Bergamottin Inhibits Bovine Viral Diarrhea Virus Replication by Suppressing ROS-Mediated Endoplasmic Reticulum Stress and Apoptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines, Viruses, and Reagents
2.2. Cytotoxicity and Antiviral Activity Assay
2.3. Real-Time Quantitative Reverse-Transcription PCR (RT-qPCR)
2.4. Virus Titration
2.5. Indirect Immunofluorescence Assay (IFA)
2.6. Western Blotting
2.7. Virus Inactivation and Replication Cycle Assay
2.8. Imaging of ER Morphology
2.9. TUNEL Staining
2.10. Determination of Mitochondrial Membrane Potential (∆Ψm)
2.11. Detection of the Antioxidant Status in Cells and Tissues
2.12. Measurement of Intracellular Reactive Oxygen Species (ROS) Levels
2.13. Animal Experiments
2.14. Immunohistochemistry (IHC)
2.15. Statistical Analysis
3. Results
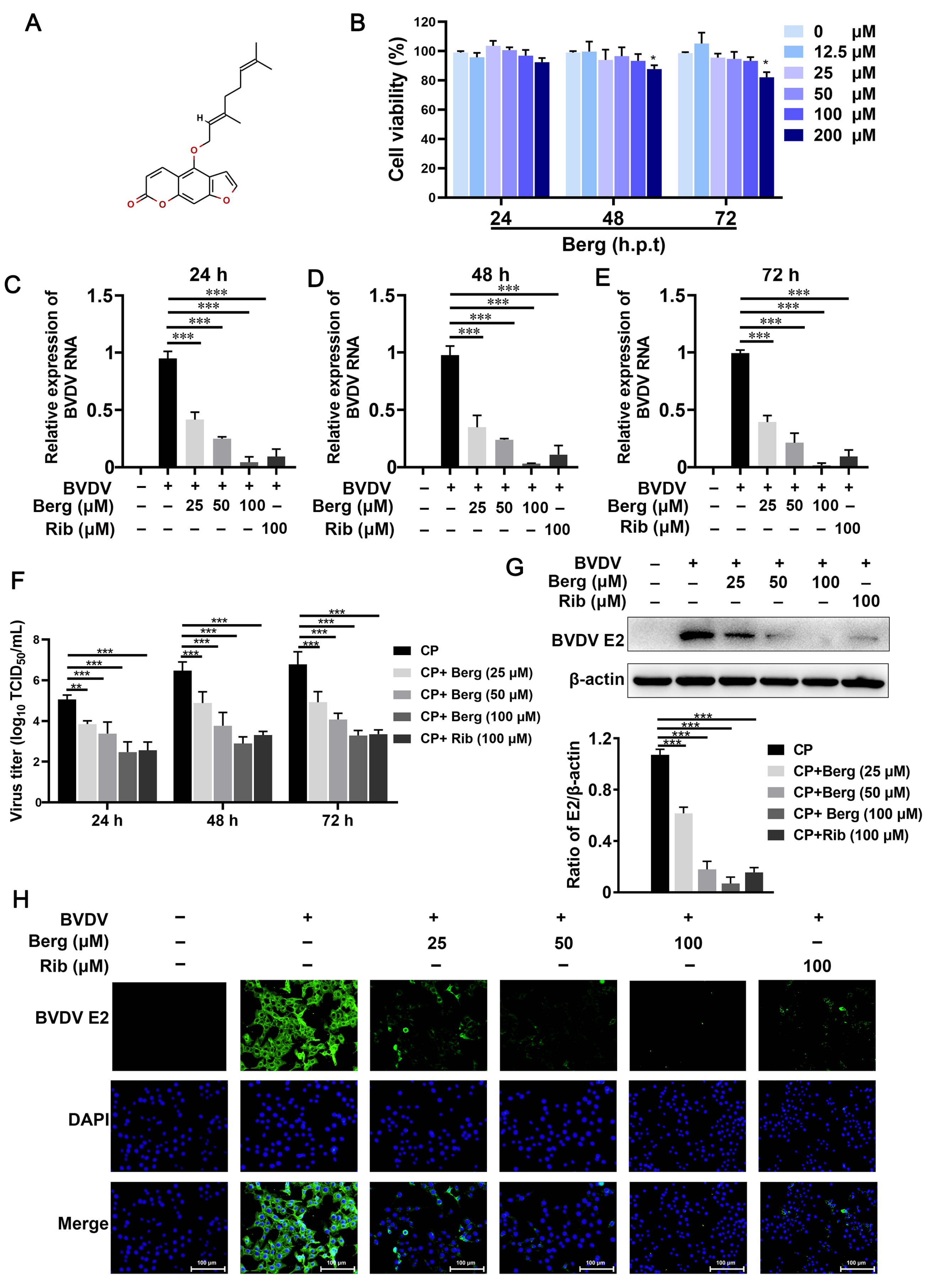
3.1. Berg Displays Antiviral Activity of BVDV in MDBK Cells
3.2. Berg Blocks the Replication and Release of BVDV, Rather Than Inactivating Virus Particles
3.3. Berg Attenuates BVDV-Induced ER Stress
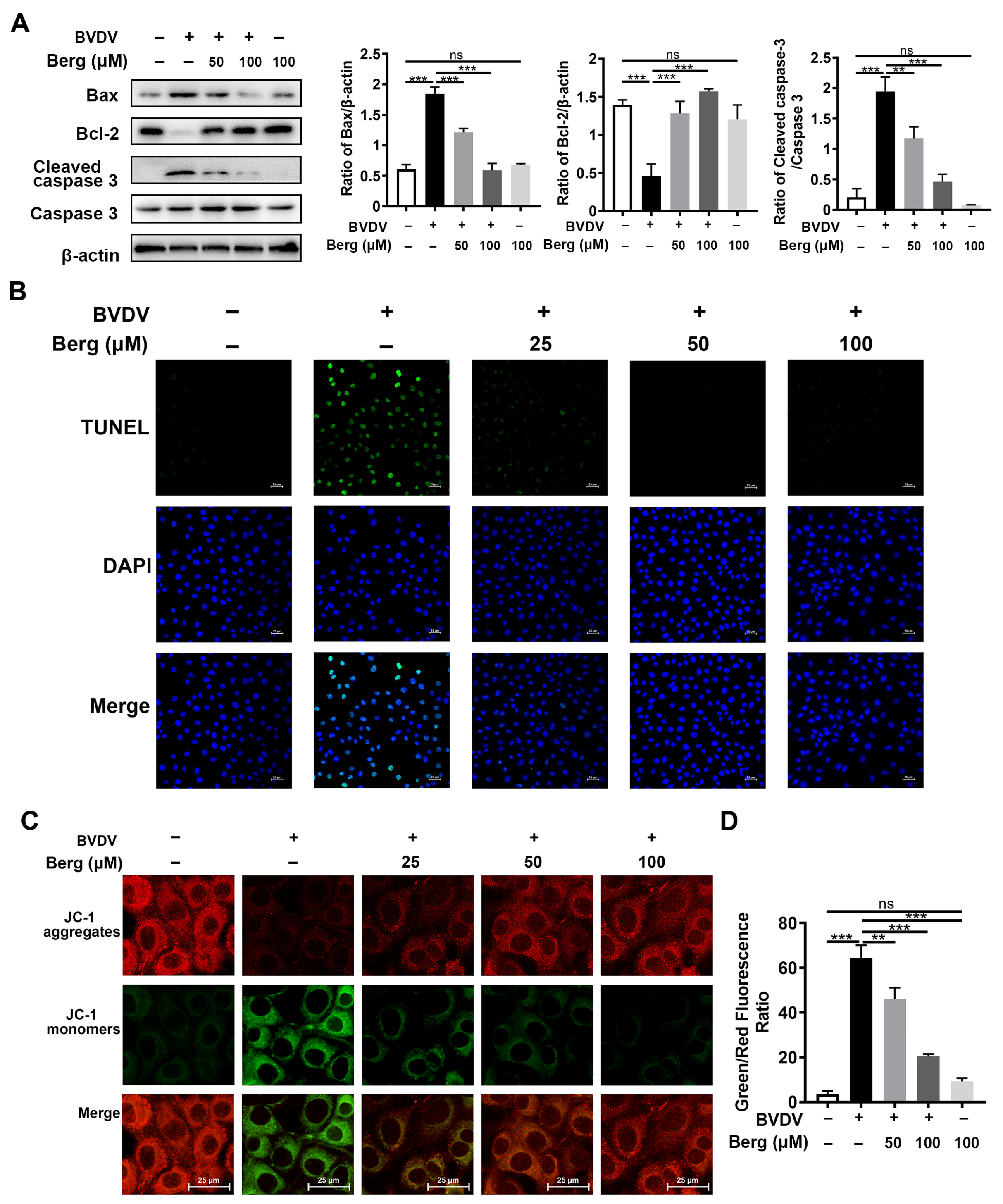
3.4. Berg Inhibits BVDV-Induced Apoptosis
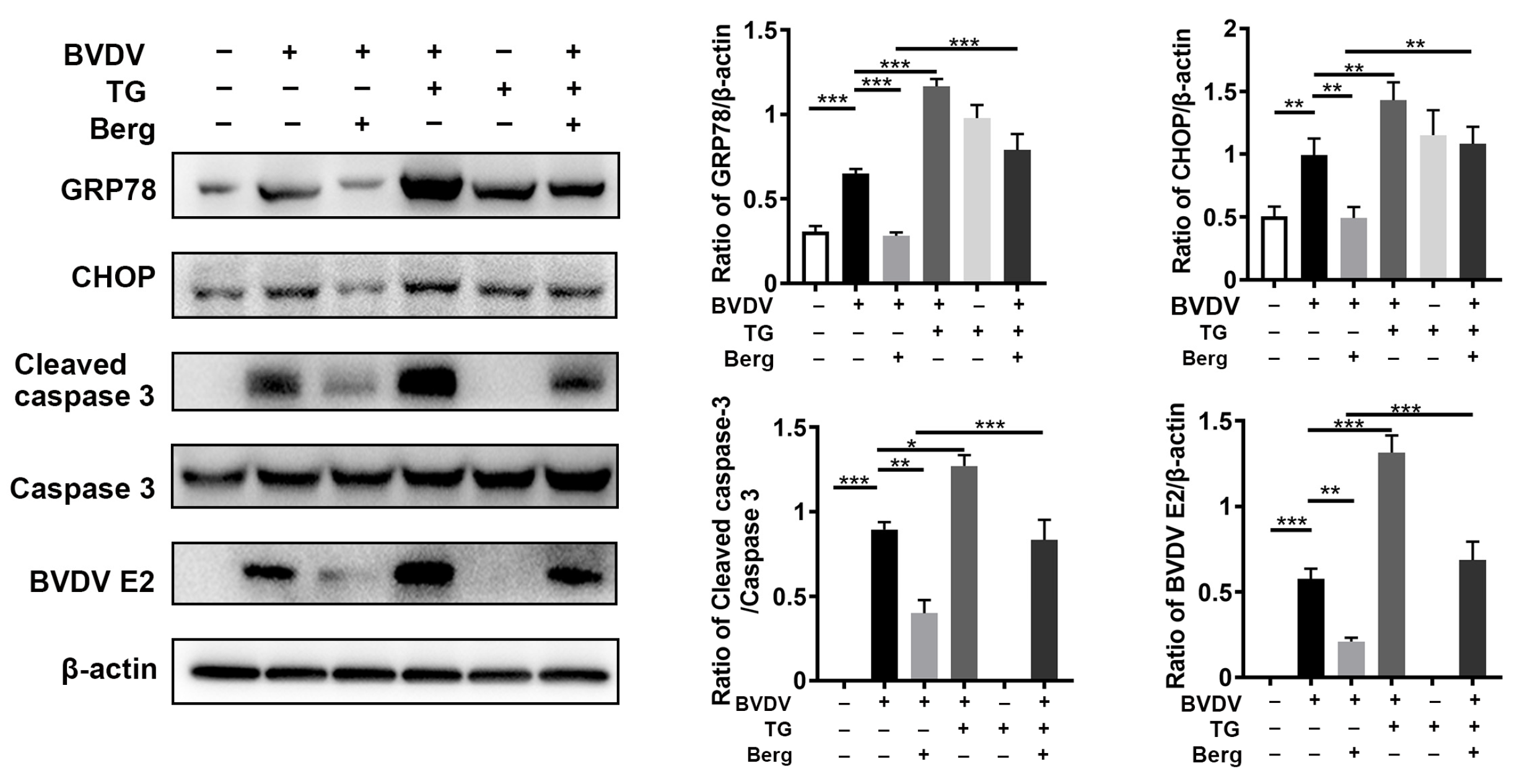
3.5. Berg Suppresses ER Stress-Mediated Apoptosis in BVDV-Infected Cells
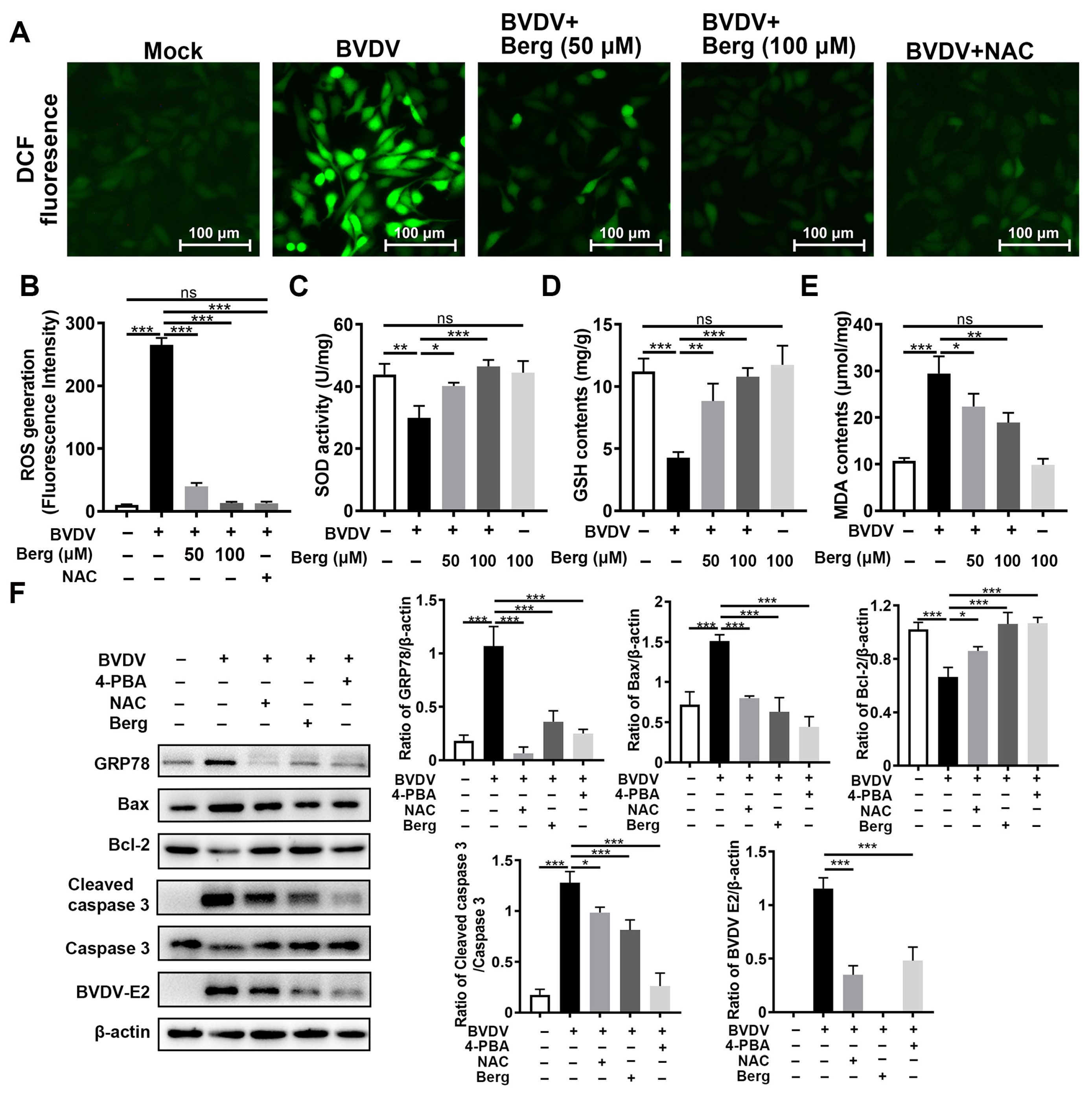
3.6. Berg Inhibits BVDV Propagation through Suppression of ER Stress-Mediated Apoptosis via Reduction in ROS Generation
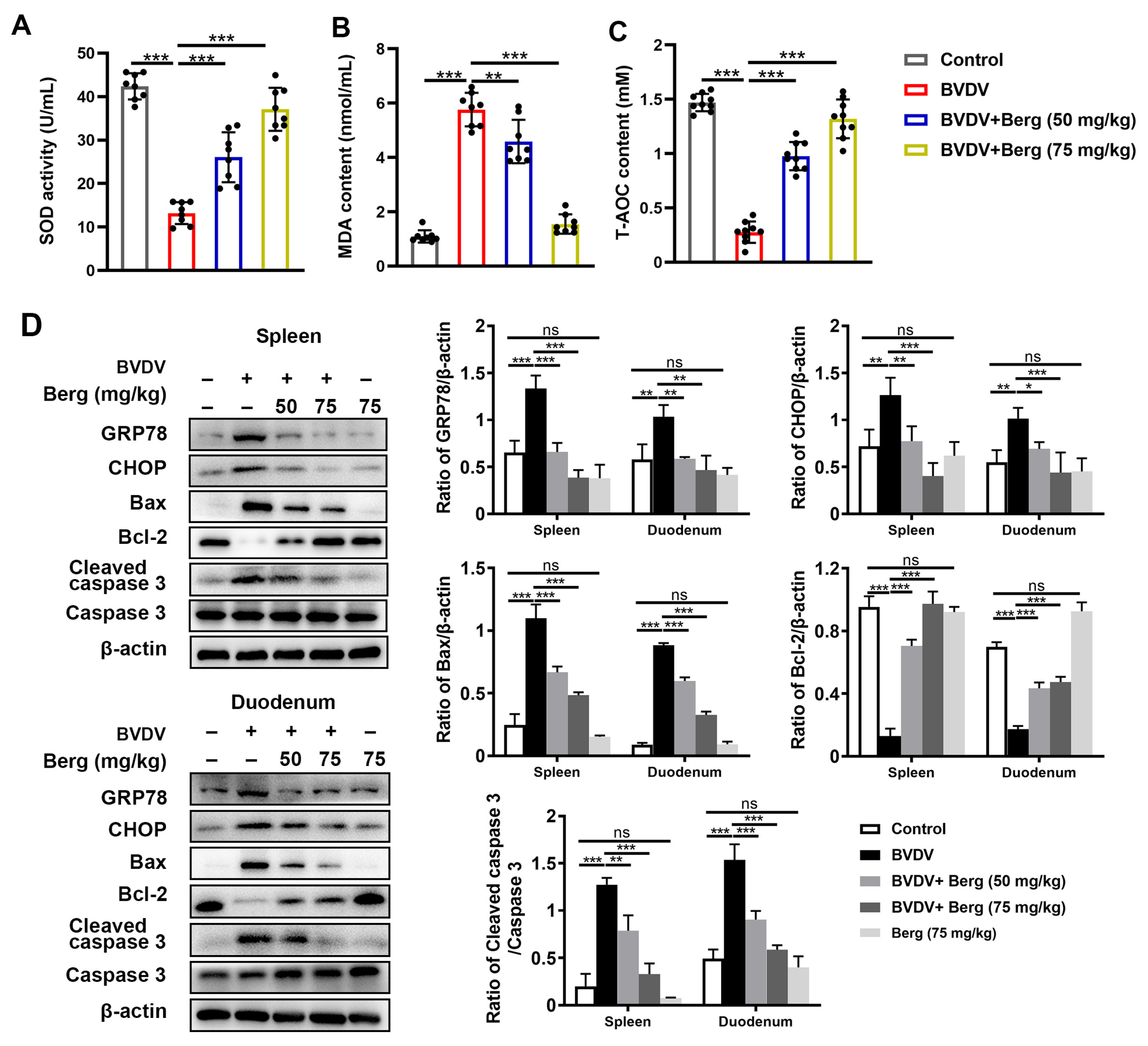
3.7. Berg Exerts Antiviral Efficacy against BVDV Infection In Vivo
3.8. Berg Weakens ER Stress-Mediated Apoptosis through Antioxidant System in BVDV-Challenged Mice
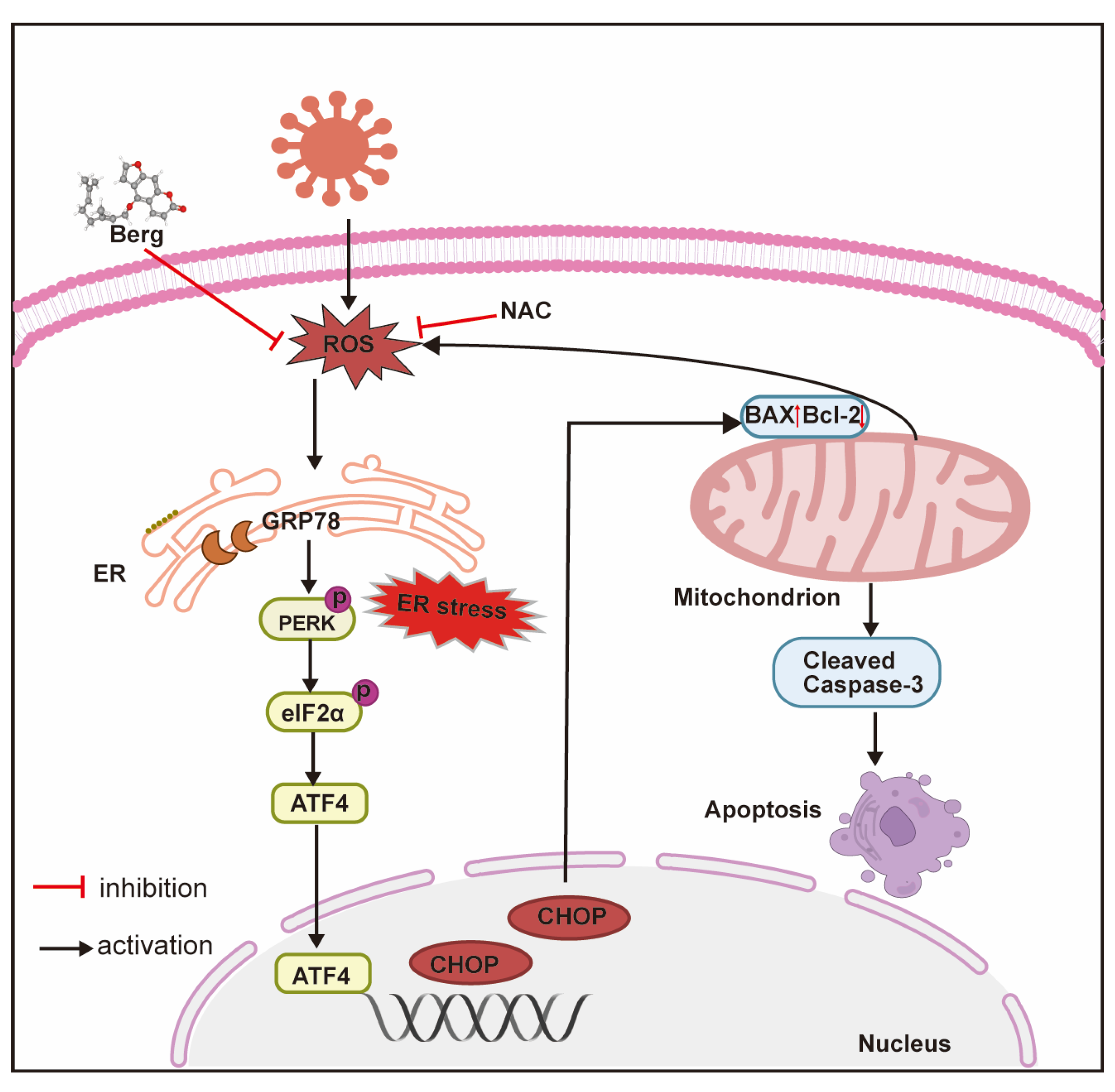
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pang, F.; Long, Q.; Wei, M. Immune evasion strategies of bovine viral diarrhea virus. Front. Cell. Infect. Microbiol. 2023, 13, 1282526. [Google Scholar] [CrossRef] [PubMed]
