Type I Interferonopathy among Non-Elderly Female Patients with Post-Acute Sequelae of COVID-19
Abstract
:1. Introduction
2. Methods
2.1. Single-Cell Transcriptome Data
2.2. Data Download and Alignment
2.3. The scRNA-seq Transcriptome Analysis
2.4. Functional Enrichment Analysis
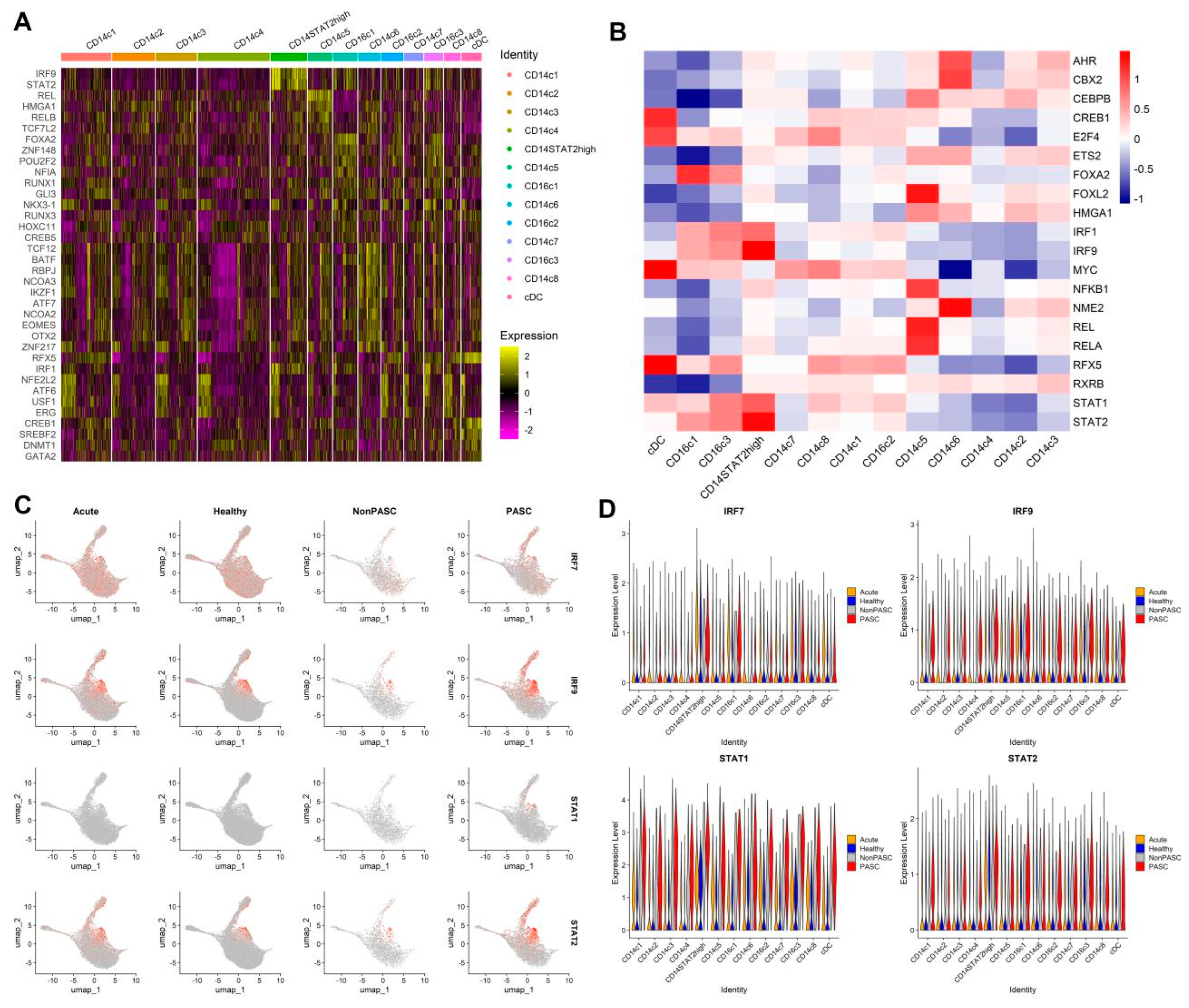
2.5. Transcription Factor (TF) Analysis
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Participants
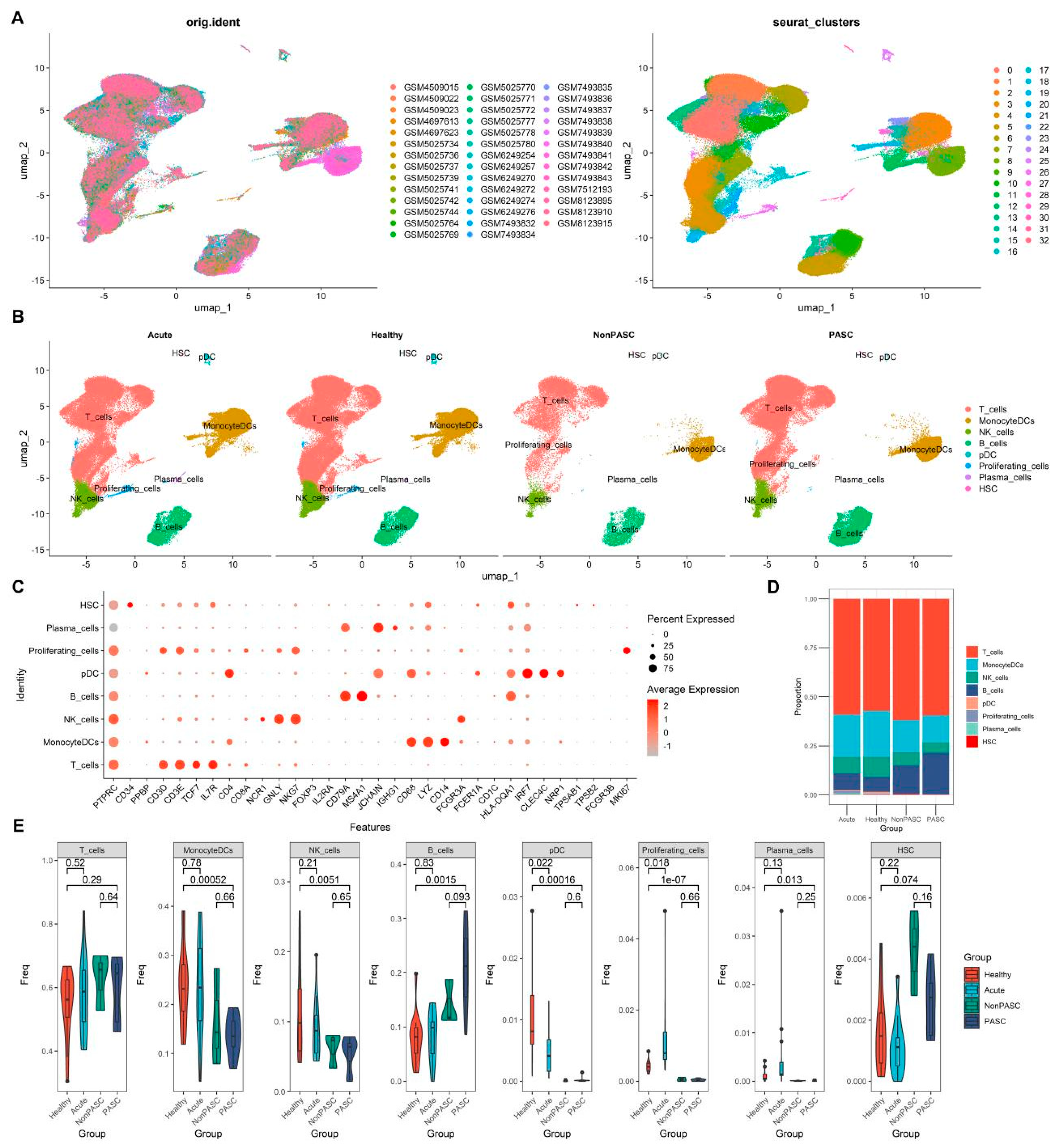
3.2. Changes in Peripheral Immune Cells among Non-Elderly Female PASC
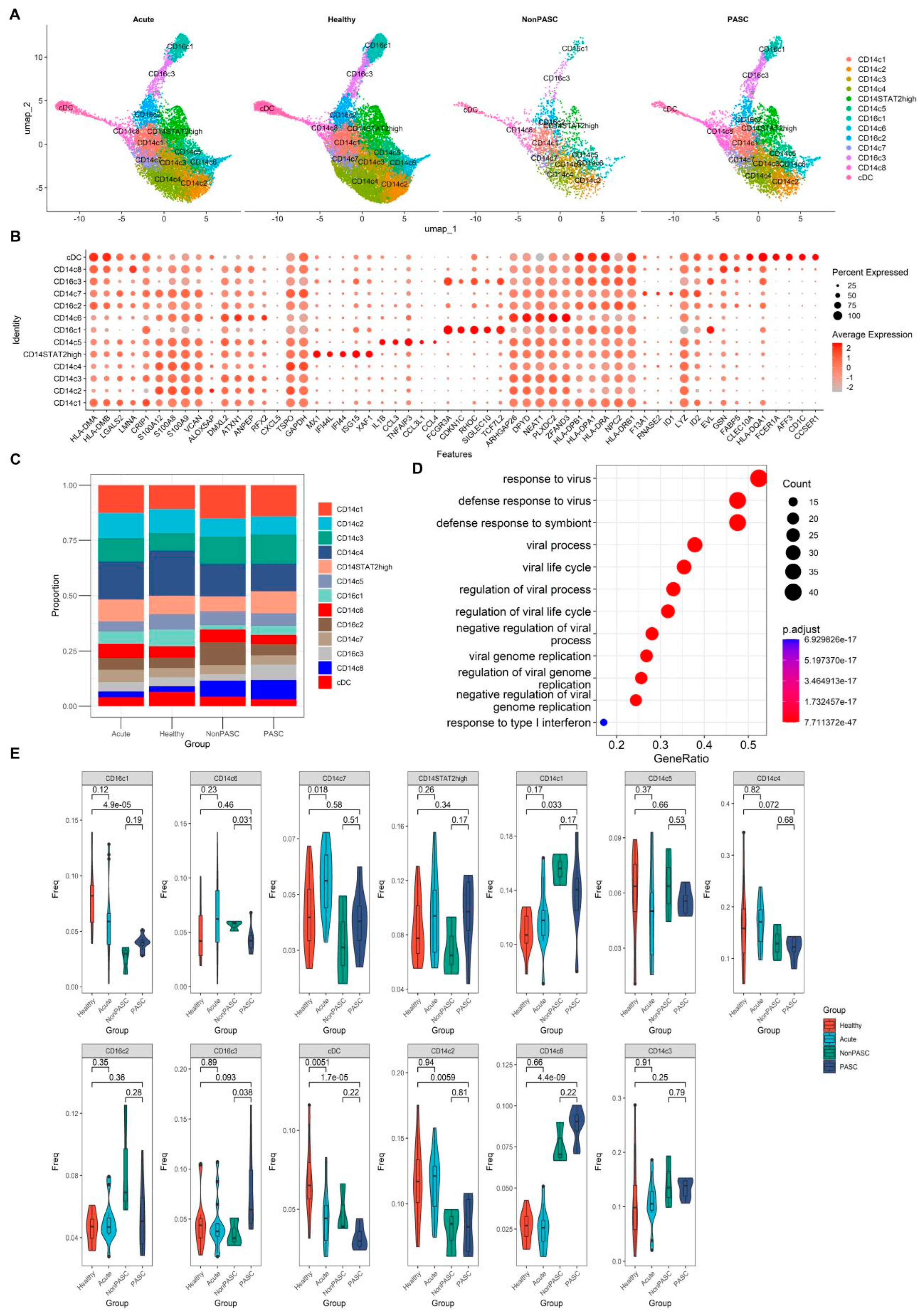
3.3. Changes in Peripheral Blood Monocytes among Non-Elderly Female PASC
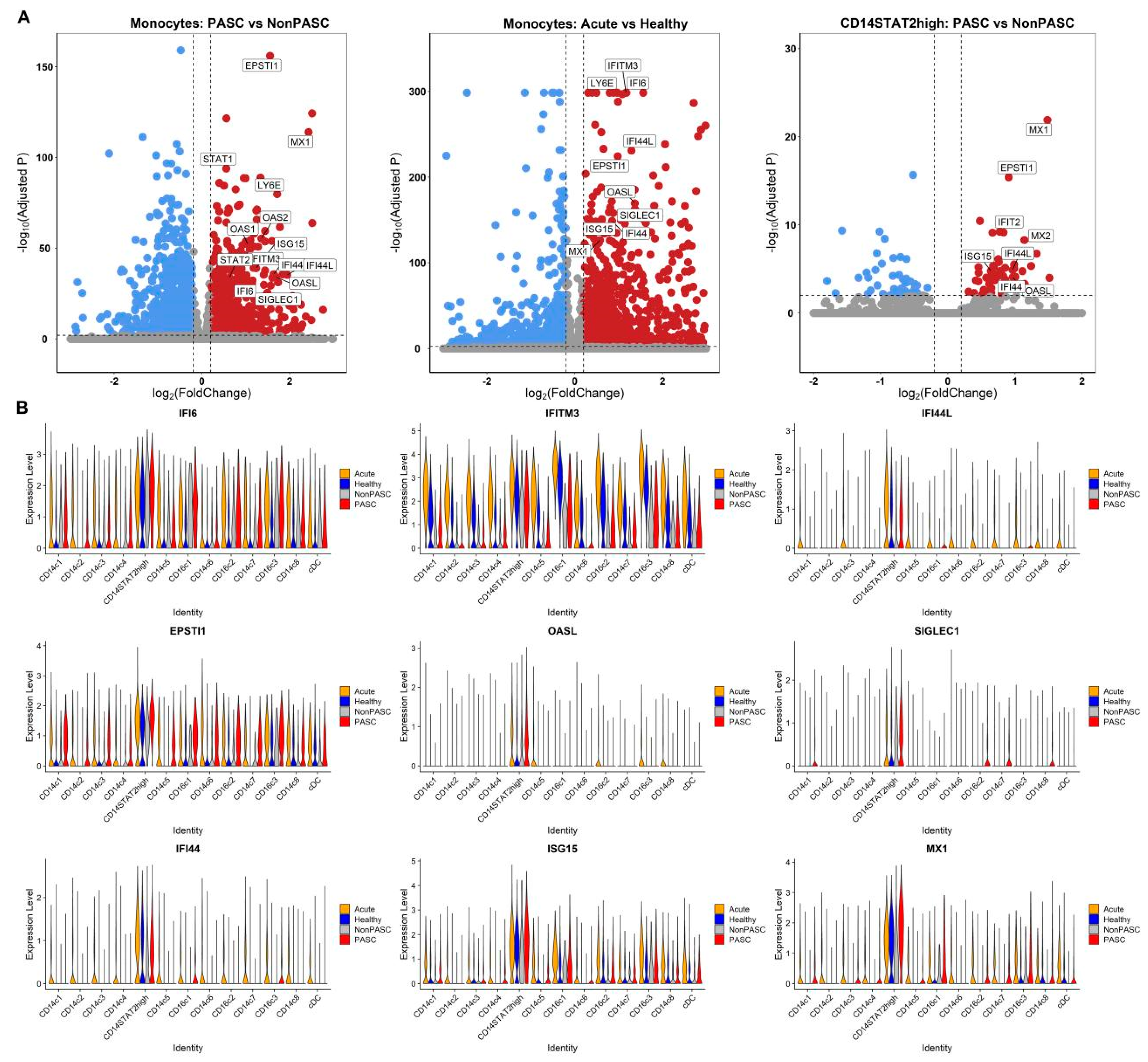
3.4. Increased Expression of Interferon-Stimulated Genes (ISGs) in Monocytes
3.5. The scRNA-Seq Transcriptome Analysis of B Cells
3.6. The scRNA-Seq Transcriptome Analysis of T Cells
3.7. The scRNA-Seq Transcriptome Analysis of NK Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Aly, Z.; Topol, E. Solving the puzzle of Long COVID. Science 2024, 383, 830–832. [Google Scholar] [CrossRef]
- Peluso, M.J.; Abdel-Mohsen, M.; Henrich, T.J.; Roan, N.R. Systems analysis of innate and adaptive immunity in Long COVID. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2024; Volume 72, p. 101873. [Google Scholar]
- Klein, J.; Wood, J.; Jaycox, J.R.; Dhodapkar, R.M.; Lu, P.; Gehlhausen, J.R.; Tabachnikova, A.; Greene, K.; Tabacof, L.; Malik, A.A.; et al. Distinguishing features of long COVID identified through immune profiling. Nature 2023, 623, 139–148. [Google Scholar] [CrossRef]
