Monitoring of Astroviruses, Brno-Hantaviruses, Coronaviruses, Influenza Viruses, Bornaviruses, Morbilliviruses, Lyssaviruses and Pestiviruses in Austrian Bats
Abstract
:1. Introduction
2. Materials and Methods
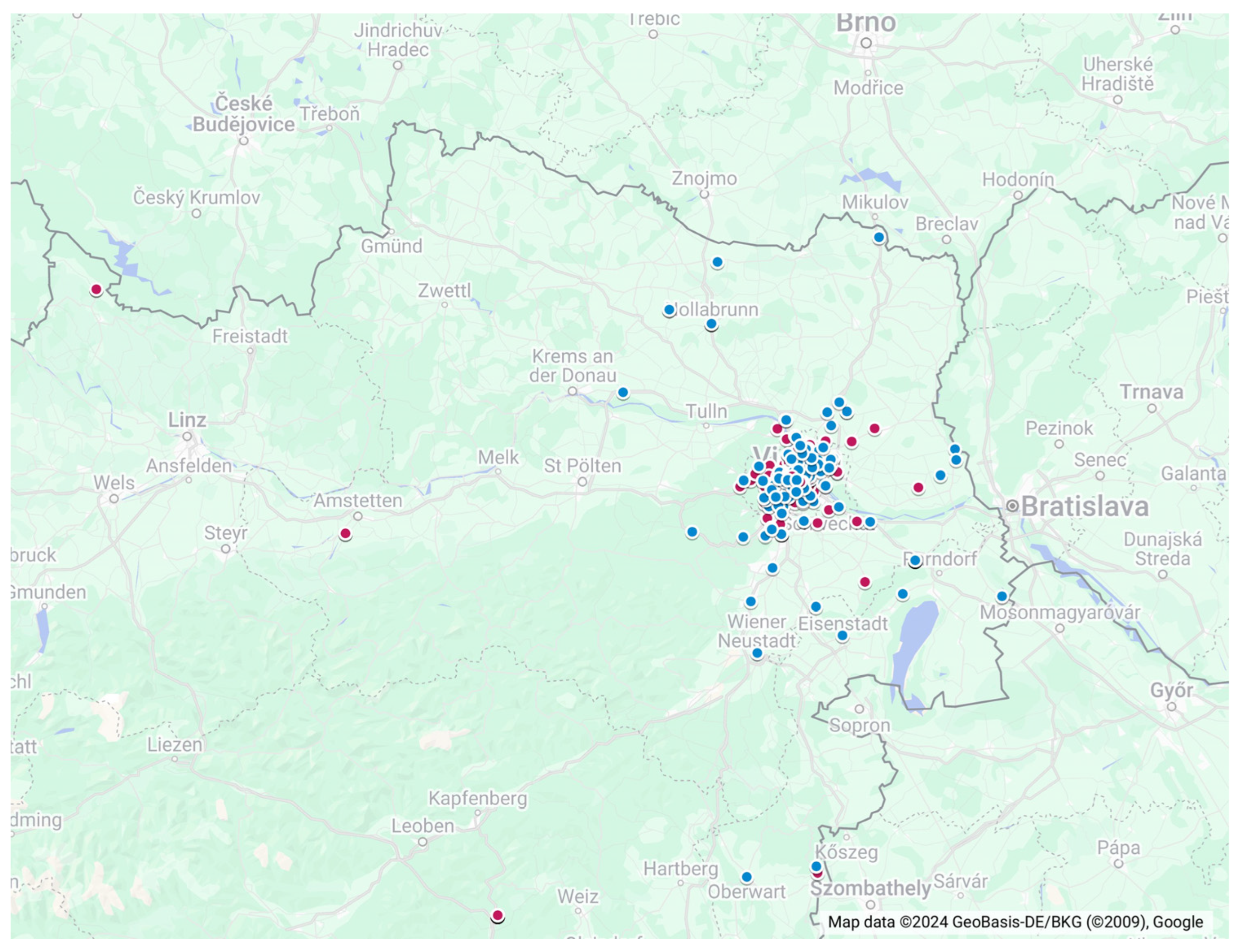
2.1. Study Plan
- (A)
- We collected 92 oropharyngeal and rectal swab samples from 46 bats. Bats were captured in the course of monitoring and inventory (population dynamic and biology–ecology) studies with mist nets. Nets were installed in front of caves during autumnal swarming, as well as in foraging areas (i.e., water bodies and woodland). Mist nets with mesh sizes of 20 × 20 mm (monofilament) and 14 × 14 mm (hair net) were used to capture the bats (Ecotone Goc, Sopon, Poland).
- (B)
- Also, 526 oropharyngeal and rectal swab samples were collected from 263 bats that were sent to a bat rehabilitation facility at the University of Veterinary Medicine, Vienna. The bats were captured after rescue activities and subsequently released after rehabilitation.
