Distrust in the Health Care System and Adherence to Direct-Acting Antiviral Therapy among People with Hepatitis C Virus Who Inject Drugs
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Medications
2.3. Measures
2.3.1. Sociodemographics
2.3.2. Substance Use-Related Characteristics
2.3.3. Health-Related Variables
2.3.4. Distrust Measures
2.3.5. Adherence Measures
2.4. Statistical Analysis
3. Results
3.1. Associations between Participants’ Baseline Characterisitics and Overall Distrust Score
3.2. Levels of Individual Distrust Scores
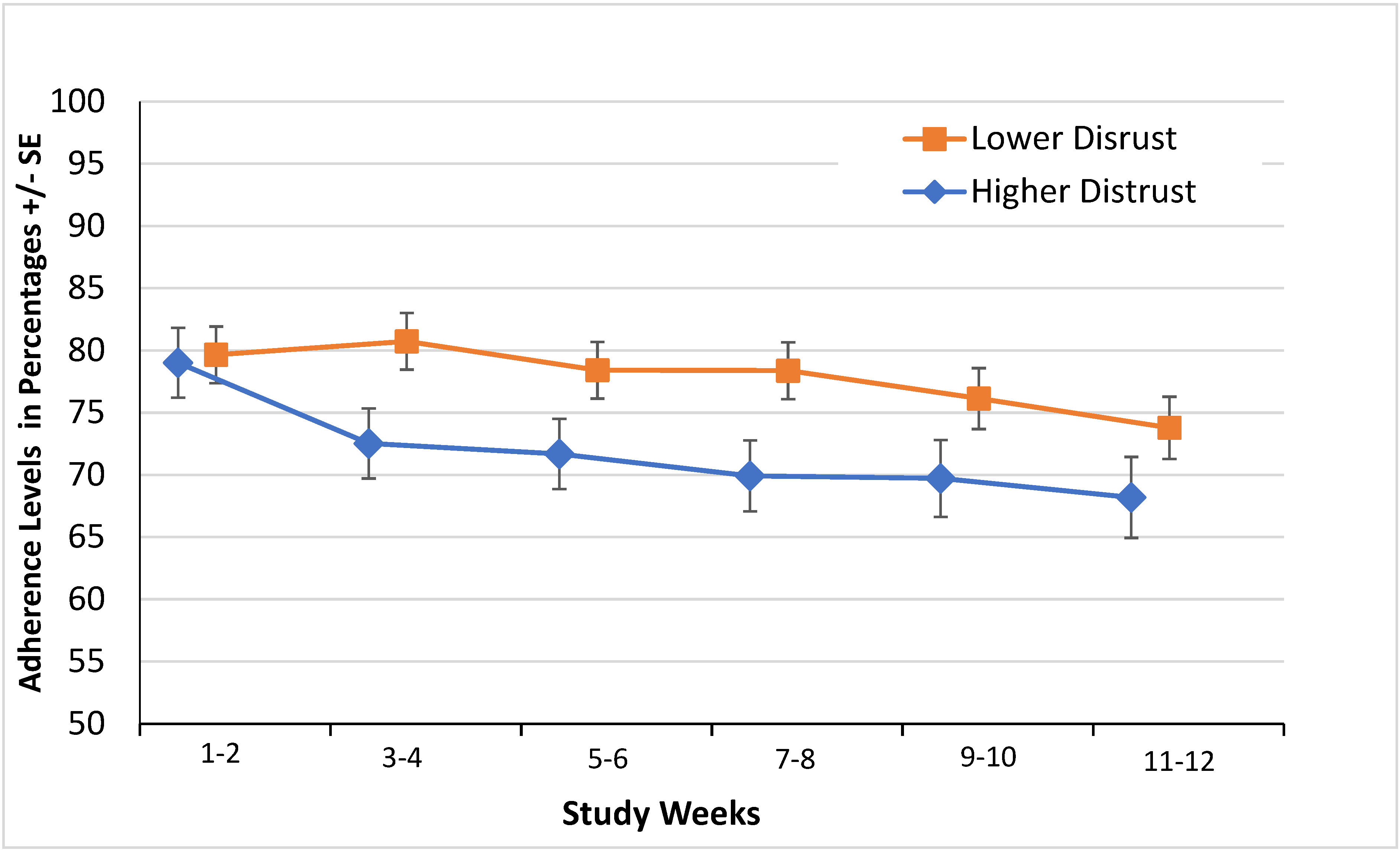
3.3. Distrust and Adherence
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hajarizadeh, B.; Kairouz, A.; Ottaviano, S.; Ireland, J.; Willing, A.; Cunningham, E.; Webb, P.; Colledge-Frisby, S.; Wheeler, A.; Leung, J.; et al. Global, regional, and country-level coverage of testing and treatment for HIV and hepatitis C infection among people who inject drugs: A systematic review. Lancet Glob. Health 2023, 11, e1885–e1898. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, L.; Webb, P.; Colledge-Frisby, S.; Ireland, J.; Wheeler, A.; Ottaviano, S.; Willing, A.; Kairouz, A.; Cunningham, E.B.; Hajarizadeh, B.; et al. Epidemiology of injecting drug use, prevalence of injecting-related harm, and exposure to behavioural and environmental risks among people who inject drugs: A systematic review. Lancet Glob Health 2023, 11, e659–e672. [Google Scholar] [CrossRef]
- Grebely, J.; Genoway, K.A.; Raffa, J.D.; Dhadwal, G.; Rajan, T.; Showler, G.; Kalousek, K.; Duncan, F.; Tyndall, M.W.; Fraser, C.; et al. Barriers associated with the treatment of hepatitis C virus infection among illicit drug users. Drug Alcohol Depend. 2008, 93, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Schackman, B.R.; Teixeira, P.A.; Beeder, A.B. Offers of hepatitis C care do not lead to treatment. J. Urban Health 2007, 84, 455–458. [Google Scholar] [CrossRef][Green Version]
- Rich, J.D.; Beckwith, C.G.; Macmadu, A.; Marshall, B.D.L.; Brinkley-Rubinstein, L.; Amon, J.J.; Milloy, M.-J.; King, M.R.F.; Sanchez, J.; Atwoli, L.; et al. Clinical care of incarcerated people with HIV, viral hepatitis, or tuberculosis. Lancet 2016, 388, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.H.; Genberg, B.L.; Astemborski, J.; Kavasery, R.; Kirk, G.D.; Vlahov, D.; Strathdee, S.A.; Thomas, D.L. Limited uptake of hepatitis C treatment among injection drug users. J. Community Health 2008, 33, 126–133. [Google Scholar] [CrossRef]
- Fierer, D.S.; Wyles, D.L. Re-treatment of Hepatitis C infection after multiple failures of direct-acting antiviral therapy. Open Forum Infect Dis. 2020, 7, ofaa095. [Google Scholar] [CrossRef]