- Wu, Y.; Sawano, M.; Wu, Y.; Shah, R.M.; Bishop, P.; Iwasaki, A.; Krumholz, H.M. Factors Associated with Long COVID: Insights from Two Nationwide Surveys. Am. J. Med. 2024, 137, 515–519. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Subramanian, A.; Nirantharakumar, K.; Hughes, S.; Myles, P.; Williams, T.; Gokhale, K.M.; Taverner, T.; Chandan, J.S.; Brown, K.; Simms-Williams, N.; et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat. Med. 2022, 28, 1706–1714. [Google Scholar] [CrossRef]
- Cho, S.M.; Premraj, L.; Battaglini, D.; Fanning, J.P.; Suen, J.; Bassi, G.L.; Fraser, J.; Robba, C.; Griffee, M.; Solomon, T.; et al. Sex differences in post-acute neurological sequelae of SARS-CoV-2 and symptom resolution in adults after coronavirus disease 2019 hospitalization: An international multi-centre prospective observational study. Brain Commun. 2024, 6, fcae036. [Google Scholar] [CrossRef] [PubMed]
- Giroux, N.S.; Ding, S.; McClain, M.T.; Burke, T.W.; Petzold, E.; Chung, H.A.; Rivera, G.O.; Wang, E.; Xi, R.; Bose, S.; et al. Differential chromatin accessibility in peripheral blood mononuclear cells underlies COVID-19 disease severity prior to seroconversion. Sci. Rep. 2022, 12, 11714. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, S.; Jeong, H.W.; Ahn, J.Y.; Choi, S.J.; Lee, H.; Choi, B.; Nam, S.K.; Sa, M.; Kwon, J.S.; et al. Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci. Immunol. 2020, 5, eabd1554. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Peluso, M.J.; Luo, X.; Thomas, R.; Shin, M.G.; Neidleman, J.; Andrew, A.; Young, K.C.; Ma, T.; Hoh, R.; et al. Long COVID manifests with T cell dysregulation, inflammation and an uncoordinated adaptive immune response to SARS-CoV-2. Nat. Immunol. 2024, 25, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bai, H.; Ma, J.; Qin, H.; Zeng, Q.; Hu, F.; Jiang, T.; Mao, W.; Zhao, Y.; Chen, X.; et al. Identification of Distinct Immune Cell Subsets Associated with Asymptomatic Infection, Disease Severity, and Viral Persistence in COVID-19 Patients. Front. Immunol. 2022, 13, 812514. [Google Scholar] [CrossRef]
