Neurological Complications of COVID-19: Unraveling the Pathophysiological Underpinnings and Therapeutic Implications
Abstract
1. Introduction
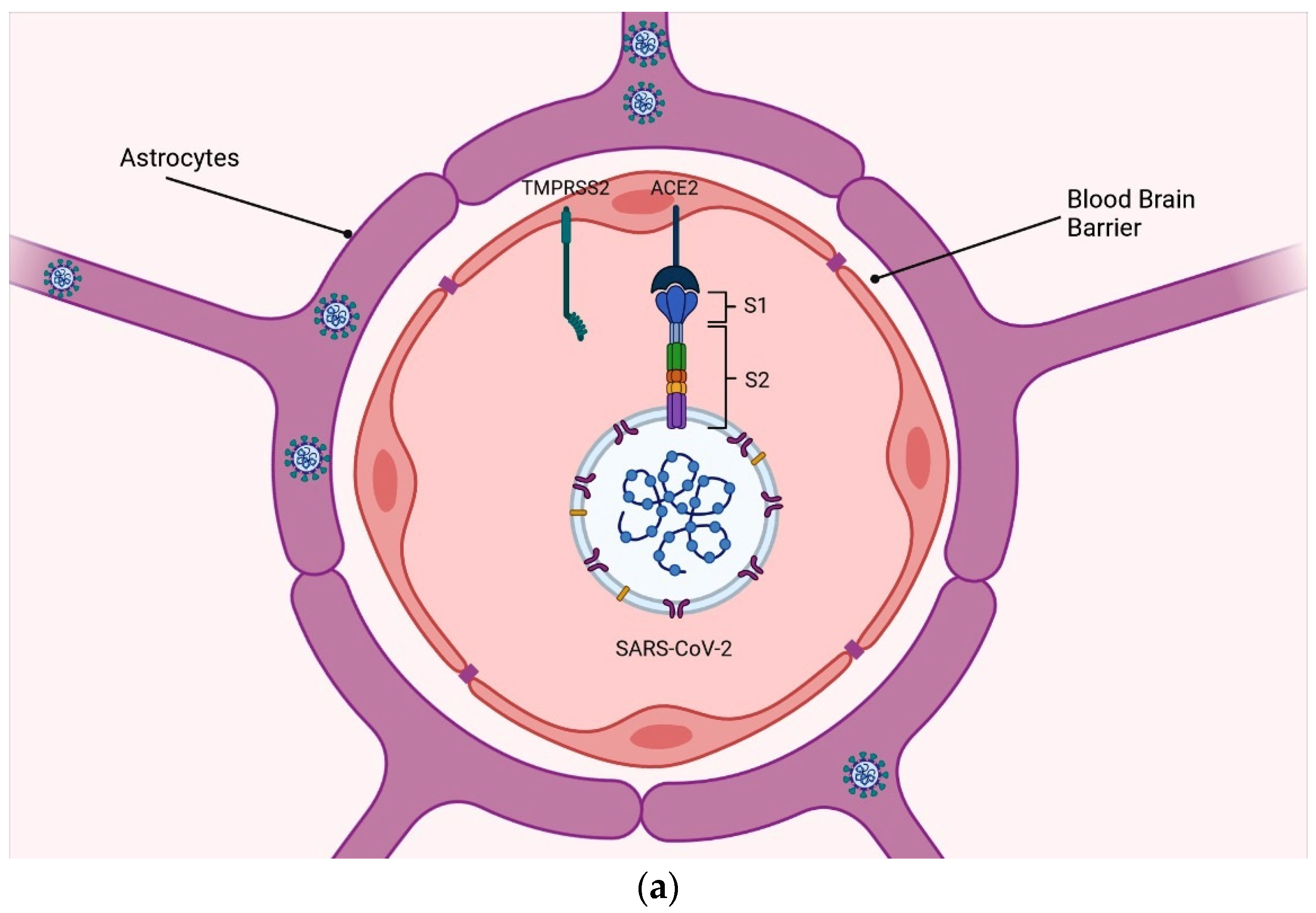
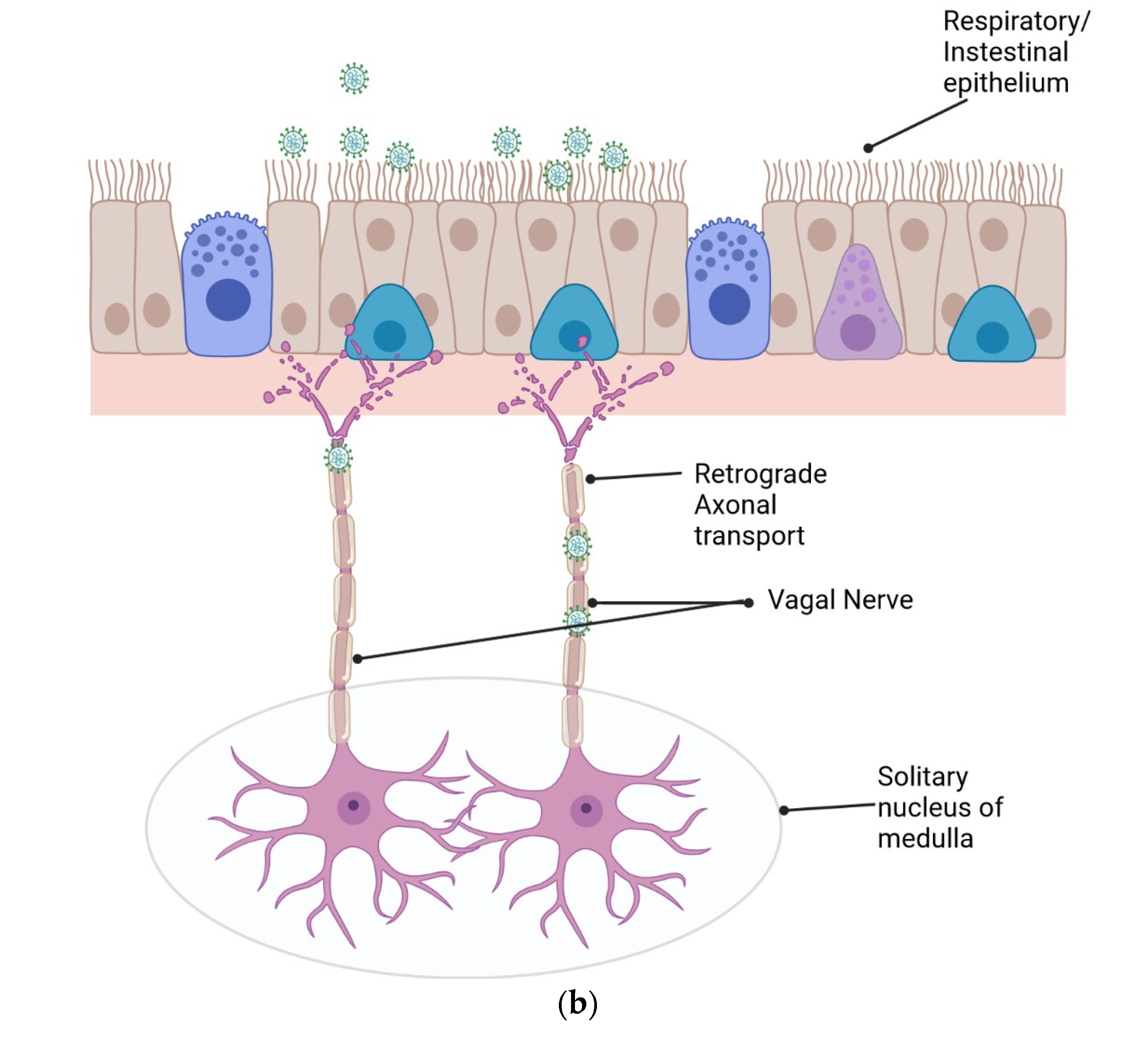
2. Neuroinvasion of SARS-CoV-2 via Potential Brain Routes
2.1. Blood–Brain Barrier
2.2. Immune Deregulation
2.3. Retrograde Axonal Transport
2.4. Via Gastrointestinal Tract
3. SARS-CoV-2 Variants and Their Neurological Manifestations
| S. No. | Patient Size | Sample Type | Type of Study | Study Design | Neurological Manifestation | Altered Markers | Potential Associated Variant | Assay Procedure | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 11 COVID-19 patients | Serum and CSF | Clinical | Retrospective | Myoclonus, oculomotor disturbance, delirium, dystonia, and epileptic seizures | Neurofilament light chain (NfL) levels in CSF were increased in all tested patients. one patient showed Yo antibodies in serum and CSF and two patients myelin antibodies in serum. One patient had high-level serum IgG NMDA receptor antibodies | SARS-CoV-2 Beta | Indirect immunofluorescence assay | [87] |
| 2 | 18 COVID-19 subjects, 14 healthy controls, and 68 non-COVID-19 neurological disease controls | CSF | Clinical | Retrospective | Stroke, encephalopathy, and headache | Anti-SARS-CoV2 antibodies were found in 77% of COVID-19 subjects’ CSF. Increase in pro-inflammatory cytokines (IL-6, TNFα, and IL-12p70) and IL-10 in the CSF of COVID-19 patients. CSF-hsCRP was present exclusively in COVID-19 cases | SARS-CoV-2 Beta | RT-PCR and ELISA | [88] |
| 3 | 35 COVID-19 patients | Serum/CSF | Clinical | Retrospective | Encephalitis and stroke | CSF tumor necrosis factor-alpha (TNFα) and IL6 levels were higher in patients presenting pronounced neuroimaging alterations compared to those who did not | SARS-CoV-2 Beta | Cytokines analysis using magnetic beads, ELISA, and LC-MS/MS | [89] |
| 4 | 844 COVID-19 patients | Blood | Clinical | Retrospective | Stroke and intracranial hemorrhage | Anticardiolipin antibodies were observed in all the patients | SARS-CoV-2 Beta | Biochemical analysis | [90] |
| 5 | 12 COVID-19 patients and 19 healthy controls | Plasma | Clinical | Longitudinal analysis/Prospective | Difficulty with memory or concentration, increased anxiety, and depression | IL-1β was significantly increased in COVID patients, both brain-derived neurotrophic factor and cortisol were significantly elevated in COVID patients | SARS-CoV-2 Epsilon, Alpha, Delta, and Omicron | Genotyping analysis, cytokine analysis, and ELISA | [91] |
| 6 | 400 COVID-19 patients | Plasma | Clinical/Autopsy | Retrospective | ischemic stroke, dementia, epilepsy, and hemorrhagic stroke | LDH, ferritin, hsTnI, and IL-6 were found to be higher in deceased patients. A concentration of hsTnI > 64 ng/L appeared to constitute a strong predictor of an unfavorable prognosis | SARS-CoV-2 Epsilon, Alpha, and Delta | RT-PCR, MR, CT, Doppler US imaging, and biochemical analysis | [92] |
| 7 | 72 COVID-19 Patients | Blood | Clinical | Prospective | Fatigue, anxiety, and depression | Elevated levels of TNF-α and IL-1β were associated with decreased episodic memory, working memory, and inhibitory control | SARS-CoV-2 Beta and Delta | Biochemical analysis | [93] |
4. Therapeutic Strategies
5. Future Perspective
- Understanding the mechanisms of COVID-19’s impact on the brain: More studies should be performed to enhance the understanding of the mechanisms by which the SARS-CoV-2 impacts the neuro system. As more time passes since the beginning of the pandemic, researchers will be able to study the long-term effects of COVID-19 on the brain and nervous system. This could provide important insights into the potential long-term consequences of COVID-19, such as increased risk of dementia or other cognitive impairments;
- Development of new diagnostic tools: Neuro-COVID research could lead to the development of new diagnostic tools to detect neurological complications of COVID-19. For example, the use of MRI and other imaging techniques to detect brain changes in COVID-19 patients should be explored;
