Update on Hepatitis C Vaccine: Results and Challenges
Abstract
:1. Introduction
2. Hepatitis C General Aspects
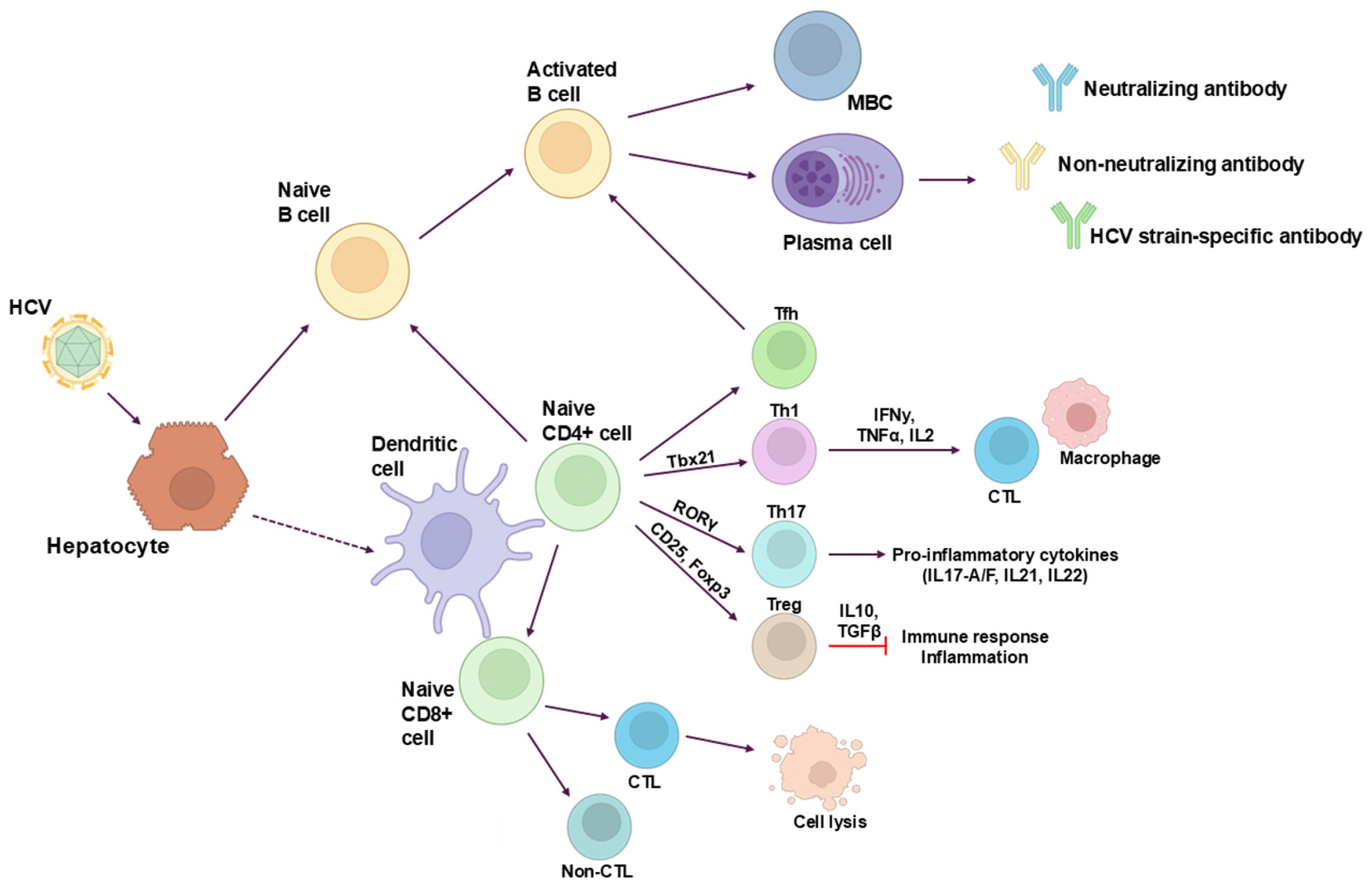
3. B-Cell Response
4. Adaptive Immune Response T Cells
5. B Cell Vaccine
6. B Cell Vaccine Optimization by Antigen Modification, Adjuvants and Formulation
7. T Cell Vaccine
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mohd Hanafiah, K.; Groeger, J.; Flaxman, A.D.; Wiersma, S.T. Global epidemiology of hepatitis C virus infection: New estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013, 57, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Blach, S.; Zeuzem, S.; Manns, M.; Altraif, I.; Duberg, A.-S.; Muljono, D.H.; Waked, I.; Alavian, S.M.; Lee, M.-H.; Negro, F.; et al. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol. Hepatol. 2017, 2, 161–176. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Hepatitis Report 2017; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Ghiglione, Y.; Polo, M.L.; Urioste, A.; Rhodes, A.; Czernikier, A.; Trifone, C.; Quiroga, M.F.; Sisto, A.; Patterson, P.; Salomón, H.; et al. Hepatitis C Virus (HCV) Clearance After Treatment With Direct-Acting Antivirals in Human Immunodeficiency Virus (HIV)-HCV Coinfection Modulates Systemic Immune Activation and HIV Transcription on Antiretroviral Therapy. Open Forum Infect. Dis. 2020, 7, ofaa115. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.-L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Petrick, J.L.; Florio, A.A.; Znaor, A.; Ruggieri, D.; Laversanne, M.; Alvarez, C.S.; Ferlay, J.; Valery, P.C.; Bray, F.; McGlynn, K.A. International trends in hepatocellular carcinoma incidence, 1978–2012. Int. J. Cancer 2020, 147, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Adee, M.; Zhong, H.; Reipold, E.I.; Zhuo, Y.; Shilton, S.; Chhatwal, J. Cost-Effectiveness of a Core Antigen-Based Rapid Diagnostic Test for Hepatitis C. Value Health 2022, 25, 1107–1115. [Google Scholar] [CrossRef]
- Tong, M.J.; El-Farra, N.S.; Reikes, A.R.; Co, R.L. Clinical Outcomes after Transfusion-Associated Hepatitis C. N. Engl. J. Med. 1995, 332, 1463–1466. [Google Scholar] [CrossRef]
- Wiese, M.; Berr, F.; Lafrenz, M.; Porst, H.; Oesen, U. Low frequency of cirrhosis in a hepatitis C(genotype 1b) single-source outbreak in germany: A 20-year multicenter study. Hepatology 2000, 32, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Thein, H.-H.; Yi, Q.; Dore, G.J.; Krahn, M.D. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: A meta-analysis and meta-regression. Hepatology 2008, 48, 418–431. [Google Scholar] [CrossRef]
- Pradat, P.; Voirin, N.; Tillmann, H.L.; Chevallier, M.; Trépo, C. Progression to cirrhosis in hepatitis C patients: An age-dependent process. Liver Int. 2007, 27, 335–339. [Google Scholar] [CrossRef]
- Jacobson, I.M.; Davis, G.L.; El-Serag, H.; Negro, F.; Trépo, C. Prevalence and challenges of liver diseases in patients with chronic hepatitis C virus infection. Clin. Gastroenterol. Hepatol. 2010, 8, 924–933; quiz e117. [Google Scholar] [CrossRef] [PubMed]
- Santiago, A.M.; da Silva Graça Amoras, E.; Queiroz, M.A.F.; da Silva Conde, S.R.S.; Cayres-Vallinoto, I.M.V.; Ishak, R.; Vallinoto, A.C.R. TNFA -308G>A and IL10 -1082A>G polymorphisms seem to be predictive biomarkers of chronic HCV infection. BMC Infect. Dis. 2021, 21, 1133. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, N.; Chiodi, D.; López, P.; Sanchez Ciceron, A.; Angulo, J.; López-Lastra, M.; Silvera, P.; Canavesi, A.; Bianchi, C.; Colistro, V.; et al. IL28B gene polymorphism rs12979860, but not rs8099917, contributes to the occurrence of chronic HCV infection in Uruguayan patients. Virol. J. 2018, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Afdhal, N.; Zeuzem, S.; Kwo, P.; Chojkier, M.; Gitlin, N.; Puoti, M.; Romero-Gomez, M.; Zarski, J.-P.; Agarwal, K.; Buggisch, P.; et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N. Engl. J. Med. 2014, 370, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Leroy, V.; Angus, P.; Bronowicki, J.-P.; Dore, G.J.; Hezode, C.; Pianko, S.; Pol, S.; Stuart, K.; Tse, E.; McPhee, F.; et al. Daclatasvir, sofosbuvir, and ribavirin for hepatitis C virus genotype 3 and advanced liver disease: A randomized phase III study (ALLY-3+). Hepatology 2016, 63, 1430–1441. [Google Scholar] [CrossRef]
- Aghemo, A.; De Francesco, R. New horizons in hepatitis C antiviral therapy with direct-acting antivirals. Hepatology 2013, 58, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Guntipalli, P.; Pakala, R.; Kumari Gara, S.; Ahmed, F.; Bhatnagar, A.; Endaya Coronel, M.-K.; Razzack, A.A.; Solimando, A.G.; Thompson, A.; Andrews, K.; et al. Worldwide prevalence, genotype distribution and management of hepatitis C. Acta Gastro-Enterol. Belg. 2021, 84, 637–656. [Google Scholar] [CrossRef]