- Unterman, A.; Sumida, T.S.; Nouri, N.; Yan, X.; Zhao, A.Y.; Gasque, V.; Schupp, J.C.; Asashima, H.; Liu, Y.; Cosme, C.; et al. Single-cell multi-omics reveals dyssynchrony of the innate and adaptive immune system in progressive COVID-19. Nat. Commun. 2022, 13, 440. [Google Scholar] [CrossRef]
- Yoon, H.; Dean, L.S.; Jiyarom, B.; Khadka, V.S.; Deng, Y.; Nerurkar, V.R.; Chow, D.C.; Shikuma, C.M.; Devendra, G.; Koh, Y.; et al. Single-cell RNA sequencing reveals characteristics of myeloid cells in post-acute sequelae of SARS-CoV-2 patients with persistent respiratory symptoms. Front. Immunol. 2023, 14, 1268510. [Google Scholar] [CrossRef] [PubMed]
- Terzoli, S.; Marzano, P.; Cazzetta, V.; Piazza, R.; Sandrock, I.; Ravens, S.; Tan, L.; Prinz, I.; Balin, S.; Calvi, M.; et al. Expansion of memory Vdelta2 T cells following SARS-CoV-2 vaccination revealed by temporal single-cell transcriptomics. NPJ Vaccines 2024, 9, 63. [Google Scholar] [CrossRef]
- Krishna, B.A.; Lim, E.Y.; Metaxaki, M.; Jackson, S.; Mactavous, L.; BioResource, N.; Lyons, P.A.; Doffinger, R.; Bradley, J.R.; Smith, K.G.C.; et al. Spontaneous, persistent, T cell-dependent IFN-gamma release in patients who progress to Long COVID. Sci. Adv. 2024, 10, eadi9379. [Google Scholar] [CrossRef] [PubMed]
- Eskandarian Boroujeni, M.; Sekrecka, A.; Antonczyk, A.; Hassani, S.; Sekrecki, M.; Nowicka, H.; Lopacinska, N.; Olya, A.; Kluzek, K.; Wesoly, J.; et al. Dysregulated Interferon Response and Immune Hyperactivation in Severe COVID-19: Targeting STATs as a Novel Therapeutic Strategy. Front. Immunol. 2022, 13, 888897. [Google Scholar] [CrossRef]
- Blaszczyk, K.; Nowicka, H.; Kostyrko, K.; Antonczyk, A.; Wesoly, J.; Bluyssen, H.A. The unique role of STAT2 in constitutive and IFN-induced transcription and antiviral responses. Cytokine Growth Factor Rev. 2016, 29, 71–81. [Google Scholar] [CrossRef]