- Treatment and management of neurological symptoms: Finally, research into the neurological effects of COVID-19 could lead to new treatments and management strategies for patients experiencing these symptoms. A study published in the Journal of Neuroimmunology in 2021 found that a combination of two monoclonal antibodies, casirivimab and imdevimab, improved neurological symptoms in hospitalized COVID-19 patients [102]. Another study published in the Journal of Clinical Investigation in 2021 found that a monoclonal antibody called CT-P59 was effective in reducing COVID-19 symptoms, including neurological symptoms, in non-hospitalized patients [103]. Also, there is a growing interest in non-pharmacological interventions for the treatment of NeuroCOVID. These interventions may include therapies such as physical rehabilitation, cognitive–behavioral therapy, and neuropsychological assessments. A study published in the Journal of Neurologic Physical Therapy in 2021 found that physical therapy was effective in improving balance and gait in COVID-19 patients with neurological complications [104].
Author Contributions
Funding
Conflicts of Interest
References
- Synowiec, A.; Szczepański, A.; Barreto-Duran, E.; Lie, L.K.; Pyrc, K. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): A systemic infection. Clin. Microbiol. Rev. 2021, 34, e00133-20. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Bhattacharya, M.; Sharma, A.R. Present variants of concern and variants of interest of severe acute respiratory syndrome coronavirus 2: Their significant mutations in S-glycoprotein, infectivity, re-infectivity, immune escape and vaccines activity. Rev. Med. Virol. 2021, 32, e2270. [Google Scholar] [CrossRef]
- Scheepers, C.; Everatt, J.; Amoako, D.G.; Mnguni, A.; Ismail, A.; Mahlangu, B.; Wibmer, C.K.; Wilkinson, E.; Tegally, H.; San, J.E.; et al. The continuous evolution of SARS-CoV-2 in South Africa: A new lineage with rapid accumulation of mutations of concern and global detection. medRxiv 2021. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef]
- Li, Z.; Liu, T.; Yang, N.; Han, D.; Mi, X.; Li, Y.; Liu, K.; Vuylsteke, A.; Xiang, H.; Guo, X. Neurological manifestations of patients with COVID-19: Potential routes of SARS-CoV-2 neuroinvasion from the periphery to the brain. Front. Med. 2020, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, D.I.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368, m1091. [Google Scholar] [CrossRef]
- Frontera, J.A.; Simon, N.M. Bridging Knowledge Gaps in the Diagnosis and Management of Neuropsychiatric Sequelae of COVID-19. JAMA Psychiatry 2022, 79, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Słyk, S.; Domitrz, I. Neurological manifestations of SARS-CoV-2—A systematic review. Neurol. I Neurochir. Pol. 2020, 54, 378–383. [Google Scholar] [CrossRef]
- He, L.; Mäe, M.A.; Sun, Y.; Muhl, L.; Nahar, K.; Liébanas, E.V.; Fagerlund, M.J.; Oldner, A.; Liu, J.; Genové, G.; et al. Pericyte-specific vascular expression of SARS-CoV-2 receptor ACE2–implications for microvascular inflammation and hypercoagulopathy in COVID-19 patients. bioRxiv 2020. [Google Scholar] [CrossRef]
- Chen, R.; Wang, K.; Yu, J.; Howard, D.; French, L.; Chen, Z.; Wen, C.; Xu, Z. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in the human and mouse brains. Front. Neurol. 2021, 11, 1860. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, W.; Zhou, Y.S.; Lian, J.Q.; Zhang, Z.; Du, P.; Gong, L.; Zhang, Y.; Cui, H.Y.; Geng, J.J.; et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. bioRxiv 2020. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; Kallio, K.; Kaya, T.; Anastasina, M.; Smura, T.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and provides a possible pathway into the central nervous system. bioRxiv 2020. [Google Scholar] [CrossRef]
- Paniz-Mondolfi, A.; Bryce, C.; Grimes, Z.; Gordon, R.E.; Reidy, J.; Lednicky, J.; Sordillo, E.M.; Fowkes, M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J. Med. Virol. 2020, 92, 699–702. [Google Scholar] [CrossRef]
