Antiviral Agents for Preventing Cytomegalovirus Disease in Recipients of Hematopoietic Cell Transplantation
Abstract
1. Introduction
1.1. Cytomegalovirus Overview
1.2. Risk Factors for CMV Viremia after HCT
1.3. Bidirectional Relationship between CMV and GVHD
2. Cytomegalovirus Diagnosis
2.1. CMV Quantitative Nucleic Acid Test
2.2. Antigen for Cytomegalovirus
2.3. Culture and Histopathology
3. Cytomegalovirus Prevention
Letermovir
4. Immune Reconstitution after Allogeneic Hematopoietic Cell Transplantation
Cytomegalovirus Cell-Mediated Immunity Assays
5. Cytomegalovirus Treatment
5.1. Ganclovir and Valganciclovir
5.2. Foscarnet
5.3. Marbavir
5.4. Cidofovir
5.5. Brincidofovir
5.6. Cytomegalovirus Immune Globulin
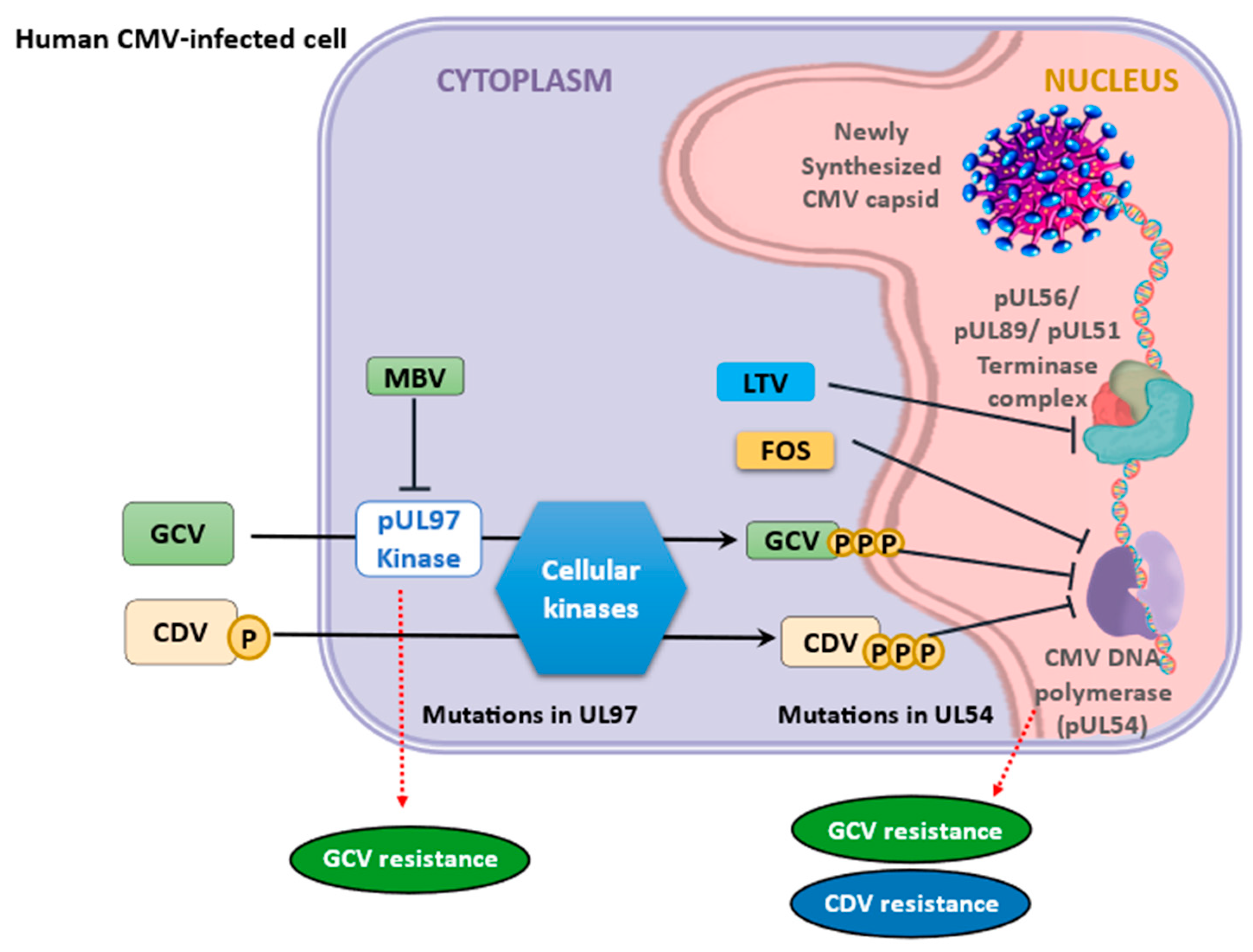
6. Cytomegalovirus Antiviral Drug Resistance
7. Use of Adoptive T-Cell Therapy as Prophylaxis
8. Cytomegalovirus Vaccines
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCV | brincidofovir |
| CD | cluster of differentiation |
| CDV | cidofovir |
| CMI | cell-mediated immunity |
| CMV | cytomegalovirus |
| DNA | deoxyribonucleic acid |
| FDA | Food and Drug Administration |
| FOS | foscarnet |
| gB | glycoprotein B |
| GCV | ganciclovir |
| GVHD | graft-versus-host disease |
| HCT | hematopoietic cell transplantation |
| HLA | human leukocyte antigen |
| Ig | immune globulin |
| LET | letermovir |
| MBV | maribavir |
| NRM | non-relapse mortality |
| OS | overall survival |
| PCR | polymerase chain reaction |
| QNAT | quantitative nucleic acid test |
| SOT | solid organ transplantation |
| UL | unique long |
| VGCV | Valganciclovir |
References
- Aldè, M.; Binda, S.; Primache, V.; Pellegrinelli, L.; Pariani, E.; Pregliasco, F.; Di Berardino, F.; Cantarella, G.; Ambrosetti, U. Congenital cytomegalovirus and hearing loss: The state of the art. J. Clin. Med. 2023, 12, 4465. [Google Scholar] [CrossRef] [PubMed]
- Bateman, C.M.; Kesson, A.; Powys, M.; Wong, M.; Blyth, E. Cytomegalovirus Infections in Children with Primary and Secondary Immune Deficiencies. Viruses 2021, 13, 2001. [Google Scholar] [CrossRef]
- Camargo, J.F.; Komanduri, K.V. Emerging concepts in cytomegalovirus infection following hematopoietic stem cell transplantation. Hematol. Oncol. Stem. Cell. Ther. 2017, 10, 233–238. [Google Scholar] [CrossRef]
- Blyth, E.; Withers, B.; Clancy, L.; Gottlieb, D. CMV-specific immune reconstitution following allogeneic stem cell transplantation. Virulence 2016, 7, 967–980. [Google Scholar] [CrossRef]
- Ljungman, P.; Hakki, M.; Boeckh, M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Hematol. Oncol. Clin. N. Am. 2011, 25, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, P.; Brand, R.; Hoek, J.; de la Camara, R.; Cordonnier, C.; Einsele, H.; Styczynski, J.; Ward, K.N.; Cesaro, S. Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Donor cytomegalovirus status influences the outcome of allogeneic stem cell transplant: A study by the European group for blood and marrow transplantation. Clin. Infect. Dis. 2014, 59, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Winston, D.J.; Razonable, R.R.; Lyon, G.M.; Silveira, F.P.; Wagener, M.M.; Stevens-Ayers, T.; Edmison, B.; Boeckh, M.; Limaye, A.P. Effect of Preemptive Therapy vs. Antiviral Prophylaxis on Cytomegalovirus Disease in Seronegative Liver Transplant Recipients with Seropositive Donors: A Randomized Clinical Trial. JAMA 2020, 323, 1378–1387. [Google Scholar] [CrossRef]
- Schmidt-Hieber, M.; Tridello, G.; Ljungman, P.; Mikulska, M.; Knelange, N.; Blaise, D.; Socié, G.; Volin, L.; Blijlevens, N.; Fegueux, N.; et al. The prognostic impact of the cytomegalovirus serostatus in patients with chronic hematological malignancies after allogeneic hematopoietic stem cell transplantation: A report from the Infectious Diseases Working Party of EBMT. Ann. Hematol. 2019, 98, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, M.; Sadowska-Krawczenko, I.; Styczynski, J. Risk Factors for Cytomegalovirus Infection after Allogeneic Hematopoietic Cell Transplantation in Malignancies: Proposal for Classification. Anticancer Res. 2017, 37, 6551–6556. [Google Scholar]
- Atilla, E.; Ataca Atilla, P.; Demirer, T. A Review of Myeloablative vs. Reduced Intensity/Non-Myeloablative Regimens in Allogeneic Hematopoietic Stem Cell Transplantations. Balkan. Med. J. 2017, 34, 1–9. [Google Scholar] [CrossRef]
