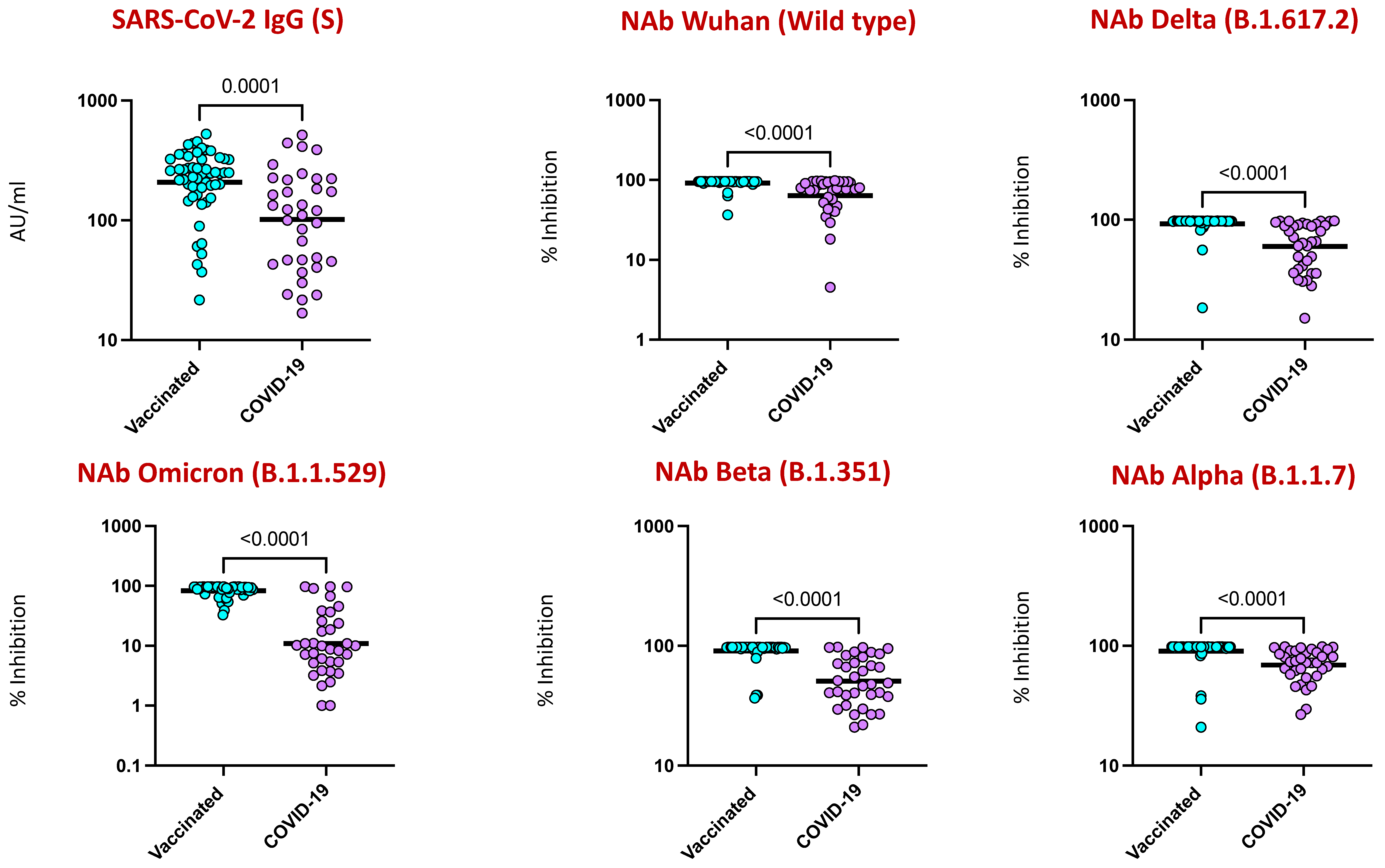
4.3. COVID-19 Vaccination Induced Enhanced IgG and Neutralizing Antibodies against SARS-CoV-2 Viral Variants
4.4. Elevated B Cell Subsets in COVID-19 Vaccinated Individuals
4.5. CD4+ and CD8+ Memory T Cell Subsets Are Altered by COVID-19 Vaccination
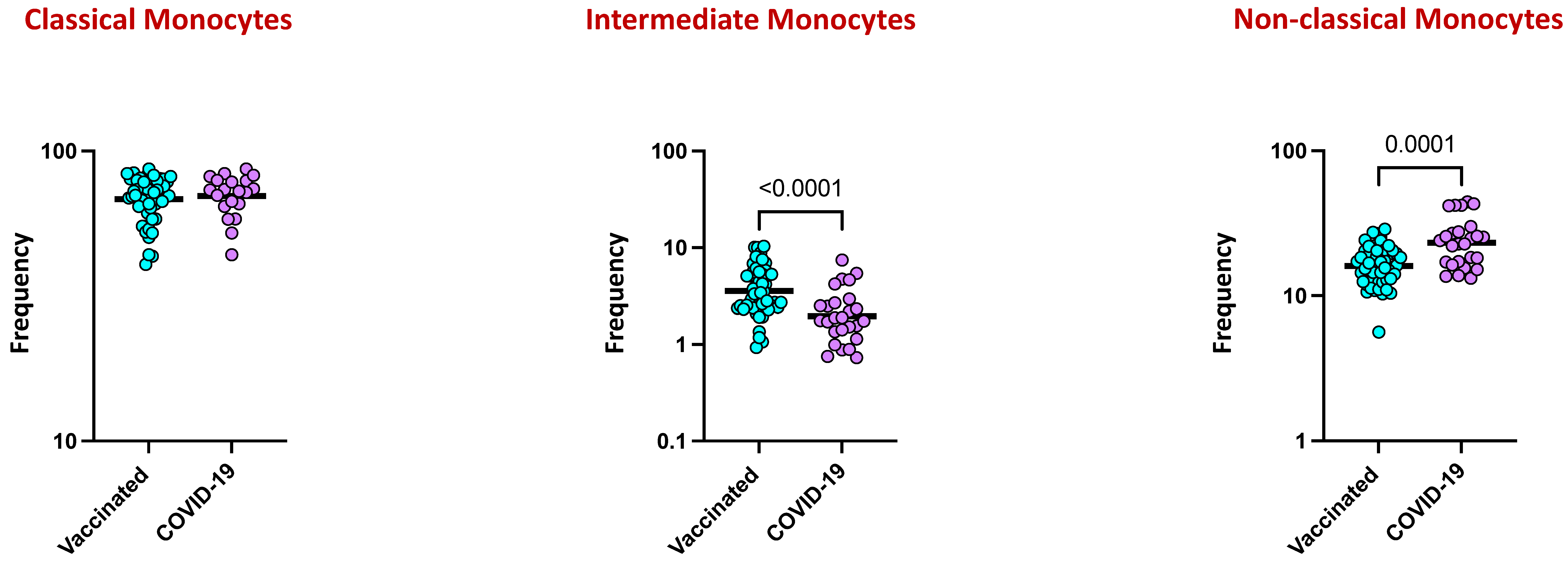
4.6. Monocyte Subsets Are Altered by COVID-19 Vaccination
4.7. COVID-19 Vaccine Induces Diminished Plasma Levels of Type 1, Type 2, Type 17, and Other Pro-Inflammatory Cytokines
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.Y.; Gu, Y.; Wheeler, B.; Young, H.; Holloway, S.; Sunny, S.K.; Moore, Z.; Zeng, D. Effectiveness of COVID-19 Vaccines over a 9-Month Period in North Carolina. N. Engl. J. Med. 2022, 386, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.G.; Stenehjem, E.; Grannis, S.; Ball, S.W.; Naleway, A.L.; Ong, T.C.; DeSilva, M.B.; Natarajan, K.; Bozio, C.H.; Lewis, N.; et al. Effectiveness of COVID-19 Vaccines in Ambulatory and Inpatient Care Settings. N. Engl. J. Med. 2021, 385, 1355–1371. [Google Scholar] [CrossRef] [PubMed]
- Hacisuleyman, E.; Hale, C.; Saito, Y.; Blachere, N.E.; Bergh, M.; Conlon, E.G.; Schaefer-Babajew, D.J.; DaSilva, J.; Muecksch, F.; Gaebler, C.; et al. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N. Engl. J. Med. 2021, 384, 2212–2218. [Google Scholar] [CrossRef] [PubMed]
- Servellita, V.; Syed, A.M.; Morris, M.K.; Brazer, N.; Saldhi, P.; Garcia-Knight, M.; Sreekumar, B.; Khalid, M.M.; Ciling, A.; Chen, P.Y.; et al. Neutralizing immunity in vaccine breakthrough infections from the SARS-CoV-2 Omicron and Delta variants. Cell 2022, 185, 1539–1548.e1535. [Google Scholar] [CrossRef] [PubMed]
- Mistry, P.; Barmania, F.; Mellet, J.; Peta, K.; Strydom, A.; Viljoen, I.M.; James, W.; Gordon, S.; Pepper, M.S. SARS-CoV-2 Variants, Vaccines, and Host Immunity. Front. Immunol. 2021, 12, 809244. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhu, Y.; Chu, M. Role of COVID-19 Vaccines in SARS-CoV-2 Variants. Front. Immunol. 2022, 13, 898192. [Google Scholar] [CrossRef] [PubMed]
- Ganneru, B.; Jogdand, H.; Daram, V.K.; Das, D.; Molugu, N.R.; Prasad, S.D.; Kannappa, S.V.; Ella, K.M.; Ravikrishnan, R.; Awasthi, A.; et al. Th1 skewed immune response of whole virion inactivated SARS-CoV-2 vaccine and its safety evaluation. iScience 2021, 24, 102298. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.D.; Ella, R.; Kumar, S.; Patil, D.R.; Mohandas, S.; Shete, A.M.; Vadrevu, K.M.; Bhati, G.; Sapkal, G.; Kaushal, H.; et al. Immunogenicity and protective efficacy of inactivated SARS-CoV-2 vaccine candidate, BBV152 in rhesus macaques. Nat. Commun. 2021, 12, 1386. [Google Scholar] [CrossRef] [PubMed]