- Ding, Y.; He, L.I.; Zhang, Q.; Huang, Z.; Che, X.; Hou, J.; Wang, H.; Shen, H.; Qiu, L.; Li, Z.; et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: Implications for pathogenesis and virus transmission pathways. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2004, 203, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Gong, E.; Zhang, B.; Zheng, J.; Gao, Z.; Zhong, Y.; Zou, W.; Zhan, J.; Wang, S.; Xie, Z.; et al. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005, 202, 415–424. [Google Scholar] [CrossRef]
- Gowrisankar, Y.V.; Clark, M.A. Angiotensin II regulation of angiotensin-converting enzymes in spontaneously hypertensive rat primary astrocyte cultures. J. Neurochem. 2016, 138, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.V.; Van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Mesci, P.; Macia, A.; Saleh, A.; Martin-Sancho, L.; Yin, X.; Snethlage, C.; Avansini, S.; Chanda, S.K.; Muotri, A. Sofosbuvir protects human brain organoids against SARS-CoV-2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ramani, A.; Müller, L.; Ostermann, P.N.; Gabriel, E.; Abida-Islam, P.; Müller-Schiffmann, A.; Mariappan, A.; Goureau, O.; Gruell, H.; Walker, A.; et al. SARS-CoV-2 targets neurons of 3D human brain organoids. EMBO J. 2020, 39, e106230. [Google Scholar] [CrossRef]
- Song, E.; Zhang, C.; Israelow, B.; Lu-Culligan, A.; Sprado, A.; Skriabine, S.; Lu, P.; Weizman, O.E.; Liu, F.; Dai, Y.; et al. Neuroinvasive potential of SARS-CoV-2 revealed in a human brain. J. Exp. Med. 2021, 218, e20202135. [Google Scholar] [CrossRef]
- Moriguchi, T.; Harii, N.; Goto, J.; Harada, D.; Sugawara, H.; Takamino, J.; Ueno, M.; Sakata, H.; Kondo, K.; Myose, N.; et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020, 94, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Schaller, T.; Hirschbühl, K.; Burkhardt, K.; Braun, G.; Trepel, M.; Märkl, B.; Claus, R. Postmortem examination of patients with COVID-19. JAMA 2020, 323, 2518–2520. [Google Scholar] [CrossRef] [PubMed]
- Cabirac, G.F.; Soike, K.F.; Butunoi, C.; Hoel, K.; Johnson, S.; Cai, G.Y.; Murray, R.S. Coronavirus JHM OMP1 pathogenesis in owl monkey CNS and coronavirus infection of owl monkey CNS via peripheral routes. In Coronaviruses; Springer: Boston, MA, USA, 1994; pp. 347–352. [Google Scholar]
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020, 323, 1843–1844. [Google Scholar] [CrossRef]
- Zheng, S.; Fan, J.; Yu, F.; Feng, B.; Lou, B.; Zou, Q.; Xie, G.; Lin, S.; Wang, R.; Yang, X.; et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: Retrospective cohort study. BMJ 2020, 369, m1443. [Google Scholar] [CrossRef] [PubMed]
- Bryce, C.; Grimes, Z.; Pujadas, E.; Ahuja, S.; Beasley, M.B.; Albrecht, R.; Hernandez, T.; Stock, A.; Zhao, Z.; Rasheed, M.A.; et al. Pathophysiology of SARS-CoV-2: Targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. The Mount Sinai COVID-19 autopsy experience. medRxiv 2020. [Google Scholar] [CrossRef]
- Erickson, M.A.; Banks, W.A. Neuroimmune axes of the blood–brain barriers and blood–brain interfaces: Bases for physiological regulation, disease states, and pharmacological interventions. Pharmacol. Rev. 2018, 70, 278–314. [Google Scholar] [CrossRef]
- Teuwen, L.A.; Geldhof, V.; Pasut, A.; Carmeliet, P. COVID-19: The vasculature unleashed. Nat. Rev. Immunol. 2020, 20, 389–391. [Google Scholar] [CrossRef]
- Kantonen, J.; Mahzabin, S.; Mäyränpää, M.I.; Tynninen, O.; Paetau, A.; Andersson, N.; Sajantila, A.; Vapalahti, O.; Carpén, O.; Kekäläinen, E.; et al. Neuropathologic features of four autopsied COVID-19 patients. Brain Pathol. 2020, 30, 1012–1016. [Google Scholar] [CrossRef]
- Reichard, R.R.; Kashani, K.B.; Boire, N.A.; Constantopoulos, E.; Guo, Y.; Lucchinetti, C.F. Neuropathology of COVID-19: A spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020, 140, 1–6. [Google Scholar] [CrossRef]
- Solomon, I.H.; Normandin, E.; Bhattacharyya, S.; Mukerji, S.S.; Keller, K.; Ali, A.S.; Adams, G.; Hornick, J.L.; Padera Jr, R.F. Neuropathological features of COVID-19. N. Engl. J. Med. 2020, 383, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Ling, E.A. The circumventricular organs. Histol. Histopathol. 2017, 32, 879–892. [Google Scholar] [PubMed]