- Nowicka, H.; Sekrecka, A.; Blaszczyk, K.; Kluzek, K.; Chang, C.Y.; Wesoly, J.; Lee, C.K.; Bluyssen, H.A.R. ISGF3 and STAT2/IRF9 Control Basal and IFN-Induced Transcription through Genome-Wide Binding of Phosphorylated and Unphosphorylated Complexes to Common ISRE-Containing ISGs. Int. J. Mol. Sci. 2023, 24, 17635. [Google Scholar] [CrossRef]
- Zhu, G.; Badonyi, M.; Franklin, L.; Seabra, L.; Rice, G.I.; Anne Boland, A.; Deleuze, J.F.; El-Chehadeh, S.; Anheim, M.; de Saint-Martin, A.; et al. Type I Interferonopathy due to a Homozygous Loss-of-Inhibitory Function Mutation in STAT2. J. Clin. Immunol. 2023, 43, 808–818. [Google Scholar] [CrossRef]
- Park, C.; Li, S.; Cha, E.; Schindler, C. Immune response in Stat2 knockout mice. Immunity 2000, 13, 795–804. [Google Scholar] [CrossRef]
- Bucciol, G.; Moens, L.; Ogishi, M.; Rinchai, D.; Matuozzo, D.; Momenilandi, M.; Kerrouche, N.; Cale, C.M.; Treffeisen, E.R.; Al Salamah, M.; et al. Human inherited complete STAT2 deficiency underlies inflammatory viral diseases. J. Clin. Investig. 2023, 133, e168321. [Google Scholar] [CrossRef]
- Duncan, C.J.A.; Thompson, B.J.; Chen, R.; Rice, G.I.; Gothe, F.; Young, D.F.; Lovell, S.C.; Shuttleworth, V.G.; Brocklebank, V.; Corner, B.; et al. Severe type I interferonopathy and unrestrained interferon signaling due to a homozygous germline mutation in STAT2. Sci. Immunol. 2019, 4, eaav7501. [Google Scholar] [CrossRef] [PubMed]
- Boudewijns, R.; Thibaut, H.J.; Kaptein, S.J.F.; Li, R.; Vergote, V.; Seldeslachts, L.; Van Weyenbergh, J.; De Keyzer, C.; Bervoets, L.; Sharma, S.; et al. STAT2 signaling restricts viral dissemination but drives severe pneumonia in SARS-CoV-2 infected hamsters. Nat. Commun. 2020, 11, 5838. [Google Scholar] [CrossRef]