- Jakharia, N.; Howard, D.; Riedel, D.J. CMV Infection in Hematopoietic Stem Cell Transplantation: Prevention and Treatment Strategies. Curr. Treat. Options. Infect. Dis. 2021, 13, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Heston, S.M.; Young, R.R.; Tanaka, J.S.; Jenkins, K.; Vinesett, R.; Saccoccio, F.M.; Martin, P.L.; Chao, N.J.; Kelly, M.S. Risk Factors for CMV Viremia and Treatment-Associated Adverse Events among Pediatric Hematopoietic Stem Cell Transplant Recipients. Open. Forum. Infect. Dis. 2021, 9, ofab639. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.L.; Ma, H.Y.; Lu, C.Y.; Chen, J.M.; Lee, P.I.; Jou, S.T.; Yang, Y.L.; Chang, H.H.; Lu, M.Y.; Chang, L.Y.; et al. Risk factors and outcomes of cytomegalovirus viremia in pediatric hematopoietic stem cell transplantation patients. J. Microbiol. Immunol. Infect. 2017, 50, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Granot, N.; Storb, R. History of hematopoietic cell transplantation: Challenges and progress. Haematologica 2020, 105, 2716–2729. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.Y.; Han, T.T.; Zuo, W.; Zhao, X.S.; Chang, Y.J.; Lv, M.; Mo, X.D.; Sun, Y.Q.; Zhang, Y.Y.; Wang, Y.; et al. CMV infection combined with acute GVHD associated with poor CD8+ T-cell immune reconstitution and poor prognosis post-HLA-matched allo-HSCT. Clin. Exp. Immunol. 2022, 208, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Cantoni, N.; Hirsch, H.H.; Khanna, N.; Gerull, S.; Buser, A.; Bucher, C.; Halter, J.; Heim, D.; Tichelli, A.; Gratwohl, A.; et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol. Blood. Marrow. Transplant. 2010, 16, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Bueno, F.; Solano, C.; Vázquez, L.; Giménez, E.; de la Cámara, R.; Albert, E.; Rovira, M.; Espigado, I.; Martín Calvo, C.; López-Jiménez, J.; et al. Assessment of the association between cytomegalovirus DNAemia and subsequent acute graft-versus-host disease in allogeneic peripheral blood stem cell transplantation: A multicenter study from the Spanish hematopoietic transplantation and cell therapy group. Transplant. Infect. Dis. 2021, 23, e13627. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhao, K.; Sun, Y.; Wen, R.; Zhang, X.; Li, X.; Long, B. Diagnosis and treatment for the early stage of cytomegalovirus infection during hematopoietic stem cell transplantation. Front. Immunol. 2022, 13, 971156. [Google Scholar] [CrossRef]
- Dong, M.Y.; Tang, B.L.; Zhu, X.Y.; Cheng, S.Q.; Fang, X.C.; Tong, J.; Wan, X.; Zheng, C.C.; Liu, H.L.; Sun, Z.M. Protective Effects of Cytomegalovirus DNA Copies ≧1000/mL for AML Patients in Complete Remission After Single Cord Blood Transplantation. Infect. Drug. Resist. 2020, 13, 373–383. [Google Scholar] [CrossRef]
- Sayyed, A.; Wilson, L.; Stavi, V.; Chen, S.; Chen, C.; Mattsson, J.; Lipton, J.H.; Kim, D.D.; Viswabandya, A.; Kumar, R.; et al. Impact of cytomegalovirus (CMV) seroconversion pre-allogeneic hematopoietic cell transplantation on posttransplant outcomes. Eur. J. Haematol. 2024. [Google Scholar] [CrossRef]
- Chorão, P.; Henriques, M.; Villalba, M.; Montoro, J.; Balaguer-Roselló, A.; González, E.M.; Gómez, M.D.; Gómez, I.; Solves, P.; Santiago, M.; et al. Cytomegalovirus Reactivations in Allogeneic Hematopoietic Stem Cell Transplantation from HLA-Matched and Haploidentical Donors with Post-Transplantation Cyclophosphamide. Transplant. Cell. Ther. 2024, 30, e1–e538. [Google Scholar] [CrossRef] [PubMed]
- Portillo, V.; Masouridi-Levrat, S.; Royston, L.; Yerly, S.; Schibler, M.; Mappoura, M.; Morin, S.; Giannotti, F.; Mamez, A.C.; van Delden, C.; et al. Revisiting cytomegalovirus serology in allogeneic hematopoietic cell transplant recipients. Clin. Infect. Dis. 2024, 78, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Natori, Y.; Alghamdi, A.; Tazari, M.; Miller, V.; Husain, S.; Komatsu, T.; Griffiths, P.; Ljungman, P.; Orchanian-Cheff, A.; Kumar, D.; et al. Use of viral load as a surrogate marker in clinical studies of cytomegalovirus in solid organ transplantation: A systematic review and meta-analysis. Clin. Infect. Dis. 2018, 66, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Erard, V.; Guthrie, K.A.; Seo, S.; Smith, J.; Huang, M.; Chien, J.; Flowers, M.E.; Corey, L.; Boeckh, M. Reduced mortality of cytomegalovirus pneumonia after hematopoietic cell transplantation due to antiviral therapy and changes in transplantation practices. Clin. Infect. Dis. 2015, 61, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kabut, T.; Weinbergerová, B.; Folber, F.; Lengerová, M.; Mayer, J. High-dose aciclovir in CMV infection prophylaxis after allogeneic HSCT: A single-center long-term experience. Bone. Marrow. Transplant. 2023, 58, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Hayden, R.T.; Sun, Y.; Tang, L.; Procop, G.W.; Hillyard, D.R.; Pinsky, B.A.; Young, S.A.; Caliendo, A.M. Progress in quantitative viral load testing: Variability and impact of the WHO Quantitative International Standards. J. Clin. Microbiol. 2017, 55, 423–430. [Google Scholar] [CrossRef]