- Postel, A.; Smith, D.B.; Becher, P. Proposed Update to the Taxonomy of Pestiviruses: Eight Additional Species within the Genus Pestivirus, Family Flaviviridae. Viruses 2021, 13, 1542. [Google Scholar] [CrossRef]
- Olafson, P.; Mac, C.A.; Fox, F.H. An apparently new transmissible disease of cattle. Cornell Vet. 1946, 36, 205–213. [Google Scholar] [PubMed]
- Zhu, J.; Wang, C.; Zhang, L.; Zhu, T.; Li, H.; Wang, Y.; Xue, K.; Qi, M.; Peng, Q.; Chen, Y.; et al. Isolation of BVDV-1a, 1m, and 1v strains from diarrheal calf in china and identification of its genome sequence and cattle virulence. Front. Vet. Sci. 2022, 9, 1008107. [Google Scholar] [CrossRef] [PubMed]
- Canal, C.W.; Strasser, M.; Hertig, C.; Masuda, A.; Peterhans, E. Detection of antibodies to bovine viral diarrhoea virus (BVDV) and characterization of genomes of BVDV from Brazil. Vet. Microbiol. 1998, 63, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Al-Khaliyfa, M.A.; Abuelzein, E.M.; Gameel, A.A. Identification of cattle persistently infected with BVDV by ear-notch testing in Saudi Arabia. Vet. Rec. 2010, 167, 660–661. [Google Scholar] [CrossRef]
- Richter, V.; Lebl, K.; Baumgartner, W.; Obritzhauser, W.; Käsbohrer, A.; Pinior, B. A systematic worldwide review of the direct monetary losses in cattle due to bovine viral diarrhoea virus infection. Vet. J. 2017, 220, 80–87. [Google Scholar] [CrossRef]
- Nugroho, W.; Silitonga, R.J.P.; Reichel, M.P.; Irianingsih, S.H.; Wicaksono, M.S. The Epidemiology and Control of Bovine Viral Diarrhoea Virus in Tropical Indonesian Cattle. Pathogens 2022, 11, 215. [Google Scholar] [CrossRef]
- Riitho, V.; Strong, R.; Larska, M.; Graham, S.P.; Steinbach, F. Bovine Pestivirus Heterogeneity and Its Potential Impact on Vaccination and Diagnosis. Viruses 2020, 12, 1134. [Google Scholar] [CrossRef]
- Fulton, R.W. Impact of species and subgenotypes of bovine viral diarrhea virus on control by vaccination. Anim. Health Res. Rev. 2015, 16, 40–54. [Google Scholar] [CrossRef]
