SARS-CoV-2 Variants and Clinical Outcomes of Special Populations: A Scoping Review of the Literature
Abstract
1. Introduction
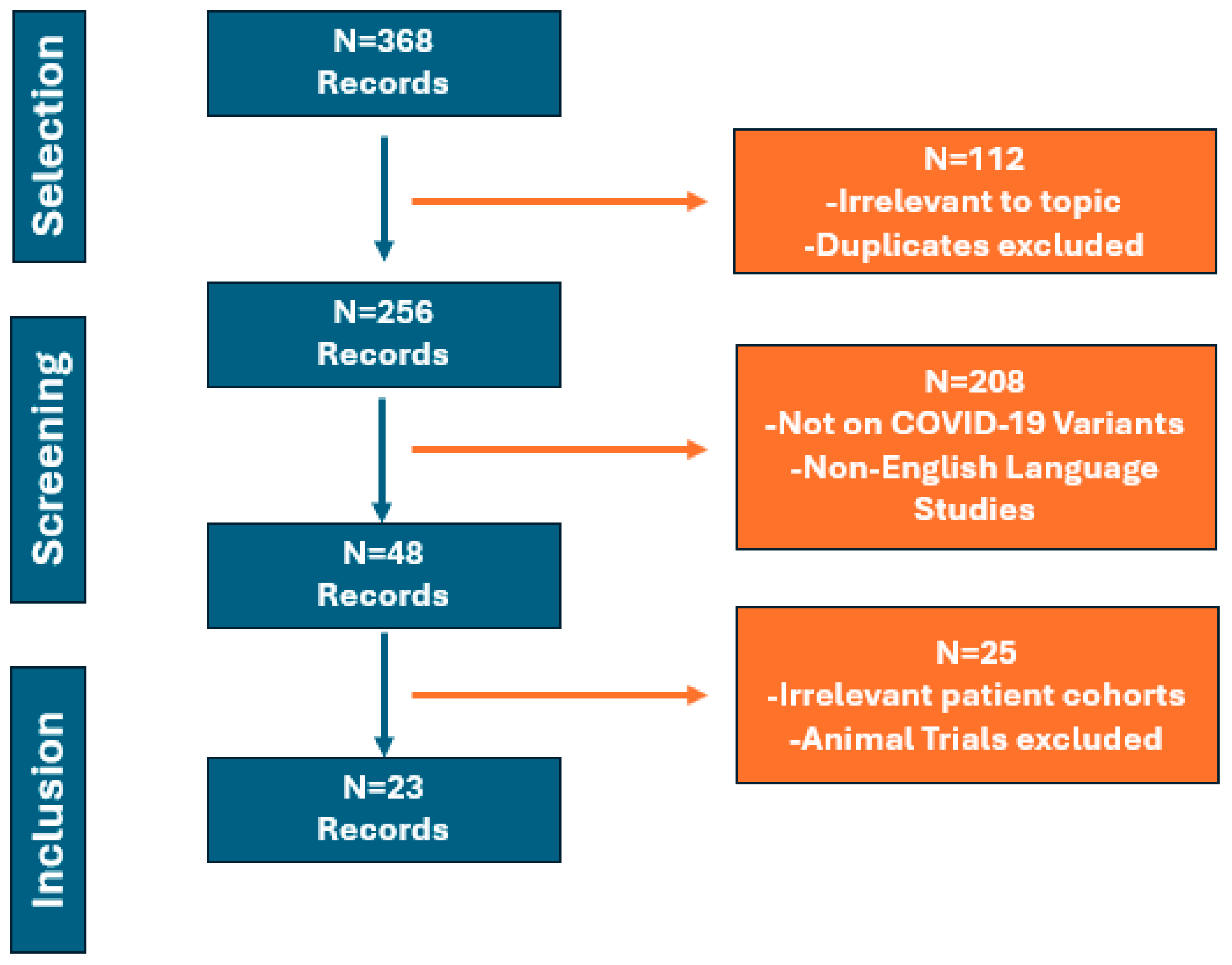
2. Materials and Methods
3. Discussion
3.1. Immunocompromised Patients
3.2. PLWHIV
3.3. CLD
3.4. Pediatric Patients
3.5. Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dai, B.; Ji, W.; Zhu, P.; Han, S.; Chen, Y.; Jin, Y. Update on Omicron variant and its threat to vulnerable populations. Public Health Pract. 2024, 7, 100494. [Google Scholar] [CrossRef] [PubMed]
- Pitsillou, E.; Yu, Y.; Beh, R.C.; Liang, J.J.; Hung, A.; Karagiannis, T.C. Chronicling the 3-year evolution of the COVID-19 pandemic: Analysis of disease management, characteristics of major variants, and impacts on pathogenicity. Clin. Exp. Med. 2023, 23, 3277–3298. [Google Scholar] [CrossRef] [PubMed]
- Myers, L.C.; Liu, V.X. The COVID-19 Pandemic Strikes Again and Again and Again. JAMA Netw. Open 2022, 5, 221760. [Google Scholar] [CrossRef] [PubMed]
- Bálint, G.; Vörös-Horváth, B.; Széchenyi, A. Omicron: Increased transmissibility and decreased pathogenicity. Signal Transduct. Target. Ther. 2022, 7, 151. [Google Scholar] [CrossRef] [PubMed]
- Kartsonaki, C.; Baillie, J.K.; Barrio, N.G.; Baruch, J.; Beane, A.; Blumberg, L.; Bozza, F.; Broadley, T.; Burrell, A.; Carson, G.; et al. Characteristics and outcomes of an international cohort of 600,000 hospitalized patients with COVID-19. Int. J. Epidemiol. 2023, 52, 355–376. [Google Scholar] [CrossRef] [PubMed]
- Veneti, L.; Seppälä, E.; Larsdatter Storm, M.; Valcarcel Salamanca, B.; Alnes Buanes, E.; Aasand, N.; Naseer, U.; Bragstad, K.; Hungnes, O.; Bøås, H.; et al. Increased risk of hospitalisation and intensive care admission associated with reported cases of SARS-CoV-2 variants B.1.1.7 and B.1.351 in Norway, December 2020–May 2021. PLoS ONE 2021, 16, 0258513. [Google Scholar] [CrossRef]
- Twohig, K.A.; Nyberg, T.; Zaidi, A.; Thelwall, S.; Sinnathamby, M.A.; Aliabadi, S.; Seaman, S.R.; Harris, R.J.; Hope, R.; Lopez-Bernal, J.; et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: A cohort study. Lancet Infect. Dis. 2022, 22, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.W.X.; Chiew, C.J.; Ang, L.W.; Mak, T.M.; Cui, L.; Toh, M.; Lim, Y.D.; Lee, P.H.; Lee, T.H.; Chia, P.Y.; et al. Clinical and Virological Features of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Variants of Concern: A Retrospective Cohort Study Comparing B.1.1.7 (Alpha), B.1.351 (Beta), and B.1.617.2 (Delta). Clin. Infect. Dis. 2022, 75, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; McMenamin, J.; Taylor, B.; Robertson, C. SARS-CoV-2 Delta VOC in Scotland: Demographics, risk of hospital admission, and vaccine effectiveness. Lancet 2021, 397, 2461–2462. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Yamasoba, D.; Kimura, I.; Wang, L.; Kishimoto, M.; Ito, J.; Morioka, Y.; Nao, N.; Nasser, H.; Uriu, K.; et al. Attenuated fusogenicity and pathogenicity of SARS-CoV-2 Omicron variant. Nature 2022, 603, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Shuai, H.; Chan, J.F.; Hu, B.; Chai, Y.; Yuen, T.T.; Yin, F.; Huang, X.; Yoon, C.; Hu, J.C.; Liu, H.; et al. Attenuated replication and pathogenicity of SARS-CoV-2 B.1.1.529 Omicron. Nature 2022, 603, 693–699. [Google Scholar] [CrossRef]
- Hyams, C.; Challen, R.; Marlow, R.; Nguyen, J.; Begier, E.; Southern, J.; King, J.; Morley, A.; Kinney, J.; Clout, M.; et al. Severity of Omicron (B.1.1.529) and Delta (B.1.617.2) SARS-CoV-2 infection among hospitalised adults: A prospective cohort study in Bristol, United Kingdom. Lancet Reg. Health Eur. 2023, 25, 100556. [Google Scholar] [CrossRef] [PubMed]
- Mendiola-Pastrana, I.R.; López-Ortiz, E.; Río de la Loza-Zamora, J.G.; González, J.; Gómez-García, A.; López-Ortiz, G. SARS-CoV-2 Variants and Clinical Outcomes: A Systematic Review. Life 2022, 12, 170. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Sears, K.; Sfetcu, R.; Currie, M.; Lisy, K.; Tufanaru, C.; Qureshi, R.; Mattis, P.; Mu, P. Conducting systematic reviews of association (etiology): The Joanna Briggs Institute’s approach. Int. J. Evid. Based Healthc. 2015, 13, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Baharom, M.; Ahmad, N.; Hod, R.; Arsad, F.S.; Tangang, F. The Impact of Meteorological Factors on Communicable Disease Incidence and Its Projection: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 1117. [Google Scholar] [CrossRef] [PubMed]