- Limaye, A.P.; Babu, T.M.; Boeckh, M. Progress and challenges in the prevention, diagnosis, and management of cytomegalovirus infection in transplantation. Clin. Microbiol. Rev. 2020, 34, e00043-19. [Google Scholar] [CrossRef] [PubMed]
- Piñana, J.L.; Giménez, E.; Gómez, M.D.; Pérez, A.; González, E.M.; Vinuesa, V.; Hernández-Boluda, J.C.; Montoro, J.; Salavert, M.; Tormo, M.; et al. Pulmonary cytomegalovirus (CMV) DNA shedding in allogeneic hematopoietic stem cell transplant recipients: Implications for the diagnosis of CMV pneumonia. J. Infect. 2019, 78, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Boeckh, M.; Stevens-Ayers, T.; Travi, G.; Huang, M.-L.; Cheng, G.-S.; Xie, H.; Leisenring, W.; Erard, V.; Seo, S.; Kimball, L.; et al. Cytomegalovirus (CMV) DNA quantitation in bronchoalveolar lavage fluid from hematopoietic stem cell transplant recipients with CMV pneumonia. J. Infect. Dis. 2017, 215, 1514–1522. [Google Scholar] [CrossRef]
- Davidson, K.R.; Ha, D.M.; Schwarz, M.I.; Chan, E.D. Bronchoalveolar lavage as a diagnostic procedure: A review of known cellular and molecular findings in various lung diseases. J. Thorac. Dis. 2020, 12, 4991–5019. [Google Scholar] [CrossRef]
- Gu, J.; Ji, H.; Liu, T.; Chen, C.; Zhao, S.; Cao, Y.; Wang, N.; Xiao, M.; Chen, L.; Cai, H. Detection of cytomegalovirus (CMV) by digital PCR in stool samples for the non-invasive diagnosis of CMV gastroenteritis. Virol. J. 2022, 19, 183. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Lledó, M.; Marcos, M.Á.; Cuatrecasas, M.; Bombi, J.A.; Fernández-Avilés, F.; Magnano, L.; Martínez-Cibrián, N.; Llobet, N.; Rosiñol, L.; Gutiérrez-García, G.; et al. Quantitative PCR is faster, more objective, and more reliable than immunohistochemistry for the diagnosis of cytomegalovirus gastrointestinal disease in allogeneic stem cell transplantation. Biol. Blood. Marrow. Transplant. 2019, 25, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Lino, K.; Trizzotti, N.; Carvalho, F.R.; Cosendey, R.I.; Souza, C.F.; Klumb, E.M.; Silva, A.A.; Almeida, J.R. Pp65 antigenemia and cytomegalovirus diagnosis in patients with lupus nephritis: Report of a series. J. Bras. Nefrol. 2018, 40, 44–52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Malouli, D.; Hansen, S.G.; Nakayasu, E.S.; Marshall, E.E.; Hughes, C.M.; Ventura, A.B.; Gilbride, R.M.; Lewis, M.S.; Xu, G.; Kreklywich, C.; et al. Cytomegalovirus pp65 limits dissemination but is dispensable for persistence. J. Clin. Investig. 2014, 124, 1928–1944. [Google Scholar] [CrossRef] [PubMed]
- Nath, P.; Kabir, M.A.; Doust, S.K.; Ray, A. Diagnosis of herpes simplex virus: Laboratory and point-of-care techniques. Infect. Dis. Rep. 2021, 13, 518–539. [Google Scholar] [CrossRef] [PubMed]
- Razonable, R.R.; Inoue, N.; Pinninti, S.G.; Boppana, S.B.; Lazzarotto, T.; Gabrielli, L.; Simonazzi, G.; Pellett, P.E.; Schmid, D.S. Clinical diagnostic testing for human cytomegalovirus infections. J. Infect. Dis. 2020, 221, S74–S85. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Cheng, M.P.; Hammond, S.P.; Einsele, H.; Marty, F.M. Antiviral prophylaxis for cytomegalovirus infection in allogeneic hematopoietic cell transplantation. Blood Adv. 2018, 2, 2159–2175. [Google Scholar] [CrossRef]
- Gagelmann, N.; Ljungman, P.; Styczynski, J.; Kröger, N. Comparative efficacy and safety of different antiviral agents for cytomegalovirus prophylaxis in allogeneic hematopoietic cell transplantation: A systematic review and meta-analysis. Biol. Blood Marrow Transplant. 2018, 24, 2101–2109. [Google Scholar] [CrossRef]
- Girmenia, C.; Lazzarotto, T.; Bonifazi, F.; Patriarca, F.; Irrera, G.; Ciceri, F.; Aversa, F.; Citterio, F.; Cillo, U.; Cozzi, E.; et al. Assessment and prevention of cytomegalovirus infection in allogeneic hematopoietic stem cell transplant and in solid organ transplant: A multidisciplinary consensus conference by the Italian GITMO, SITO, and AMCLI societies. Clin. Transplant. 2019, 33, e13666. [Google Scholar] [CrossRef]