- Song, Q.J.; Weng, X.G.; Cai, D.J.; Zhang, W.; Wang, J.F. Forsythoside A Inhibits BVDV Replication via TRAF2-Dependent CD28-4-1BB Signaling in Bovine PBMCs. PLoS ONE 2016, 11, e0162791. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Liu, Y.; Bai, T.; Chen, J.; Zhao, Z.; Li, J.; Shao, B.; Zhang, Z.; Zhou, Y.; Wang, X.; et al. Quercetin Inhibits Hsp70 Blocking of Bovine Viral Diarrhea Virus Infection and Replication in the Early Stage of Virus Infection. Viruses 2022, 14, 2365. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Shen, Z.; Tian, B.; Chen, J.; Zhang, Y.; Shen, L.; Wang, Y.; Ma, X.; Zuo, Z. Matrine and icariin can inhibit bovine viral diarrhoea virus replication by promoting type I interferon response in vitro. J. Vet. Res. 2024, 68, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Finkielsztein, L.M.; Moltrasio, G.Y.; Caputto, M.E.; Castro, E.F.; Cavallaro, L.V.; Moglioni, A.G. What is known about the antiviral agents active against bovine viral diarrhea virus (BVDV)? Curr. Med. Chem. 2010, 17, 2933–2955. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ma, L.; Jiang, D.; Zhu, S.; Yan, F.; Xie, Y.; Xie, Z.; Guo, W.; Deng, X. Content evaluation of 4 furanocoumarin monomers in various citrus germplasms. Food Chem. 2015, 187, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Arfuso, F.; Sethi, G.; Ahn, K.S. Pharmacological Utilization of Bergamottin, Derived from Grapefruits, in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2018, 19, 4048. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.L.; Suh, J.H.; Wang, Y. Chemistry and health effects of furanocoumarins in grapefruit. J. Food Drug Anal. 2017, 25, 71–83. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, Y.; Cao, J.; Dong, S.; Hou, Y.; Yu, Y.; Zhang, Q.; Zhang, Y.; Jia, X.; Zhang, B.; et al. Bergamottin, a bioactive component of bergamot, inhibits SARS-CoV-2 infection in golden Syrian hamsters. Antiviral Res. 2022, 204, 105365. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Y.; Zeb, A.; Wu, Y.; Cheng, L. Bergamottin and PAP-1 Induced ACE2 Degradation to Alleviate Infection of SARS-CoV-2. Int. J. Mol. Sci. 2022, 23, 12565. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, J.; Cao, J.; Zhang, G.; Jia, X.; Wang, P.; Xiao, G.; Wang, W. Screening of Botanical Drugs against Lassa Virus Entry. J. Virol. 2021, 95, e02429-20. [Google Scholar] [CrossRef]
- Prasad, V.; Greber, U.F. The endoplasmic reticulum unfolded protein response—Homeostasis, cell death and evolution in virus infections. FEMS Microbiol. Rev. 2021, 45, fuab016. [Google Scholar] [CrossRef]
- Hetz, C.; Papa, F.R. The Unfolded Protein Response and Cell Fate Control. Mol. Cell 2018, 69, 169–181. [Google Scholar] [CrossRef]
- Li, S.; Kong, L.; Yu, X. The expanding roles of endoplasmic reticulum stress in virus replication and pathogenesis. Crit. Rev. Microbiol. 2015, 41, 150–164. [Google Scholar] [CrossRef]
- Hu, T.; Wang, J.; Li, W.; Liu, M.; Han, N.; Yuan, M.; Du, L.; Tang, H. Endoplasmic Reticulum Stress in Hepatitis B Virus and Hepatitis C Virus Infection. Viruses 2022, 14, 2630. [Google Scholar] [CrossRef]
- Yu, C.Y.; Hsu, Y.W.; Liao, C.L.; Lin, Y.L. Flavivirus infection activates the XBP1 pathway of the unfolded protein response to cope with endoplasmic reticulum stress. J. Virol. 2006, 80, 11868–11880. [Google Scholar] [CrossRef]
- Jordan, R.; Wang, L.; Graczyk, T.M.; Block, T.M.; Romano, P.R. Replication of a cytopathic strain of bovine viral diarrhea virus activates PERK and induces endoplasmic reticulum stress-mediated apoptosis of MDBK cells. J. Virol. 2002, 76, 9588–9599. [Google Scholar] [CrossRef]
- Wang, J.; Chen, K.Y.; Wang, S.H.; Liu, Y.; Zhao, Y.Q.; Yang, L.; Yang, G.H.; Wang, X.J.; Zhu, Y.H.; Yin, J.H.; et al. Effects of Spatial Expression of Activating Transcription Factor 4 on the Pathogenicity of Two Phenotypes of Bovine Viral Diarrhea Virus by Regulating the Endoplasmic Reticulum-Mediated Autophagy Process. Microbiol. Spectr. 2023, 11, e04225-22. [Google Scholar] [CrossRef]