- Hoang, V.T.; Colson, P.; Levasseur, A.; Delerce, J.; Lagier, J.C.; Parola, P.; Million, M.; Fournier, P.E.; Raoult, D.; Gautret, P. Clinical outcomes in patients infected with different SARS-CoV-2 variants at one hospital during three phases of the COVID-19 epidemic in Marseille, France. Infect. Genet. Evol. 2021, 95, 105092. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.P.; Luo, C.H.; Amadi, A.; Schwartz, M.; Gallagher, N.; Ray, S.C.; Pekosz, A.; Mostafa, H.H. An Update on Severe Acute Respiratory Syndrome Coronavirus 2 Diversity in the US National Capital Region: Evolution of Novel and Variants of Concern. Clin. Infect. Dis. 2022, 74, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Patone, M.; Thomas, K.; Hatch, R.; Tan, P.S.; Coupland, C.; Liao, W.; Mouncey, P.; Harrison, D.; Rowan, K.; Horby, P.; et al. Mortality and critical care unit admission associated with the SARS-CoV-2 lineage B.1.1.7 in England: An observational cohort study. Lancet Infect. Dis. 2021, 21, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A.; Patel, K.; Pham, H.; Whitaker, M.; Anglin, O.; Kambhampati, A.K.; Milucky, J.; Chai, S.J.; Kirley, P.D.; Alden, N.B.; et al. Severity of Disease Among Adults Hospitalized with Laboratory-Confirmed COVID-19 Before and During the Period of SARS-CoV-2 B.1.617.2 (Delta) Predominance-COVID-NET, 14 States, January-August 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Corsi Decenti, E.; Salvatore, M.A.; Mandolini, D.; Donati, S. Vaccination against SARS-CoV-2 in pregnancy during the Omicron wave: The prospective cohort study of the Italian obstetric surveillance system. Clin. Microbiol. Infect. 2023, 29, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J.; Moffatt, C.R.; Aaron, L.T.; Beaverson, G.; Chaw, K.; Curtis, C.; Freeman-Lamb, R.; Judd, D.; Khatry, K.; Li, Y.S.; et al. Factors associated with hospitalisations and deaths of residential aged care residents with COVID-19 during the Omicron (BA.1) wave in Queensland. Med. J. Aust. 2023, 218, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Zhang, Y.; Zhang, H.; Ai, J.; He, L.; Yuan, X.; Bao, S.; Chen, X.; Wang, H.; Cai, J.; et al. Geriatric risk and protective factors for serious COVID-19 outcomes among older adults in Shanghai Omicron wave. Emerg. Microbes Infect. 2022, 11, 2045–2054. [Google Scholar] [CrossRef] [PubMed]
- Yusof, M.Y.; Arnold, J.; Saleem, B.; Vandevelde, C.; Dass, S.; Savic, S.; Vital, E.M.; Emery, P. Breakthrough SARS-CoV-2 infections and prediction of moderate-to-severe outcomes during rituximab therapy in patients with rheumatic and musculoskeletal diseases in the UK: A single-centre cohort study. Lancet Rheumatol. 2023, 5, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Mupanomunda, M.; Fakih, M.G.; Miller, C.; Ottenbacher, A.; Winegar, A.L.; Roberts, P.; Kimathi, M.; Gianopoulos, J.G.; Cahill, A.G.; Cacchione, J.G.; et al. Comparison of Severe Maternal Morbidities Associated With Delivery During Periods of Circulation of Specific SARS-CoV-2 Variants. JAMA Netw. Open 2022, 5, 2226436. [Google Scholar] [CrossRef] [PubMed]
- Bhalala, U.S.; Gist, K.M.; Tripathi, S.; Boman, K.; Kumar, V.K.; Retford, L.; Chiotos, K.; Blatz, A.M.; Dapul, H.; Verma, S.; et al. Characterization and Outcomes of Hospitalized Children With Coronavirus Disease 2019: A Report From a Multicenter, Viral Infection and Respiratory Illness Universal Study (Coronavirus Disease 2019) Registry. Crit. Care Med. 2022, 50, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Brewster, R.C.; Parsons, C.; Laird-Gion, J.; Hilker, S.; Irwin, M.; Sommerschield, A.; Michaelis, K.A.; Lam, M.; Parsons, A.; Mansbach, J.M. COVID-19-Associated Croup in Children. Pediatrics 2022, 149, 056492. [Google Scholar] [CrossRef] [PubMed]
- Antinori, A.; Bausch-Jurken, M. The Burden of COVID-19 in the Immunocompromised Patient: Implications for Vaccination and Needs for the Future. J. Infect. Dis. 2023, 228, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Meyts, I.; Bousfiha, A.; Duff, C.; Singh, S.; Lau, Y.L.; Condino-Neto, A.; Bezrodnik, L.; Ali, A.; Adeli, M.; Drabwell, J. Primary Immunodeficiencies: A Decade of Progress and a Promising Future. Front. Immunol. 2020, 11, 625753. [Google Scholar] [CrossRef] [PubMed]