- Douglas, G.; Yong, M.K.; Tio, S.Y.; Chau, M.; Prabahran, A.; Sasadeusz, J.; Slavin, M.; Ritchie, D.; Chee, L. Effective CMV prophylaxis with high-dose valaciclovir in allogeneic hematopoietic stem-cell recipients at a high risk of CMV infection. Transplant. Infect. Dis. 2023, 25, e13994. [Google Scholar] [CrossRef]
- Marty, F.M.; Ljungman, P.; Chemaly, R.F.; Maertens, J.; Dadwal, S.S.; Duarte, R.F.; Haider, S.; Ullmann, A.J.; Katayama, Y.; Brown, J.; et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N. Engl. J. Med. 2017, 377, 2433–2444. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, P.; Schmitt, M.; Marty, F.M.; Maertens, J.; Chemaly, R.F.; Kartsonis, N.A.; Butterton, J.R.; Wan, H.; Teal, V.L.; Sarratt, K.; et al. A Mortality Analysis of letermovir prophylaxis for cytomegalovirus (CMV) in CMV-seropositive recipients of allogeneic hematopoietic cell transplantation. Clin. Infect. Dis. 2019, 70, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; Raja, M.; Vazquez, N.; Morris, M.; Komanduri, K.; Camargo, J. Clinical “real-world” experience with letermovir for prevention of cytomegalovirus infection in allogeneic hematopoietic cell transplant recipients. Clin. Transplant. 2020, 34, e13866. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Maloy, M.; Su, Y.; Bhatt, V.; Derespiris, L.; Griffin, M.; Lau, C.; Proli, A.; Barker, J.; Shaffer, B.; et al. Letermovir for primary and secondary cytomegalovirus prevention in allogeneic hematopoietic cell transplant recipients: Real-world experience. Transplant. Infect. Dis. 2019, 21, e13187. [Google Scholar] [CrossRef] [PubMed]
- Robin, C.; Thiebaut, A.; Alain, S.; Sicre de Fontbrune, F.; Berceanu, A.; D’Aveni, M.; Ceballos, P.; Redjoul, R.; Nguyen-Quoc, S.; Bénard, N.; et al. Letermovir for Secondary Prophylaxis of Cytomegalovirus Infection and Disease after Allogeneic Hematopoietic Cell Transplantation: Results from the French Compassionate Program. Biol. Blood Marrow Transplant. 2020, 26, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; John, L.S.S.; Wieder, E.D.; Khalili, J.; Ma, Q.; Komanduri, K.V. Human late memory CD8+ T cells have a distinct cytokine signature characterized by CC chemokine production without IL-2 production. J. Immunol. 2009, 183, 6167–6174. [Google Scholar] [CrossRef] [PubMed]
- Ciáurriz, M.; Zabalza, A.; Beloki, L.; Mansilla, C.; Pérez-Valderrama, E.; Lachén, M.; Bandrés, E.; Olavarría, E.; Ramírez, N. The immune response to cytomegalovirus in allogeneic hematopoietic stem cell transplant recipients. Cell. Mol. Life. Sci. 2015, 72, 4049–4062. [Google Scholar] [CrossRef]
- Zamora, D.; Duke, E.R.; Xie, H.; Edmison, B.C.; Akoto, B.; Kiener, R.; Stevens-Ayers, T.; Wagner, R.; Mielcarek, M.; Leisenring, W.M.; et al. Cytomegalovirus-specific T-cell reconstitution following letermovir prophylaxis after hematopoietic cell transplantation. Blood 2021, 138, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, J.; Zheng, R.; Yu, M.; Lin, Z.; Wang, C.; McCluskey, J.; Yang, J.; Chen, Z.; Corbett, A.J.; et al. The establishment of a cytomegalovirus-specific CD8+ T-cell threshold by kinetic modeling for the prediction of post-hemopoietic stem cell transplant reactivation. iScience 2022, 25, 105340. [Google Scholar] [CrossRef]
- Prockop, S.E.; Hasan, A.; Doubrovina, E.; Dahi, P.B.; Rodriguez-Sanchez, I.; Curry, M.; Mauguen, A.; Papanicolaou, G.A.; Su, Y.; Yao, J.; et al. Third-party cytomegalovirus-specific T cells improved survival in refractory cytomegalovirus viremia after hematopoietic transplant. J. Clin. Investig. 2023, 133, e165476. [Google Scholar] [CrossRef]
- El Jurdi, N.; Hoover, A.; O’Leary, D.; Cao, Q.; Gupta, A.; Ebens, C.; Maakaron, J.; Betts, B.C.; Rashidi, A.; Juckett, M.; et al. Phase II study of myeloablative 8/8- or 7/8-matched allotransplantation with post-transplant cyclophosphamide, tacrolimus, and mycophenolate mofetil: Marked reduction in GVHD risk without increased relapse risk compared to historical cyclosporine/methotrexate. medRxiv 2023, preprint. [Google Scholar]
- Chen, G.L.; Shpall, E.J. Another tool against cytomegalovirus after allogeneic hematopoietic cell transplantation. J. Clin. Investig. 2023, 133, e170282. [Google Scholar] [CrossRef] [PubMed]
- Ogonek, J.; Kralj Juric, M.; Ghimire, S.; Varanasi, P.R.; Holler, E.; Greinix, H.; Weissinger, E. Immune Reconstitution after Allogeneic Hematopoietic Stem Cell Transplantation. Front. Immunol. 2016, 7, 507. [Google Scholar] [CrossRef] [PubMed]