- Yazıcı, E.; Şahin, E.; Alvuroğlu, E.; Yuluğ, E.; Menteşe, A. Bergamottin reduces liver damage by suppressing inflammation, endoplasmic reticulum and oxidative stress in cafeteria diet-fed mice. Food Biosci. 2023, 52, 102371. [Google Scholar] [CrossRef]
- Shen, Y.; Shenk, T.E. Viruses and apoptosis. Curr. Opin. Genet. Dev. 1995, 5, 105–111. [Google Scholar] [CrossRef]
- Zhang, G.; Aldridge, S.; Clarke, M.C.; McCauley, J.W. Cell death induced by cytopathic bovine viral diarrhoea virus is mediated by apoptosis. J. Gen. Virol. 1996, 77 Pt 8, 1677–1681. [Google Scholar] [CrossRef]
- Grummer, B.; Bendfeldt, S.; Wagner, B.; Greiser-Wilke, I. Induction of the intrinsic apoptotic pathway in cells infected with cytopathic bovine virus diarrhoea virus. Virus Res. 2002, 90, 143–153. [Google Scholar] [CrossRef]
- Bendfeldt, S.; Grummer, B.; Greiser-Wilke, I. No caspase activation but overexpression of Bcl-2 in bovine cells infected with noncytopathic bovine virus diarrhoea virus. Vet. Microbiol. 2003, 96, 313–326. [Google Scholar] [CrossRef]
- Yamane, D.; Kato, K.; Tohya, Y.; Akashi, H. The relationship between the viral RNA level and upregulation of innate immunity in spleen of cattle persistently infected with bovine viral diarrhea virus. Vet. Microbiol. 2008, 129, 69–79. [Google Scholar] [CrossRef]
- Schweizer, M.; Peterhans, E. Oxidative stress in cells infected with bovine viral diarrhoea virus: A crucial step in the induction of apoptosis. J. Gen. Virol. 1999, 80 Pt 5, 1147–1155. [Google Scholar] [CrossRef]
- Yuan, N.; Song, Q.; Jin, Y.; Zhang, Z.; Wu, Z.; Sheng, X.; Qi, X.; Xing, K.; Xiao, L.; Wang, X. Replication of standard bovine viral diarrhea strain OregonC24Va induces endoplasmic reticulum stress-mediated apoptosis of bovine trophoblast cells. Cell Stress. Chaperones 2023, 28, 49–60. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, A. Virus-induced ER stress and the unfolded protein response. Front. Plant Sci. 2012, 3, 293. [Google Scholar] [CrossRef]
