Proliferating Cell Nuclear Antigen in the Era of Oncolytic Virotherapy
Abstract
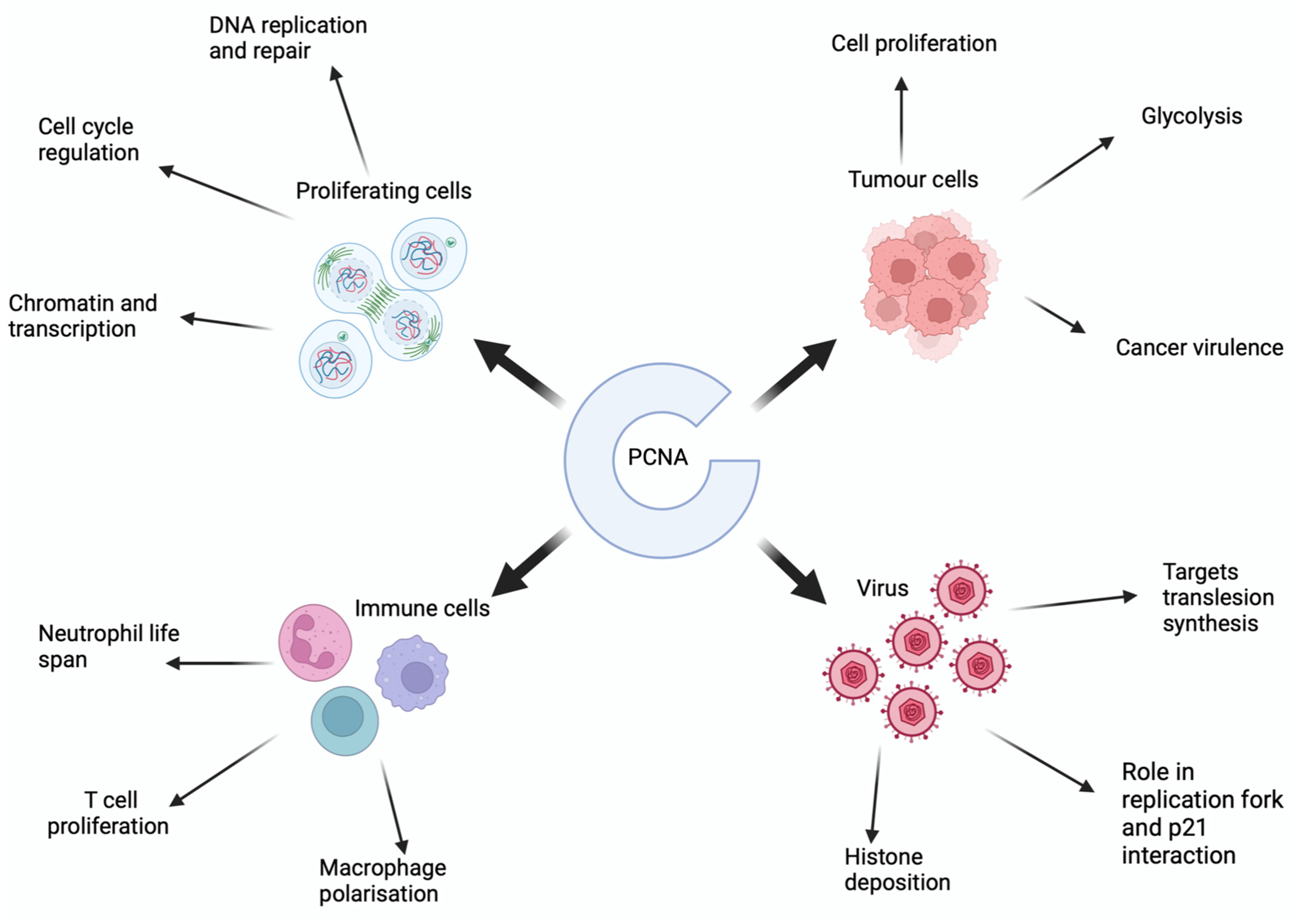
:1. Introduction
2. The Structure of PCNA
3. What Is the Role of PCNA in Eukaryotic Cells?
4. What Is the Role of PCNA in Cancer?
5. What Is Oncolytic Virotherapy?
6. What Is Role of PCNA in Viral Replication?
7. How Does PCNA Regulate Immune Cell Functioning?
7.1. Neutrophils
7.2. NK Cells
7.3. T Lymphocytes
7.4. Macrophages
8. Can PCNA Overexpression Be Targeted?
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, S.Y.; Jeong, M.S.; Han, C.W.; Yu, H.S.; Jang, S.B. Structural and Functional Insight into Proliferating Cell Nuclear Antigen. J. Microbiol. Biotechnol. 2016, 26, 637–647. [Google Scholar] [CrossRef] [PubMed]
- De Biasio, A.; Blanco, F.J. Proliferating Cell Nuclear Antigen Structure and Interactions. Adv. Protein Chem. Struct. Biol. 2013, 91, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Maga, G.; Hübscher, U. Proliferating cell nuclear antigen (PCNA): A dancer with many partners. J. Cell Sci. 2003, 116, 3051–3060. [Google Scholar] [CrossRef] [PubMed]
- Naryzhny, S.N.; Lee, H. Characterization of proliferating cell nuclear antigen (PCNA) isoforms in normal and cancer cells: There is no cancer-associated form of PCNA. FEBS Lett. 2007, 581, 4917–4920. [Google Scholar] [CrossRef] [PubMed]
- Malkas, L.H.; Herbert, B.S.; Abdel-Aziz, W.; Dobrolecki, L.E.; Liu, Y.; Agarwal, B.; Hoelz, D.; Badve, S.; Schnaper, L.; Arnold, R.J.; et al. A cancer-associated PCNA expressed in breast cancer has implications as a potential biomarker. Proc. Natl. Acad. Sci. USA 2006, 103, 19472–19477. [Google Scholar] [CrossRef]
- Gu, L.; Lingeman, R.G.; Yakushijin, F.; Sun, E.; Cui, Q.; Chao, J.; Hu, W.; Li, H.; Hickey, R.J.; Stark, J.M.; et al. The Anticancer Activity of a First-in-class Small-molecule Targeting PCNA. Clin. Cancer Res. 2018, 24, 6053–6065. [Google Scholar] [CrossRef]
- Gu, L.; Li, M.; Li, C.M.; Haratipour, P.; Lingeman, R.; Jossart, J.; Gutova, M.; Flores, L.; Hyde, C.; Kenjić, N.; et al. Small molecule targeting of transcription-replication conflict for selective chemotherapy. Cell Chem. Biol. 2023, 30, 1235–1247.e6. [Google Scholar] [CrossRef]
- Kwan, A.; Winder, N.; Atkinson, E.; Al-Janabi, H.; Allen, R.J.; Hughes, R.; Moamin, M.; Louie, R.; Evans, D.; Hutchinson, M.; et al. Macrophages Mediate the Antitumor Effects of the Oncolytic Virus HSV1716 in Mammary Tumors. Mol. Cancer Ther. 2021, 20, 589–601. [Google Scholar] [CrossRef]
- Kelman, Z.; O’Donnell, M. Structural and functional similarities of prokaryotic and eukaryotic DNA polymerase sliding clamps. Nucleic Acids Res. 1995, 23, 3613–3620. [Google Scholar] [CrossRef]