- Islam, N.; Krajden, M.; Shoveller, J.; Gustafson, P.; Gilbert, M.; Buxton, J.A.; Wong, J.; Tyndall, M.W.; Janjua, N.Z. Incidence, risk factors, and prevention of hepatitis C reinfection: A population-based cohort study. Lancet Gastroenterol. Hepatol. 2017, 2, 200–210. [Google Scholar] [CrossRef]
- Craine, N.; Hickman, M.; Parry, J.V.; Smith, J.; Walker, A.M.; Russell, D.; Nix, B.; May, M.; McDonald, T.; Lyons, M. Incidence of hepatitis C in drug injectors: The role of homelessness, opiate substitution treatment, equipment sharing, and community size. Epidemiol. Infect. 2009, 137, 1255–1265. [Google Scholar] [CrossRef]
- Falade-Nwulia, O.; Sulkowski, M.S.; Merkow, A.; Latkin, C.; Mehta, S.H. Understanding and addressing hepatitis C reinfection in the oral direct-acting antiviral era. J. Viral Hepat. 2018, 25, 220–227. [Google Scholar] [CrossRef]
- Singal, A.G.; Lim, J.K.; Kanwal, F. AGA Clinical Practice Update on Interaction Between Oral Direct-Acting Antivirals for Chronic Hepatitis C Infection and Hepatocellular Carcinoma: Expert Review. Gastroenterology 2019, 156, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Mariño, Z.; Perelló, C.; Iñarrairaegui, M.; Ribeiro, A.; Lens, S.; Díaz, A.; Vilana, R.; Darnell, A.; Varela, M.; et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J. Hepatol. 2016, 65, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Moradpour, D.; Penin, F.; Rice, C.M. Replication of hepatitis C virus. Nat. Rev. Microbiol. 2007, 5, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Lapa, D.; Garbuglia, A.R.; Capobianchi, M.R.; Del Porto, P. Hepatitis C Virus Genetic Variability, Human Immune Response, and Genome Polymorphisms: Which Is the Interplay? Cells 2019, 8, 305. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.C.; Lemon, S.M.; Zuckerman, A.J. Viral Hepatitis, 3rd ed.; Blackwell Publishing: Malden, MA, USA, 2005; ISBN 978-0-470-98713-1. [Google Scholar]
- Cuevas, J.M.; González-Candelas, F.; Moya, A.; Sanjuán, R. Effect of ribavirin on the mutation rate and spectrum of hepatitis C virus in vivo. J. Virol. 2009, 83, 5760–5764. [Google Scholar] [CrossRef]
- Sanjuán, R.; Nebot, M.R.; Chirico, N.; Mansky, L.M.; Belshaw, R. Viral mutation rates. J. Virol. 2010, 84, 9733–9748. [Google Scholar] [CrossRef]
- Wang, G.P.; Sherrill-Mix, S.A.; Chang, K.-M.; Quince, C.; Bushman, F.D. Hepatitis C virus transmission bottlenecks analyzed by deep sequencing. J. Virol. 2010, 84, 6218–6228. [Google Scholar] [CrossRef]
- Nakano, T.; Lau, G.M.G.; Lau, G.M.L.; Sugiyama, M.; Mizokami, M. An updated analysis of hepatitis C virus genotypes and subtypes based on the complete coding region. Liver Int. 2012, 32, 339–345. [Google Scholar] [CrossRef]
- Smith, D.B.; Bukh, J.; Kuiken, C.; Muerhoff, A.S.; Rice, C.M.; Stapleton, J.T.; Simmonds, P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: Updated criteria and genotype assignment web resource. Hepatology 2014, 59, 318–327. [Google Scholar] [CrossRef]
- Choo, Q.L.; Richman, K.H.; Han, J.H.; Berger, K.; Lee, C.; Dong, C.; Gallegos, C.; Coit, D.; Medina-Selby, R.; Barr, P.J. Genetic organization and diversity of the hepatitis C virus. Proc. Natl. Acad. Sci. USA 1991, 88, 2451–2455. [Google Scholar] [CrossRef]
- Simmonds, P.; Bukh, J.; Combet, C.; Deléage, G.; Enomoto, N.; Feinstone, S.; Halfon, P.; Inchauspé, G.; Kuiken, C.; Maertens, G.; et al. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology 2005, 42, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Kuiken, C.; Yusim, K.; Boykin, L.; Richardson, R. The Los Alamos hepatitis C sequence database. Bioinformatics 2005, 21, 379–384. [Google Scholar] [CrossRef]
- Hedskog, C.; Parhy, B.; Chang, S.; Zeuzem, S.; Moreno, C.; Shafran, S.D.; Borgia, S.M.; Asselah, T.; Alric, L.; Abergel, A.; et al. Identification of 19 Novel Hepatitis C Virus Subtypes-Further Expanding HCV Classification. Open Forum Infect. Dis. 2019, 6, ofz076. [Google Scholar] [CrossRef] [PubMed]
- Kuiken, C.; Combet, C.; Bukh, J.; Shin-I, T.; Deleage, G.; Mizokami, M.; Richardson, R.; Sablon, E.; Yusim, K.; Pawlotsky, J.-M.; et al. A comprehensive system for consistent numbering of HCV sequences, proteins and epitopes. Hepatology 2006, 44, 1355–1361. [Google Scholar] [CrossRef]
- Spach, D.H. HCV Epidemiology in the United States-Lesson I-Hepatitis C. Available online: https://www.hepatitisc.uw.edu/go/screening-diagnosis/epidemiology-us (accessed on 17 July 2024).