- González Vicent, M.; Molina, B.; de Pablo, J.G.; Castillo, A.; Díaz, M.Á. Ruxolitinib treatment for steroid refractory acute and chronic graft vs host disease in children: Clinical and immunological results. Am. J. Hematol. 2019, 94, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Chemaly, R.F.; Haddad, L.E.; Winston, D.J.; Rowley, S.D.; Mulane, K.M.; Chandrasekar, P.; Avery, R.K.; Hari, P.; Peggs, K.S.; Kumar, D.; et al. Cytomegalovirus (CMV) Cell-mediated immunity and CMV infection after allogeneic hematopoietic cell transplantation: The REACT Study. Clin. Infect. Dis. 2020, 71, 2365–2374. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.G.; Humar, A.; Kumar, D. Utility of cytomegalovirus cell-mediated immunity assays in solid organ transplantation. J. Clin. Microbiol. 2022, 60, e0171621. [Google Scholar] [CrossRef] [PubMed]
- Sester, M.; Leboeuf, C.; Schmidt, T.; Hirsch, H.H. The “ABC” of Virus-Specific T Cell Immunity in Solid Organ Transplantation. Am. J. Transplant. 2016, 16, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Bestard, O.; Kaminski, H.; Couzi, L.; Fernández-Ruiz, M.; Manuel, O. Cytomegalovirus Cell-Mediated Immunity: Ready for Routine Use? Transplant. Int. 2023, 36, 11963. [Google Scholar] [CrossRef]
- Lee, H.; Oh, E.J. Laboratory diagnostic testing for cytomegalovirus infection in solid organ transplant patients. Korean J. Transplant. 2022, 36, 15–28. [Google Scholar] [CrossRef]
- Callister, S.M.; Jobe, D.A.; Stuparic-Stancic, A.; Miyamasu, M.; Boyle, J.; Dattwyler, R.J.; Arnaboldi, P.M. Detection of IFN-γ Secretion by T Cells Collected Before and After Successful Treatment of Early Lyme Disease. Clin. Infect. Dis. 2016, 62, 1235–1241. [Google Scholar] [CrossRef]
- Yang, F.; Patton, K.; Kasprzyk, T.; Long, B.; Gupta, S.; Zoog, S.J.; Tracy, K.; Vettermann, C. Validation of an IFN-gamma ELISpot assay to measure cellular immune responses against viral antigens in non-human primates. Gene. Ther. 2022, 29, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ruiz, M.; Redondo, N.; Parra, P.; Ruiz-Merlo, T.; Rodríguez-Goncer, I.; Polanco, N.; González, E.; López-Medrano, F.; San Juan, R.; Navarro, D.; et al. Comparison of intracellular cytokine staining versus an ELISA-based assay to assess CMV-specific cell-mediated immunity in high-risk kidney transplant recipients. J. Med. Virol. 2023, 95, e28733. [Google Scholar] [CrossRef] [PubMed]
- García-Ríos, E.; Nuévalos, M.; Mancebo, F.J.; Pérez-Romero, P. Is it feasible to use CMV-specific T-cell adoptive transfer as treatment against infection in SOT recipients? Front. Immunol. 2021, 12, 657144. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, S.L. From the food and drug administration. JAMA 1991, 266, 3263. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baker, R. FDA approves oral ganciclovir as first drug to prevent CMV disease. Food and Drug Administration. BETA 1995, 8. [Google Scholar]
- Schwetz, B.A. Oral Therapy for CMV Retinitis. JAMA 2001, 285, 2705. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, S.L. From the Food and Drug Administration. JAMA 1996, 276, 1710. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bowman, L.J.; Melaragno, J.I.; Brennan, D.C. Letermovir for the management of cytomegalovirus infection. Expert. Opin. Investig. Drugs 2017, 26, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.G.; Kotton, C.N. Evaluating the safety of maribavir for the treatment of cytomegalovirus. Ther. Clin. Risk. Manag. 2022, 18, 223–232. [Google Scholar] [CrossRef]
- Saullo, J.L.; Miller, R.A. Cytomegalovirus Therapy: Role of Letermovir in Prophylaxis and Treatment in Transplant Recipients. Annu. Rev. Med. 2023, 74, 89–105. [Google Scholar] [CrossRef]
- Vaziri, S.; Pezhman, Z.; Sayyad, B.; Mansouri, F.; Janbakhsh, A.; Afsharian, M.; Najafi, F. Efficacy of valganciclovir and ganciclovir for cytomegalovirus disease in solid organ transplants: A meta-analysis. J. Res. Med. Sci. 2014, 19, 1185–1192. [Google Scholar] [PubMed]
- Chawla, J.S.; Ghobadi, A.; Mosley, J., 3rd; Verkruyse, L.; Trinkaus, K.; Abboud, C.N.; Cashen, A.F.; Stockerl-Goldstein, K.E.; Uy, G.L.; Westervelt, P.; et al. Oral valganciclovir versus ganciclovir as delayed pre-emptive therapy for patients after allogeneic hematopoietic stem cell transplant: A pilot trial (04-0274) and review of the literature. Transplant. Infect. Dis. 2012, 14, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heiden, P.L.J.; Kalpoe, J.S.; Barge, R.M.; Willemze, R.; Kroes, A.C.M.; Schippers, E.F. Oral valganciclovir as pre-emptive therapy has similar efficacy on cytomegalovirus DNA load reduction as intravenous ganciclovir in allogeneic stem cell transplantation recipients. Bone Marrow Transplant. 2006, 37, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.K.; Arav-Boger, R.; Marr, K.A.; Kraus, E.; Shoham, S.; Lees, L.; Trollinger, B.; Shah, P.; Ambinder, R.; Neofytos, D.; et al. Outcomes in Transplant Recipients Treated with Foscarnet for Ganciclovir-Resistant or Refractory Cytomegalovirus Infection. Transplantation 2016, 100, e74–e80. [Google Scholar] [CrossRef] [PubMed]