- (C)
- Lastly, 155 pooled tissue samples were collected between November 2015 and February 2018 during the necropsy of dead-found bats. The brain samples were removed and separately collected for passive surveillance and tested negative for rabies using the FAT method by AGES. The internal organ samples were stored as a pool per individual at −80 °C in plastic containers and were kindly provided for this study.
2.2. Sample Preparation and Nucleic Acid Extraction
2.3. Diagnostic Procedure
2.4. RT-qPCR
| Virus Genus/Species | Protocol Name | Oligo Name | Oligo Sequence (5′ → 3′) | Fragment Size (Gene) | AC | References |
|---|---|---|---|---|---|---|
| Influenza A virus | IAV-PB1 | IAV-PB1_120F | CAT TTG AAT GGA YGT CAA YCC GA | 152 bp (PB1) | A | [63,64] |
| IAV-PB1_271R | CTG TTD ACY GTG TCC ATD GTG TA | |||||
| IAV-PB1_247FAM | FAM-CCW GTY CCY GTY CCA TGG CTG TA-BHQ1 | |||||
| Influenza C/D virus | Pan-IVC/D-Mix1 | IVC/D PB1-713F | GCC AAA GAY GGR GAA AGA GG | 152 bp (PB1) | A | in-house |
| IVC/D PB1-864R | TTC TCA TTD CCV CCA ACA GG | |||||
| IVC/D PB1-779FAM | FAM-CCA TTY TCA AAA ATT GTD GAA ACT KTA GCA CA-BHQ1 | |||||
| Bornavirus | Pan-Borna-Mix7.2 | Borna-1319F | CGC GAC CMT CGA GYC TRG T | 211 bp (P) | A | [65] |
| Borna-1529R | GAC ARC TGY TCC CTT CCK GT | |||||
| Borna-1471.2FAM | FAM-AAG AAY CCH TCC ATG ATC TCM GAY CMA GA-BHQ1 | |||||
| Hantavirus | Hanta-Brno-Mix1 | Hanta-Brno-234F | GAT TAG TTA GAG GTA ATT GGT TAC A | 109 bp (POL) | B | [66] |
| Hanta-Brno-342R | GTT AGA GGT AAT TGG TTA CAA GGA | |||||
| Hanta-Brno-274FAM | FAM-TCM GAG AAT AAT TCA CTC CAA ACT-BHQ1 | |||||
| Morbillivirus | Pan-Morbilli-Mix21 | Morbilli-1072F | TCA RTT CAG AAC AAR TTY AGT GC | 103 bp (NP) | C | In-house |
| Morbilli-1174R | CAA GTT YAA ICC YCC CAT KGA | |||||
| Morbilli-1111FAM | FAM-CTM TGG AGY TAT GCB ATG GGW GT-BHQ1 | |||||
| Lyssavirus | Lyssa-N-MGB-FAM-Mix1v3+LBV | Lyssa_N_1-F-a | ACG CTT AAC AAC AAA ATC ATA AAA | 165 bp (NP) | D | In-house |
| Lyssa_N_1-F-b | ACG CTT AAC AAC AAA ACC AAA GAA | |||||
| Lyssa_N_1-F-c | ACG CTT AAC AAC AAG ATC AAA GAA | |||||
| Lyssa_N_1-F-d | ACG CTT AAC AAC AAA ATC ATA GAA | |||||
| Lyssa_N_1-F-e | ACG CTT AAC AAC AAA ATC AGA GAA | |||||
| Lyssa_N_1-F-f | ACG CTT AAC AAC CAG ATC AAA GAA | |||||
| Lyssa_N_1-F-g | ACG CTT AAC AAC AAA ATC AAA GAA | |||||
| Lyssa_N_1-F-h | ACG CTT AAC GAC AAA ATC AGA GAA | |||||
| Lyssa_N_1-F-i | ACG CTT AAC AAC AAA AAC AAA AAA | |||||
| Lyssa_N_1-F-j | ACG CTT AAC GAC AAA ACC AGA AAA | |||||
| Lyssa_N165-146R | GCA GGG TAY TTR TAC TCA TA | |||||
| Lyssa_N_143-R-a | GCA GGA TAT TTG TAC TCA TA | |||||
| Lyssa_N_143-R-b | GCA GGG TAC TTG TAC TCA TA | |||||
| Lyssa_N_143-R-c | GCA GGG TAC TTG TAC TCA TG | |||||
| Lyssa_N_143-R-d | GCA GGG TAT TTG TAC TCA TA | |||||
| Lyssa_N_143-R-e | GCG GGG TAT TTG TAC TCA TA | |||||
| Lyssa_N_143-R-f | AGC CGG GTA TTT GTA CTC GTA | |||||
| Lyssa_N_85_LBV_FAM-Taq | FAM-GAT TGT KTT YAA RGT TCR TAA TCA G-BHQ1 | |||||
| Lyssa_N_53-FAM-MGB | FAM-AAA TGT AAC ACC YCT ACA ATG GA-MGBNFQ | |||||
| Pestivirus | Pan-Pesti-NS5B-Assay 2 | Pesti-11453-F | ACA GCM ATR CCA AAR AAT GAG AA | 154 bp (POL) | E | [67] |