- Tangye, S.G.; Al-Herz, W.; Bousfiha, A.; Chatila, T.; Cunningham-Rundles, C.; Etzioni, A.; Franco, J.L.; Holland, S.M.; Klein, C.; Morio, T.; et al. Human Inborn Errors of Immunity: 2019 Update on the Classification from the International Union of Immunological Societies Expert Committee. J. Clin. Immunol. 2020, 40, 24–64. [Google Scholar] [CrossRef] [PubMed]
- Tuano, K.S.; Seth, N.; Chinen, J. Secondary immunodeficiencies: An overview. Ann. Allergy Asthma Immunol. 2021, 127, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.P.K.; Desai, S.; Marti, M.; Nohynek, H.; Kaslow, D.C.; Kochhar, S.; O’Brien, K.L.; Hombach, J.; Wilder-Smith, A. Response to additional COVID-19 vaccine doses in people who are immunocompromised: A rapid review. Lancet Glob. Health 2022, 10, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.M.; Farraye, F.A.; Winthrop, K.L.; Willer, D.O.; Vink, P.; Tavares-Da-Silva, F. Safety and efficacy of recombinant and live herpes zoster vaccines for prevention in at-risk adults with chronic diseases and immunocompromising conditions. Vaccine 2023, 41, 36–48. [Google Scholar] [CrossRef] [PubMed]
- DeWolf, S.; Laracy, J.C.; Perales, M.A.; Kamboj, M.; van den Brink, M.R.M.; Vardhana, S. SARS-CoV-2 in immunocompromised individuals. Immunity 2022, 55, 1779–1798. [Google Scholar] [CrossRef] [PubMed]
- Singson, J.R.C.; Kirley, P.D.; Pham, H.; Rothrock, G.; Armistead, I.; Meek, J.; Anderson, E.J.; Reeg, L.; Lynfield, R.; Ropp, S.; et al. Factors Associated with Severe Outcomes Among Immunocompromised Adults Hospitalized for COVID-19-COVID-NET, 10 States, March 2020-February 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Garg, S.; O’Halloran, A.; Whitaker, M.; Pham, H.; Anderson, E.J.; Armistead, I.; Bennett, N.M.; Billing, L.; Como-Sabetti, K.; et al. Risk Factors for Intensive Care Unit Admission and In-hospital Mortality Among Hospitalized Adults Identified through the US Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). Clin. Infect. Dis. 2021, 72, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, J.P.; Tideman, S.; French, T.; Wright, B.; Parsons, G.; Diaz, G.; Robicsek, A. Odds of Hospitalization for COVID-19 After 3 vs 2 Doses of mRNA COVID-19 Vaccine by Time Since Booster Dose. JAMA 2022, 328, 1559–1561. [Google Scholar] [CrossRef] [PubMed]
- Yadaw, A.S.; Sahner, D.K.; Sidky, H.; Afzali, B.; Hotaling, N.; Pfaff, E.R.; Mathé, E.A. Preexisting Autoimmunity Is Associated With Increased Severity of Coronavirus Disease 2019: A Retrospective Cohort Study Using Data From the National COVID Cohort Collaborative (N3C). Clin. Infect. Dis. 2023, 77, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Inciarte, A.; Gonzalez-Cordon, A.; Rojas, J.; Torres, B.; de Lazzari, E.; de la Mora, L.; Martinez-Rebollar, M.; Laguno, M.; Callau, P.; Gonzalez-Navarro, A.; et al. Clinical characteristics, risk factors, and incidence of symptomatic coronavirus disease 2019 in a large cohort of adults living with HIV: A single-center, prospective observational study. AIDS 2020, 34, 1775–1780. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, J.; Patel, R.C.; Zhang, J.; Guo, S.; Zheng, Q.; Olex, A.L.; Olatosi, B.; Weissman, S.B.; Islam, J.Y.; et al. Associations between HIV infection and clinical spectrum of COVID-19: A population level analysis based on US National COVID Cohort Collaborative (N3C) data. Lancet HIV 2021, 8, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Dandachi, D.; Geiger, G.; Montgomery, M.W.; Karmen-Tuohy, S.; Golzy, M.; Antar, A.A.R.; Llibre, J.M.; Camazine, M.; Díaz-De Santiago, A.; Carlucci, P.M.; et al. Characteristics, Comorbidities, and Outcomes in a Multicenter Registry of Patients With Human Immunodeficiency Virus and Coronavirus Disease 2019. Clin. Infect. Dis. 2021, 73, 1964–1972. [Google Scholar] [CrossRef] [PubMed]