- Naryzhny, S.N.; Lee, H. Proliferating cell nuclear antigen in the cytoplasm interacts with components of glycolysis and cancer. FEBS Lett. 2010, 584, 4292–4298. [Google Scholar] [CrossRef]
- Naryzhny, S.N.; Lee, H. Observation of multiple isoforms and specific proteolysis patterns of proliferating cell nuclear antigen in the context of cell cycle compartments and sample preparations. Proteomics 2003, 3, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Strzalka, W.; Ziemienowicz, A. Proliferating cell nuclear antigen (PCNA): A key factor in DNA replication and cell cycle regulation. Ann. Bot. 2011, 107, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shahar, T.R.; Castillo, A.G.; Osborne, M.J.; Borden, K.L.B.; Kornblatt, J.; Verreault, A. Two fundamentally distinct PCNA interaction peptides contribute to chromatin assembly factor 1 function. Mol. Cell. Biol. 2009, 29, 6353–6365. [Google Scholar] [CrossRef] [PubMed]
- Choe, K.N.; Moldovan, G.-L. Forging ahead through darkness: PCNA, still the principal conductor at the replication fork. Mol. Cell 2002, 65, 380–392. [Google Scholar] [CrossRef] [PubMed]
- De March, M.; De Biasio, A. The dark side of the ring: Role of the DNA sliding surface of PCNA. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Boehm, E.M.; Gildenberg, M.S.; Washington, M.T. The many roles of PCNA in eukaryotic DNA replication. Enzymes 2016, 39, 231–254. [Google Scholar] [PubMed]
- Matsumoto, Y.; Brooks, R.C.; Sverzhinsky, A.; Pascal, J.M.; Tomkinson, A.E. Dynamic DNA-bound PCNA complexes co-ordinate Okazaki fragment synthesis, processing and ligation. J. Mol. Biol. 2020, 432, 166698. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bozza, W.; Zhuang, Z. Ubiquitination of PCNA and its essential role in eukaryotic translesion synthesis. Cell Biochem. Biophys. 2011, 60, 47–60. [Google Scholar] [CrossRef]
- Zhao, L.; Washington, M.T. Translesion synthesis: Insights into the selection and switching of DNA polymerases. Genes 2017, 8, 24. [Google Scholar] [CrossRef]
- Dieckman, L.M.; Freudenthal, B.D.; Washington, M.T. PCNA structure and function: Insights from structures of PCNA complexes and post-translationally modified PCNA. In The Eukaryotic Replisome: A Guide to Protein Structure and Function; MacNeill, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 62, pp. 281–299. [Google Scholar]
- Prosperi, E. The fellowship of the rings: Distinct pools of proliferating cell nuclear antigen trimer at work. FASEB J. 2006, 20, 833–837. [Google Scholar] [CrossRef]
- Naryzhny, S.N.; Zhao, H.; Lee, H. Proliferating Cell Nuclear Antigen (PCNA) May Function as a Double Homotrimer Complex in the Mammalian Cell. J. Biol. Chem. 2005, 280, 13888–13894. [Google Scholar] [CrossRef] [PubMed]