- Maertens, J.; Cordonnier, C.; Jaksch, P.; Poiré, X.; Uknis, M.; Wu, J.; Wijatyk, A.; Saliba, F.; Witzke, O.; Villano, S.; et al. Maribavir for preemptive treatment of cytomegalovirus reactivation. N. Engl. J. Med. 2019, 381, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Papanicolaou, G.A.; Silveira, F.P.; Langston, A.A.; Pereira, M.R.; Avery, R.K.; Uknis, M.; Wijatyk, A.; Wu, J.; Boeckh, M.; Marty, F.M.; et al. Maribavir for refractory or resistant cytomegalovirus infections in hematopoietic-cell or solid-organ transplant recipients: A randomized, dose-ranging, double-blind, phase 2 study. Clin. Infect. Dis. 2018, 68, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Ude, I.N.; Yeh, S.; Shantha, J.G. Cytomegalovirus retinitis in the highly active anti-retroviral therapy era. Ann. Eye. Sci. 2022, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.M.; Hay, M.W.; Emami-Naeini, P. Medication-induced Uveitis: An Update. J. Ophthalmic Vis. Res. 2021, 16, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Marty, F.M.; Winston, D.J.; Chemaly, R.F.; Mullane, K.M.; Shore, T.B.; Papanicolaou, G.A.; Chittick, G.; Brundage, T.M.; Wilson, C.; Morrison, M.E.; et al. A randomized, double-blind, placebo-controlled phase 3 trial of oral brincidofovir for cytomegalovirus prophylaxis in allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2019, 25, 369–381. [Google Scholar] [CrossRef]
- Einsele, H.; Ljungman, P.; Boeckh, M. How I treat CMV reactivation after allogeneic hematopoietic stem cell transplantation. Blood 2020, 135, 1619–1629. [Google Scholar] [CrossRef]
- Yong, M.K.; Shigle, T.L.; Kim, Y.J.; Carpenter, P.A.; Chemaly, R.F.; Papanicolaou, G.A. American Society for Transplantation and Cellular Therapy Series: #4—Cytomegalovirus treatment and management of resistant or refractory infections after hematopoietic cell transplantation. Transplant. Cell. Ther. 2021, 27, 957–967. [Google Scholar]
- Chaer, F.E.; Shah, D.P.; Chemaly, R.F. How I treat resistant cytomegalovirus infection in hematopoietic cell transplantation recipients. Blood 2016, 128, 2624–2636. [Google Scholar] [CrossRef]
- Campos, A.B.; Ribeiro, J.; Boutolleau, D.; Sousa, H. Human cytomegalovirus antiviral drug resistance in hematopoietic stem cell transplantation: Current state of the art. Rev. Med. Virol. 2016, 26, 161–182. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Lee, D.G.; Kim, H.J. Cytomegalovirus infections after hematopoietic stem cell transplantation: Current status and future immunotherapy. Int. J. Mol. Sci. 2019, 20, 2666. [Google Scholar] [CrossRef] [PubMed]
- Panda, K.; Parashar, D.; Viswanathan, R. An update on current antiviral strategies to combat human cytomegalovirus infection. Viruses 2023, 15, 1358. [Google Scholar] [CrossRef]
- Pierini, A.; Ruggeri, L.; Carotti, A.; Falzetti, F.; Saldi, S.; Terenzi, A.; Zucchetti, C.; Ingrosso, G.; Zei, T.; Iacucci Ostini, R.; et al. Haploidentical age-adapted myeloablative transplant and regulatory and effector T cells for acute myeloid leukemia. Blood Adv. 2021, 5, 1199–1208. [Google Scholar] [CrossRef]
- Kaeuferle, T.; Krauss, R.; Blaeschke, F.; Willier, S.; Feuchtinger, T. Strategies of adoptive T-cell transfer to treat refractory viral infections post allogeneic stem cell transplantation. J. Hematol. Oncol. 2019, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Shafat, M.S.; Mehra, V.; Peggs, K.S.; Roddie, C. Therapeutic Approaches to Cytomegalovirus Infection Following Allogeneic Stem Cell Transplantation. Front. Immunol. Front. Immunol. 2020, 11, 1694. [Google Scholar] [CrossRef]
- Tzannou, I.; Papadopoulou, A.; Naik, S.; Leung, K.; Martinez, C.A.; Ramos, C.A.; Carrum, G.; Sasa, G.; Lulla, P.; Watanabe, A.; et al. Off-the-shelf virus-specific T cells to treat BK virus, human herpesvirus 6, cytomegalovirus, Epstein-Barr virus, and adenovirus infections after allogeneic hematopoietic stem-cell transplantation. J. Clin. Oncol. 2017, 35, 3547–3557. [Google Scholar] [CrossRef]