- Pramod, S.; Govindan, D.; Ramasubramani, P.; Kar, S.S.; Aggarwal, R.; JIPMER Vaccine Effectiveness Study Group. Effectiveness of Covishield vaccine in preventing COVID-19—A test-negative case-control study. Vaccine 2022, 40, 3294–3297. [Google Scholar] [CrossRef]
- Das, S.; Kar, S.S.; Samanta, S.; Banerjee, J.; Giri, B.; Dash, S.K. Immunogenic and reactogenic efficacy of Covaxin and Covishield: A comparative review. Immunol. Res. 2022, 70, 289–315. [Google Scholar] [CrossRef] [PubMed]
- Maecker, H.T.; McCoy, J.P.; Nussenblatt, R. Standardizing immunophenotyping for the Human Immunology Project. Nat. Rev. Immunol. 2012, 12, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Portugal, S.; Obeng-Adjei, N.; Moir, S.; Crompton, P.D.; Pierce, S.K. Atypical memory B cells in human chronic infectious diseases: An interim report. Cell Immunol. 2017, 321, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.P.; Moideen, K.; Dhakshinraj, S.D.; Banurekha, V.V.; Nair, D.; Dolla, C.; Kumaran, P.; Babu, S. Profiling leucocyte subsets in tuberculosis-diabetes co-morbidity. Immunology 2015, 146, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Rajamanickam, A.; Munisankar, S.; Dolla, C.K.; Babu, S. Undernutrition is associated with perturbations in T cell-, B cell-, monocyte- and dendritic cell- subsets in latent Mycobacterium tuberculosis infection. PLoS ONE 2019, 14, e0225611. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D.; Zumla, A.; Locatelli, F.; Ippolito, G.; Kroemer, G. Coronavirus infections: Epidemiological, clinical and immunological features and hypotheses. Cell Stress 2020, 4, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Abufares, H.I.; Oyoun Alsoud, L.; Alqudah, M.A.Y.; Shara, M.; Soares, N.C.; Alzoubi, K.H.; El-Huneidi, W.; Bustanji, Y.; Soliman, S.S.M.; Semreen, M.H. COVID-19 Vaccines, Effectiveness, and Immune Responses. Int. J. Mol. Sci. 2022, 23, 15415. [Google Scholar] [CrossRef] [PubMed]
- Kanesa-thasan, N.; Sun, W.; Kim-Ahn, G.; Van Albert, S.; Putnak, J.R.; King, A.; Raengsakulsrach, B.; Christ-Schmidt, H.; Gilson, K.; Zahradnik, J.M.; et al. Safety and immunogenicity of attenuated dengue virus vaccines (Aventis Pasteur) in human volunteers. Vaccine 2001, 19, 3179–3188. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.G.; Bertholet, S.; Coler, R.N.; Friede, M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009, 30, 23–32. [Google Scholar] [CrossRef]