| Pesti-11607-R | TTT CTG CTT TAC CCA VTT RTA CAT | |||||
| Coronavirus | Pan Corona-beta Mix 10 (SYBR Green) | CoV-beta-17106F | TCC WCA MCT TAT GGG TTG GGA | 454 bp 447 bp 238 bp 231 bp (POL) | F | In house |
| CoV-beta-17113F | CCT TAT GGG TTG GGA YTA YCC | |||||
| CoV-beta-17344R | TAG CAT AAG CAG TDG TDG CAT C | |||||
| CoV-beta-17560R | GCC ATC ATC ASA MAR AAT CAT CAT | |||||
| Astrovirus | Pan-Astro-K22 (SYBR Green) | Astro-K22-3162-F | CAC GTT TTG ATG GBA CVA THC C | 411 bp (ORF1b) 170 bp (ORF2) | G | In house |
| Astro-K22-3573-R | TCA GGY TTR ACC CAC ATD CCR AA | |||||
| Pan-Astro-K24 (SYBR Green) | Astro-K24-3714-F | AAT TWG CCC TCT RTG GGA ARC T | ||||
| Astro-K24-3884-R | TCT TTG GTC CKC CCC TCC A |
2.5. Sequencing
2.6. Fluorescent Antibody Test (FAT)
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kunz, T.H. Ecology of Bats; Springer: New York, NY, USA, 1982; ISBN 978-1-4613-3423-1. [Google Scholar] [CrossRef]
- Dietz, C.; Kiefer, A. Die Fledermäuse Europas. Kennen, Bestimmen, Schützen; Kosmos: Stuttgart, Germany, 2014; 394p. [Google Scholar]
- Alcalde, J.T.; Jiménez, M.; Brila, I.; Vintulis, V.; Voigt, C.C.; Pētersons, G. Transcontinental 2200 km migration of a Nathusius’ pipistrelle (Pipistrellus nathusii) across Europe. Mammalia 2020, 85, 161–163. [Google Scholar] [CrossRef]
- Vasenkov, D.; Desmet, J.; Popov, I.; Sidorchuk, N. Bats can migrate farther than it was previously known: A new longest migration record by Nathusius’ pipistrelle Pipistrellus nathusii (Chiroptera: Vespertilionidae). Mammalia 2022, 86, 524–526. [Google Scholar] [CrossRef]
- Lyman, C.P. Chapter 9—Thermoregulation and Metabolism in Bats. In Biology of Bats; Wimsatt, W.A., Ed.; Academic Press: Cambridge, MA, USA, 1970; pp. 301–330. ISBN 9780127580012. [Google Scholar] [CrossRef]
- Boyles, J.G.; Cryan, P.M.; McCracken, G.F.; Kunz, T.H. Conservation. Economic importance of bats in agriculture. Science 2011, 332, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Kunz, T.H.; Fenton, M.B. Bat Ecology; University of Chicago Press: Chicago, IL, USA, 2003; 779p. [Google Scholar]
- Maas, B.; Clough, Y.; Tscharntke, T. Bats and birds increase crop yield in tropical agroforestry landscapes. Ecol. Lett. 2013, 16, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Calisher, C.H.; Childs, J.E.; Field, H.E.; Holmes, K.V.; Schountz, T. Bats: Important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006, 19, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.E.; Mittermeier, R.A. (Eds.) Handbook of the Mammals of the World; Lynx Edicions: Barcelona, Spain, 2019; Volume 9, pp. 1–1008. ISBN 978-84-16728-19-0. [Google Scholar]
- Froidevaux, J.S.P.; Toshkova, N.; Barbaro, L.; Benítez-López, A.; Kerbiriou, C.; Le Viol, I.; Pacifici, M.; Santini, L.; Stawski, C.; Russo, D.; et al. A species-level trait dataset of bats in Europe and beyond. Sci. Data 2023, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Fledermausschutz, Koordinationsstelle für Fledermausschutz- und Forschung in Österreich. Available online: www.fledermausschutz.at (accessed on 30 September 2018).