- Liew, F.; Efstathiou, C.; Fontanella, S.; Richardson, M.; Saunders, R.; Swieboda, D.; Sidhu, J.K.; Ascough, S.; Moore, S.C.; Mohamed, N.; et al. Large-scale phenotyping of patients with long COVID post-hospitalization reveals mechanistic subtypes of disease. Nat. Immunol. 2024, 25, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.A.F.; Brito, W.; Pereira, K.A.S.; Pereira, L.M.S.; Amoras, E.; Lima, S.S.; Santos, E.F.D.; Costa, F.P.D.; Sarges, K.M.L.; Cantanhede, M.H.D.; et al. Severe COVID-19 and long COVID are associated with high expression of STING, cGAS and IFN-alpha. Sci. Rep. 2024, 14, 4974. [Google Scholar] [CrossRef]
- Gallucci, S.; Meka, S.; Gamero, A.M. Abnormalities of the type I interferon signaling pathway in lupus autoimmunity. Cytokine 2021, 146, 155633. [Google Scholar] [CrossRef]
- Guery, J.C. Sex Differences in Primary HIV Infection: Revisiting the Role of TLR7-Driven Type 1 IFN Production by Plasmacytoid Dendritic Cells in Women. Front. Immunol. 2021, 12, 729233. [Google Scholar] [CrossRef]
- Spiering, A.E.; de Vries, T.J. Why Females Do Better: The X Chromosomal TLR7 Gene-Dose Effect in COVID-19. Front. Immunol. 2021, 12, 756262. [Google Scholar] [CrossRef] [PubMed]
- Chanana, N.; Palmo, T.; Sharma, K.; Kumar, R.; Graham, B.B.; Pasha, Q. Sex-derived attributes contributing to SARS-CoV-2 mortality. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E562–E567. [Google Scholar] [CrossRef]
- Umiker, B.R.; Andersson, S.; Fernandez, L.; Korgaokar, P.; Larbi, A.; Pilichowska, M.; Weinkauf, C.C.; Wortis, H.H.; Kearney, J.F.; Imanishi-Kari, T. Dosage of X-linked Toll-like receptor 8 determines gender differences in the development of systemic lupus erythematosus. Eur. J. Immunol. 2014, 44, 1503–1516. [Google Scholar] [CrossRef]