- Gurwith, M.; Lock, M.; Taylor, E.M.; Ishioka, G.; Alexander, J.; Mayall, T.; Ervin, J.E.; Greenberg, R.N.; Strout, C.; Treanor, J.J.; et al. Safety and immunogenicity of an oral, replicating adenovirus serotype 4 vector vaccine for H5N1 influenza: A randomised, double-blind, placebo-controlled, phase 1 study. Lancet Infect. Dis. 2013, 13, 238–250. [Google Scholar] [CrossRef]
- Xiang, Z.; Li, Y.; Cun, A.; Yang, W.; Ellenberg, S.; Switzer, W.M.; Kalish, M.L.; Ertl, H.C. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg. Infect. Dis. 2006, 12, 1596–1599. [Google Scholar] [CrossRef] [PubMed]
- Vespa, S.; Simeone, P.; Catitti, G.; Buca, D.; De Bellis, D.; Pierdomenico, L.; Pieragostino, D.; Cicalini, I.; Del Boccio, P.; Natale, L.; et al. SARS-CoV-2 and Immunity: Natural Infection Compared with Vaccination. Int. J. Mol. Sci. 2022, 23, 8982. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.T.; Garcia-Carreras, B.; Hitchings, M.D.T.; Yang, B.; Katzelnick, L.C.; Rattigan, S.M.; Borgert, B.A.; Moreno, C.A.; Solomon, B.D.; Trimmer-Smith, L.; et al. A systematic review of antibody mediated immunity to coronaviruses: Kinetics, correlates of protection, and association with severity. Nat. Commun. 2020, 11, 4704. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.P.; Banurekha, V.V.; Kumar, C.P.G.; Nancy, A.; Padmapriyadarsini, C.; Shankar, S.; Hanna, L.E.; Murhekar, M.; Devi, K.R.U.; Babu, S. Inactivated COVID-19 vaccines: Durability of Covaxin/BBV152 induced immunity against variants of concern. J. Travel. Med. 2022, 29, taac088. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.B.; Montefiori, D.C.; McDermott, A.B.; Fong, Y.; Benkeser, D.; Deng, W.; Zhou, H.; Houchens, C.R.; Martins, K.; Jayashankar, L.; et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022, 375, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef] [PubMed]
- Kedzierska, K.; Thomas, P.G. Count on us: T cells in SARS-CoV-2 infection and vaccination. Cell Rep. Med. 2022, 3, 100562. [Google Scholar] [CrossRef]
- Chen, S.; Guan, F.; Candotti, F.; Benlagha, K.; Camara, N.O.S.; Herrada, A.A.; James, L.K.; Lei, J.; Miller, H.; Kubo, M.; et al. The role of B cells in COVID-19 infection and vaccination. Front. Immunol. 2022, 13, 988536. [Google Scholar] [CrossRef]