- Nampoothiri, S.; Sauve, F.; Ternier, G.; Fernandois, D.; Coelho, C.; Imbernon, M.; Deligia, E.; Perbet, R.; Florent, V.; Baroncini, M.; et al. The hypothalamus as a hub for putative SARS-CoV-2 brain infection. bioRxiv 2020. [Google Scholar] [CrossRef]
- Bergmann, C.C.; Lane, T.E.; Stohlman, S.A. Coronavirus infection of the central nervous system: Host–virus stand-off. Nat. Rev. Microbiol. 2006, 4, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, B.; Vajkoczy, P.; Weller, R.O. The movers and shapers in immune privilege of the CNS. Nat. Immunol. 2017, 18, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef]
- Feng, Z.; Diao, B.; Wang, R.; Wang, G.; Wang, C.; Tan, Y.; Liu, L.; Wang, C.; Liu, Y.; Liu, Y.; et al. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes. medRxiv 2020. [Google Scholar] [CrossRef]
- Bost, P.; Giladi, A.; Liu, Y.; Bendjelal, Y.; Xu, G.; David, E.; Blecher-Gonen, R.; Cohen, M.; Medaglia, C.; Li, H.; et al. Host-viral infection maps reveal signatures of severe COVID-19 patients. Cell 2020, 181, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Bai, W.Z.; Hirano, N.; Hayashida, T.; Taniguchi, T.; Sugita, Y.; Tohyama, K.; Hashikawa, T. Neurotropic virus tracing suggests a membranous-coating-mediated mechanism for transsynaptic communication. J. Comp. Neurol. 2013, 521, 203–212. [Google Scholar] [CrossRef]
- Biswas, K.; Das Sarma, J. Effect of microtubule disruption on neuronal spread and replication of demyelinating and nondemyelinating strains of mouse hepatitis virus in vitro. J. Virol. 2014, 88, 3043–3047. [Google Scholar] [CrossRef]
- Matsuda, K.; Park, C.H.; Sunden, Y.; Kimura, T.; Ochiai, K.; Kida, H.; Umemura, T. The vagus nerve is one route of transneural invasion for intranasally inoculated influenza a virus in mice. Vet. Pathol. 2004, 41, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Fenrich, M.; Mrdenovic, S.; Balog, M.; Tomic, S.; Zjalic, M.; Roncevic, A.; Mandic, D.; Debeljak, Z.; Heffer, M. SARS-CoV-2 dissemination through peripheral nerves explains multiple organ injury. Front. Cell. Neurosci. 2020, 14, 229. [Google Scholar] [CrossRef] [PubMed]
- Papatsiros, V.G.; Stylianaki, I.; Papakonstantinou, G.; Papaioannou, N.; Christodoulopoulos, G. Case report of transmissible gastroenteritis coronavirus infection associated with small intestine and brain lesions in piglets. Viral Immunol. 2019, 32, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Sanchis-Gomar, F.; Lavie, C.J.; Perez-Quilis, C.; Henry, B.M.; Lippi, G. Angiotensin-converting enzyme 2 and antihypertensives (angiotensin receptor blockers and angiotensin-converting enzyme inhibitors) in coronavirus disease 2019. Mayo Clin. Proc. 2020, 95, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Zuckerkandl, E.; Linus, P. Evolutionary divergence and convergence in proteins. In Evolving Genes and Proteins; Academic Press: Cambridge, MA, US, 1965; pp. 97–166. [Google Scholar]
- Beacon, T.H.; Su, R.C.; Lakowski, T.M.; Delcuve, G.P.; Davie, J.R. SARS-CoV-2 multifaceted interaction with the human host. Part II: Innate immunity response, immunopathology, and epigenetics. IUBMB Life 2020, 72, 2331–2354. [Google Scholar] [CrossRef] [PubMed]
- Sundar, U.S.; Shah, M.H.; Merchant, S.A.; Asole, D.C. Acute ataxia and myoclonus in COVID-19: A case series. Ann. Indian Acad. Neurol. 2021, 24, 807–809. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.; Kremer, S.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Kummerlen, C.; Collange, O.; Boulay, C.; Fafi-Kremer, S.; Ohana, M.; et al. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020, 382, 2268–2270. [Google Scholar] [CrossRef]
- Liotta, E.M.; Batra, A.; Clark, J.R.; Shlobin, N.A.; Hoffman, S.C.; Orban, Z.S.; Koralnik, I.J. Frequent neurologic manifestations and encephalopathy-associated morbidity in COVID-19 patients. Ann. Clin. Transl. Neurol. 2020, 7, 2221–2230. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, C.; Tang, L.; Hong, Z.; Zhou, J.; Dong, X.; Yin, H.; Xiao, Q.; Tang, Y.; Qu, X.; et al. Prolonged presence of SARS-CoV-2 viral RNA in fecal samples. Lancet Gastroenterol. Hepatol. 2020, 5, 434–435. [Google Scholar] [CrossRef]