- Withers, B.; Blyth, E.; Clancy, L.E.; Yong, A.; Fraser, C.; Burgess, J.; Simms, R.; Brown, R.; Kliman, D.; Dubosq, M.C.; et al. Long-term control of recurrent or refractory viral infections after allogeneic HSCT with third-party virus-specific T cells. Blood. Adv. 2017, 1, 2193–2205. [Google Scholar] [CrossRef]
- Neuenhahn, M.; Albrecht, J.; Odendahl, M.; Schlott, F.; Dössinger, G.; Schiemann, M.; Lakshmipathi, S.; Martin, K.; Bunjes, D.; Harsdorf, S.; et al. Transfer of minimally manipulated CMV-specific T cells from stem cell or third-party donors to treat CMV infection after allo-HSCT. Leukemia 2017, 31, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.Y.; Zhao, X.Y.; Chang, Y.J.; Liu, J.; Xu, L.P.; Wang, Y.; Zhang, X.H.; Han, W.; Chen, Y.H.; Huang, X.J. Cytomegalovirus-Specific T-Cell Transfer for Refractory Cytomegalovirus Infection after Haploidentical Stem Cell Transplantation: The Quantitative and Qualitative Immune Recovery for Cytomegalovirus. J. Infect. Dis. 2017, 216, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.A.; John, T.D.; Keller, M.D.; Cruz, C.R.N.; Salem, B.; Roesch, L.; Liu, H.; Hoq, F.; Grilley, B.J.; Gee, A.P.; et al. Safety and feasibility of virus-specific T cells derived from umbilical cord blood in cord blood transplant recipients. Blood Adv. 2019, 3, 2057–2068. [Google Scholar] [CrossRef] [PubMed]
- Blyth, E.; Clancy, L.; Simms, R.; Ma, C.K.K.; Burgess, J.; Deo, S.; Byth, K.; Dubosq, M.C.; Shaw, P.J.; Micklethwaite, K.P.; et al. Donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood 2013, 121, 3745–3758. [Google Scholar] [CrossRef] [PubMed]
- Maertens, J.; Logan, A.C.; Jang, J.; Long, G.; Tang, J.L.; Hwang, W.Y.K.; Koh, L.P.; Chemaly, R.; Gerbitz, A.; Winkler, J.; et al. Phase 2 study of anti-human cytomegalovirus monoclonal antibodies for prophylaxis in hematopoietic cell transplantation. Antimicrob. Agents. Chemother. 2020, 64, e02467-19. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; Baraniak, I.A.; Lankina, A.; Moulder, Z.; Holenya, P.; Atkinson, C.; Tang, G.; Mahungu, T.; Kern, F.; Griffiths, P.D.; et al. The cytomegalovirus gB/MF59 vaccine candidate induces antibodies against an antigenic domain controlling cell-to-cell spread. Nat. Commun. 2023, 14, 1041. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; La Rosa, C.; Longmate, J.; Drake, J.; Slape, C.; Zhou, Q.; Lampa, M.G.; O’Donnell, M.; Cai, J.L.; Farol, L.; et al. Viraemia, immunogenicity, and survival outcomes of cytomegalovirus chimeric epitope vaccine supplemented with PF03512676 (CMVPepVax) in allogeneic haemopoietic stem-cell transplantation: Randomised phase 1b trial. Lancet Haematol. 2016, 3, e87–e98. [Google Scholar] [CrossRef] [PubMed]
- Diamond, D.J.; La Rosa, C.; Chiuppesi, F.; Contreras, H.; Dadwal, S.; Wussow, F.; Bautista, S.; Nakamura, R.; Zaia, J.A. A fifty-year odyssey: Prospects for a cytomegalovirus vaccine in transplant and congenital infection. Expert. Rev. Vaccines 2018, 17, 889–911. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.I.; Munoz, F.M.; Callahan, S.T.; Rupp, R.; Wootton, S.H.; Edwards, K.M.; Turley, C.B.; Stanberry, L.R.; Patel, S.M.; Mcneal, M.M.; et al. Safety and efficacy of a cytomegalovirus glycoprotein B (gB) vaccine in adolescent girls: A randomized clinical trial. Vaccine 2016, 34, 313–319. [Google Scholar] [CrossRef]
- Pass, R.F.; Zhang, C.; Evans, A.; Simpson, T.; Andrews, W.; Huang, M.L.; Corey, L.; Hill, J.; Davis, E.; Flanigan, C.; et al. Vaccine prevention of maternal cytomegalovirus infection. N. Engl. J. Med. 2009, 360, 1191–1199. [Google Scholar] [CrossRef]
- Fierro, C.; Brune, D.; Shaw, M.; Schwartz, H.; Knightly, C.; Lin, J.; Carfi, A.; Natenshon, A.; Kalidindi, S.; Reuter, C.; et al. Safety and Immunogenicity of a Messenger RNA-Based Cytomegalovirus Vaccine in Healthy Adults: Results from a Phase 1, Randomized, Clinical Trial. J. Infect. Dis. 2024, jiae114. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Karthigeyan, K.P.; Herbek, S.; Valencia, S.M.; Jenks, J.A.; Webster, H.; Miller, I.G.; Connors, M.; Pollara, J.; Andy, C.; et al. Human cytomegalovirus mRNA-1647 vaccine candidate elicits potent and broad neutralization and higher antibody-dependent cellular cytotoxicity responses than the gB/MF59 vaccine. J. Infect. Dis. 2024, jiad593. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.; Schmitt, M.; Pilorge, S.; Stelljes, M.; Kawakita, T.; Teal, V.L.; Haber, B.; Bopp, C.; Dadwal, S.S.; Badshah, C. Efficacy and safety of extended duration letermovir prophylaxis in recipients of haematopoietic stem-cell transplantation at risk of cytomegalovirus infection: A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Haematol. 2024, 11, e127–e135. [Google Scholar] [CrossRef] [PubMed]