- Geretti, A.M.; Stockdale, A.J.; Kelly, S.H.; Cevik, M.; Collins, S.; Waters, L.; Villa, G.; Docherty, A.; Harrison, E.M.; Turtle, L.; et al. Outcomes of Coronavirus Disease 2019 (COVID-19) Related Hospitalization Among People With Human Immunodeficiency Virus (HIV) in the ISARIC World Health Organization (WHO) Clinical Characterization Protocol (UK): A Prospective Observational Study. Clin. Infect. Dis. 2021, 73, 2095–2106. [Google Scholar] [CrossRef] [PubMed]
- Bertagnolio, S.; Thwin, S.S.; Silva, R.; Nagarajan, S.; Jassat, W.; Fowler, R.; Haniffa, R.; Reveiz, L.; Ford, N.; Doherty, M.; et al. Clinical features of, and risk factors for, severe or fatal COVID-19 among people living with HIV admitted to hospital: Analysis of data from the WHO Global Clinical Platform of COVID-19. Lancet HIV 2022, 9, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Esper, F.P.; Adhikari, T.M.; Tu, Z.J.; Cheng, Y.W.; El-Haddad, K.; Farkas, D.H.; Bosler, D.; Rhoads, D.; Procop, G.W.; Ko, J.S.; et al. Alpha to Omicron: Disease Severity and Clinical Outcomes of Major SARS-CoV-2 Variants. J. Infect. Dis. 2023, 227, 344–352. [Google Scholar] [CrossRef] [PubMed]
- de Prost, N.; Audureau, E.; Heming, N.; Gault, E.; Pham, T.; Chaghouri, A.; de Montmollin, N.; Voiriot, G.; Morand-Joubert, L.; Joseph, A.; et al. Clinical phenotypes and outcomes associated with SARS-CoV-2 variant Omicron in critically ill French patients with COVID-19. Nat. Commun. 2022, 13, 6025. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.A.; Dube, S.; Lu, Y.; Yates, M.; Arnetorp, S.; Barnes, E.; Bell, S.; Carty, L.; Evans, K.; Graham, S.; et al. Impact of COVID-19 on immunocompromised populations during the Omicron era: Insights from the observational population-based INFORM study. Lancet Reg. Health Eur. 2023, 35, 100747. [Google Scholar] [CrossRef] [PubMed]
- Ketkar, A.; Willey, V.; Pollack, M.; Glasser, L.; Dobie, C.; Wenziger, C.; Teng, C.C.; Dube, C.; Cunningham, D.; Verduzco-Gutierrez, M. Assessing the risk and costs of COVID-19 in immunocompromised populations in a large United States commercial insurance health plan: The EPOCH-US Study. Curr. Med. Res. Opin. 2023, 39, 1103–1118. [Google Scholar] [CrossRef] [PubMed]
- Malahe, S.R.K.; Hoek, R.A.S.; Dalm, V.; Broers, A.E.C.; den Hoed, C.M.; Manintveld, O.C.; Baan, C.C.; van Deuzen, C.M.; Papageorgiou, G.; Bax, H.I.; et al. Clinical Characteristics and Outcomes of Immunocompromised Patients With Coronavirus Disease 2019 Caused by the Omicron Variant: A Prospective, Observational Study. Clin. Infect. Dis. 2023, 76, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Huygens, S.; GeurtsvanKessel, C.; Gharbharan, A.; Bogers, S.; Worp, N.; Boter, M.; Bax, H.I.; Kampschreur, L.M.; Hassing, R.J.; Fiets, R.B.; et al. Clinical and Virological Outcome of Monoclonal Antibody Therapies Across Severe Acute Respiratory Syndrome Coronavirus 2 Variants in 245 Immunocompromised Patients: A Multicenter Prospective Cohort Study. Clin. Infect. Dis. 2024, 78, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Turtle, L.; Thorpe, M.; Drake, T.M.; Swets, M.; Palmieri, C.; Russell, C.D.; Ho, A.; Aston, S.; Wootton, D.G.; Richter, A.; et al. Outcome of COVID-19 in hospitalised immunocompromised patients: An analysis of the WHO ISARIC CCP-UK prospective cohort study. PLoS Med. 2023, 20, 1004086. [Google Scholar] [CrossRef] [PubMed]
- Basoulis, D.; Mastrogianni, E.; Voutsinas, P.M.; Psichogiou, M. HIV and COVID-19 Co-Infection: Epidemiology, Clinical Characteristics, and Treatment. Viruses 2023, 15, 577. [Google Scholar] [CrossRef] [PubMed]
- Vizcarra, P.; Pérez-Elías, M.J.; Quereda, C.; Moreno, A.; Vivancos, M.J.; Dronda, F.; Casado, J.L. Description of COVID-19 in HIV-infected individuals: A single-centre, prospective cohort. Lancet HIV 2020, 7, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Karmen-Tuohy, S.; Carlucci, P.M.; Zervou, F.N.; Zacharioudakis, I.M.; Rebick, G.; Klein, E.; Reich, J.; Jones, S.; Rahimian, J. Outcomes Among HIV-Positive Patients Hospitalized With COVID-19. J. Acquir. Immune Defic. Syndr. 2020, 85, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Gervasoni, C.; Meraviglia, P.; Riva, A.; Giacomelli, A.; Oreni, L.; Minisci, D.; Atzori, C.; Ridolfo, A.; Cattaneo, D. Clinical Features and Outcomes of Patients With Human Immunodeficiency Virus With COVID-19. Clin. Infect. Dis. 2020, 71, 2276–2278. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, K.; Rentsch, C.T.; MacKenna, B.; Schultze, A.; Mehrkar, A.; Bates, C.J.; Eggo, R.M.; Morton, C.E.; Bacon, S.C.J.; Inglesby, P.; et al. HIV infection and COVID-19 death: A population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV 2021, 8, 24–32. [Google Scholar] [CrossRef]