- Sverzhinsky, A.; Tomkinson, A.E.; Pascal, J.M. Cryo-EM structures and biochemical insights into heterotrimeric PCNA regulation of DNA ligase. Structure 2022, 30, 371–385.e5. [Google Scholar] [CrossRef] [PubMed]
- Aihara, H. DNA ligase and PCNA: Double-ring down to seal a break in DNA. Structure 2022, 30, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, D.; De Chiara, A.; Chapuis, N.; Candalh, C.; Mocek, J.; Ribeil, J.-A.; Haddaoui, L.; Ifrah, N.; Hermine, O.; Bouillaud, F.; et al. Cytoplasmic proliferating cell nuclear antigen connects glycolysis and cell survival in acute myeloid leukemia. Sci. Rep. 2016, 6, 35561. [Google Scholar] [CrossRef] [PubMed]
- Revollo, J.R.; Grimm, A.A.; Imai, S.-I. The NAD Biosynthesis Pathway Mediated by Nicotinamide Phosphoribosyltransferase Regulates Sir2 Activity in Mammalian Cells. J. Biol. Chem. 2004, 279, 50754–50763. [Google Scholar] [CrossRef] [PubMed]
- Dennis, P.A.; Kastan, M.B. Cellular survival pathways and resistance to cancer therapy. Drug Resist. Updat. 1998, 1, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Palanivelu, L.; Liu, C.-H.; Lin, L.-T. Immunogenic cell death: The cornerstone of oncolytic viro-immunotherapy. Front. Immunol. 2023, 13, 1038226. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ramachandran, M.; Jin, C.; Quijano-Rubio, C.; Martikainen, M.; Yu, D.; Essand, M. Characterization of virus-mediated immunogenic cancer cell death and the consequences for oncolytic virus-based immunotherapy of cancer. Cell Death Dis. 2020, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-Y.; Sun, T.-K.; Chen, M.-S.; Munir, M.; Liu, H.-J. Oncolytic viruses-modulated immunogenic cell death, apoptosis and autophagy linking to virotherapy and cancer immune response. Front. Cell. Infect. Microbiol. 2023, 13, 1142172. [Google Scholar] [CrossRef]
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients with Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Zheng, M.; Huang, J.; Tong, A.; Yang, H. Oncolytic Viruses for Cancer Therapy: Barriers and Recent Advances. Mol. Ther. Oncolytics 2019, 15, 234–247. [Google Scholar] [CrossRef]
- Kwan, A.; Winder, N.; Muthana, M. Oncolytic Virotherapy Treatment of Breast Cancer: Barriers and Recent Advances. Viruses 2021, 13, 1128. [Google Scholar] [CrossRef]
- Kang, B.Y.; You, H.; Bandyopadhyay, S.; Agrawal, N.; Melchert, R.B.; Basnakian, A.G.; Liu, Y.; Hermonat, P.L. Cervical cancer isolate PT3, super-permissive for adeno-associated virus replication, over-expresses DNA polymerase δ, PCNA, RFC and RPA. BMC Microbiol. 2009, 9, 79. [Google Scholar] [CrossRef]
- Postigo, A.; Ramsden, A.E.; Howell, M.; Way, M. Cytoplasmic ATR Activation Promotes Vaccinia Virus Genome Replication. Cell Rep. 2017, 19, 1022–1032. [Google Scholar] [CrossRef]