- Rajamanickam, A.; Kumar, N.P.; Nancy, P.A.; Selvaraj, N.; Munisankar, S.; Renji, R.M.; V, V.; Murhekar, M.; Thangaraj, J.W.V.; Kumar, M.S.; et al. Recovery of Memory B-cell Subsets and Persistence of Antibodies in Convalescent COVID-19 Patients. Am. J. Trop. Med. Hyg. 2021, 105, 1255–1260. [Google Scholar] [CrossRef]
- Rajamanickam, A.; Kumar, N.P.; Padmapriyadarsini, C.; Nancy, A.; Selvaraj, N.; Karunanithi, K.; Munisankar, S.; Bm, S.; Renji, R.M.; Ambu, T.C.; et al. Latent tuberculosis co-infection is associated with heightened levels of humoral, cytokine and acute phase responses in seropositive SARS-CoV-2 infection. J. Infect. 2021, 83, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Muecksch, F.; Schaefer-Babajew, D.; Finkin, S.; Viant, C.; Gaebler, C.; Hoffmann, H.H.; Barnes, C.O.; Cipolla, M.; Ramos, V.; et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 2021, 595, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naive and recovered individuals following mRNA vaccination. Sci. Immunol. 2021, 6, eabi6950. [Google Scholar] [CrossRef] [PubMed]
- Gazit, S.; Shlezinger, R.; Perez, G.; Lotan, R.; Peretz, A.; Ben-Tov, A.; Herzel, E.; Alapi, H.; Cohen, D.; Muhsen, K.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Naturally Acquired Immunity versus Vaccine-induced Immunity, Reinfections versus Breakthrough Infections: A Retrospective Cohort Study. Clin. Infect. Dis. 2022, 75, e545–e551. [Google Scholar] [CrossRef]
- Mateus, J.; Dan, J.M.; Zhang, Z.; Rydyznski Moderbacher, C.; Lammers, M.; Goodwin, B.; Sette, A.; Crotty, S.; Weiskopf, D. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science 2021, 374, eabj9853. [Google Scholar] [CrossRef]
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 2022, 185, 847–859.e811. [Google Scholar] [CrossRef]
- Chang, J.T.; Wherry, E.J.; Goldrath, A.W. Molecular regulation of effector and memory T cell differentiation. Nat. Immunol. 2014, 15, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Rydyznski Moderbacher, C.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012.e1019. [Google Scholar] [CrossRef]
- Peng, Y.; Mentzer, A.J.; Liu, G.; Yao, X.; Yin, Z.; Dong, D.; Dejnirattisai, W.; Rostron, T.; Supasa, P.; Liu, C.; et al. Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 2020, 21, 1336–1345. [Google Scholar] [CrossRef]
- Prame Kumar, K.; Nicholls, A.J.; Wong, C.H.Y. Partners in crime: Neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res. 2018, 371, 551–565. [Google Scholar] [CrossRef]