- Bourlière, M.; Gordon, S.C.; Flamm, S.L.; Cooper, C.L.; Ramji, A.; Tong, M.; Ravendhran, N.; Vierling, J.M.; Tran, T.T.; Pianko, S.; et al. Sofosbuvir, Velpatasvir, and Voxilaprevir for Previously Treated HCV Infection. N. Engl. J. Med. 2017, 376, 2134–2146. [Google Scholar] [CrossRef]
- Mravcik, V.; Strada, L.; Štolfa, J.; Bencko, B.; Groshkova, T.; Reimer, J.; Schulte, B. Factors associated with uptake, adherence, and efficacy of hepatitis C treatment in people who inject drugs: A literature review. Patient Prefer. Adherence 2013, 7, 1067–1075. [Google Scholar] [CrossRef]
- Rich, Z.C.; Chu, C.; Mao, J.; Zhou, K.; Cai, W.; Ma, Q.; Volberding, P.; Tucker, J.D. Facilitators of HCV treatment adherence among people who inject drugs: A systematic qualitative review and implications for scale up of direct acting antivirals. BMC Public Health 2016, 16, 994. [Google Scholar] [CrossRef]
- Tofighi, B.; Sindhu, S.S.; Lee, J.D.; Chemi, C.; Leonard, N.R. Engagement in the Hepatitis C care continuum among people who use drugs. J. Subst. Use 2020, 25, 343–349. [Google Scholar] [CrossRef]
- Harris, M.; Rhodes, T.; Hepatitis, C. treatment access and uptake for people who inject drugs: A review mapping the role of social factors. Harm Reduct. J. 2013, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, M.; Lindstrom, M. Social capital, trust in the health-care system and self-rated health: The role of access to health care in a population-based study. Soc. Sci. Med. 2007, 64, 1373–1383. [Google Scholar] [CrossRef]
- Hall, M.A.; Dugan, E.; Zheng, B.; Mishra, A.K. Trust in Physicians and Medical Institutions: What Is It, Can It Be Measured, and Does It Matter? Milbank Q. 2001, 79, 613–639. [Google Scholar] [CrossRef]
- Laveist, T.A.; Isaac, L.A.; Williams, K.P. Mistrust of health care organizations is associated with underutilization of health services. Health Serv. Res. 2009, 44, 2093–2105. [Google Scholar] [CrossRef]
- Shoff, C.; Yang, T.C. Untangling the associations among distrust, race, and neighborhood social environment: A social disorganization perspective. Soc. Sci. Med. 2012, 74, 1342–1352. [Google Scholar] [CrossRef]
- Treloar, C.; Rance, J.; Backmund, M. Understanding barriers to hepatitis c virus care and stigmatization from a social perspective. Clin. Infect. Dis. 2013, 57 (Suppl. S2), S51–S55. [Google Scholar] [CrossRef] [PubMed]
- Dale, S.K.; Bogart, L.M.; Wagner, G.J.; Galvan, F.H.; Klein, D.J. Medical mistrust is related to lower longitudinal medication adherence among African-American males with HIV. J. Health Psychol. 2016, 21, 1311–1321. [Google Scholar] [CrossRef]