- Dong, X.; Guan, J.; Zheng, C.; Zheng, X. The herpes simplex virus 1 UL36USP deubiquitinase suppresses DNA repair in host cells via deubiquitination of proliferating cell nuclear antigen. J. Biol. Chem. 2017, 292, 8472–8483. [Google Scholar] [CrossRef]
- Sanders, I.; Boyer, M.; Fraser, N.W. Early nucleosome deposition on, and replication of, HSV DNA requires cell factor PCNA. J. NeuroVirology 2015, 21, 358–369. [Google Scholar] [CrossRef]
- Oh, J.; Ruskoski, N.; Fraser, N.W. Chromatin Assembly on Herpes Simplex Virus 1 DNA Early during a Lytic Infection Is Asf1a Dependent. J. Virol. 2012, 86, 12313–12321. [Google Scholar] [CrossRef]
- Dembowski, J.A.; Dremel, S.E.; DeLuca, N.A. Replication-Coupled Recruitment of Viral and Cellular Factors to Herpes Simplex Virus Type 1 Replication Forks for the Maintenance and Expression of Viral Genomes. PLoS Pathog. 2017, 13, e1006166. [Google Scholar] [CrossRef]
- Packard, J.E.; Williams, M.R.; Fromuth, D.P.; Dembowski, J.A. Proliferating cell nuclear antigen inhibitors block distinct stages of herpes simplex virus infection. PLoS Pathog. 2023, 19, e1011539. [Google Scholar] [CrossRef]
- Lu, S.; Dong, Z. Additive effects of a small molecular PCNA inhibitor PCNA-I1S and DNA damaging agents on growth inhibition and DNA damage in prostate and lung cancer cells. PLoS ONE 2019, 14, e0223894. [Google Scholar] [CrossRef]
- Inoue, A.; Kikuchi, S.; Hishiki, A.; Shao, Y.; Heath, R.; Evison, B.J.; Actis, M.; Canman, C.E.; Hashimoto, H.; Fujii, N. A Small Molecule Inhibitor of Monoubiquitinated Proliferating Cell Nuclear Antigen (PCNA) Inhibits Repair of Interstrand DNA Cross-link, Enhances DNA Double Strand Break, and Sensitizes Cancer Cells to Cisplatin. J. Biol. Chem. 2014, 289, 7109–7120. [Google Scholar] [CrossRef]
- Detta, A.; Harland, J.; Hanif, I.; Brown, S.M.; Cruickshank, G. Proliferative activity and in vitro replication of HSV1716 in human metastatic brain tumours. J. Gene Med. 2003, 5, 681–689. [Google Scholar] [CrossRef]
- Gartel, A.L.; Radhakrishnan, S.K. Lost in Transcription: p21 Repression, Mechanisms, and Consequences. Cancer Res. 2005, 65, 3980–3985. [Google Scholar] [CrossRef]
- Bashir, T.; Hörlein, R.; Rommelaere, J.; Willwand, K. Cyclin A activates the DNA polymerase δ-dependent elongation machinery in vitro: A parvovirus DNA replication model. Proc. Natl. Acad. Sci. USA 2000, 97, 5522–5527. [Google Scholar] [CrossRef]
- Adeyemi, R.O.; Fuller, M.S.; Pintel, D.J. Efficient Parvovirus Replication Requires CRL4Cdt2-Targeted Depletion of p21 to Prevent Its Inhibitory Interaction with PCNA. PLoS Pathog. 2014, 10, e1004055. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Ohayon, D. Proliferating cell nuclear antigen in neutrophil fate. Immunol. Rev. 2016, 273, 344–356. [Google Scholar] [CrossRef]