- Milchram, M.; Dietz, C.; Mayer, F.; Gurke, M.; Krainer, K.; Mixanig, M.; Wieser, D.; Reiter, G. Moving north: Morphometric traits facilitate monitoring of the expanding steppe whiskered bat Myotis davidii in Europe. Hystrix Ital. J. Mammal. 2023, 34, 19–23. [Google Scholar] [CrossRef]
- Spitzenberger, F.; Weiss, E.; Sackl, P. First record of the Mediterranean horseshoe bat Rhinolophus euryale (Blasius, 1853) in Austria (Chiroptera, Rhinolophidae). Nyctalus 2022, 20, 68–70. [Google Scholar]
- Vasenkov, D.; Vasiliev, N.; Sidorchuk, N.; Rozhnova, V. Autumn Migration of Greater Noctule Bat (Nyctalus Lasiopterus): Through Countries and over Mountains to a New Migration Flight Record in Bats. Dokl. Biol. Sci. 2023, 513, 395–399. [Google Scholar] [CrossRef]
- Allen, T.; Murray, K.A.; Zambrana-Torrelio, C.; Morse, S.S.; Rondinini, C.; Di Marco, M.; Breit, N.; Olival, K.J.; Daszak, P. Global hotspots and correlates of emerging zoonotic diseases. Nat. Commun. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Allocati, N.; Petrucci, A.G.; Di Giovanni, P.; Masulli, M.; Di Ilio, C.; De Laurenzi, V. Bat-man disease transmission: Zoonotic pathogens from wildlife reservoirs to human populations. Cell Death Discov. 2016, 2, 16048. [Google Scholar] [CrossRef] [PubMed]
- Olival, J.; Weekley, C.C.; Daszak, P. Are Bats Really “Special” as Viral Reservoirs? What We Know and Need to Know. In Bats and Viruses: A New Frontier of Emerging Infectious Diseases, 1st ed.; Wang, L., Cowled, C., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Hayman, D.T. Bats as Viral Reservoirs. Annu. Rev. Virol. 2016, 3, 77–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Walker, P.J.; Poon, L.L. Mass extinctions, biodiversity and mitochondrial function: Are bats ‘special’ as reservoirs for emerging viruses? Curr. Opin. Virol. 2011, 1, 649–657. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, T.J.; Cryan, P.M.; Cunningham, A.A.; Fooks, A.R.; Hayman, D.T.; Luis, A.D.; Peel, A.J.; Plowright, R.K.; Wood, J.L. Bat flight and zoonotic viruses. Emerg. Infect. Dis. 2014, 20, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.L.; Schountz, T.; Wang, L.F. Antiviral immune responses of bats: A review. Zoonoses Public Health 2013, 60, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.B.; Bellini, W.J.; Rota, P.A.; Harcourt, B.H.; Tamin, A.; Lam, S.K.; Ksiazek, T.G.; Rollin, P.E.; Zaki, S.R.; Shieh, W.; et al. Nipah virus: A recently emergent deadly paramyxovirus. Science 2000, 288, 1432–1435. [Google Scholar] [CrossRef]
- Halpin, K.; Young, P.L.; Field, H.E.; Mackenzie, J.S. Isolation of Hendra virus from pteropid bats: A natural reservoir of Hendra virus. J. Gen. Virol. 2000, 81, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Leroy, E.M.; Kumulungui, B.; Pourrut, X.; Rouquet, P.; Hassanin, A.; Yaba, P.; Délicat, A.; Paweska, J.T.; Gonzalez, J.P.; Swanepoel, R.; et al. Fruit bats as reservoirs of Ebola virus. Nature 2005, 438, 575–576. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Victoria, J.G.; Wang, C.; Jones, M.; Fellers, G.M.; Kunz, T.H.; Delwart, E. Bat guano virome: Predominance of dietary viruses from insects and plants plus novel mammalian viruses. J. Virol. 2010, 84, 6955–6965. [Google Scholar] [CrossRef]
- Rahman, S.A.; Hassan, S.S.; Olival, K.J.; Mohamed, M.; Chang, L.Y.; Hassan, L.; Saad, N.M.; Shohaimi, S.A.; Mamat, Z.C.; Naim, M.S.; et al. Characterization of Nipah virus from naturally infected Pteropus vampyrus bats, Malaysia. Emerg. Infect. Dis. 2010, 16, 1990–1993. [Google Scholar] [CrossRef]
- Swanepoel, R.; Smit, S.B.; Rollin, P.E.; Formenty, P.; Leman, P.A.; Kemp, A.; Burt, F.J.; Grobbelaar, A.A.; Croft, J.; Bausch, D.G.; et al. Studies of reservoir hosts for Marburg virus. Emerg. Infect. Dis. 2007, 13, 1847–1851. [Google Scholar] [CrossRef] [PubMed]