- Tesoriero, J.M.; Swain, C.E.; Pierce, J.L.; Zamboni, L.; Wu, M.; Holtgrave, D.R.; Gonzalez, C.J.; Udo, T.; Morne, J.E.; Hart-Malloy, R.; et al. COVID-19 Outcomes Among Persons Living With or Without Diagnosed HIV Infection in New York State. JAMA Netw. Open 2021, 4, e2037069. [Google Scholar] [CrossRef] [PubMed]
- Mellor, M.M.; Bast, A.C.; Jones, N.R.; Roberts, N.W.; Ordóñez-Mena, J.M.; Reith, A.J.M.; Butler, C.C.; Matthews, P.C.; Dorward, J. Risk of adverse coronavirus disease 2019 outcomes for people living with HIV. AIDS 2021, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hariyanto, T.I.; Rosalind, J.; Christian, K.; Kurniawan, A. Human immunodeficiency virus and mortality from coronavirus disease 2019: A systematic review and meta-analysis. S. Afr. J. HIV. Med. 2021, 22, 1220. [Google Scholar] [CrossRef] [PubMed]
- Ssentongo, P.; Heilbrunn, E.S.; Ssentongo, A.E.; Advani, S.; Chinchilli, V.M.; Nunez, J.J.; Du, P. Epidemiology and outcomes of COVID-19 in HIV-infected individuals: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 6283. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, Y.; Hu, S.; Ai, W.; Tao, Y.; Tang, H.; Jing, F.; Tang, W. Systematic Review and Meta-Analyses of The Interaction Between HIV Infection And COVID-19: Two Years’ Evidence Summary. Front. Immunol. 2022, 13, 864838. [Google Scholar] [CrossRef] [PubMed]
- Dzinamarira, T.; Murewanhema, G.; Chitungo, I.; Ngara, B.; Nkambule, S.J.; Madziva, R.; Herrera, H.; Mukwenha, S.; Cuadros, D.F.; Iradukunda, P.G.; et al. Risk of mortality in HIV-infected COVID-19 patients: A systematic review and meta-analysis. J. Infect. Public Health 2022, 15, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Cabello, A.; Zamarro, B.; Nistal, S.; Victor, V.; Hernández, J.; Prieto-Pérez, L.; Carrillo, I.; Álvarez, B.; Fernández-Roblas, R.; Hernández-Segurado, M.; et al. COVID-19 in people living with HIV: A multicenter case-series study. Int. J. Infect. Dis. 2021, 102, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, J.; Polo, R.; Moreno, S.; Díaz, A.; Martínez, E.; Arribas, J.R.; Jarrín, I.; Hernán, M.A. Incidence and Severity of COVID-19 in HIV-Positive Persons Receiving Antiretroviral Therapy: A Cohort Study. Ann. Intern. Med. 2020, 173, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Puyat, J.H.; Fowokan, A.; Wilton, J.; Janjua, N.Z.; Wong, J.; Grennan, T.; Chambers, C.; Kroch, A.; Costiniuk, C.T.; Cooper, C.L.; et al. Risk of COVID-19 hospitalization in people living with HIV and HIV-negative individuals and the role of COVID-19 vaccination: A retrospective cohort study. Int. J. Infect. Dis. 2023, 135, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Danwang, C.; Noubiap, J.J.; Robert, A.; Yombi, J.C. Outcomes of patients with HIV and COVID-19 co-infection: A systematic review and meta-analysis. AIDS Res. Ther. 2022, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Barbera, L.K.; Kamis, K.F.; Rowan, S.E.; Davis, A.J.; Shehata, S.; Carlson, J.J.; Johnson, S.C.; Erlandson, K.M. HIV and COVID-19: Review of clinical course and outcomes. HIV Res. Clin. Pract. 2021, 22, 102–118. [Google Scholar] [CrossRef] [PubMed]
- Hadi, Y.B.; Naqvi, S.F.Z.; Kupec, J.T.; Sarwari, A.R. Characteristics and outcomes of COVID-19 in patients with HIV: A multicentre research network study. AIDS 2020, 34, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Marjot, T.; Moon, A.M.; Cook, J.A.; Abd-Elsalam, S.; Aloman, C.; Armstrong, M.J.; Pose, E.; Brenner, E.J.; Cargill, T.; Catana, M.A.; et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J. Hepatol. 2021, 74, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Pletcher, M.J.; Lai, J.C. Outcomes of SARS-CoV-2 Infection in Patients With Chronic Liver Disease and Cirrhosis: A National COVID Cohort Collaborative Study. Gastroenterology 2021, 161, 1487–1501. [Google Scholar] [CrossRef] [PubMed]