- Kowalska, E.; Bartnicki, F.; Fujisawa, R.; Bonarek, P.; Hermanowicz, P.; Tsurimoto, T.; Muszyńska, K.; Strzalka, W. Inhibition of DNA replication by an anti-PCNA aptamer/PCNA complex. Nucleic Acids Res. 2018, 46, 25–41. [Google Scholar] [CrossRef]
- Knaneh, J.; Hodak, E.; Fedida-Metula, S.; Edri, A.; Eren, R.; Yoffe, Y.; Amitay-Laish, I.; Naveh, H.P.; Lubin, I.; Porgador, A.; et al. mAb14, a Monoclonal Antibody against Cell Surface PCNA: A Potential Tool for Sezary Syndrome Diagnosis and Targeted Immunotherapy. Cancers 2023, 15, 4421. [Google Scholar] [CrossRef]
- Kundu, K.; Ghosh, S.; Sarkar, R.; Edri, A.; Brusilovsky, M.; Gershoni-Yahalom, O.; Yossef, R.; Shemesh, A.; Soria, J.-C.; Lazar, V.; et al. Inhibition of the NKp44-PCNA Immune Checkpoint Using a mAb to PCNA. Cancer Immunol. Res. 2019, 7, 1120–1134. [Google Scholar] [CrossRef]
- Lei, Q.; Gao, F.; Liu, T.; Ren, W.; Chen, L.; Cao, Y.; Chen, W.; Guo, S.; Zhang, Q.; Chen, W.; et al. Extracellular vesicles deposit PCNA to rejuvenate aged bone marrow–derived mesenchymal stem cells and slow age-related degeneration. Sci. Transl. Med. 2021, 13, eaaz8697. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Mocek, J.; Bouayad, D.; Tamassia, N.; Ribeil, J.-A.; Candalh, C.; Davezac, N.; Reuter, N.; Mouthon, L.; Hermine, O.; et al. Proliferating cell nuclear antigen acts as a cytoplasmic platform controlling human neutrophil survival. J. Exp. Med. 2010, 207, 2631–2645. [Google Scholar] [CrossRef] [PubMed]
- McCracken, J.M.; Allen, L.-A.H. Regulation of Human Neutrophil Apoptosis and Lifespan in Health and Disease. J. Cell Death 2014, 7, JCD.S11038–23. [Google Scholar] [CrossRef] [PubMed]
- Bouayad, D.; Pederzoli-Ribeil, M.; Mocek, J.; Candalh, C.; Arlet, J.-B.; Hermine, O.; Reuter, N.; Davezac, N.; Witko-Sarsat, V. Nuclear-to-cytoplasmic Relocalization of the Proliferating Cell Nuclear Antigen (PCNA) during Differentiation Involves a Chromosome Region Maintenance 1 (CRM1)-dependent Export and Is a Prerequisite for PCNA Antiapoptotic Activity in Mature Neutrophils. J. Biol. Chem. 2012, 287, 33812–33825. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y. Caspase activation, inhibition, and reactivation: A mechanistic view. Protein Sci. 2004, 13, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- De Chiara, A.; Pederzoli-Ribeil, M.; Mocek, J.; Candalh, C.; Mayeux, P.; Millet, A.; Witko-Sarsat, V. Characterization of cytosolic proliferating cell nuclear antigen (PCNA) in neutrophils: Antiapoptotic role of the monomer. J. Leukoc. Biol. 2013, 94, 723–731. [Google Scholar] [CrossRef]