- Messina, J.P.; Humphreys, I.; Flaxman, A.; Brown, A.; Cooke, G.S.; Pybus, O.G.; Barnes, E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology 2015, 61, 77–87. [Google Scholar] [CrossRef]
- Tsukiyama-Kohara, K.; Kohara, M. Hepatitis C Virus: Viral Quasispecies and Genotypes. Int. J. Mol. Sci. 2017, 19, 23. [Google Scholar] [CrossRef]
- Murphy, D.G.; Sablon, E.; Chamberland, J.; Fournier, E.; Dandavino, R.; Tremblay, C.L. Hepatitis C virus genotype 7, a new genotype originating from central Africa. J. Clin. Microbiol. 2015, 53, 967–972. [Google Scholar] [CrossRef]
- Borgia, S.M.; Hedskog, C.; Parhy, B.; Hyland, R.H.; Stamm, L.M.; Brainard, D.M.; Subramanian, M.G.; McHutchison, J.G.; Mo, H.; Svarovskaia, E.; et al. Identification of a Novel Hepatitis C Virus Genotype From Punjab, India: Expanding Classification of Hepatitis C Virus Into 8 Genotypes. J. Infect. Dis. 2018, 218, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, O.; Norder, H.; Magnius, L.O. Full-length open reading frame of a recombinant hepatitis C virus strain from St Petersburg: Proposed mechanism for its formation. J. Gen. Virol. 2004, 85, 1853–1857. [Google Scholar] [CrossRef]
- Kalinina, O.; Norder, H.; Mukomolov, S.; Magnius, L.O. A natural intergenotypic recombinant of hepatitis C virus identified in St. Petersburg. J. Virol. 2002, 76, 4034–4043. [Google Scholar] [CrossRef] [PubMed]
- Colina, R.; Casane, D.; Vasquez, S.; García-Aguirre, L.; Chunga, A.; Romero, H.; Khan, B.; Cristina, J. Evidence of intratypic recombination in natural populations of hepatitis C virus. J. Gen. Virol. 2004, 85, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, D.T.; Rizzetto, M.; Manns, M.P. Management of chronic hepatitis C patients who have relapsed or not responded to pegylated interferon alfa plus ribavirin. J. Viral Hepat. 2009, 16, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Keck, M.-L.; Wrensch, F.; Pierce, B.G.; Baumert, T.F.; Foung, S.K.H. Mapping Determinants of Virus Neutralization and Viral Escape for Rational Design of a Hepatitis C Virus Vaccine. Front. Immunol. 2018, 9, 1194. [Google Scholar] [CrossRef] [PubMed]
- Goffard, A.; Dubuisson, J. Glycosylation of hepatitis C virus envelope proteins. Biochimie 2003, 85, 295–301. [Google Scholar] [CrossRef]
- Benedicto, I.; Molina-Jiménez, F.; Barreiro, O.; Maldonado-Rodríguez, A.; Prieto, J.; Moreno-Otero, R.; Aldabe, R.; López-Cabrera, M.; Majano, P.L. Hepatitis C virus envelope components alter localization of hepatocyte tight junction-associated proteins and promote occludin retention in the endoplasmic reticulum. Hepatology 2008, 48, 1044–1053. [Google Scholar] [CrossRef]
- Meertens, L.; Bertaux, C.; Cukierman, L.; Cormier, E.; Lavillette, D.; Cosset, F.-L.; Dragic, T. The tight junction proteins claudin-1, -6, and -9 are entry cofactors for hepatitis C virus. J. Virol. 2008, 82, 3555–3560. [Google Scholar] [CrossRef]
- Aebi-Popp, K.; Wandeler, G.; Salazar-Vizcaya, L.; Metzner, K.; Stöckle, M.; Cavassini, M.; Hoffmann, M.; Lüthi, A.; Suter, F.; Bernasconi, E.; et al. Rapid decline of anti-hepatitis C virus (HCV) antibodies following early treatment of incident HCV infections in HIV-infected men who have sex with men. HIV Med. 2018, 19, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Strasak, A.M.; Kim, A.Y.; Lauer, G.M.; de Sousa, P.S.; Ginuino, C.F.; Fernandes, C.A.; Velloso, C.E.; de Almeida, A.J.; de Oliveira, J.M.; Yoshida, C.F.; et al. Antibody dynamics and spontaneous viral clearance in patients with acute hepatitis C infection in Rio de Janeiro, Brazil. BMC Infect. Dis. 2011, 11, 15. [Google Scholar] [CrossRef]
- Dahari, H.; Feinstone, S.M.; Major, M.E. Meta-analysis of hepatitis C virus vaccine efficacy in chimpanzees indicates an importance for structural proteins. Gastroenterology 2010, 139, 965–974. [Google Scholar] [CrossRef]
- Law, M. Antibody Responses in Hepatitis C Infection. Cold Spring Harb. Perspect. Med. 2021, 11, a036962. [Google Scholar] [CrossRef] [PubMed]
- Tzarum, N.; Wilson, I.A.; Law, M. The Neutralizing Face of Hepatitis C Virus E2 Envelope Glycoprotein. Front. Immunol. 2018, 9, 1315. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Giang, E.; Nieusma, T.; Kadam, R.U.; Cogburn, K.E.; Hua, Y.; Dai, X.; Stanfield, R.L.; Burton, D.R.; Ward, A.B.; et al. Hepatitis C virus E2 envelope glycoprotein core structure. Science 2013, 342, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Ruwona, T.B.; Giang, E.; Nieusma, T.; Law, M. Fine mapping of murine antibody responses to immunization with a novel soluble form of hepatitis C virus envelope glycoprotein complex. J. Virol. 2014, 88, 10459–10471. [Google Scholar] [CrossRef] [PubMed]
- Sevvana, M.; Keck, Z.; Foung, S.K.; Kuhn, R.J. Structural perspectives on HCV humoral immune evasion mechanisms. Curr. Opin. Virol. 2021, 49, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Tarr, A.W.; Owsianka, A.M.; Timms, J.M.; McClure, C.P.; Brown, R.J.P.; Hickling, T.P.; Pietschmann, T.; Bartenschlager, R.; Patel, A.H.; Ball, J.K. Characterization of the hepatitis C virus E2 epitope defined by the broadly neutralizing monoclonal antibody AP33. Hepatology 2006, 43, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Keck, Z.; Xia, J.; Wang, Y.; Wang, W.; Krey, T.; Prentoe, J.; Carlsen, T.; Li, A.Y.-J.; Patel, A.H.; Lemon, S.M.; et al. Human Monoclonal Antibodies to a Novel Cluster of Conformational Epitopes on HCV E2 with Resistance to Neutralization Escape in a Genotype 2a Isolate. PLoS Pathog. 2012, 8, e1002653. [Google Scholar] [CrossRef] [PubMed]