| Agent | Indications in Transplant Recipients | Dosing for CMV Prevention in Pediatric Transplant | Dosing for CMV Treatment in Pediatric Transplant | Adverse Effects | Major Resistance Mutations |
|---|---|---|---|---|---|
| Valganciclovir | First line for treatment and prevention of CMV DNAemia and disease | 7 × BSA × CrCLS with an upper limit of creatinine clearance of 150 mL/min PO daily (max dose 900 mg PO daily) or 16 mg/kg PO daily (max dose 900 mg PO daily) | 7 × BSA × CrCLS with an upper limit of creatinine clearance of 150 mL/min PO BID (max dose 900 mg PO BID) or 16 mg/kg PO BID (max dose 900 mg PO BID) | Hematologic toxicity, infertility, fetal toxicity, nephrotoxicity, diarrhea | UL97 > UL54 |
| Ganciclovir | First line for treatment and prevention of CMV DNAemia and disease | 5 mg/kg IV once daily | 5 mg/kg IV every 12 h | Hematological toxicity, infertility, fetal toxicity, nephrotoxicity, diarrhea, headache | UL97 > UL54 |
| Foscarnet | Second-line agent for therapy in SOT recipients First-line agent for HCT recipients who cannot tolerate marrow suppression from Ganciclovir/Valganciclovir | Currently not indicated for prevention in SOT recipients 90 mg/kg IV every 24 h for prevention in HCT | Induction: 60 mg/kg/dose q8h or 90 mg/kg q12 h for 7–14 days. Maintenance: 90 mg/kg/dose once daily until the indicator test is negative. Renal dose adjustment is required; no hepatic adjustment is needed | Seizures, nephrotoxicity, headache, hypokalemia, hypocalcemia, hypomagnesemia, hypophosphatemia, nausea, vomiting, diarrhea, anemia, granulocytopenia | UL54 |
| Cidofovir | A third-line drug used to treat CMV DNAemia and disease in SOT patients second-line drug for treating and preventing CMV DNAemia and disease in people who have had a heart transplant Second-line treatment for CMV DNAemia or illness that is resistant to ganciclovir UL97 mutation | Not recommended for prevention in SOT users at this time 5 mg/kg/dose IV once a week for two weeks in a row, then 5 mg/kg/dose IV once every two weeks, along with probenecid in HCT | 5 mg/kg/dose IV once a week for two weeks in a row, then 5 mg/kg/dose IV once every two weeks, along with probenecid | Nephrotoxicity, neutropenia, carcinogenic and teratogenic, infusion reactions, headache, nausea | UL54 |
| Maribavir | Refractory CMV DNAemia or disease (with or without resistance to traditional agents) age 12 and older | Currently not indicated for CMV prevention | 400 mg PO twice daily for patients ≧12 years of age and ≧35 kg | Changes in taste, diarrhea, loss of taste, nausea, unusual tiredness or weakness, and vomiting. Recommend reviewing contaminant medications as it has numerous other drug-drug interactions Appropriate studies have not been performed on the relationship of age to the effects of MBV in children younger than 12 years of age. | UL97 |
| Letermovir | Prophylaxis of CMV infection and disease in adult CMV-seropositive recipients [R+] of an allogeneic hematopoietic stem cell transplant | 480 mg PO or IV administered once daily (over 1 h) through 100 days posttransplant; dose should be reduced to 240 mg if coadministered with cyclosporine | Currently not indicated for treatment | Headache, nausea, peripheral edema, diarrhea When coadministered with CYP3A4 inhibitors, including cyclosporine, tacrolimus, and statins, dose modifications and constant monitoring are needed. | UL56 > UL51 and UL89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaing, T.-H.; Wang, Y.-L.; Chiu, C.-C. Antiviral Agents for Preventing Cytomegalovirus Disease in Recipients of Hematopoietic Cell Transplantation. Viruses 2024, 16, 1268. https://doi.org/10.3390/v16081268
Jaing T-H, Wang Y-L, Chiu C-C. Antiviral Agents for Preventing Cytomegalovirus Disease in Recipients of Hematopoietic Cell Transplantation. Viruses. 2024; 16(8):1268. https://doi.org/10.3390/v16081268
Chicago/Turabian StyleJaing, Tang-Her, Yi-Lun Wang, and Chia-Chi Chiu. 2024. "Antiviral Agents for Preventing Cytomegalovirus Disease in Recipients of Hematopoietic Cell Transplantation" Viruses 16, no. 8: 1268. https://doi.org/10.3390/v16081268
APA StyleJaing, T.-H., Wang, Y.-L., & Chiu, C.-C. (2024). Antiviral Agents for Preventing Cytomegalovirus Disease in Recipients of Hematopoietic Cell Transplantation. Viruses, 16(8), 1268. https://doi.org/10.3390/v16081268