- Altice, F.L.; Mostashari, F.; Friedland, G. Trust and the acceptance of and adherence to antirtroviraltherapy. JAIDS J. Acquir. Immune Defic. Syndr. 2001, 28, 47–58. [Google Scholar] [CrossRef]
- Brown, P.R.; Calnan, M.W. Chains of (dis)trust: Exploring the underpinnings of knowledge-sharing and quality care across mental health services. Sociol. Health Illn. 2016, 38, 286–305. [Google Scholar] [CrossRef]
- Armstrong, K.; Rose, A.; Peters, N.; Long, J.A.; McMurphy, S.; Shea, J.A. Distrust of the health care system and self-reported health in the United States. J. Gen. Intern. Med. 2006, 21, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Arnett, M.J.; Thorpe, R.J.; Gaskin, D.J.; Bowie, J.V.; LaVeist, T.A. Race, Medical Mistrust, and Segregation in Primary Care as Usual Source of Care: Findings from the Exploring Health Disparities in Integrated Communities Study. J. Urban Health 2016, 93, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Hammond, W.P.; Matthews, D.; Mohottige, D.; Agyemang, A.; Corbie-Smith, G. Masculinity, medical mistrust, and preventive health services delays among community-dwelling african-american men. J. Gen. Intern. Med. 2010, 25, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Ostertag, S.; Wright, B.R.E.; Broadhead, R.S.; Altice, F.L. Trust and other characteristics associated with health care utilization by injection drug users. J. Drug Issues 2006, 36, 953–974. [Google Scholar] [CrossRef]
- Muncan, B.; Walters, S.M.; Ezell, J.; Ompad, D.C. “They look at us like junkies”: Influences of drug use stigma on the healthcare engagement of people who inject drugs in New York City. Harm Reduct. J. 2020, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Masson, C.L.; Fokuo, J.K.; Anderson, A.; Powell, J.; Zevin, B.; Bush, D.; Khalili, M. Clients’ perceptions of barriers and facilitators to implementing hepatitis C virus care in homeless shelters. BMC Infect. Dis. 2020, 20, 386. [Google Scholar] [CrossRef] [PubMed]
- Celeste-Villalvir, A.; Wilkerson, J.M.; Markham, C.; Rodriguez, L.; Schick, V. A qualitative investigation of the barriers and facilitators to Hepatitis C virus (HCV) screening among individuals experiencing homelessness in Houston, Texas. Dialogues Health 2022, 1, 100058. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.O.; Sohler, N.L.; Cooperman, N.A.; Berg, K.M.; Litwin, A.H.; Arnsten, J.H. Strategies to improve access to and utilization of health care services and adherence to antiretroviral therapy among HIV-infected drug users. Subst. Use Misuse 2011, 46, 218–232. [Google Scholar] [CrossRef]