- Keck, Z.-Y.; Saha, A.; Xia, J.; Wang, Y.; Lau, P.; Krey, T.; Rey, F.A.; Foung, S.K.H. Mapping a region of hepatitis C virus E2 that is responsible for escape from neutralizing antibodies and a core CD81-binding region that does not tolerate neutralization escape mutations. J. Virol. 2011, 85, 10451–10463. [Google Scholar] [CrossRef]
- Colbert, M.D.; Flyak, A.I.; Ogega, C.O.; Kinchen, V.J.; Massaccesi, G.; Hernandez, M.; Davidson, E.; Doranz, B.J.; Cox, A.L.; Crowe, J.E.; et al. Broadly Neutralizing Antibodies Targeting New Sites of Vulnerability in Hepatitis C Virus E1E2. J. Virol. 2019, 93, e02070-18. [Google Scholar] [CrossRef]
- Shimizu, Y.K.; Hijikata, M.; Iwamoto, A.; Alter, H.J.; Purcell, R.H.; Yoshikura, H. Neutralizing antibodies against hepatitis C virus and the emergence of neutralization escape mutant viruses. J. Virol. 1994, 68, 1494–1500. [Google Scholar] [CrossRef]
- Kato, N.; Sekiya, H.; Ootsuyama, Y.; Nakazawa, T.; Hijikata, M.; Ohkoshi, S.; Shimotohno, K. Humoral immune response to hypervariable region 1 of the putative envelope glycoprotein (gp70) of hepatitis C virus. J. Virol. 1993, 67, 3923–3930. [Google Scholar] [CrossRef]
- Weiner, A.J.; Geysen, H.M.; Christopherson, C.; Hall, J.E.; Mason, T.J.; Saracco, G.; Bonino, F.; Crawford, K.; Marion, C.D.; Crawford, K.A. Evidence for immune selection of hepatitis C virus (HCV) putative envelope glycoprotein variants: Potential role in chronic HCV infections. Proc. Natl. Acad. Sci. USA 1992, 89, 3468–3472. [Google Scholar] [CrossRef] [PubMed]
- Dowd, K.A.; Netski, D.M.; Wang, X.-H.; Cox, A.L.; Ray, S.C. Selection pressure from neutralizing antibodies drives sequence evolution during acute infection with hepatitis C virus. Gastroenterology 2009, 136, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Prentoe, J.; Velázquez-Moctezuma, R.; Augestad, E.H.; Galli, A.; Wang, R.; Law, M.; Alter, H.; Bukh, J. Hypervariable region 1 and N-linked glycans of hepatitis C regulate virion neutralization by modulating envelope conformations. Proc. Natl. Acad. Sci. USA 2019, 116, 10039–10047. [Google Scholar] [CrossRef]
- Prentoe, J.; Velázquez-Moctezuma, R.; Foung, S.K.H.; Law, M.; Bukh, J. Hypervariable region 1 shielding of hepatitis C virus is a main contributor to genotypic differences in neutralization sensitivity. Hepatology 2016, 64, 1881–1892. [Google Scholar] [CrossRef]
- Wang, Y.; Keck, Z.-Y.; Foung, S.K.H. Neutralizing Antibody Response to Hepatitis C Virus. Viruses 2011, 3, 2127–2145. [Google Scholar] [CrossRef] [PubMed]
- Helle, F.; Goffard, A.; Morel, V.; Duverlie, G.; McKeating, J.; Keck, Z.-Y.; Foung, S.; Penin, F.; Dubuisson, J.; Voisset, C. The neutralizing activity of anti-hepatitis C virus antibodies is modulated by specific glycans on the E2 envelope protein. J. Virol. 2007, 81, 8101–8111. [Google Scholar] [CrossRef] [PubMed]
- Falkowska, E.; Kajumo, F.; Garcia, E.; Reinus, J.; Dragic, T. Hepatitis C virus envelope glycoprotein E2 glycans modulate entry, CD81 binding, and neutralization. J. Virol. 2007, 81, 8072–8079. [Google Scholar] [CrossRef] [PubMed]
- Thomssen, R.; Bonk, S.; Propfe, C.; Heermann, K.H.; Köchel, H.G.; Uy, A. Association of hepatitis C virus in human sera with beta-lipoprotein. Med. Microbiol. Immunol. 1992, 181, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, C.G.; Mihalik, K.; Virata, M.L.; Yu, M.-Y.W.; Alter, H.J.; Feinstone, S.M. Hepatitis C virus epitope-specific neutralizing antibodies in Igs prepared from human plasma. Proc. Natl. Acad. Sci. USA 2007, 104, 8449–8454. [Google Scholar] [CrossRef]
- Zhang, P.; Zhong, L.; Struble, E.B.; Watanabe, H.; Kachko, A.; Mihalik, K.; Virata, M.L.; Alter, H.J.; Feinstone, S.; Major, M. Depletion of interfering antibodies in chronic hepatitis C patients and vaccinated chimpanzees reveals broad cross-genotype neutralizing activity. Proc. Natl. Acad. Sci. USA 2009, 106, 7537–7541. [Google Scholar] [CrossRef]
- Walker, M.R.; Leung, P.; Eltahla, A.A.; Underwood, A.; Abayasingam, A.; Brasher, N.A.; Li, H.; Wu, B.-R.; Maher, L.; Luciani, F.; et al. Clearance of hepatitis C virus is associated with early and potent but narrowly-directed, Envelope-specific antibodies. Sci. Rep. 2019, 9, 13300. [Google Scholar] [CrossRef] [PubMed]
- Tarr, A.W.; Urbanowicz, R.A.; Hamed, M.R.; Albecka, A.; McClure, C.P.; Brown, R.J.P.; Irving, W.L.; Dubuisson, J.; Ball, J.K. Hepatitis C patient-derived glycoproteins exhibit marked differences in susceptibility to serum neutralizing antibodies: Genetic subtype defines antigenic but not neutralization serotype. J. Virol. 2011, 85, 4246–4257. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.; Morey, S.; Hamoodi, A.; Thompson, C.; Jones, D.; Hewett, M.; Hunter, E.; Taha, Y.; McPherson, S. High rate of hepatitis C reinfection following antiviral treatment in the North East England Prisons. J. Viral Hepat. 2020, 27, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Brasher, N.A.; Eltahla, A.A.; Underwood, A.; Boo, I.; Rizzetto, S.; Walker, M.R.; Rodrigo, C.; Luciani, F.; Maher, L.; Drummer, H.E.; et al. B cell immunodominance in primary hepatitis C virus infection. J. Hepatol. 2020, 72, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Kinchen, V.J.; Massaccesi, G.; Flyak, A.I.; Mankowski, M.C.; Colbert, M.D.; Osburn, W.O.; Ray, S.C.; Cox, A.L.; Crowe, J.E.; Bailey, J.R. Plasma deconvolution identifies broadly neutralizing antibodies associated with hepatitis C virus clearance. J. Clin. Investig. 2019, 129, 4786–4796. [Google Scholar] [CrossRef] [PubMed]
- Thimme, R.; Oldach, D.; Chang, K.M.; Steiger, C.; Ray, S.C.; Chisari, F.V. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 2001, 194, 1395–1406. [Google Scholar] [CrossRef]