- Latinne, A.; Hu, B.; Olival, K.J.; Zhu, G.; Zhang, L.; Li, H.; Chmura, A.A.; Field, H.E.; Zambrana-Torrelio, C.; Epstein, J.H. Origin and cross-species transmission of bat coronaviruses in China. Nat. Commun. 2020, 11, 4235. [Google Scholar] [CrossRef] [PubMed]
- Nokireki, T.; Sironen, T.; Smura, T.; Karkamo, V.; Sihvonen, L.; Gadd, T. Second case of European bat lyssavirus type 2 detected in a Daubenton’s bat in Finland. Acta Vet. Scand. 2017, 59, 62. [Google Scholar] [CrossRef] [PubMed]
- Nokireki, T.; Tammiranta, N.; Kokkonen, U.M.; Kantala, T.; Gadd, T. Tentative novel lyssavirus in a bat in Finland. Transbound. Emerg. Dis. 2018, 65, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Aznar-Lopez, C.; Vazquez-Moron, S.; Marston, D.A.; Juste, J.; Ibáñez, C.; Berciano, J.M.; Salsamendi, E.; Aihartza, J.; Banyard, A.C.; McElhinney, L. Detection of rhabdovirus viral RNA in oropharyngeal swabs and ectoparasites of Spanish bats. J. Gen. Virol. 2013, 94, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Lelli, D.; Prosperi, A.; Moreno, A.; Chiapponi, C.; Gibellini, A.M.; De Benedictis, P.; Leopardi, S.; Sozzi, E.; Lavazza, A. Isolation of a novel Rhabdovirus from an insectivorous bat (Pipistrellus kuhlii) in Italy. Virol. J. 2018, 15, 37. [Google Scholar] [CrossRef] [PubMed]
- Černe, D.; Hostnik, P.; Toplak, I.; Presetnik, P.; Maurer-Wernig, J.; Kuhar, U. Discovery of a novel bat lyssavirus in a Long-fingered bat (Myotis capaccinii) from Slovenia. PLoS Negl Trop Dis. 2023, 17, e0011420. [Google Scholar] [CrossRef] [PubMed]
- Racey, P.A.; Hutson, A.M.; Lina, P.H. Bat rabies, public health and European bat conservation. Zoonoses Public Health 2013, 60, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Cadar, D.; Becker, N.; Campos Rde, M.; Börstler, J.; Jöst, H.; Schmidt-Chanasit, J. Usutu virus in bats, Germany, 2013. Emerg Infect. Dis. 2014, 20, 1771–1773. [Google Scholar] [CrossRef]
- Pozo, F.; Juste, J.; Vázquez-Morón, S.; Aznar-López, C.; Ibáñez, C.; Garin, I.; Aihartza, J.; Casas, I.; Tenorio, A.; Echevarría, J.E. Identification of Novel Betaherpesviruses in Iberian Bats Reveals Parallel Evolution. PLoS ONE 2016, 11, e0169153. [Google Scholar] [CrossRef]
- Bányai, K.; Kemenesi, G.; Budinski, I.; Földes, F.; Zana, B.; Marton, S.; Varga-Kugler, R.; Oldal, M.; Kurucz, K.; Jakab, F. Candidate new rotavirus species in Schreiber’s bats, Serbia. Infect. Genet. Evol. 2017, 48, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Dufkova, L.; Straková, P.; Širmarová, J.; Salát, J.; Moutelíková, R.; Chrudimský, T.; Bartonička, T.; Nowotny, N.; Růžek, D. Detection of Diverse Novel Bat Astrovirus Sequences in the Czech Republic. Vector Borne Zoonotic Dis. 2015, 15, 518–521. [Google Scholar] [CrossRef] [PubMed]
- Kemenesi, G.; Dallos, B.; Görföl, T.; Boldogh, S.; Estók, P.; Kurucz, K.; Oldal, M.; Németh, V.; Madai, M.; Bányai, K.; et al. Novel European lineages of bat astroviruses identified in Hungary. Acta Virol. 2014, 58, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Kemenesi, G.; Kurucz, K.; Dallos, B.; Zana, B.; Földes, F.; Boldogh, S.; Görföl, T.; Carroll, M.W.; Jakab, F. Re-emergence of Lloviu virus in Miniopterus schreibersii bats, Hungary, 2016. Emerg. Microbes Infect. 2018, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, M.G.; Russo, D.; Lanave, G.; Cistrone, L.; Pratelli, A.; Martella, V.; Galiero, G.; Decaro, N.; Fusco, G. Detection and phylogenetic characterization of astroviruses in insectivorous bats from Central-Southern Italy. Zoonoses Public Health 2018, 65, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Pinho Dos Reis, V.; Balkema-Buschmann, A. Bat Astroviruses: Towards Understanding the Transmission Dynamics of a Neglected Virus Family. Viruses 2017, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Pauly, M.; Pir, J.B.; Loesch, C.; Sausy, A.; Snoeck, C.J.; Hübschen, J.M.; Muller, C.P. Novel Alphacoronaviruses and Paramyxoviruses Cocirculate with Type 1 and Severe Acute Respiratory System (SARS)-Related Betacoronaviruses in Synanthropic Bats of Luxembourg. Appl. Environ. Microbiol. 