- Aleem, A.; Akbar Samad, A.B.; Vaqar, S. Emerging Variants of SARS-CoV-2 and Novel Therapeutics against Coronavirus (COVID-19). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Beretta, S.; Cristillo, V.; Camera, G.; Morotti Colleoni, C.; Pellitteri, G.; Viti, B.; Bianchi, E.; Gipponi, S.; Grimoldi, M.; Valente, M.; et al. Incidence and long-term functional outcome of neurologic disorders in hospitalized patients with COVID-19 infected with pre-omicron variants. Neurology 2023, 101, e892–e903. [Google Scholar] [CrossRef]
- de Melo, G.D.; Perraud, V.; Alvarez, F.; Vieites-Prado, A.; Kim, S.; Kergoat, L.; Coleon, A.; Trüeb, B.S.; Tichit, M.; Piazza, A.; et al. Neuroinvasion and anosmia are independent phenomena upon infection with SARS-CoV-2 and its variants. Nat. Commun. 2023, 14, 4485. [Google Scholar] [CrossRef] [PubMed]
- TTaquet, M.; Sillett, R.; Zhu, L.; Mendel, J.; Camplisson, I.; Dercon, Q.; Harrison, P.J. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: An analysis of 2-year retrospective cohort studies including 1,284,437 patients. Lancet Psychiatry 2022, 9, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Proust, A.; Queval, C.J.; Harvey, R.; Adams, L.; Bennett, M.; Wilkinson, R.J. Differential effects of SARS-CoV-2 variants on central nervous system cells and blood–brain barrier functions. J. Neuroinflamm. 2023, 20, 184. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B. 1.1. 529) and delta (B. 1.617. 2) variants in England: A cohort study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Paterson, R.W.; Brown, R.L.; Benjamin, L.; Nortley, R.; Wiethoff, S.; Bharucha, T.; Jayaseelan, D.L.; Kumar, G.; Raftopoulos, R.E.; Zambreanu, L.; et al. The emerging spectrum of COVID-19 neurology: Clinical, radiological and laboratory findings. Brain 2020, 143, 3104–3120. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Oto-Rhino Laryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Garcia-Telles, N.; Aggarwal, G.; Lavie, C.; Lippi, G.; Henry, B.M. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): Early report from the United States. Diagnosis 2020, 7, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Massion, S.P.; Howa, A.C.; Zhu, Y.; Kim, A.; Halasa, N.; Chappell, J.; McGonigle, T.; Mellis, A.M.; Deyoe, J.E.; Reed, C.; et al. Sex differences in COVID-19 symptom severity and trajectories among ambulatory adults. Influenza Other Respir. Viruses 2023, 17, e13235. [Google Scholar] [CrossRef] [PubMed]
- Vashisht, A.; Ahluwalia, P.; Mondal, A.K.; Singh, H.; Sahajpal, N.S.; Fulzele, S.; Kota, V.; Gahlay, G.K.; Kolhe, R. Immune Factors Drive Expression of SARS-CoV-2 Receptor Genes Amid Sexual Disparity. Viruses 2023, 15, 657. [Google Scholar] [CrossRef] [PubMed]
- Spinato, G.; Fabbris, C.; Polesel, J.; Cazzador, D.; Borsetto, D.; Hopkins, C.; Boscolo-Rizzo, P. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA 2020, 323, 2089–2090. [Google Scholar] [CrossRef]
- Beltrán-Corbellini, Á.; Chico-García, J.L.; Martínez-Poles, J.; Rodríguez-Jorge, F.; Natera-Villalba, E.; Gómez-Corral, J.; Gómez-López, A.; Monreal, E.; Parra-Díaz, P.; Cortés-Cuevas, J.L.; et al. Acute-onset smell and taste disorders in the context of COVID-19: A pilot multicentre polymerase chain reaction based case–control study. Eur. J. Neurol. 2020, 27, 1738–1741. [Google Scholar] [CrossRef] [PubMed]
- Moein, S.T.; Hashemian, S.M.; Mansourafshar, B.; Khorram-Tousi, A.; Tabarsi, P.; Doty, R.L. Smell dysfunction: A biomarker for COVID-19. Int. Forum Allergy Rhinol. 2020, 10, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Oxley, T.J.; Mocco, J.; Majidi, S.; Kellner, C.P.; Shoirah, H.; Singh, I.P.; De Leacy, R.A.; Shigematsu, T.; Ladner, T.R.; Yaeger, K.A.; et al. Large-vessel stroke as a presenting feature of COVID-19 in the young. N. Engl. J. Med. 2020, 382, e60. [Google Scholar] [CrossRef] [PubMed]