- Thimme, R.; Bukh, J.; Spangenberg, H.C.; Wieland, S.; Pemberton, J.; Steiger, C.; Govindarajan, S.; Purcell, R.H.; Chisari, F.V. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc. Natl. Acad. Sci. USA 2002, 99, 15661–15668. [Google Scholar] [CrossRef]
- Holz, L.; Rehermann, B. T cell responses in hepatitis C virus infection: Historical overview and goals for future research. Antivir. Res. 2015, 114, 96–105. [Google Scholar] [CrossRef]
- Schulze Zur Wiesch, J.; Ciuffreda, D.; Lewis-Ximenez, L.; Kasprowicz, V.; Nolan, B.E.; Streeck, H.; Aneja, J.; Reyor, L.L.; Allen, T.M.; Lohse, A.W.; et al. Broadly directed virus-specific CD4+ T cell responses are primed during acute hepatitis C infection, but rapidly disappear from human blood with viral persistence. J. Exp. Med. 2012, 209, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.L.; Mosbruger, T.; Lauer, G.M.; Pardoll, D.; Thomas, D.L.; Ray, S.C. Comprehensive Analyses of CD8+ T Cell Responses during Longitudinal Study of Acute Human Hepatitis C. Hepatology 2005, 42, 104–112. [Google Scholar] [CrossRef]
- Raziorrouh, B.; Ulsenheimer, A.; Schraut, W.; Heeg, M.; Kurktschiev, P.; Zachoval, R.; Jung, M.-C.; Thimme, R.; Neumann-Haefelin, C.; Horster, S.; et al. Inhibitory molecules that regulate expansion and restoration of HCV-specific CD4+ T cells in patients with chronic infection. Gastroenterology 2011, 141, 1422–1431.e6. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, N.; Ueno, H. Regulation of human helper T cell subset differentiation by cytokines. Curr. Opin. Immunol. 2015, 34, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Barbi, J.; Pan, F. The regulation of immune tolerance by FOXP3. Nat. Rev. Immunol. 2017, 17, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Claassen, M.A.A.; de Knegt, R.J.; Tilanus, H.W.; Janssen, H.L.A.; Boonstra, A. Abundant numbers of regulatory T cells localize to the liver of chronic hepatitis C infected patients and limit the extent of fibrosis. J. Hepatol. 2010, 52, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, M. Interleukin 17 is a chief orchestrator of immunity. Nat. Immunol. 2017, 18, 612–621. [Google Scholar] [CrossRef]
- Rios, D.A.; Casciato, P.C.; Caldirola, M.S.; Gaillard, M.I.; Giadans, C.; Ameigeiras, B.; De Matteo, E.N.; Preciado, M.V.; Valva, P. Chronic Hepatitis C Pathogenesis: Immune Response in the Liver Microenvironment and Peripheral Compartment. Front. Cell. Infect. Microbiol. 2021, 11, 712105. [Google Scholar] [CrossRef] [PubMed]
- Raziorrouh, B.; Sacher, K.; Tawar, R.G.; Emmerich, F.; Neumann-Haefelin, C.; Baumert, T.F.; Thimme, R.; Boettler, T. Virus-Specific CD4+ T Cells Have Functional and Phenotypic Characteristics of Follicular T-Helper Cells in Patients With Acute and Chronic HCV Infections. Gastroenterology 2016, 150, 696–706.e3. [Google Scholar] [CrossRef] [PubMed]
- Salinas, E.; Boisvert, M.; Upadhyay, A.A.; Bédard, N.; Nelson, S.A.; Bruneau, J.; Derdeyn, C.A.; Marcotrigiano, J.; Evans, M.J.; Bosinger, S.E.; et al. Early T follicular helper cell activity accelerates hepatitis C virus-specific B cell expansion. J. Clin. Investig. 2021, 131, e140590. [Google Scholar] [CrossRef]
- Heim, M.H.; Thimme, R. Innate and adaptive immune responses in HCV infections. J. Hepatol. 2014, 61, S14–S25. [Google Scholar] [CrossRef]
- Kang, W.; Sung, P.S.; Park, S.-H.; Yoon, S.; Chang, D.-Y.; Kim, S.; Han, K.H.; Kim, J.K.; Rehermann, B.; Chwae, Y.-J.; et al. Hepatitis C Virus Attenuates Interferon-Induced MHC Class I Expression and Decreases CD8+ T-Cell Effector Functions. Gastroenterology 2014, 146, 1351–1360.e4. [Google Scholar] [CrossRef]
- Kared, H.; Fabre, T.; Bédard, N.; Bruneau, J.; Shoukry, N.H. Galectin-9 and IL-21 mediate cross-regulation between Th17 and Treg cells during acute hepatitis C. PLoS Pathog. 2013, 9, e1003422. [Google Scholar] [CrossRef] [PubMed]
- McLane, L.M.; Abdel-Hakeem, M.S.; Wherry, E.J. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu. Rev. Immunol. 2019, 37, 457–495. [Google Scholar] [CrossRef] [PubMed]
- Bull, R.A.; Leung, P.; Gaudieri, S.; Deshpande, P.; Cameron, B.; Walker, M.; Chopra, A.; Lloyd, A.R.; Luciani, F. Transmitted/Founder Viruses Rapidly Escape from CD8+ T Cell Responses in Acute Hepatitis C Virus Infection. J. Virol. 2015, 89, 5478–5490. [Google Scholar] [CrossRef]
- Callendret, B.; Bukh, J.; Eccleston, H.B.; Heksch, R.; Hasselschwert, D.L.; Purcell, R.H.; Hughes, A.L.; Walker, C.M. Transmission of clonal hepatitis C virus genomes reveals the dominant but transitory role of CD8+ T cells in early viral evolution. J. Virol. 2011, 85, 11833–11845. [Google Scholar] [CrossRef] [PubMed]
- von Hahn, T.; Yoon, J.C.; Alter, H.; Rice, C.M.; Rehermann, B.; Balfe, P.; McKeating, J.A. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology 2007, 132, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Wölfl, M.; Rutebemberwa, A.; Mosbruger, T.; Mao, Q.; Li, H.; Netski, D.; Ray, S.C.; Pardoll, D.; Sidney, J.; Sette, A.; et al. Hepatitis C virus immune escape via exploitation of a hole in the T cell repertoire. J. Immunol. 2008, 181, 6435–6446. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, M.; Knuschke, T.; Schewior, K.; Glavinic, L.; Neumann-Haefelin, C.; Chang, D.-I.; Klein, M.; Heinemann, F.M.; Tenckhoff, H.; Wiese, M.; et al. CD8+ T-cell response promotes evolution of hepatitis C virus nonstructural proteins. Gastroenterology 2011, 140, 2064–2073. [Google Scholar] [CrossRef] [PubMed]