- Griggs, E.P.; Mitchell, P.K.; Lazariu, V.; Gaglani, M.; McEvoy, C.; Klein, N.P.; Valvi, N.R.; Irving, S.A.; Kojima, N.; Stenehjem, E.; et al. Clinical Epidemiology and Risk Factors for Critical Outcomes Among Vaccinated and Unvaccinated Adults Hospitalized With COVID-19-VISION Network, 10 States, June 2021–March 2023. Clin. Infect. Dis. 2024, 78, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Velásquez García, H.A.; Adu, P.A.; Okonkwo-Dappa, A.; Makuza, J.D.; Cua, G.; Binka, M.; Wilton, J.; Sbihi, H.; Janjua, N.Z. Risk of Severe COVID-19-Related Outcomes among Patients with Cirrhosis: A Population-Based Cohort Study in Canada. Viruses 2024, 16, 351. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Cabrera, A.; Colin-Vilchis, J.A.; Haque, U.; Velazquez, C.; Alvarez Villaseñor, A.S.; Magdaleno-Márquez, L.E.; Calleros-Muñoz, C.I.; Figueroa-Enríquez, K.F.; Angulo-Molina, A.; Gallego-Hernández, A.L. SARS-CoV-2 Variants of Concern and Clinical Severity in the Mexican Pediatric Population. Infect. Dis. Rep. 2023, 15, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Marks, K.J.; Whitaker, M.; Anglin, O.; Milucky, J.; Patel, K.; Pham, H.; Chai, S.J.; Kirley, P.D.; Armistead, I.; McLafferty, S.; et al. Hospitalizations of Children and Adolescents with Laboratory-Confirmed COVID-19-COVID-NET, 14 States, July 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Marks, K.J.; Whitaker, M.; Agathis, N.T.; Anglin, O.; Milucky, J.; Patel, K.; Pham, H.; Kirley, P.D.; Kawasaki, B.; Meek, J.; et al. Hospitalization of Infants and Children Aged 0-4 Years with Laboratory-Confirmed COVID-19-COVID-NET, 14 States, March 2020-February 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Ho, R.M.; Bowen, A.C.; Blyth, C.C.; Imrie, A.; Kollmann, T.R.; Stick, S.M.; Kicic, A. Defining the pediatric response to SARS-CoV-2 variants. Front. Immunol. 2023, 14, 1200456. [Google Scholar] [CrossRef] [PubMed]
- Delahoy, M.J.; Ujamaa, D.; Whitaker, M.; O’Halloran, A.; Anglin, O.; Burns, E.; Cummings, C.; Holstein, R.; Kambhampati, A.K.; Milucky, J.; et al. Hospitalizations Associated with COVID-19 Among Children and Adolescents-COVID-NET, 14 States, March 1, 2020-August 14, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.A.; Reses, H.E.; Cool, A.J.; Shapiro, C.N.; Hsu, J.; Boehmer, T.K.; Cornwell, C.R.; Gray, E.B.; Henley, S.J.; Lochner, K.; et al. Trends in COVID-19 Cases, Emergency Department Visits, and Hospital Admissions Among Children and Adolescents Aged 0–17 Years-United States, August 2020-August 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Molteni, E.; Sudre, C.H.; Canas, L.D.S.; Bhopal, S.S.; Hughes, R.C.; Chen, L.; Deng, J.; Murray, B.; Kerfoot, E.; Antonelli, M.; et al. Illness Characteristics of COVID-19 in Children Infected with the SARS-CoV-2 Delta Variant. Children 2022, 9, 652. [Google Scholar] [CrossRef] [PubMed]
- Edward, P.R.; Lorenzo-Redondo, R.; Reyna, M.E.; Simons, L.M.; Hultquist, J.F.; Patel, A.B.; Ozer, E.A.; Muller, W.J.; Heald-Sargent, T.; McHugh, M.; et al. Severity of Illness Caused by Severe Acute Respiratory Syndrome Coronavirus 2 Variants of Concern in Children: A Single-Center Retrospective Cohort Study. J. Pediatric. Infect. Dis. Soc. 2022, 11, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Dowell, A.C.; Butler, M.S.; Jinks, E.; Tut, G.; Lancaster, T.; Sylla, P.; Begum, J.; Bruton, R.; Pearce, H.; Verma, K.; et al. Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 40–49. [Google Scholar] [CrossRef]
- Khemiri, H.; Ayouni, K.; Triki, H.; Haddad-Boubaker, S. SARS-CoV-2 infection in pediatric population before and during the Delta (B.1.617.2) and Omicron (B.1.1.529) variants era. Virol. J. 2022, 19, 144. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Guo, W.; Guo, T.; Li, J.; He, W.; Ni, S.; Ouyang, X.; Liu, J.; Xie, Y.; Tan, X.; et al. Novel coronavirus infection in children outside of Wuhan, China. Pediatr. Pulmonol. 2020, 55, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, Q.; Chen, J.; Xiang, R.; Song, H.; Shu, S.; Chen, L.; Liang, L.; Zhou, J.; You, L.; et al. Detection of Covid-19 in Children in Early January 2020 in Wuhan, China. N. Engl. J. Med. 2020, 382, 1370–1371. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, L.; Du, H.; Zhang, J.; Li, Y.Y.; Qu, J.; Zhang, W.; Wang, Y.; Bao, S.; Li, Y.; et al. SARS-CoV-2 Infection in Children. N. Engl. J. Med. 2020, 382, 1663–1665. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Wu, J.; Hong, L.; Luo, Y.; Song, Q.; Chen, D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: An observational cohort study. Lancet Infect. Dis. 2020, 20, 689–696. [Google Scholar] [CrossRef]