- Varghese, G.M.; John, R.; Manesh, A.; Karthik, R.; Abraham, O.C. Clinical management of COVID-19. Indian J. Med. Res. 2020, 151, 401. [Google Scholar] [CrossRef] [PubMed]
- Favas, T.T.; Dev, P.; Chaurasia, R.N.; Chakravarty, K.; Mishra, R.; Joshi, D.; Mishra, V.N.; Kumar, A.; Singh, V.K.; Pandey, M.; et al. Neurological manifestations of COVID-19: A systematic review and meta-analysis of proportions. Neurol. Sci. 2020, 41, 3437–3470. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, M.; Wang, M.; Zhou, Y.; Chang, J.; Xian, Y.; Wang, D.; Mao, L.; Jin, H.; Hu, B. Acute cerebrovascular disease following COVID-19: A single center, retrospective, observational study. Stroke Vasc. Neurol. 2020, 5, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Heo, J.H.; Kim, H.O.; Song, S.H.; Park, S.S.; Park, T.H.; Ahn, J.Y.; Kim, M.K.; Choi, J.P. Neurological complications during treatment of Middle East respiratory syndrome. J. Clin. Neurol. 2017, 13, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.K.; Hsieh, S.T.; Chao, C.C.; Chen, Y.C.; Lin, Y.H.; Chang, S.C.; Chang, Y.C. Neuromuscular disorders in severe acute respiratory syndrome. Arch. Neurol. 2004, 61, 1669–1673. [Google Scholar] [CrossRef] [PubMed]
- Jacomy, H.; Fragoso, G.; Almazan, G.; Mushynski, W.E.; Talbot, P.J. Human coronavirus OC43 infection induces chronic encephalitis leading to disabilities in BALB/C mice. Virology 2006, 349, 335–346. [Google Scholar] [CrossRef]
- Fierz, W. Multiple sclerosis: An example of pathogenic viral interaction? Virol. J. 2017, 14, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Crow, M.K.; Olferiev, M.; Kirou, K.A. Type I interferons in autoimmune disease. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 369–393. [Google Scholar] [CrossRef] [PubMed]
- Arbour, N.; Day, R.; Newcombe, J.; Talbot, P.J. Neuroinvasion by human respiratory coronaviruses. J. Virol. 2000, 74, 8913–8921. [Google Scholar] [CrossRef] [PubMed]
- Boucher, A.; Desforges, M.; Duquette, P.; Talbot, P.J. Long-term human coronavirus-myelin cross-reactive T-cell clones derived from multiple sclerosis patients. Clin. Immunol. 2007, 123, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kwon, S.H.; Kam, T.I.; Panicker, N.; Karuppagounder, S.S.; Lee, S.; Lee, J.H.; Kim, W.R.; Kook, M.; Foss, C.A.; et al. Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson’s disease. Neuron 2019, 103, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Srikantha, P.; Mohajeri, M.H. The possible role of the microbiota-gut-brain-axis in autism spectrum disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef]
- Mavrikaki, M.; Lee, J.D.; Solomon, I.H.; Slack, F.J. Severe COVID-19 induces molecular signatures of aging in the human brain. Nat. Aging 2022, 2, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Samudyata, N.; Oliveira, A.O.; Malwade, S.; Rufino de Sousa, N.; Goparaju, S.K.; Gracias, J.; Orhan, F.; Steponaviciute, L.; Schalling, M.; Sheridan, S.D.; et al. SARS-CoV-2 promotes microglial synapse elimination in human brain organoids. Mol. Psychiatry 2022, 27, 3939–3950. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, E.; Musich, P.R.; Lin, F. Mitochondrial dysfunction in neurodegenerative diseases and the potential countermeasure. CNS Neurosci. Ther. 2019, 25, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Ganji, R.; Reddy, P.H. Impact of COVID-19 on mitochondrial-based immunity in aging and age-related diseases. Front. Aging Neurosci. 2021, 12, 614650. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, Y.A.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Wang, Y.; Zhang, X.; Luo, W.; Zhou, C. Association of frailty status with adverse clinical outcomes in patients with COVID-19: Protocol for a systematic review and dose–response meta-analysis. BMJ Open 2021, 11, e046980. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.S.; Martins, T.B.; Shah, K.S.; Hill, H.R.; Coiras, M.; Spivak, A.M.; Planelles, V. Cytokine Deficiencies in Patients with Long-COVID. J. Clin. Cell. Immunol. 2022, 13, 672. [Google Scholar] [PubMed]