2017, 83, e01326-17. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Lelli, D.; de Sabato, L.; Zaccaria, G.; Boni, A.; Sozzi, E.; Prosperi, A.; Lavazza, A.; Cella, E.; Castrucci, M.R.; et al. Detection and full genome characterization of two beta CoV viruses related to Middle East respiratory syndrome from bats in Italy. Virol. J. 2017, 14, 239. [Google Scholar] [CrossRef] [PubMed]
- Monchatre-Leroy, E.; Boué, F.; Boucher, J.M.; Renault, C.; Moutou, F.; Ar Gouilh, M.; Umhang, G. Identification of Alpha and Beta Coronavirus in Wildlife Species in France: Bats, Rodents, Rabbits, and Hedgehogs. Viruses 2017, 9, 364. [Google Scholar] [CrossRef]
- Ar Gouilh, M.; Puechmaille, S.J.; Diancourt, L.; Vandenbogaert, M.; Serra-Cobo, J.; Lopez Roïg, M.; Brown, P.; Moutou, F.; Caro, V.; Vabret, A.; et al. SARS-CoV related Betacoronavirus and diverse Alphacoronavirus members found in western old-world. Virology 2018, 517, 88–97. [Google Scholar] [CrossRef]
- Reusken, C.B.; Lina, P.H.; Pielaat, A.; de Vries, A.; Dam-Deisz, C.; Adema, J.; Drexler, J.F.; Drosten, C.; Kooi, E. Circulation of group 2 coronaviruses in a bat species common to urban areas in Western Europe. Vector Borne Zoonotic Dis. 2010, 10, 785–791. [Google Scholar] [CrossRef]
- Annan, A.; Baldwin, H.J.; Corman, V.M.; Klose, S.M.; Owusu, M.; Nkrumah, E.E.; Badu, E.K.; Anti, P.; Agbenyega, O.; Meyer, B.; et al. Human betacoronavirus 2c EMC/2012-related viruses in bats, Ghana and Europe. Emerg. Infect. Dis. 2013, 19, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Crook, J.M.; Murphy, I.; Carter, D.P.; Pullan, S.T.; Carroll, M.; Vipond, R.; Cunningham, A.A.; Bell, D. Metagenomic identification of a new sarbecovirus from horseshoe bats in Europe. Sci. Rep. 2021, 11, 14723. [Google Scholar] [CrossRef] [PubMed]
- Alkhovsky, S.; Lenshin, S.; Romashin, A.; Vishnevskaya, T.; Vyshemirsky, O.; Bulycheva, Y.; Lvov, D.; Gitelman, A. SARS-like Coronaviruses in Horseshoe Bats (Rhinolophus spp.) in Russia, 2020. Viruses 2022, 14, 113. [Google Scholar] [CrossRef] [PubMed]
- Straková, P.; Dufkova, L.; Širmarová, J.; Salát, J.; Bartonička, T.; Klempa, B.; Pfaff, F.; Höper, D.; Hoffmann, B.; Ulrich, R.G.; et al. Novel hantavirus identified in European bat species Nyctalus noctula. Infect. Genet. Evol. 2017, 48, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Kivistö, I.; Tidenberg, E.M.; Lilley, T.; Suominen, K.; Forbes, K.M.; Vapalahti, O.; Huovilainen, A.; Sironen, T. First Report of Coronaviruses in Northern European Bats. Vector Borne Zoonotic Dis. 2020, 20, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Zeus, V.; Kwasnitschka, L.; Kerth, G.; Haase, M.; Groschup, M.H.; Balkema-Buschmann, A. Insectivorous bats carry host specific astroviruses and coronaviruses across different regions in Germany. Infect. Genet. Evol. 2016, 37, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Donato, C.; Vijaykrishna, D. The Broad Host Range and Genetic Diversity of Mammalian and Avian Astroviruses. Viruses 2017, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Han, H.J.; Wen, H.L.; Zhou, C.M.; Chen, F.F.; Luo, L.M.; Liu, J.W.; Yu, X.J. Bats as reservoirs of severe emerging infectious diseases. Virus Res. 2015, 205, 1–6. [Google Scholar] [CrossRef]
- Kohl, C.; Kurth, A. European bats as carriers of viruses with zoonotic potential. Viruses 2014, 6, 3110–3128. [Google Scholar] [CrossRef]
- Fereidouni, S.R.; Globig, A.; Starick, E.; Harder, T.C. Effect of swab matrix, storage time, and temperature on detection of avian influenza virus RNA in swab samples. Avian Dis. 2012, 56, 955–958. [Google Scholar] [CrossRef]