- Malta, M.; Strathdee, S.A.; Magnanini, M.M.F.; Bastros, F.L. Adherence to antiretroviral therapy for human immunodeficiency virus/acquired immune deficiency syndrome among drug users: A systematic review. Addiction 2008, 103, 1242–1257. [Google Scholar] [CrossRef]
- Escudero, D.J.; Lurie, M.N.; Mayer, K.H.; King, M.; Galea, S.; Friedman, S.R.; Marshall, B.D.L. The risk of HIV transmission at each step of the HIV care continuum among people who inject drugs: A modeling study. BMC Public Health 2017, 17, 614. [Google Scholar] [CrossRef]
- Akiyama, M.J.; Agyemang, L.; Arnsten, J.H.; Heo, M.; Norton, B.L.; Schackman, B.R.; Linas, B.P.; Litwin, A.H. Rationale, design, and methodology of a trial evaluating three models of care for HCV treatment among injection drug users on opioid agonist therapy. BMC Infect Dis. 2018, 18, 74. [Google Scholar] [CrossRef]
- Akiyama, M.J.; Norton, B.L.; Arnsten, J.H.; Agyemang, L.; Heo, M.; Litwin, A.H. Intensive models of hepatitis C care for people who inject drugs receiving opioid agonist therapy a randomized controlled trial. Ann. Intern. Med. 2019, 170, 594–603. [Google Scholar] [CrossRef]
- Cunningham, C.O.; Sohler, N.L.; Korin, L.; Gao, W.; Anastos, K. HIV status, trust in health care providers, and distrust in the healthcare system among Bronx women. AIDS Care 2007, 19, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Norton, B.L.; Akiyama, M.J.; Agyemang, L.; Heo, M.; Pericot-Valverde, I.; Litwin, A.H. Low adherence achieves high HCV cure rates among people who inject drugs treated with direct-acting antiviral agents. Open Forum Infect Dis. 2020, 7, ofaa377. [Google Scholar] [CrossRef]
- Earl, T.R.; Saha, S.; Lombe, M.; Korthuis, P.T.; Sharp, V.; Cohn, J.; Moore, R.; Beach, M.C. Race, relationships, and trust in providers among black patients with HIV/AIDS. Soc. Work Res. 2013, 37, 219–226. [Google Scholar] [CrossRef]
- Casagrande, S.S.; Gary, T.L.; Laveist, T.A.; Gaskin, D.J.; Cooper, L.A. Perceived discrimination and adherence to medical care in a racially integrated community. J. Gen. Intern. Med. 2007, 22, 389–395. [Google Scholar] [CrossRef]
- Galvan, F.H.; Bogart, L.M.; Klein, D.J.; Wagner, G.J.; Chen, Y.T. Medical mistrust as a key mediator in the association between perceived discrimination and adherence to antiretroviral therapy among HIV-positive Latino men. J. Behav. Med. 2017, 40, 784–793. [Google Scholar] [CrossRef]
- Thrasher, A.D.; Earp, J.A.L.; Golin, C.E.; Zimmer, C.R. Discrimination, Distrust, and Racial/Ethnic Disparities in Antiretroviral Therapy Adherence Among a National Sample of HIV-Infected Patients. Am. J. Ther. 2008, 49, 84–93. [Google Scholar] [CrossRef]