- Kroy, D.C.; Ciuffreda, D.; Cooperrider, J.H.; Tomlinson, M.; Hauck, G.D.; Aneja, J.; Berger, C.; Wolski, D.; Carrington, M.; Wherry, E.J.; et al. Liver environment and HCV replication affect human T-cell phenotype and expression of inhibitory receptors. Gastroenterology 2014, 146, 550–561. [Google Scholar] [CrossRef]
- Owusu Sekyere, S.; Suneetha, P.V.; Kraft, A.R.M.; Zhang, S.; Dietz, J.; Sarrazin, C.; Manns, M.P.; Schlaphoff, V.; Cornberg, M.; Wedemeyer, H. A heterogeneous hierarchy of co-regulatory receptors regulates exhaustion of HCV-specific CD8 T cells in patients with chronic hepatitis C. J. Hepatol. 2015, 62, 31–40. [Google Scholar] [CrossRef]
- Godfrey, D.I.; Uldrich, A.P.; McCluskey, J.; Rossjohn, J.; Moody, D.B. The burgeoning family of unconventional T cells. Nat. Immunol. 2015, 16, 1114–1123. [Google Scholar] [CrossRef]
- Dusseaux, M.; Martin, E.; Serriari, N.; Péguillet, I.; Premel, V.; Louis, D.; Milder, M.; Le Bourhis, L.; Soudais, C.; Treiner, E.; et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 2011, 117, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Hengst, J.; Theorell, J.; Deterding, K.; Potthoff, A.; Dettmer, A.; Ljunggren, H.-G.; Wedemeyer, H.; Björkström, N.K. High-resolution determination of human immune cell signatures from fine-needle liver aspirates. Eur. J. Immunol. 2015, 45, 2154–2157. [Google Scholar] [CrossRef]
- Hengst, J.; Strunz, B.; Deterding, K.; Ljunggren, H.-G.; Leeansyah, E.; Manns, M.P.; Cornberg, M.; Sandberg, J.K.; Wedemeyer, H.; Björkström, N.K. Nonreversible MAIT cell-dysfunction in chronic hepatitis C virus infection despite successful interferon-free therapy. Eur. J. Immunol. 2016, 46, 2204–2210. [Google Scholar] [CrossRef] [PubMed]
- Bolte, F.J.; O’Keefe, A.C.; Webb, L.M.; Serti, E.; Rivera, E.; Liang, T.J.; Ghany, M.; Rehermann, B. Intra-Hepatic Depletion of Mucosal-Associated Invariant T Cells in Hepatitis C Virus-Induced Liver Inflammation. Gastroenterology 2017, 153, 1392–1403.e2. [Google Scholar] [CrossRef]
- Ghosh, A.; Mondal, R.K.; Romani, S.; Bagchi, S.; Cairo, C.; Pauza, C.D.; Kottilil, S.; Poonia, B. Persistent gamma delta T-cell dysfunction in chronic HCV infection despite direct-acting antiviral therapy induced cure. J. Viral Hepat. 2019, 26, 1105–1116. [Google Scholar] [CrossRef]
- Yin, W.; Tong, S.; Zhang, Q.; Shao, J.; Liu, Q.; Peng, H.; Hu, H.; Peng, M.; Hu, P.; Ren, H.; et al. Functional dichotomy of Vδ2 γδ T cells in chronic hepatitis C virus infections: Role in cytotoxicity but not for IFN-γ production. Sci. Rep. 2016, 6, 26296. [Google Scholar] [CrossRef] [PubMed]
- Osburn, W.O.; Fisher, B.E.; Dowd, K.A.; Urban, G.; Liu, L.; Ray, S.C.; Thomas, D.L.; Cox, A.L. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology 2010, 138, 315–324. [Google Scholar] [CrossRef]
- Klenerman, P.; Thimme, R. T cell responses in hepatitis C: The good, the bad and the unconventional. Gut 2012, 61, 1226–1234. [Google Scholar] [CrossRef]
- Shin, E.-C.; Sung, P.S.; Park, S.-H. Immune responses and immunopathology in acute and chronic viral hepatitis. Nat. Rev. Immunol. 2016, 16, 509–523. [Google Scholar] [CrossRef]
- Shoukry, N.H.; Grakoui, A.; Houghton, M.; Chien, D.Y.; Ghrayeb, J.; Reimann, K.A.; Walker, C.M. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J. Exp. Med. 2003, 197, 1645–1655. [Google Scholar] [CrossRef]
- Grakoui, A.; Shoukry, N.H.; Woollard, D.J.; Han, J.-H.; Hanson, H.L.; Ghrayeb, J.; Murthy, K.K.; Rice, C.M.; Walker, C.M. HCV persistence and immune evasion in the absence of memory T cell help. Science 2003, 302, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Farci, P.; Shimoda, A.; Wong, D.; Cabezon, T.; De Gioannis, D.; Strazzera, A.; Shimizu, Y.; Shapiro, M.; Alter, H.J.; Purcell, R.H. Prevention of hepatitis C virus infection in chimpanzees by hyperimmune serum against the hypervariable region 1 of the envelope 2 protein. Proc. Natl. Acad. Sci. USA 1996, 93, 15394–15399. [Google Scholar] [CrossRef]
- Choo, Q.L.; Kuo, G.; Ralston, R.; Weiner, A.; Chien, D.; Van Nest, G.; Han, J.; Berger, K.; Thudium, K.; Kuo, C. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc. Natl. Acad. Sci. USA 1994, 91, 1294–1298. [Google Scholar] [CrossRef] [PubMed]
- Lavillette, D.; Morice, Y.; Germanidis, G.; Donot, P.; Soulier, A.; Pagkalos, E.; Sakellariou, G.; Intrator, L.; Bartosch, B.; Pawlotsky, J.-M.; et al. Human serum facilitates hepatitis C virus infection, and neutralizing responses inversely correlate with viral replication kinetics at the acute phase of hepatitis C virus infection. J. Virol. 2005, 79, 6023–6034. [Google Scholar] [CrossRef]
- Pestka, J.M.; Zeisel, M.B.; Bläser, E.; Schürmann, P.; Bartosch, B.; Cosset, F.-L.; Patel, A.H.; Meisel, H.; Baumert, J.; Viazov, S.; et al. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc. Natl. Acad. Sci. USA 2007, 104, 6025–6030. [Google Scholar] [CrossRef]
- Osburn, W.O.; Snider, A.E.; Wells, B.L.; Latanich, R.; Bailey, J.R.; Thomas, D.L.; Cox, A.L.; Ray, S.C. Clearance of hepatitis C infection is associated with the early appearance of broad neutralizing antibody responses. Hepatology 2014, 59, 2140–2151. [Google Scholar] [CrossRef] [PubMed]
- Meunier, J.-C.; Gottwein, J.M.; Houghton, M.; Russell, R.S.; Emerson, S.U.; Bukh, J.; Purcell, R.H. Vaccine-induced cross-genotype reactive neutralizing antibodies against hepatitis C virus. J. Infect. Dis. 2011, 204, 1186–1190. [Google Scholar] [CrossRef]
- Houghton, M.; Abrignani, S. Prospects for a vaccine against the hepatitis C virus. Nature 2005, 436, 961–966. [Google Scholar] [CrossRef]