- Zhang, C.; Pu, F.; Zhang, A.; Xu, L.; Li, N.; Yan, Y.; Gao, J.; Liu, H.; Zhang, G.; Goodfellow, I.G.; et al. Heme Oxygenase-1 Suppresses Bovine Viral Diarrhoea Virus Replication in vitro. Sci. Rep. 2015, 5, 15575. [Google Scholar] [CrossRef]
- Wang, S.; Yang, G.; Nie, J.; Yang, R.; Du, M.; Su, J.; Wang, J.; Wang, J.; Zhu, Y. Recombinant E(rns)-E2 protein vaccine formulated with MF59 and CPG-ODN promotes T cell immunity against bovine viral diarrhea virus infection. Vaccine 2020, 38, 3881–3891. [Google Scholar] [CrossRef]
- Neill, J.D. Molecular biology of bovine viral diarrhea virus. Biologicals 2013, 41, 2–7. [Google Scholar] [CrossRef]
- Grummer, B.; Grotha, S.; Greiser-Wilke, I. Bovine viral diarrhoea virus is internalized by clathrin-dependent receptor-mediated endocytosis. J. Vet. Med. B Infect. Dis. Vet. Public Health 2004, 51, 427–432. [Google Scholar] [CrossRef]
- Krey, T.; Himmelreich, A.; Heimann, M.; Menge, C.; Thiel, H.J.; Maurer, K.; Rümenapf, T. Function of bovine CD46 as a cellular receptor for bovine viral diarrhea virus is determined by complement control protein 1. J. Virol. 2006, 80, 3912–3922. [Google Scholar] [CrossRef]
- Lecot, S.; Belouzard, S.; Dubuisson, J.; Rouillé, Y. Bovine viral diarrhea virus entry is dependent on clathrin-mediated endocytosis. J. Virol. 2005, 79, 10826–10829. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; He, M.; Xiong, S.; Xia, X. Endoplasmic reticulum stress: Molecular mechanism and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 352. [Google Scholar] [CrossRef]
- Zhou, X.; Jiang, W.; Liu, Z.; Liu, S.; Liang, X. Virus Infection and Death Receptor-Mediated Apoptosis. Viruses 2017, 9, 316. [Google Scholar] [CrossRef]