- Saha, S.; Jacobs, E.A.; Moore, R.D.; Beach, M.C. Trust in physicians and racial disparities in HIV care. AIDS Patient Care STDS 2010, 24, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Bogart, L.M.; Wagner, G.J.; Green, H.D.; Mutchler, M.G.; Klein, D.J.; McDavitt, B.; Lawrence, S.J.; Hilliard, C.L. Medical mistrust among social network members may contribute to antiretroviral treatment nonadherence in African Americans living with HIV. Soc. Sci. Med. 2016, 164, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Salvalaggio, G.; McKim, R.; Taylor, M.; Cameron Wild, T. Patient–provider rapport in the health care of people who inject drugs. Sage Open 2013, 3, 2158244013509252. [Google Scholar] [CrossRef]
- Eckhardt, B.J.; Scherer, M.; Winkelstein, E.; Marks, K.; Edlin, B.R. Hepatitis C treatment outcomes for people who inject drugs treated in an accessible care program located at a syringe service program. Open Forum Infect. Dis. 2018, 5, ofy048. [Google Scholar] [CrossRef] [PubMed]
| Distrust in the Healthcare System * (N = 150) | |||
|---|---|---|---|
| Characteristic at Baseline | Summary Score > 3 n = 60 (40.0%) | Summary Score ≤ 3 n = 90 (60.0%) | p |
| Study Arm ** | 0.239 | ||
| mDOT | 16 (31.4%) | 35 (68.6%) | |
| GT | 23 (47.9%) | 25 (52.1%) | |
| SIT | 21 (41.2%) | 30 (58.8%) | |
| Age (median-split) | 0.616 | ||
| ≥52 years old | 30 (37.5%) | 50 (62.5%) | |
| <52 years old | 30 (42.9%) | 40 (57.1%) | |
| Sex at birth | 1 | ||
| Male | 39 (40.2%) | 58 (59.8%) | |
| Female | 21 (39.6%) | 32 (60.4%) | |
| Race/Ethnicity | 0.633 | ||
| Black | 16 (40.0%) | 24 (60.0%) | |
| Hispanic/Latino | 34 (40.5%) | 50 (59.5%) | |
| White | 3 (25.0%) | 9 (75.0%) | |
| Other | 7 (50.0%) | 7 (50.0%) | |
| Education | 0.098 | ||
| Lower than High School | 31 (48.4%) | 33 (51.6%) | |
| ≥High School graduate | 29 (33.7%) | 57 (66.3%) | |
| Marital status | 0.663 | ||
| Married/cohabitaion | 29 (42.6%) | 39 (57.4%) | |
| Other | 31 (37.8%) | 51 (62.2%) | |
| Current health insurance | 1 | ||
| Yes | 56 (41.2%) | 80 (58.8%) | |
| No | 4 (40.0%) | 6 (60.0%) | |
| Monthly income(median-split) | 1 | ||
| ≥$840 | 30 (39.5%) | 46 (60.5%) | |
| <$840 | 30 (40.5%) | 44 (59.5%) | |
| Employment | 0.489 | ||
| Employed | 2 (16.7%) | 10 (83.3%) | |
| Unemployed | 30 (41.1%) | 43 (58.9%) | |
| Retired | 7 (50.0%) | 7 (50.0%) | |
| Disabled | 18 (41.9%) | 25 (58.1%) | |
| Other | 3 (37.5%) | 5 (62.5%) | |
| Homelessness | 0.449 | ||
| Yes | 16 (47.1%) | 18 (52.9%) | |
| No | 44 (37.9%) | 72 (62.1%) | |
| Experience of living in a controlled environment *** | 1 | ||