- Hoffmann, B.; Depner, K.; Schirrmeier, H.; Beer, M. A universal heterologous internal control system for duplex real-time RT-PCR assays used in a detection system for pestiviruses. J. Virol. Methods 2006, 136, 200–209. [Google Scholar] [CrossRef]
- Toussaint, J.F.; Sailleau, C.; Breard, E.; Zientara, S.; De Clercq, K. Bluetongue virus detection by two real-time RT-qPCRs targeting two different genomic segments. J. Virol. Methods 2007, 140, 115–123. [Google Scholar] [CrossRef]
- Wakeley, P.R.; Johnson, N.; McElhinney, L.M.; Marston, D.; Sawyer, J.; Fooks, A.R. Development of a real-time, TaqMan reverse transcription-PCR assay for detection and differentiation of lyssavirus genotypes 1, 5, and 6. J. Clin. Microbiol. 2005, 43, 2786–2792. [Google Scholar] [CrossRef] [PubMed]
- Wernike, K.; Hoffmann, B.; Kalthoff, D.; König, P.; Beer, M. Development and validation of a triplex real-time PCR assay for the rapid detection and differentiation of wild-type and glycoprotein E-deleted vaccine strains of Bovine herpesvirus type 1. J. Virol. Methods 2011, 174, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Grund, C.; Hoffmann, D.; Ulrich, R.; Naguib, M.; Schinköthe, J.; Hoffmann, B.; Harder, T.; Saenger, S.; Zscheppang, K.; Tönnies, M.; et al. A novel European H5N8 influenza A virus has increased virulence in ducks but low zoonotic potential. Emerg. Microbes Infect. 2018, 7, 132. [Google Scholar] [CrossRef]
- Halwe, N.J.; Gorka, M.; Hoffmann, B.; Rissmann, M.; Breithaupt, A.; Schwemmle, M.; Beer, M.; Kandeil, A.; Ali, M.A.; Kayali, G.; et al. Egyptian Fruit Bats (Rousettus aegyptiacus) Were Resistant to Experimental Inoculation with Avian-Origin Influenza A Virus of Subtype H9N2, But Are Susceptible to Experimental Infection with Bat-Borne H9N2 Virus. Viruses 2021, 13, 672. [Google Scholar] [CrossRef]
- Schlottau, K.; Forth, L.; Angstwurm, K.; Höper, D.; Zecher, D.; Liesche, F.; Hoffmann, B.; Kegel, V.; Seehofer, D.; Platen, S.; et al. Fatal Encephalitic Borna Disease Virus 1 in Solid-Organ Transplant Recipients. N. Engl. J. Med. 2018, 379, 1377–1379. [Google Scholar] [CrossRef] [PubMed]
- Dafalla, M.; Orłowska, A.; Keleş, S.J.; Straková, P.; Schlottau, K.; Jeske, K.; Hoffmann, B.; Wibbelt, G.; Smreczak, M.; Müller, T.; et al. Hantavirus Brno loanvirus is highly specific to the common noctule bat (Nyctalus noctula) and widespread in Central Europe. Virus Genes 2023, 59, 323–332. [Google Scholar] [CrossRef]
- Beer, M.; Wernike, K.; Dräger, C.; Höper, D.; Pohlmann, A.; Bergermann, C.; Schröder, C.; Klinkhammer, S.; Blome, S.; Hoffmann, B. High Prevalence of Highly Variable Atypical Porcine Pestiviruses Found in Germany. Transbound Emerg. Dis. 2017, 64, e22–e26. [Google Scholar] [CrossRef]
- World Organisation for Animal Health. Chapter 3.1.18.—Rabies (Infection with Rabies Virus and Other Lyssaviruses); Terrestrial Manual; World Organisation for Animal Health: Paris, France, 2023. [Google Scholar]
- Nobach, D.; Herden, C. No evidence for European bats serving as reservoir for Borna disease virus 1 or other known mammalian orthobornaviruses. Virol. J. 2020, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Li, Y.; Rivailler, P.; Conrardy, C.; Castillo, D.A.; Chen, L.M.; Recuenco, S.; Ellison, J.A.; Davis, C.T.; York, I.A.; et al. A distinct lineage of influenza A virus from bats. Proc. Natl. Acad. Sci. USA 2012, 109, 4269–4274. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Zhu, X.; Li, Y.; Shi, M.; Zhang, J.; Bourgeois, M.; Yang, H.; Chen, X.; Recuenco, S.; Gomez, J.; et al. New world bats harbor diverse influenza A viruses. PLoS Pathog. 2013, 9, e1003657. [Google Scholar] [CrossRef] [PubMed]