- Aymonnier, K.; Bosetta, E.; Leborgne, N.G.F.; Ullmer, A.; Le Gall, M.; De Chiara, A.; Salnot, V.; Many, S.; Scapini, P.; Wicks, I.; et al. G-CSF reshapes the cytosolic PCNA scaffold and modulates glycolysis in neutrophils. J. Leukoc. Biol. 2024, 115, 205–221. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Formiga, R.; Pesenti, L.; Ladjemi, M.Z.; Frachet, P.; Andrieu, M.; Many, S.; Karunanithy, V.; Bailly, K.; Dhôte, T.; Castel, M.; et al. Cytosolic PCNA interacts with S100A8 and controls an inflammatory subset of neutrophils in COVID-19. medRxiv 2022. [Google Scholar] [CrossRef]
- De Chiara, A.; Pederzoli-Ribeil, M.; Burgel, P.-R.; Danel, C.; Witko-Sarsat, V. Targeting cytosolic proliferating cell nuclear antigen in neutrophil-dominated inflammation. Front. Immunol. 2012, 3, 311. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Rosental, B.; Brusilovsky, M.; Hadad, U.; Oz, D.; Appel, M.Y.; Afergan, F.; Yossef, R.; Rosenberg, L.A.; Aharoni, A.; Cerwenka, A.; et al. Proliferating Cell Nuclear Antigen Is a Novel Inhibitory Ligand for the Natural Cytotoxicity Receptor NKp44. J. Immunol. 2011, 187, 5693–5702. [Google Scholar] [CrossRef]
- Garzetti, G.G.; Ciavattini, A.; Goteri, G.; Tranquilli, A.L.; Muzzioli, M.; Fabris, N.; De Nictolis, M.; Romanini, C. Natural Killer Cell Activity in Stage I Endometrial Carcinoma: Correlation with Nuclear Grading, Myometrial Invasion, and Immunoreactivity of Proliferating Cell Nuclear Antigen. Gynecol. Oncol. 1994, 55, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Marrufo, A.M.; Mathew, S.O.; Chaudhary, P.; Malaer, J.D.; Ahmed, N.; Vishwanatha, J.K.; Mathew, P.A. Blocking PCNA interaction with NKp44 enhances primary natural killer cell-mediated lysis of triple-negative breast cancer cells. Am. J. Cancer Res. 2023, 13, 1082–1090. [Google Scholar] [PubMed]
- Kurki, P.; Lotz, M.; Ogata, K.; Tan, E.M. Proliferating cell nuclear antigen (PCNA)/cyclin in activated human T lymphocytes. J. Immunol. 1987, 138, 4114–4120. [Google Scholar] [CrossRef]
- Szepesi, A.; Gelfand, E.; Lucas, J. Association of proliferating cell nuclear antigen with cyclin-dependent kinases and cyclins in normal and transformed human T lymphocytes. Blood 1994, 84, 3413–3421. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y. Tumor-associated macrophages: From basic research to clinical application. J. Hematol. Oncol. 2017, 10, 58. [Google Scholar] [CrossRef]
- Campbell, M.J.; Wolf, D.; Mukhtar, R.A.; Tandon, V.; Yau, C.; Au, A.; Baehner, F.; Veer, L.V.; Berry, D.; Esserman, L.J. The Prognostic Implications of Macrophages Expressing Proliferating Cell Nuclear Antigen in Breast Cancer Depend on Immune Context. PLoS ONE 2013, 8, e79114. [Google Scholar] [CrossRef]