| Yes | 10 (40.0%) | 15 (60.0%) | |
| No | 50 (40.0%) | 75 (60.0%) | |
| Perceived general health status | 0.077 | ||
| Excellent/Very Good/Good | 23 (31.9%) | 49 (68.1%) | |
| Fair/Poor | 37 (47.4%) | 41 (52.6%) | |
| Depression | 0.150 | ||
| BDI-II **** < 17 | 28 (34.1%) | 54 (65.9%) | |
| BDI-II ≥ 17 | 32 (47.1%) | 36 (52.9%) | |
| Curret psychiatric illnesses | 0.283 | ||
| Yes | 30 (44.8%) | 37 (55.2%) | |
| No | 30 (36.1%) | 53 (63.9%) | |
| HIV co-infection | 0.848 | ||
| Yes | 8 (38.1%) | 13 (61.9%) | |
| No | 52 (40.3%) | 77 (59.7%) | |
| Distrust in Healthcare System * (N = 150) | |||
|---|---|---|---|
| Drug Use in the Past 30 Days | Summary Score > 3 n = 60 (40.0%) | Summary Score ≤ 3 n = 90 (60.0%) | p |
| Heroin | 0.764 | ||
| Yes | 10 (35.7%) | 18 (64.3%) | |
| No | 50 (41.0%) | 72 (59.0%) | |
| Other Opiates/Analgesics | 0.903 | ||
| Yes | 14 (42.4%) | 19 (57.6%) | |
| No | 46 (39.3%) | 71 (60.7%) | |
| Cocaine | 0.725 | ||
| Yes | 13 (36.1%) | 23 (63.9%) | |
| No | 47 (41.2%) | 67 (58.8%) | |
| Alcohol | 0.640 | ||
| Yes | 17 (36.2%) | 30 (63.8%) | |
| No | 43 (41.7%) | 60 (58.3%) | |
| Alcohol Intoxication | 0.969 | ||
| Yes | 15 (41.7%) | 21 (58.3%) | |
| No | 45 (39.5%) | 69 (60.5%) | |
| Methadone ** | 1 | ||
| Yes | 59 (39.9%) | 89 (60.1%) | 1 |
| No | 1 (50.0%) | 1 (50.0%) | |
| Barbiturates | |||
| Yes | 2 (33.3%) | 4 (66.7%) | 1 |
| No | 58 (40.3%) | 86 (56.7%) | |
| Sedatives/Hypnotics/Tranquilizers | 0.355 | ||
| Yes | 16 (48.5%) | 17 (51.5%) | |
| No | 44 (37.6%) | 73 (62.4%) | |
| Amphetamines | 0.150 | ||
| Yes | 0 (0.0%) | 4 (100.0%) | |
| No | 60 (41.1%) | 86 (58.9%) | |
| Cannabis | 0.288 | ||
| Yes | 21 (47.7%) | 23 (52.3%) | |
| No | 39 (36.8%) | 67 (63.2%) | |
| Hallucinogens | 1 | ||
| Yes | 2 (40.0%) | 3 (60.0%) | |
| No | 58 (40.0%) | 87 (60.0%) | |
| Inhalants | 0.400 | ||
| Yes | 1 (100.0%) | 0 (0.0%) | |
| No | 59 (39.6%) | 90 (60.4%) | |
| Distrust in the Healthcare System Questionnaire Item (1 to 5; 5 Highest Distrust) | N (%) with Individual Item Scores > 3 | Score M (SD) |
|---|---|---|
| I think that I have been experimented on in hospitals without being told | 21 (14.0%) | 2.23 (1.08) |
| I think that drug companies don’t tell me all the bad things that can happen with their medication | 68 (45.3%) | 3.11 (1.16) |
| I think there is a cure for AIDS, but the government is keeping it from us | 57 (38.0%) | 3.10 (1.16) |
| Despite what the government would have you believe, I think that HIV was made in a laboratory | 65 (43.3%) | 3.21 (1.11) |
| I suspect that my blood or urine is being tested for things I’m not told about | 43 (28.7%) | 2.81 (1.06) |