- Christensen, P.A.; Olsen, R.J.; Long, S.W.; Subedi, S.; Davis, J.J.; Hodjat, P.; Walley, D.R.; Kinskey, J.C.; Ojeda Saavedra, M.; Pruitt, L.; et al. Delta Variants of SARS-CoV-2 Cause Significantly Increased Vaccine Breakthrough COVID-19 Cases in Houston, Texas. Am. J. Pathol. 2022, 192, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Livieratos, A.; Gogos, C.; Akinosoglou, K. Impact of Prior COVID-19 Immunization and/or Prior Infection on Immune Responses and Clinical Outcomes. Viruses 2024, 16, 685. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; DeWitt, P.E.; Russell, S.; Anand, A.; Bradwell, K.R.; Bremer, C.; Gabriel, D.; Girvin, A.T.; Hajagos, J.G.; McMurry, J.A.; et al. Characteristics, Outcomes, and Severity Risk Factors Associated With SARS-CoV-2 Infection Among Children in the US National COVID Cohort Collaborative. JAMA Netw. Open 2022, 5, 2143151. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.A.; Dargham, S.R.; Loka, S.; Shaik, R.M.; Chemaitelly, H.; Tang, P.; Hasan, M.R.; Coyle, P.V.; Yassine, H.M.; Al-Khatib, H.A.; et al. Coronavirus Disease 2019 Disease Severity in Children Infected With the Omicron Variant. Clin. Infect. Dis. 2022, 75, 361–367. [Google Scholar] [CrossRef] [PubMed]
| Included Studies | Type of Study | Population Size (N) | Type of Variant | Clinical Outcomes (% of Patients) * |
|---|---|---|---|---|
| Singson J.R.C. et al., 2022 (USA) [35] | Observational Study | N = 22,345 | 54.2% pre-Delta, 20.0% Delta, 25.8% Omicron for immunocompromised | ICU admission (OR): 1.26; 95% CIs: [1.08–1.49] Mortality (OR): 1.34; 95% CIs: [1.05–1.70] |
| Yadaw A.S. et al., 2023 (USA) [38] | Retrospective Cohort Study | N = 2,453,799 | Ancestral, Alpha, Beta, Gamma, Delta, and Omicron | Hospitalization: 16.1% (AID), 16.5% (IS) Life-threatening: 4.5%(AID), 4.9% (IS) |
| Esper F.P. et al., 2023 (USA) [44] | Retrospective Cohort Study | N = 2779 | 48.8% Alpha, 5.2% Gamma, 34.2% Delta, 25% Omicron Any immune co-morbidity: 15.7% Alpha, 13.9% Gamma, 19.4% Delta, 14.3% Omicron | ICU admission: 2.7% (Alpha), 3.3% (Gamma), 3.6% (Delta), 1.0% (Omicron) Mortality: 1.3% (Alpha), 1.6% (Gamma), 1.0% (Delta), 0,4% (Omicron) |
| de Prost N. et al., 2022 (France) [45] | Observational Study | N = 259 | N = 111 Delta (86.4% immunosuppressed), N = 148 Omicron (56.8% immunosuppressed) | Invasive MV: 56.8% (Delta), 46.6% (Omicron), 51.6% (Omicron, only immunosuppressed) No differences among Omicron sub-lineages on 28-day mortality |
| Evans R.A. et al., 2023 (UK) [46] | Observational Study | N = 11,990,730 | Omicron | ICU Admission: 16.5% Mortality: 7.4% |
| Ketkar A. et al., 2023 (USA) [47] | Retrospective Cohort Study | N = 16,873,161 | 2.7% immunocompromised Alpha, Beta, Gamma, Delta, and Omicron | Hospitalization: 23.5% |
| Malahe S.R.K. et al., 2023 (Netherlands) [48] | Prospective Observational Study | N = 114 | Omicron | Hospitalization: 20% Mortality: 1 patient |
| Huygens S. et al., 2024 (Netherlands) [49] | Prospective Cohort Study | N = 245 | 50% Omicron, 50% Delta | MV or Mortality: 27% |
| Turtle L. et al., 2023 (UK) [50] | Prospective Cohort Study | N = 156,552 | 14% immunocompromised Ancestral, Alpha, Delta, and Omicron | Ancestral Strain Mortality (OR): 1.28; 95% CIs: [1.20–1.36] Alpha Strain Mortality (OR): 1.19; 95% CIs: [1.12–1.26] Delta Strain Mortality (OR): 0.83; 95% CIs: [0.76–0.91] Omicron Strain Mortality (OR): 0.66; 95% CIs: [0.54–0.80], |
| Included Studies | Type of Study | Population Size (N) | Type of Variant | Clinical Outcomes (% of Patients) * |
|---|---|---|---|---|
| Yang X. et al., 2021 (USA) [40] | Retrospective Cohort Study | N = 1,436,622 | Ancestral, Alpha, Gamma | Hospitalization (OR): 1.20; 95% CIs: [1.15–1.26] Mortality (OR): 1.29; 95% CIs: [1.16–1.44] |
| Bertagnolio S. et al., 2022 (Switzerland) [43] | Prospective Cohort Study | N = 197,479 | Ancestral, Alpha, Gamma | Hospitalization: 38.4% Mortality: 24.3% |