- Park, J.; Dean, L.S.; Jiyarom, B.; Gangcuangco, L.M.; Shah, P.; Awamura, T.; Ching, L.L.; Nerurkar, V.R.; Chow, D.C.; Igno, F.; et al. Elevated circulating monocytes and monocyte activation in COVID-19 convalescent individuals. Front. Immunol. 2023, 14, 1151780. [Google Scholar] [CrossRef] [PubMed]
- Rajamanickam, A.; Kumar, N.P.; Pandiarajan, A.N.; Selvaraj, N.; Munisankar, S.; Renji, R.M.; Venkatramani, V.; Murhekar, M.; Thangaraj, J.W.V.; Kumar, M.S.; et al. Dynamic alterations in monocyte numbers, subset frequencies and activation markers in acute and convalescent COVID-19 individuals. Sci. Rep. 2021, 11, 20254. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Ge, Y.; Wu, B.; Zhang, W.; Wu, T.; Wen, T.; Liu, J.; Guo, X.; Huang, C.; Jiao, Y.; et al. Serum Cytokine and Chemokine Profile in Relation to the Severity of Coronavirus Disease 2019 in China. J. Infect. Dis. 2020, 222, 746–754. [Google Scholar] [CrossRef]
- Kumar, N.P.; Banurekha, V.V.; C. P., G.K.; Nancy, A.; Padmapriyadarsini, C.; Mary, A.S.; Devi, K.R.U.; Murhekar, M.; Babu, S. Prime-Boost Vaccination with Covaxin/BBV152 Induces Heightened Systemic Cytokine and Chemokine Responses. Front. Immunol. 2021, 12, 752397. [Google Scholar] [CrossRef]



| S. No | Characteristics of Study Population | Vaccinated (n = 53) | COVID-19-Positive Cases (n = 26) |
|---|---|---|---|
| 1 | Age | 41.32 (18–59) | 35 (21–58) |
| 2 | Male | 25 | 15 |
| 3 | Female | 28 | 11 |
| 4 | COVID-19 | Negative | Positive |
| 5 | Tuberculosis | Negative | Negative |
| 6 | Diabetes mellitus | 15/38 | 9/17 |
| 7 | Hypertension | 9/44 | 5/21 |
| 8 | Cardiovascular disease | 5/48 | Nil |
| 9 | Chronic obstructive pulmonary disease (COPD) | Nil | Nil |
| 10 | Dyslipidemia | Nil | Nil |
| 11 | Immunological disease | Nil | Nil |
| 12 | Autoimmune disease | Nil | Nil |
| 13 | SARS-CoV-2 IgG (S) (AU/ML) | 255.58 (21.68–526.62) | 115.285 (16.82–514.97) |
| 14 | NAb_Wild type (% of inhibition) | 92.54 (36.38–97.75) | 76.305 (4.52–96.77) |
| 15 | NAb_Delta (% of inhibition) | 94.19 (18.47–97.88) | 65.755 (28.18–97.93) |
| 16 | NAb_Omicron (% of inhibition) | 81.47 (0.2–97.39) | 8.12 (−17.75–97.44) |
| 17 | NAb_SA (% of inhibition) | 89.64 (13.3–97.7) | 48.375 (20.9–97.6) |
| 18 | NAb_UK (% of inhibition) | 93.13 (20.95–98.27) | 72.05 (29.55–98.19) |
| S. No | Hematology | Vaccinated (n = 53) | COVID-19 (n = 26) | p-Value |
|---|---|---|---|---|
| 1 | WBC 103/mL | 8.52 (4.47–14.13) | 7.65 (2.12–10.5) | 0.1153 |
| 2 | RBC 106/mL | 4.85 (3.13–6.11) | 4.93 (3.71–7.26) | 0.7126 |
| 3 | HGB g/dL | 13.61 (9.42–18.06) | 14.165 (9.95–19.9) | 0.4695 |