- Hagen, S.H.; Henseling, F.; Hennesen, J.; Savel, H.; Delahaye, S.; Richert, L.; Ziegler, S.M.; Altfeld, M. Heterogeneous Escape from X Chromosome Inactivation Results in Sex Differences in Type I IFN Responses at the Single Human pDC Level. Cell Rep. 2020, 33, 108485. [Google Scholar] [CrossRef]
- Gomez-Carballa, A.; Pardo-Seco, J.; Pischedda, S.; Rivero-Calle, I.; Butler-Laporte, G.; Richards, J.B.; Viz-Lasheras, S.; Martinon-Torres, F.; Salas, A.; Network, G. Sex-biased expression of the TLR7 gene in severe COVID-19 patients: Insights from transcriptomics and epigenomics. Env. Res. 2022, 215 Pt 2, 114288. [Google Scholar] [CrossRef]
- Fallerini, C.; Daga, S.; Mantovani, S.; Benetti, E.; Picchiotti, N.; Francisci, D.; Paciosi, F.; Schiaroli, E.; Baldassarri, M.; Fava, F.; et al. Association of Toll-like receptor 7 variants with life-threatening COVID-19 disease in males: Findings from a nested case-control study. Elife 2021, 10, e67569. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Boisson, B.; Onodi, F.; Matuozzo, D.; Moncada-Velez, M.; Maglorius Renkilaraj, M.R.L.; Zhang, P.; Meertens, L.; Bolze, A.; Materna, M.; et al. X-linked recessive TLR7 deficiency in ~1% of men under 60 years old with life-threatening COVID-19. Sci. Immunol. 2021, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Dai, C.; Cai, P.; Wang, J.; Xu, L.; Li, J.; Hu, G.; Wang, Z.; Zheng, F.; Wang, L. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: A possible reason underlying different outcome between sex. J. Med. Virol. 2020, 92, 2050–2054. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A. Exploring the Pathophysiology of Long COVID: The Central Role of Low-Grade Inflammation and Multisystem Involvement. Int. J. Mol. Sci. 2024, 25, 6389. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Whitehead, C.; Surrao, K.; Pillai, A.; Madeshiya, A.; Li, Y.; Khodadadi, H.; Ahmed, A.O.; Turecki, G.; Baban, B.; et al. Type 1 interferon mediates chronic stress-induced neuroinflammation and behavioral deficits via complement component 3-dependent pathway. Mol. Psychiatry 2021, 26, 3043–3059. [Google Scholar] [CrossRef]