- Mukhtar, R.A.; Moore, A.P.; Tandon, V.J.; Nseyo, O.; Twomey, P.; Adisa, C.A.; Eleweke, N.; Au, A.; Baehner, F.L.; Moore, D.H.; et al. Elevated Levels of Proliferating and Recently Migrated Tumor-associated Macrophages Confer Increased Aggressiveness and Worse Outcomes in Breast Cancer. Ann. Surg. Oncol. 2012, 19, 3979–3986. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, R.A.; Moore, A.P.; Nseyo, O.; Baehner, F.L.; Au, A.; Moore, D.H.; Twomey, P.; Campbell, M.J.; Esserman, L.J. Elevated PCNA+ tumor-associated macrophages in breast cancer are associated with early recurrence and non-Caucasian ethnicity. Breast Cancer Res. Treat. 2011, 130, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Lemech, C.R.; Kichenadasse, G.; Marschner, J.-P.; Alevizopoulos, K.; Otterlei, M.; Millward, M. ATX-101, a cell-penetrating protein targeting PCNA, can be safely administered as intravenous infusion in patients and shows clinical activity in a Phase 1 study. Oncogene 2023, 42, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Punchihewa, C.; Inoue, A.; Hishiki, A.; Fujikawa, Y.; Connelly, M.; Evison, B.; Shao, Y.; Heath, R.; Kuraoka, I.; Rodrigues, P.; et al. Identification of Small Molecule Proliferating Cell Nuclear Antigen (PCNA) Inhibitor That Disrupts Interactions with PIP-box Proteins and Inhibits DNA Replication. J. Biol. Chem. 2012, 287, 14289–14300. [Google Scholar] [CrossRef]
- Dillehay, K.L.; Seibel, W.L.; Zhao, D.; Lu, S.; Dong, Z. Target validation and structure–activity analysis of a series of novel PCNA inhibitors. Pharmacol. Res. Perspect. 2015, 3, e00115. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Wortman, M.; Dillehay, K.L.; Seibel, W.L.; Evelyn, C.R.; Smith, S.J.; Malkas, L.H.; Zheng, Y.; Lu, S.; Dong, Z. Small-Molecule Targeting of Proliferating Cell Nuclear Antigen Chromatin Association Inhibits Tumor Cell Growth. Mol. Pharmacol. 2012, 81, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peters, R.; Saha, P.; Lee, P.; Theodoras, A.; Pagano, M.; Wagner, G.; Dutta, A. A 39 Amino Acid Fragment of the Cell Cycle Regulator p21 Is Sufficient to Bind PCNA and Partially Inhibit DNA Replication in vivo. Nucleic Acids Res. 1996, 24, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Warbrick, E.; Lane, D.P.; Glover, D.M.; Cox, L.S. A small peptide inhibitor of DNA replication defines the site of interaction between the cyclin-dependent kinase inhibitor p21WAF1 and proliferating cell nuclear antigen. Curr. Biol. 1995, 5, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Lo, Y.-H.; Ma, L.; Waltz, S.E.; Gray, J.K.; Hung, M.-C.; Wang, S.-C. Targeting Tyrosine Phosphorylation of PCNA Inhibits Prostate Cancer Growth. Mol. Cancer Ther. 2011, 10, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.C.; Gopal, P.; Lim, S.; Wei, X.; Chandramohan, A.; Mangadu, R.; Smith, J.; Ng, S.; Gindy, M.; Phan, U.; et al. Targeted degradation of PCNA outperforms stoichiometric inhibition to result in programed cell death. Cell Chem. Biol. 2022, 29, 1601–1615.e7. [Google Scholar] [CrossRef] [PubMed]