- Gao, P.; Zhou, L.; Wu, J.; Weng, W.; Wang, H.; Ye, M.; Qu, Y.; Hao, Y.; Zhang, Y.; Ge, X.; et al. Riding apoptotic bodies for cell-cell transmission by African swine fever virus. Proc. Natl. Acad. Sci. USA 2023, 120, e2309506120. [Google Scholar] [CrossRef]
- Sun, D.; Chen, S.; Cheng, A.; Wang, M. Roles of the Picornaviral 3C Proteinase in the Viral Life Cycle and Host Cells. Viruses 2016, 8, 82. [Google Scholar] [CrossRef]
- Yang, M.; Ma, J.; Chu, Z.; Cao, X.; Lu, K.; Shi, X.; Tong, L.; Yan, C.; Liu, H.; Wang, X.; et al. Musashi1 inhibit the release of Newcastle disease viruses through preventing apoptosis of DF-1 cells. Poult. Sci. 2021, 100, 101105. [Google Scholar] [CrossRef]
- Deszcz, L.; Seipelt, J.; Vassilieva, E.; Roetzer, A.; Kuechler, E. Antiviral activity of caspase inhibitors: Effect on picornaviral 2A proteinase. FEBS Lett. 2004, 560, 51–55. [Google Scholar] [CrossRef]
- Wei, X.; Li, R.; Qiao, S.; Chen, X.X.; Xing, G.; Zhang, G. Porcine Reproductive and Respiratory Syndrome Virus Utilizes Viral Apoptotic Mimicry as an Alternative Pathway To Infect Host Cells. J. Virol. 2020, 94, e00709-20. [Google Scholar] [CrossRef]
- Amara, A.; Mercer, J. Viral apoptotic mimicry. Nat. Rev. Microbiol. 2015, 13, 461–469. [Google Scholar] [CrossRef]
- Atkin-Smith, G.K.; Duan, M.; Zanker, D.J.; Loh, L.; Nguyen, T.H.O.; Koutsakos, M.; Nguyen, T.; Jiang, X.; Carrera, J.; Phan, T.K.; et al. Monocyte apoptotic bodies are vehicles for influenza A virus propagation. Commun. Biol. 2020, 3, 223. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, B.; Zhang, Y.; Fan, W.; Xue, Q.; Chen, X.; Wang, J.; Qi, X. Mitochondria-mediated ferroptosis contributes to the inflammatory responses of bovine viral diarrhea virus (BVDV) in vitro. J. Virol. 2024, 98, e0188023. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhao, B.; Xue, Q.; Wang, C.; Wan, S.; Wang, J.; Chen, X.; Qi, X. Non-cytopathic bovine viral diarrhea virus (BVDV) inhibits innate immune responses via induction of mitophagy. Vet. Res. 2024, 55, 27. [Google Scholar] [CrossRef]