| Bhaskaran K. et al., 2021 (UK) [55] | Prospective Cohort Study | N = 17,282,905 | Ancestral | Mortality (HR): 2.90; 95% CIs: [1.96–4.30] |
| Tesoriero J.M. et al., 2021 (USA) [56] | Retrospective Cohort Study | N = 2988 | Ancestral | Hospitalization (sRR): 1.38; 95% Cls: [1.29–1.47] Mortality (sRR): 1.23; 95% Cls: [1.07–1.40] |
| Del Amo J. et al., 2020 (Spain) [63] | Retrospective Cohort Study | N = 77,590 | Ancestral | Hospitalization (SR): 17.8; 95% CIs: [17.7–18.0] Mortality (SR): 3.7; 95% CIs: [3.6–3.8] |
| Puyat J.H. et al., 2023 (Canada) [64] | Retrospective Cohort Study | N = 253,129 | Alpha, Gamma, Delta, Omicron | Hospitalization: 17.5% Mortality: 1.2% |
| Included Studies | Type of Study | Population Size (N) | Type of Variant | Clinical Outcomes (% of Patients) |
|---|---|---|---|---|
| Marjot T. et al., 2020 (UK) [68] | Retrospective Cohort Study | N = 745 | Ancestral | Mortality: 8% without cirrhosis Mortality: 32% with cirrhosis Mortality: 19% with Child-Pugh class A Mortality: 35% Child-Pugh class B Mortality: 51% Child-Pugh class C |
| Ge J. et al., 2021 (USA) [69] | Retrospective Cohort Study | N = 220,727 | Ancestral, Alpha, Gamma, Delta | Hospitalization by day 90: 22.9% (Non-cirrhosis) Hospitalization by day 90: 50.1% (Cirrhosis) Mortality by day 90: 2.3% (Non-cirrhosis) Mortality by day 90: 12.7% (Cirrhosis) |
| Griggs E.P. et al., 2024 (USA) [70] | Retrospective Cohort Study | N = 60,488 | Delta, Omicron | Hospitalization (Delta): 6.6% Hospitalization (BA.1): 8.1% Hospitalization (BA.2): 7.2% Hospitalization (BA.4/BA.5): 7.5% Hospitalization (Post-BA.4/BA.5): 6.8% |
| Velásquez Garcia H.A.V.et al., 2024 (Canada) [71] | Retrospective Cohort Study | N = 162,509 | 11.2% Alpha, 8.6% Gamma, 25.7% Delta, 4.3% Omicron | Hospitalization (No Cirrhosis): 3.6% Hospitalization (Cirrhosis): 17.2% ICU admission (No Cirrhosis): 1.5% ICU admission (Cirrhosis): 12.8% |
| Included Studies | Type of Study | Population Size (N) | Type of Variant | Clinical Outcomes (% of Patients) * |
|---|---|---|---|---|
| Maldonado-Cabrera A. et al., 2023 (Mexico) [72] | Retrospective Cohort Study | N = 372,989 | Alpha, Gamma, Delta, Omicron | Hospitalization (IRR): 1.69 (Alpha); 95% CIs: [1.54–1.86], 2.67 (Gamma); 95% CIs: [2.52–2.82], 0.97 (Delta); 95% CIs: [0.96–0.98], 0.95 (Omicron); 95%: [0.94–0.96] Mortality (IRR): 1.68 (Alpha); 95% CIs: [1.52–1.85], 2.7 (Gamma); 95% CIs: [2.54–2.85], 0.99 (Delta); 95% CIs: [0.98–1.00], 0.97 (Omicron); 95% CIs: [0.96–0.98] |
| Edward P.R. et al., 2022 (USA) [79] | Retrospective Cohort Study] | N = 714 | 13.4% Alpha, 5.3% Gamma, 16.7% Delta, 30.1% Omicron | Hospitalization (OR): 2.2 (Alpha); 95% CIs: [0.73–6.6], 6.7 (Gamma); 95% CIs: [2.0–22.1], 2.8 (Delta); 95% CIs: [0.95–8.0], 0.79 (Omicron); 95% CIs: [0.20–2.7] ICU Admission (OR): 2.5 (Alpha); 95% CIs: [0.53–13.6], 5.4 (Gamma); 95% CIs: [0.92–31.4], 2.7 (Delta); 95% CIs: [0.56–14.8] 4.5 (Omicron); 95% CIs: [0.94–24.1] |
| Martin B. et al., 2022 (USA) [88] | Prospective Cohort Study | N = 167,262 | Delta, Pre-Delta | IMV: 6% (Delta) IMV: 8% (Pre-Delta) |
| Butt A.A. et al., 2022 (Qatar) [89] | Retrospective Cohort Study | N = 1735 | Delta, Omicron | Moderate Disease: 15.7% (Delta) Moderate Disease: 2.2% (Omicron) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Livieratos, A.; Gogos, C.; Akinosoglou, K. SARS-CoV-2 Variants and Clinical Outcomes of Special Populations: A Scoping Review of the Literature. Viruses 2024, 16, 1222. https://doi.org/10.3390/v16081222
Livieratos A, Gogos C, Akinosoglou K. SARS-CoV-2 Variants and Clinical Outcomes of Special Populations: A Scoping Review of the Literature. Viruses. 2024; 16(8):1222. https://doi.org/10.3390/v16081222
Chicago/Turabian StyleLivieratos, Achilleas, Charalambos Gogos, and Karolina Akinosoglou. 2024. "SARS-CoV-2 Variants and Clinical Outcomes of Special Populations: A Scoping Review of the Literature" Viruses 16, no. 8: 1222. https://doi.org/10.3390/v16081222
APA StyleLivieratos, A., Gogos, C., & Akinosoglou, K. (2024). SARS-CoV-2 Variants and Clinical Outcomes of Special Populations: A Scoping Review of the Literature. Viruses, 16(8), 1222. https://doi.org/10.3390/v16081222