- Kandeil, A.; Gomaa, M.R.; Shehata, M.M.; El Taweel, A.N.; Mahmoud, S.H.; Bagato, O.; Moatasim, Y.; Kutkat, O.; Kayed, A.S.; Dawson, P.; et al. Isolation and Characterization of a Distinct Influenza A Virus from Egyptian Bats. J. Virol. 2019, 93, e01059-18. [Google Scholar] [CrossRef]
- Fereidouni, S.; Kwasnitschka, L.; Balkema Buschmann, A.; Müller, T.; Freuling, C.; Schatz, J.; Pikula, J.; Bandouchova, H.; Hoffmann, R.; Ohlendorf, B.; et al. No virological evidence for an influenza A-like virus in European bats. Zoonoses Public Health 2015, 62, 187–189. [Google Scholar] [CrossRef]
| Bat Species | English Name | Swab Sampling | Tissue Sampling | No. of Animals |
|---|---|---|---|---|
| Rhinolophus ferrumequinum | Greater horseshoe | 3 | 0 | 3 |
| Rhinolophus hipposideros | Lesser horseshoe | 2 | 0 | 2 |
| Myotis daubentonii | Daubenton’s bat | 2 | 0 | 2 |
| Myotis mystacinus | Whiskered bat | 2 | 4 | 6 |
| Myotis emarginatus | Geoffroy’s bat | 0 | 2 | 2 |
| Myotis myotis | Greater mouse-eared | 0 | 1 | 1 |
| Nyctalus noctula | Common noctule | 173 | 34 | 207 |
| Nyctalus leisleri | Lesser noctule | 1 | 0 | 1 |
| Eptesicus serotinus | Serotine bat | 5 | 2 | 7 |
| Vespertilio murinus | Parti-coloured bat | 26 | 17 | 43 |
| Pipistrellus pipistrellus | Common pipistrelle | 13 | 17 | 30 |
| Pipistrellus pygmaeus | Soprano pipistrelle | 3 | 0 | 3 |
| Pipistrellus nathusii | Nathusius’s pipistrelle | 17 | 16 | 33 |
| Pipistrellus kuhlii | Kuhl’s pipistrelle | 31 | 14 | 45 |
| Hypsugo savii | Savi’s pipistrelle | 10 | 41 | 51 |
| Plecotus austriacus | Grey long-eared bat | 6 | 5 | 11 |
| Barbastella barbastellus | Western barbastelle | 10 | 1 | 11 |
| Miniopterus schreibersii | Common bent-wing bat | 5 | 0 | 5 |
| Unknown species | 0 | 1 | 1 | |
| 309 | 155 | 464 |
| Viruses | Bat Species | Swab Samples | Organ Samples | ||||
|---|---|---|---|---|---|---|---|
| RT-qPCR Positive | Ct Values | Confirmed by Sequencing | RT-qPCR Positive | Ct Values | Confirmed by Sequencing | ||
| Hantaviruses | N. noctula | - | - | - | 1 | 27.6 | 1 |
| Coronaviruses | P. kuhlii | 2 | 30.9, 34.2 | 2 | 2 | 33.4, 33.9 | - |
| P. nathusii | 1 | 36.5 | 1 | 8 | 31.2, 31.4, 31.9, 33.3, 33.6, 33.7, 34.3, 34.6 | - | |
| H. savii | - | - | - | 5 | 31.5, 32.1, 32.6, 34.1, 35.2 | - | |
| V. murinus | - | - | - | 2 | 33.4, 35.7 | - | |
| N. noctula | - | - | - | 1 | 36.9 | - | |
| P. austriacus | - | - | - | 1 | 36.5 | - | |
| B. barbastellus | - | - | - | 1 | 35.1 | - | |
| M. emarginatus | - | - | - | 1 | 33.1 | - | |
| Astroviruses | N. noctula | 1 | 37.2 | 1 | 2 | 35.2, 32.4 | - |
| P. kuhlii | - | - | - | 3 | 32.3, 31.9, 35.7 | - | |
| P. nathusii | - | - | - | 1 | 31.3 | - | |
| H. savii | - | - | - | 1 | 32 | - | |
| V. murinus | - | - | - | 1 | 33 | - | |
| P. pipistrellus | - | - | - | 1 | 28.6 | - | |
| E. serotinus | - | - | - | 1 | 32.6 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fereidouni, S.; Keleş, S.J.; Schlottau, K.; Bagó, Z.; Reiter, G.; Milchram, M.; Hoffmann, B. Monitoring of Astroviruses, Brno-Hantaviruses, Coronaviruses, Influenza Viruses, Bornaviruses, Morbilliviruses, Lyssaviruses and Pestiviruses in Austrian Bats. Viruses 2024, 16, 1232. https://doi.org/10.3390/v16081232
Fereidouni S, Keleş SJ, Schlottau K, Bagó Z, Reiter G, Milchram M, Hoffmann B. Monitoring of Astroviruses, Brno-Hantaviruses, Coronaviruses, Influenza Viruses, Bornaviruses, Morbilliviruses, Lyssaviruses and Pestiviruses in Austrian Bats. Viruses. 2024; 16(8):1232. https://doi.org/10.3390/v16081232
Chicago/Turabian StyleFereidouni, Sasan, Sinan Julian Keleş, Kore Schlottau, Zoltán Bagó, Guido Reiter, Markus Milchram, and Bernd Hoffmann. 2024. "Monitoring of Astroviruses, Brno-Hantaviruses, Coronaviruses, Influenza Viruses, Bornaviruses, Morbilliviruses, Lyssaviruses and Pestiviruses in Austrian Bats" Viruses 16, no. 8: 1232. https://doi.org/10.3390/v16081232