- Franke, C.; Ferse, C.; Kreye, J.; Reincke, S.M.; Sanchez-Sendin, E.; Rocco, A.; Steinbrenner, M.; Angermair, S.; Treskatsch, S.; Zickler, D.; et al. High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Behav. Immun. 2021, 93, 415–419. [Google Scholar] [CrossRef] [PubMed]
- GGarcia, M.A.; Barreras, P.V.; Lewis, A.; Pinilla, G.; Sokoll, L.J.; Kickler, T.; Mostafa, H.; Caturegli, M.; Moghekar, A.; Fitzgerald, K.C.; et al. Cerebrospinal fluid in COVID-19 neurological complications: No cytokine storm or neuroinflammation. medRxiv 2021. [Google Scholar] [CrossRef]
- Barros-Aragão, F.G.; Pinto, T.P.; Carregari, V.C.; Rezende, N.B.; Pinheiro, T.L.; Reis-de-Oliveira, G.; Cabral-Castro, M.J.; Queiroz, D.C.; Fonseca, P.L.; Gonçalves, A.L.; et al. Changes in neuroinflammatory biomarkers correlate with disease severity and neuroimaging alterations in patients with COVID-19 neurological complications. Brain Behav. Immun. Health 2024, 39, 100805. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, A.; Oldridge, O.; Schwennesen, H.; Do, D.; Cucchiara, B.L. Acute cerebrovascular events in hospitalized COVID-19 patients. Stroke 2020, 51, e219–e222. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Kido, T.; Shi, J.; McCafferty, E.; Ford, J.M.; Dal Bon, K.; Pulliam, L. Blood markers show neural consequences of longCOVID-19. Cells 2024, 13, 478. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Turek, J.; Turek, G.; Lyson, T.; Gajewski, J.; Ząbek, M.; Dorobek, M. Inflammatory proteins as strong predictors of death in COVID-19 patients with coexisting neurological diseases. medRxiv 2024. [Google Scholar] [CrossRef]
- Sudo, F.K.; Pinto, T.P.; Barros-Aragão, F.G.; Bramati, I.; Marins, T.F.; Monteiro, M.; Meireles, F.; Soares, R.; Erthal, P.; Calil, V.; et al. Cognitive, behavioral, neuroimaging and inflammatory biomarkers after hospitalization for COVID-19 in Brazil. Brain Behav. Immun. 2024, 115, 434–447. [Google Scholar] [CrossRef]
- Wang, A.; Mandigo, G.K.; Yim, P.D.; Meyers, P.M.; Lavine, S.D. Stroke and mechanical thrombectomy in patients with COVID-19: Technical observations and patient characteristics. J. Neurointerventional Surg. 2020, 12, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Escalard, S.; Maïer, B.; Redjem, H.; Delvoye, F.; Hébert, S.; Smajda, S.; Ciccio, G.; Desilles, J.P.; Mazighi, M.; Blanc, R.; et al. Treatment of acute ischemic stroke due to large vessel occlusion with COVID-19: Experience from Paris. Stroke 2020, 51, 2540–2543. [Google Scholar] [CrossRef] [PubMed]
- Siow, I.; Lee, K.S.; Zhang, J.J.; Saffari, S.E.; Ng, A. Encephalitis as neurological complication of COVID-19: A systematic review and meta-analysis of incidence, outcomes, and predictors. Eur. J. Neurol. 2021, 28, 3491–3502. [Google Scholar] [CrossRef] [PubMed]
- Aladawi, M.; Elfil, M.; Abu-Esheh, B.; Jazar, D.A.; Armouti, A.; Bayoumi, A.; Piccione, E. Guillain Barre syndrome as a complication of COVID-19: A systematic review. Can. J. Neurol. Sci. 2022, 49, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Puntambekar, S.S.; Hinton, D.R.; Yin, X.; Savarin, C.; Bergmann, C.C.; Trapp, B.D.; Stohlman, S.A. Interleukin-10 is a critical regulator of white matter lesion containment following viral induced demyelination. Glia 2015, 63, 2106–2120. [Google Scholar] [CrossRef] [PubMed]
- Glezer, I.; Rivest, S. Oncostatin M is a novel glucocorticoid-dependent neuroinflammatory factor that enhances oligodendrocyte precursor cell activity in demyelinated sites. Brain Behav. Immun. 2010, 24, 695–704. [Google Scholar] [CrossRef]
- Liu, S.; Jin, R.; Xiao, A.Y.; Zhong, W.; Li, G. Inhibition of CD147 improves oligodendrogenesis and promotes white matter integrity and functional recovery in mice after ischemic stroke. Brain Behav. Immun. 2019, 82, 13–24. [Google Scholar] [CrossRef]
- Haber, P.; Sejvar, J.; Mikaeloff, Y.; DeStefano, F. Vaccines and guillain-barre syndrome. Drug Saf. 2009, 32, 309–323. [Google Scholar] [CrossRef]