- Jodele, S.; Medvedovic, M.; Luebbering, N.; Chen, J.; Dandoy, C.E.; Laskin, B.L.; Davies, S.M. Interferon-complement loop in transplant-associated thrombotic microangiopathy. Blood Adv. 2020, 4, 1166–1177. [Google Scholar] [CrossRef]
- Iba, T.; Connors, J.M.; Levy, J.H. What Role Does Microthrombosis Play in Long COVID? In Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers: New York, NY, USA, 2024; Volume 50, pp. 527–536. [Google Scholar]



| GSMID | Gender | Age (Year) | Group | Hospitalization | Sample Type | Reference |
|---|---|---|---|---|---|---|
| GSM7493832 | Female | 37 | Non-PASC | No | PBMC | PMID: 38212464 [10] |
| GSM7493834 | Female | 38 | Non-PASC | No | PBMC | PMID: 38212464 [10] |
| GSM7493835 | Female | 50 | Non-PASC | No | PBMC | PMID: 38212464 [10] |
| GSM7493836 | Female | 57 | PASC | No | PBMC | PMID: 38212464 [10] |
| GSM7493837 | Female | 46 | PASC | Yes | PBMC | PMID: 38212464 [10] |
| GSM7493838 | Female | 49 | PASC | Yes | PBMC | PMID: 38212464 [10] |
| GSM7493839 | Female | 33 | PASC | No | PBMC | PMID: 38212464 [10] |
| GSM7493840 | Female | 43 | PASC | Yes | PBMC | PMID: 38212464 [10] |
| GSM7493841 | Female | 48 | PASC | No | PBMC | PMID: 38212464 [10] |
| GSM7493842 | Female | 26 | PASC | No | PBMC | PMID: 38212464 [10] |
| GSM7493843 | Female | 46 | PASC | Yes | PBMC | PMID: 38212464 [10] |
| GSM6249254 | Female | 29 | Acute COVID-19 | Not provided | PBMC | PMID: 35810186 [8] |
| GSM6249257 | Female | 32 | Acute COVID-19 | Not provided | PBMC | PMID: 35810186 [8] |