- Singh, M.; O’Hagan, D. Advances in vaccine adjuvants. Nat. Biotechnol. 1999, 17, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.E.; Houghton, M.; Coates, S.; Abrignani, S.; Chien, D.; Rosa, D.; Pileri, P.; Ray, R.; Di Bisceglie, A.M.; Rinella, P.; et al. Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults. Vaccine 2010, 28, 6367–6373. [Google Scholar] [CrossRef] [PubMed]
- Stamataki, Z.; Coates, S.; Abrignani, S.; Houghton, M.; McKeating, J.A. Immunization of human volunteers with hepatitis C virus envelope glycoproteins elicits antibodies that cross-neutralize heterologous virus strains. J. Infect. Dis. 2011, 204, 811–813. [Google Scholar] [CrossRef]
- Law, J.L.M.; Chen, C.; Wong, J.; Hockman, D.; Santer, D.M.; Frey, S.E.; Belshe, R.B.; Wakita, T.; Bukh, J.; Jones, C.T.; et al. A hepatitis C virus (HCV) vaccine comprising envelope glycoproteins gpE1/gpE2 derived from a single isolate elicits broad cross-genotype neutralizing antibodies in humans. PLoS ONE 2013, 8, e59776. [Google Scholar] [CrossRef]
- Chen, F.; Nagy, K.; Chavez, D.; Willis, S.; McBride, R.; Giang, E.; Honda, A.; Bukh, J.; Ordoukhanian, P.; Zhu, J.; et al. Antibody Responses to Immunization With HCV Envelope Glycoproteins as a Baseline for B-Cell-Based Vaccine Development. Gastroenterology 2020, 158, 1058–1071.e6. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Le, H.T.; Logan, M.; Hockman, D.; Landi, A.; Crawford, K.; Wininger, M.; Johnson, J.; Kundu, J.K.; Tiffney, E.A.; et al. Recombinant H77C gpE1/gpE2 heterodimer elicits superior HCV cross-neutralisation than H77C gpE2 alone. J. Hepatol. 2024, S0168-8278(24)02335-3. [Google Scholar] [CrossRef]
- Law, J.L.M.; Logan, M.; Wong, J.; Kundu, J.; Hockman, D.; Landi, A.; Chen, C.; Crawford, K.; Wininger, M.; Johnson, J.; et al. Role of the E2 Hypervariable Region (HVR1) in the Immunogenicity of a Recombinant Hepatitis C Virus Vaccine. J. Virol. 2018, 92, e02141-17. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, K.; Boo, I.; Poumbourios, P.; Drummer, H.E. Expression and characterization of a minimal hepatitis C virus glycoprotein E2 core domain that retains CD81 binding. J. Virol. 2007, 81, 9584–9590. [Google Scholar] [CrossRef] [PubMed]
- Vietheer, P.T.; Boo, I.; Gu, J.; McCaffrey, K.; Edwards, S.; Owczarek, C.; Hardy, M.P.; Fabri, L.; Center, R.J.; Poumbourios, P.; et al. The core domain of hepatitis C virus glycoprotein E2 generates potent cross-neutralizing antibodies in guinea pigs. Hepatology 2017, 65, 1117–1131. [Google Scholar] [CrossRef]
- Center, R.J.; Boo, I.; Phu, L.; McGregor, J.; Poumbourios, P.; Drummer, H.E. Enhancing the antigenicity and immunogenicity of monomeric forms of hepatitis C virus E2 for use as a preventive vaccine. J. Biol. Chem. 2020, 295, 7179–7192. [Google Scholar] [CrossRef]
- Lavie, M.; Hanoulle, X.; Dubuisson, J. Glycan Shielding and Modulation of Hepatitis C Virus Neutralizing Antibodies. Front. Immunol. 2018, 9, 910. [Google Scholar] [CrossRef]
- Ren, Y.; Min, Y.-Q.; Liu, M.; Chi, L.; Zhao, P.; Zhang, X.-L. N-glycosylation-mutated HCV envelope glycoprotein complex enhances antigen-presenting activity and cellular and neutralizing antibody responses. Biochim. Biophys. Acta 2016, 1860, 1764–1775. [Google Scholar] [CrossRef]
- Urbanowicz, R.A.; Wang, R.; Schiel, J.E.; Keck, Z.-Y.; Kerzic, M.C.; Lau, P.; Rangarajan, S.; Garagusi, K.J.; Tan, L.; Guest, J.D.; et al. Antigenicity and Immunogenicity of Differentially Glycosylated Hepatitis C Virus E2 Envelope Proteins Expressed in Mammalian and Insect Cells. J. Virol. 2019, 93, e01403-18. [Google Scholar] [CrossRef]
- Vijayamahantesh, V.; Patra, T.; Meyer, K.; Alameh, M.-G.; Reagan, E.K.; Weissman, D.; Ray, R. Modified E2 Glycoprotein of Hepatitis C Virus Enhances Proinflammatory Cytokines and Protective Immune Response. J. Virol. 2022, 96, e0052322. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.B.; Agnew, B.J.; Deschatelets, L.; Fleming, L.P.; Sprott, G.D. In vitro assessment of archaeosome stability for developing oral delivery systems. Int. J. Pharm. 2000, 194, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Benvegnu, T.; Lemiegre, L.; Cammas-Marion, S. New Generation of Liposomes Called Archaeosomes Based on Natural or Synthetic Archaeal Lipids as Innovative Formulations for Drug Delivery. Recent Pat. Drug Deliv. Formul. 2009, 3, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Landi, A.; Law, J.; Hockman, D.; Logan, M.; Crawford, K.; Chen, C.; Kundu, J.; Ebensen, T.; Guzman, C.A.; Deschatelets, L.; et al. Superior immunogenicity of HCV envelope glycoproteins when adjuvanted with cyclic-di-AMP, a STING activator or archaeosomes. Vaccine 2017, 35, 6949–6956. [Google Scholar] [CrossRef] [PubMed]
- Akache, B.; Deschatelets, L.; Harrison, B.A.; Dudani, R.; Stark, F.C.; Jia, Y.; Landi, A.; Law, J.L.M.; Logan, M.; Hockman, D.; et al. Effect of Different Adjuvants on the Longevity and Strength of Humoral and Cellular Immune Responses to the HCV Envelope Glycoproteins. Vaccines 2019, 7, 204. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, X.; Lou, P.; Hu, Z.; Qu, P.; Li, D.; Li, Q.; Xu, Y.; Niu, J.; He, Y.; et al. A Nanoparticle-Based Hepatitis C Virus Vaccine With Enhanced Potency. J. Infect. Dis. 2020, 221, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Tzarum, N.; Lin, X.; Shapero, B.; Sou, C.; Mann, C.J.; Stano, A.; Zhang, L.; Nagy, K.; Giang, E.; et al. Proof of concept for rational design of hepatitis C virus E2 core nanoparticle vaccines. Sci. Adv. 2020, 6, eaaz6225. [Google Scholar] [CrossRef]
- Sliepen, K.; Radić, L.; Capella-Pujol, J.; Watanabe, Y.; Zon, I.; Chumbe, A.; Lee, W.-H.; de Gast, M.; Koopsen, J.; Koekkoek, S.; et al. Induction of cross-neutralizing antibodies by a permuted hepatitis C virus glycoprotein nanoparticle vaccine candidate. Nat. Commun. 2022, 13, 7271. [Google Scholar] [CrossRef]