- Taghdisi, S.M.; Danesh, N.M.; Nameghi, M.A.; Bahreyni, A.; Ramezani, M.; Alibolandi, M.; Emrani, A.S.; Abnous, K. Co-delivery of doxorubicin and α-PCNA aptamer using AS1411-modified pH-responsive nanoparticles for cancer synergistic therapy. J. Drug Deliv. Sci. Technol. 2020, 58, 101816. [Google Scholar] [CrossRef]
- Shemesh, A.; Kundu, K.; Peleg, R.; Yossef, R.; Kaplanov, I.; Ghosh, S.; Khrapunsky, Y.; Gershoni-Yahalom, O.; Rabinski, T.; Cerwenka, A.; et al. NKp44-Derived Peptide Binds Proliferating Cell Nuclear Antigen and Mediates Tumor Cell Death. Front. Immunol. 2018, 9, 1114. [Google Scholar] [CrossRef]


| Name | Mechanism of Action | Key Information | References |
|---|---|---|---|
| T2AA | Blocks protein interactions between the PCNA IDCL and proteins containing the PIP-box peptide motif Inhibits PCNA–Pol δ interaction Inhibits de novo DNA synthesis |
| [43,72] |
| PCNA-I1 | PCNA-I1 binds at the interface between PCNA monomers, stabilizes the homotrimer, and may interfere with protein–protein interactions |
| [73,74] |
| AOH1160 | Small molecule target against caPCNA |
| [6] |
| AOH1996 | Small molecule target against caPCNA |
| [7] |
| p21C2 | p21-PCNA interaction |
| [75] |
| p21PBP | p21-PCNA interaction |
| [76] |
| Y211F CPPP | Y211F peptide blocks phosphorylation of PCNA tyrosine 211 |
| [77] |
| Con1-Spop | Acts directly on PCNA to cause impaired mitotic division and mitochondria dysfunction |
| [78] |
| ATX-101 | Disrupts PCNA from interacting with APIM-containing proteins |
| [71] |
| α-PCNA aptamer | Blocks replication of the DNA template by forming an α-PCNA aptamer/PCNA/DNA pol complex that is unable to bind the primer-template DNA |
| [49,79] |
| 14-25-9 | Inhibition of NKp44-PCNA immune checkpoint, as 14-25-9 is a checkpoint-blocking mAb against proliferating cell nuclear antigen (PCNA) |
| [51] |
| NKp44-pep8 | Partly block the NKp44–PCNA interaction to target intracellular PCNA |
| [79] |
| mAb14 | Monoclonal antibody to PCNA |
| [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwan, A.; Mcdermott-Brown, I.; Muthana, M. Proliferating Cell Nuclear Antigen in the Era of Oncolytic Virotherapy. Viruses 2024, 16, 1264. https://doi.org/10.3390/v16081264
Kwan A, Mcdermott-Brown I, Muthana M. Proliferating Cell Nuclear Antigen in the Era of Oncolytic Virotherapy. Viruses. 2024; 16(8):1264. https://doi.org/10.3390/v16081264
Chicago/Turabian StyleKwan, Amy, India Mcdermott-Brown, and Munitta Muthana. 2024. "Proliferating Cell Nuclear Antigen in the Era of Oncolytic Virotherapy" Viruses 16, no. 8: 1264. https://doi.org/10.3390/v16081264