- Foo, J.; Bellot, G.; Pervaiz, S.; Alonso, S. Mitochondria-mediated oxidative stress during viral infection. Trends Microbiol. 2022, 30, 679–692. [Google Scholar] [CrossRef]
- Zeeshan, H.M.; Lee, G.H.; Kim, H.R.; Chae, H.J. Endoplasmic Reticulum Stress and Associated ROS. Int. J. Mol. Sci. 2016, 17, 327. [Google Scholar] [CrossRef]
- Wan, Y.; Yang, L.; Jiang, S.; Qian, D.; Duan, J. Excessive Apoptosis in Ulcerative Colitis: Crosstalk Between Apoptosis, ROS, ER Stress, and Intestinal Homeostasis. Inflamm. Bowel Dis. 2022, 28, 639–648. [Google Scholar] [CrossRef]
- Fraternale, A.; Paoletti, M.F.; Casabianca, A.; Nencioni, L.; Garaci, E.; Palamara, A.T.; Magnani, M. GSH and analogs in antiviral therapy. Mol. Asp. Med. 2009, 30, 99–110. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, J.; Zhang, J.; Liu, Y.; Duan, C.; Wang, J. Bergamottin Inhibits Bovine Viral Diarrhea Virus Replication by Suppressing ROS-Mediated Endoplasmic Reticulum Stress and Apoptosis. Viruses 2024, 16, 1287. https://doi.org/10.3390/v16081287
Yin J, Zhang J, Liu Y, Duan C, Wang J. Bergamottin Inhibits Bovine Viral Diarrhea Virus Replication by Suppressing ROS-Mediated Endoplasmic Reticulum Stress and Apoptosis. Viruses. 2024; 16(8):1287. https://doi.org/10.3390/v16081287
Chicago/Turabian StyleYin, Jinhua, Jialu Zhang, Yi Liu, Cong Duan, and Jiufeng Wang. 2024. "Bergamottin Inhibits Bovine Viral Diarrhea Virus Replication by Suppressing ROS-Mediated Endoplasmic Reticulum Stress and Apoptosis" Viruses 16, no. 8: 1287. https://doi.org/10.3390/v16081287
APA StyleYin, J., Zhang, J., Liu, Y., Duan, C., & Wang, J. (2024). Bergamottin Inhibits Bovine Viral Diarrhea Virus Replication by Suppressing ROS-Mediated Endoplasmic Reticulum Stress and Apoptosis. Viruses, 16(8), 1287. https://doi.org/10.3390/v16081287