| Medical scientists know more about HIV than they’re letting on | 66 (44.0%) | 3.26 (1.05) |
| I think that hospitals are more concerned with making money than healing me | 54 (36.0%) | 2.93 (1.17) |
| Despite what the government would have you believe, I think that hepatitis C was made in a laboratory | 22 (14.7%) | 2.67 (1.01) |
| Medical scientists know more about hepatitis C than they’re letting on | 39 (26.0%) | 2.85 (1.11) |
| Overal Summary Score | 60 (40.0%) | 2.91 (0.77) |
| Unadjusted Estimates | Adjusted Estimates | |||
|---|---|---|---|---|
| Effects | Estimates (95% CI) | p | Estimates (95% CI) | p |
| Overall Distrust (Higher vs. Lower) * | −5.9% (−11.2%, −0.7%) | 0.027 | −6.0% (−11.3%, −0.8%) | 0.024 |
| Time (Discrete) | *** | 0.030 **** | ||
| Distrust × Time Interaction | *** | 0.137 **** | ||
| Study Arm ** | 0.040 **** | |||
| mDOT vs. GT | 4.1% (−1.9%, 10.1%) | 0.179 | ||
| mDOT vs. SIT | 7.7% (1.8%, 13.7%) | 0.011 | ||
| GT vs. SIT | 3.6% (−2.4%, 9.7%) | 0.237 | ||
| Alcohol Intoxication (Yes vs. No) | −7.1% (−12.8%, −1.4%) | 0.015 | ||
| Psychiatric Illnesses (Yes vs. No) | −4.4% (−9.3%, 0.6%) | 0.082 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padi, A.; Pericot-Valverde, I.; Heo, M.; Dotherow, J.E.; Niu, J.; Martin, M.; Norton, B.L.; Akiyama, M.J.; Arnsten, J.H.; Litwin, A.H. Distrust in the Health Care System and Adherence to Direct-Acting Antiviral Therapy among People with Hepatitis C Virus Who Inject Drugs. Viruses 2024, 16, 1304. https://doi.org/10.3390/v16081304
Padi A, Pericot-Valverde I, Heo M, Dotherow JE, Niu J, Martin M, Norton BL, Akiyama MJ, Arnsten JH, Litwin AH. Distrust in the Health Care System and Adherence to Direct-Acting Antiviral Therapy among People with Hepatitis C Virus Who Inject Drugs. Viruses. 2024; 16(8):1304. https://doi.org/10.3390/v16081304
Chicago/Turabian StylePadi, Akhila, Irene Pericot-Valverde, Moonseong Heo, James Edward Dotherow, Jiajing Niu, Madhuri Martin, Brianna L. Norton, Matthew J. Akiyama, Julia H. Arnsten, and Alain H. Litwin. 2024. "Distrust in the Health Care System and Adherence to Direct-Acting Antiviral Therapy among People with Hepatitis C Virus Who Inject Drugs" Viruses 16, no. 8: 1304. https://doi.org/10.3390/v16081304
APA StylePadi, A., Pericot-Valverde, I., Heo, M., Dotherow, J. E., Niu, J., Martin, M., Norton, B. L., Akiyama, M. J., Arnsten, J. H., & Litwin, A. H. (2024). Distrust in the Health Care System and Adherence to Direct-Acting Antiviral Therapy among People with Hepatitis C Virus Who Inject Drugs. Viruses, 16(8), 1304. https://doi.org/10.3390/v16081304