- Garrone, P.; Fluckiger, A.-C.; Mangeot, P.E.; Gauthier, E.; Dupeyrot-Lacas, P.; Mancip, J.; Cangialosi, A.; Du Chéné, I.; LeGrand, R.; Mangeot, I.; et al. A prime-boost strategy using virus-like particles pseudotyped for HCV proteins triggers broadly neutralizing antibodies in macaques. Sci. Transl. Med. 2011, 3, 94ra71. [Google Scholar] [CrossRef] [PubMed]
- Elmowalid, G.A.; Qiao, M.; Jeong, S.-H.; Borg, B.B.; Baumert, T.F.; Sapp, R.K.; Hu, Z.; Murthy, K.; Liang, T.J. Immunization with hepatitis C virus-like particles results in control of hepatitis C virus infection in chimpanzees. Proc. Natl. Acad. Sci. USA 2007, 104, 8427–8432. [Google Scholar] [CrossRef]
- Christiansen, D.; Earnest-Silveira, L.; Chua, B.; Meuleman, P.; Boo, I.; Grubor-Bauk, B.; Jackson, D.C.; Keck, Z.Y.; Foung, S.K.H.; Drummer, H.E.; et al. Immunological responses following administration of a genotype 1a/1b/2/3a quadrivalent HCV VLP vaccine. Sci. Rep. 2018, 8, 6483. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, D.; Earnest-Silveira, L.; Grubor-Bauk, B.; Wijesundara, D.K.; Boo, I.; Ramsland, P.A.; Vincan, E.; Drummer, H.E.; Gowans, E.J.; Torresi, J. Pre-clinical evaluation of a quadrivalent HCV VLP vaccine in pigs following microneedle delivery. Sci. Rep. 2019, 9, 9251. [Google Scholar] [CrossRef]
- Akazawa, D.; Moriyama, M.; Yokokawa, H.; Omi, N.; Watanabe, N.; Date, T.; Morikawa, K.; Aizaki, H.; Ishii, K.; Kato, T.; et al. Neutralizing antibodies induced by cell culture-derived hepatitis C virus protect against infection in mice. Gastroenterology 2013, 145, 447–455.e4. [Google Scholar] [CrossRef]
- Yokokawa, H.; Higashino, A.; Suzuki, S.; Moriyama, M.; Nakamura, N.; Suzuki, T.; Suzuki, R.; Ishii, K.; Kobiyama, K.; Ishii, K.J.; et al. Induction of humoural and cellular immunity by immunisation with HCV particle vaccine in a non-human primate model. Gut 2018, 67, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Folgori, A.; Capone, S.; Ruggeri, L.; Meola, A.; Sporeno, E.; Ercole, B.B.; Pezzanera, M.; Tafi, R.; Arcuri, M.; Fattori, E.; et al. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat. Med. 2006, 12, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Shin, E.-C.; Capone, S.; Caggiari, L.; De Re, V.; Nicosia, A.; Folgori, A.; Rehermann, B. Successful vaccination induces multifunctional memory T-cell precursors associated with early control of hepatitis C virus. Gastroenterology 2012, 143, 1048–1060.e4. [Google Scholar] [CrossRef]
- Drane, D.; Maraskovsky, E.; Gibson, R.; Mitchell, S.; Barnden, M.; Moskwa, A.; Shaw, D.; Gervase, B.; Coates, S.; Houghton, M.; et al. Priming of CD4+ and CD8+ T cell responses using a HCV core ISCOMATRIX vaccine: A phase I study in healthy volunteers. Hum. Vaccin. 2009, 5, 151–157. [Google Scholar] [CrossRef]
- Colloca, S.; Barnes, E.; Folgori, A.; Ammendola, V.; Capone, S.; Cirillo, A.; Siani, L.; Naddeo, M.; Grazioli, F.; Esposito, M.L.; et al. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci. Transl. Med. 2012, 4, 115ra2. [Google Scholar] [CrossRef]
- Barnes, E.; Folgori, A.; Capone, S.; Swadling, L.; Aston, S.; Kurioka, A.; Meyer, J.; Huddart, R.; Smith, K.; Townsend, R.; et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci. Transl. Med. 2012, 4, 115ra1. [Google Scholar] [CrossRef]
- Swadling, L.; Capone, S.; Antrobus, R.D.; Brown, A.; Richardson, R.; Newell, E.W.; Halliday, J.; Kelly, C.; Bowen, D.; Fergusson, J.; et al. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Sci. Transl. Med. 2014, 6, 261ra153. [Google Scholar] [CrossRef] [PubMed]
- Page, K.; Melia, M.T.; Veenhuis, R.T.; Winter, M.; Rousseau, K.E.; Massaccesi, G.; Osburn, W.O.; Forman, M.; Thomas, E.; Thornton, K.; et al. Randomized Trial of a Vaccine Regimen to Prevent Chronic HCV Infection. N. Engl. J. Med. 2021, 384, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Donnison, T.; McGregor, J.; Chinnakannan, S.; Hutchings, C.; Center, R.J.; Poumbourios, P.; Klenerman, P.; Drummer, H.E.; Barnes, E. A pan-genotype hepatitis C virus viral vector vaccine generates T cells and neutralizing antibodies in mice. Hepatology 2022, 76, 1190–1202. [Google Scholar] [CrossRef] [PubMed]
- Rappuoli, R.; Bottomley, M.J.; D’Oro, U.; Finco, O.; De Gregorio, E. Reverse vaccinology 2.0: Human immunology instructs vaccine antigen design. J. Exp. Med. 2016, 213, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Byrne, P.O.; McLellan, J.S. Principles and practical applications of structure-based vaccine design. Curr. Opin. Immunol. 2022, 77, 102209. [Google Scholar] [CrossRef]
- Guest, J.D.; Pierce, B.G. Structure-Based and Rational Design of a Hepatitis C Virus Vaccine. Viruses 2021, 13, 837. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garbuglia, A.R.; Pauciullo, S.; Zulian, V.; Del Porto, P. Update on Hepatitis C Vaccine: Results and Challenges. Viruses 2024, 16, 1337. https://doi.org/10.3390/v16081337
Garbuglia AR, Pauciullo S, Zulian V, Del Porto P. Update on Hepatitis C Vaccine: Results and Challenges. Viruses. 2024; 16(8):1337. https://doi.org/10.3390/v16081337
Chicago/Turabian StyleGarbuglia, Anna Rosa, Silvia Pauciullo, Verdiana Zulian, and Paola Del Porto. 2024. "Update on Hepatitis C Vaccine: Results and Challenges" Viruses 16, no. 8: 1337. https://doi.org/10.3390/v16081337
APA StyleGarbuglia, A. R., Pauciullo, S., Zulian, V., & Del Porto, P. (2024). Update on Hepatitis C Vaccine: Results and Challenges. Viruses, 16(8), 1337. https://doi.org/10.3390/v16081337