| 4 | MCV fL | 84.94 (68.4–104.1) | 85.6 (59–94.4) | 0.4764 |
| 5 | MCH Pg | 28.06 (20.6–37.7) | 28.6 (18.7–32.6) | 0.7937 |
| 6 | MCHC g/dL | 32.99 (30.1–39.5) | 33.3 (31.1–34.6) | 0.3167 |
| 7 | HCT% | 41.19 (29.9–53.3) | 42.2 (31.6–59) | 0.4905 |
| 8 | PLT 103/mL | 287.24 (127.9–455.1) | 241.4 (129.8–350.9) | 0.0256 |
| 9 | Neutrophil 103/mL | 4.60 (2.08–10.68) | 3.035 (0.76–5.8) | 0.0048 |
| 10 | Lymphocyte 103/mL | 3.15 (1.77–4.98) | 3.095 (1.17–5.75) | 0.6917 |
| 11 | Monocyte 103/mL | 0.47 (0.24–0.88) | 0.53 (0.02–2.16) | 0.0633 |
| 12 | Eosinophil 103/mL | 0.21 (0.04–0.71) | 0.15 (0.05–0.55) | 0.5524 |
| 13 | Basophil 103/mL | 0.07 (0.03–0.15) | 0.05 (0–0.1) | 0.0023 |
| 14 | Neutrophil% | 52.90 (35.01–76.48) | 38.245 (20.7–65.47) | 0.0070 |
| 15 | Lymphocyte% | 37.87 (19.94–54.14) | 42.245 (25.99–64.6) | 0.0359 |
| 16 | Monocyte% | 5.76 (2.7–8.95) | 6.82 (1.15–28) | 0.0078 |
| 17 | Eosinophil% | 2.53 (0.33–7.37) | 1.8 (0.7–14.06) | 0.8682 |
| 18 | Basophil% | 0.93 (0.36–1.84) | 0.645 (0–1.8) | 0.0434 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanamudhu, A.; Devi Arumugam, R.; Nancy, A.; Selvaraj, N.; Moiden, K.; Hissar, S.; Ranganathan, U.D.; Bethunaickan, R.; Babu, S.; Kumar, N.P. Elucidating the Immune Response to SARS-CoV-2: Natural Infection versus Covaxin/Covishield Vaccination in a South Indian Population. Viruses 2024, 16, 1178. https://doi.org/10.3390/v16081178
Vanamudhu A, Devi Arumugam R, Nancy A, Selvaraj N, Moiden K, Hissar S, Ranganathan UD, Bethunaickan R, Babu S, Kumar NP. Elucidating the Immune Response to SARS-CoV-2: Natural Infection versus Covaxin/Covishield Vaccination in a South Indian Population. Viruses. 2024; 16(8):1178. https://doi.org/10.3390/v16081178
Chicago/Turabian StyleVanamudhu, Agalya, Renuka Devi Arumugam, Arul Nancy, Nandhini Selvaraj, Kadar Moiden, Syed Hissar, Uma Devi Ranganathan, Ramalingam Bethunaickan, Subash Babu, and Nathella Pavan Kumar. 2024. "Elucidating the Immune Response to SARS-CoV-2: Natural Infection versus Covaxin/Covishield Vaccination in a South Indian Population" Viruses 16, no. 8: 1178. https://doi.org/10.3390/v16081178
APA StyleVanamudhu, A., Devi Arumugam, R., Nancy, A., Selvaraj, N., Moiden, K., Hissar, S., Ranganathan, U. D., Bethunaickan, R., Babu, S., & Kumar, N. P. (2024). Elucidating the Immune Response to SARS-CoV-2: Natural Infection versus Covaxin/Covishield Vaccination in a South Indian Population. Viruses, 16(8), 1178. https://doi.org/10.3390/v16081178