- O’Brien, M.P.; Forleo-Neto, E.; Sarkar, N.; Isa, F.; Hou, P.; Chan, K.C.; Musser, B.J.; Bar, K.J.; Barnabas, R.V.; Barouch, D.H.; et al. Effect of subcutaneous casirivimab and imdevimab antibody combination vs placebo on development of symptomatic COVID-19 in early asymptomatic SARS-CoV-2 infection: A randomized clinical trial. JAMA 2022, 327, 432–441. [Google Scholar] [CrossRef]
- Streinu-Cercel, A.; Săndulescu, O.; Preotescu, L.L.; Kim, J.Y.; Kim, Y.S.; Cheon, S.; Jang, Y.R.; Lee, S.J.; Kim, S.H.; Chang, I.; et al. Efficacy and safety of regdanvimab (CT-P59): A phase 2/3 randomized, double-blind, placebo-controlled trial in outpatients with mild-to-moderate coronavirus disease 2019. In Open Forum Infectious Diseases; Oxford University Press: Oxford, MA, USA, 2022; Volume 9, p. ofac053. [Google Scholar]
- Cuenca-Zaldivar, J.N.; Monroy Acevedo, Á.; Fernández-Carnero, J.; Sánchez-Romero, E.A.; Villafañe, J.H.; Barragán Carballar, C. Effects of a Multicomponent Exercise Program on Improving Frailty in Post-COVID-19 Older Adults after Intensive Care Units: A Single-Group Retrospective Cohort Study. Biology 2022, 11, 1084. [Google Scholar] [CrossRef]
| Aspect of Management | Treatment Details |
|---|---|
| Acute Stroke Therapies | Acute stroke therapies (thrombolysis and thrombectomy) not specifically tested in COVID-19 population; standard care applies as for non-COVID-19 patients. Evaluation for candidacy should be timely. |
| Seizure Management | No specific data on seizure management in COVID-19 patients; general risk–benefit ratio applies. |
| Encephalopathy Treatment Options | Reported options include monotherapy or combinations of corticosteroids (e.g., methylprednisolone 1 g daily for 5–10 days), intravenous immunoglobulin, plasma exchange, and rituximab. |
| Ischemic and Hemorrhagic Stroke Management | Follow same standards of care as non-COVID-19 patients, including timely evaluation for acute medical and interventional stroke therapies. |
| Guillain-Barré Syndrome (GBS) | Consider if respiratory insufficiency is disproportionate to pulmonary findings in COVID-19 patients. Intravenous immunoglobulin and plasma exchange are reported therapies. |
| Autonomic Nervous System | Immunomodulatory actions may help prevent the hyper-inflammatory state, leading to severe COVID-19 symptoms. Maintaining an efficient immune response may minimize tissue damage, including CNS involvement. |
| IL-10 Therapy | No study comprising human samples so far but in MHV-infected mice, IL-10 therapy causes astrocytes to produce glial scars that restrict demyelination regions. |
| CD147 Inhibition | In mouse models of ischemic stroke, the inhibition of CD147 (a SARS-CoV-2 receptor) helps preserve oligodendrocytes and white matter. However, antibodies against CD147 can increase brain inflammation, presenting a double-edged sword effect. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vashisht, A.; Vashisht, V.; Singh, H.; Ahluwalia, P.; Mondal, A.K.; Williams, C.; Farmaha, J.; Woodall, J.; Kolhe, R. Neurological Complications of COVID-19: Unraveling the Pathophysiological Underpinnings and Therapeutic Implications. Viruses 2024, 16, 1183. https://doi.org/10.3390/v16081183
Vashisht A, Vashisht V, Singh H, Ahluwalia P, Mondal AK, Williams C, Farmaha J, Woodall J, Kolhe R. Neurological Complications of COVID-19: Unraveling the Pathophysiological Underpinnings and Therapeutic Implications. Viruses. 2024; 16(8):1183. https://doi.org/10.3390/v16081183
Chicago/Turabian StyleVashisht, Ashutosh, Vishakha Vashisht, Harmanpreet Singh, Pankaj Ahluwalia, Ashis K. Mondal, Colin Williams, Jaspreet Farmaha, Jana Woodall, and Ravindra Kolhe. 2024. "Neurological Complications of COVID-19: Unraveling the Pathophysiological Underpinnings and Therapeutic Implications" Viruses 16, no. 8: 1183. https://doi.org/10.3390/v16081183
APA StyleVashisht, A., Vashisht, V., Singh, H., Ahluwalia, P., Mondal, A. K., Williams, C., Farmaha, J., Woodall, J., & Kolhe, R. (2024). Neurological Complications of COVID-19: Unraveling the Pathophysiological Underpinnings and Therapeutic Implications. Viruses, 16(8), 1183. https://doi.org/10.3390/v16081183