| GSM6249270 | Female | 45 | Acute COVID-19 | Not provided | PBMC | PMID: 35810186 [8] |
| GSM6249272 | Female | 27 | Healthy | -- | PBMC | PMID: 35810186 [8] |
| GSM6249274 | Female | 44 | Healthy | -- | PBMC | PMID: 35810186 [8] |
| GSM6249276 | Female | 60 | Healthy | -- | PBMC | PMID: 35810186 [8] |
| GSM4509015 | Female | 63 | Healthy | -- | PBMC | PMID: 32651212 [9] |
| GSM4509022 | Female | 38 | Acute COVID-19 | Not provided | PBMC | PMID: 32651212 [9] |
| GSM4509023 | Female | 54 | Healthy | -- | PBMC | PMID: 32651212 [9] |
| GSM4697613 | Female | 53 | Acute COVID-19 | Yes | PBMC | PMID: 35064122 [12] |
| GSM4697623 | Female | 56 | Acute COVID-19 | Yes | PBMC | PMID: 35064122 [12] |
| GSM5025734 | Female | 46 | Acute COVID-19 | Not provided | PBMC | PMID: 35281000 [11] |
| GSM5025736 | Female | 53 | Acute COVID-19 | Not provided | PBMC | PMID: 35281000 [11] |
| GSM5025737 | Female | 39 | Acute COVID-19 | Not provided | PBMC | PMID: 35281000 [11] |
| GSM5025739 | Female | 47 | Acute COVID-19 | Not provided | PBMC | PMID: 35281000 [11] |
| GSM5025741 | Female | 53 | Acute COVID-19 | Not provided | PBMC | PMID: 35281000 [11] |
| GSM5025742 | Female | 61 | Acute COVID-19 | Not provided | PBMC | PMID: 35281000 [11] |
| GSM5025744 | Female | 46 | Acute COVID-19 | Not provided | PBMC | PMID: 35281000 [11] |
| GSM5025764 | Female | 53 | Acute COVID-19 | Yes | PBMC | PMID: 35281000 [11] |
| GSM5025769 | Female | 38 | Acute COVID-19 | Yes | PBMC | PMID: 35281000 [11] |
| GSM5025770 | Female | 44 | Healthy | -- | PBMC | PMID: 35281000 [11] |
| GSM5025771 | Female | 34 | Healthy | -- | PBMC | PMID: 35281000 [11] |
| GSM5025772 | Female | 39 | Healthy | -- | PBMC | PMID: 35281000 [11] |
| GSM5025777 | Female | 50 | Healthy | -- | PBMC | PMID: 35281000 [11] |
| GSM5025778 | Female | 52 | Healthy | -- | PBMC | PMID: 35281000 [11] |
| GSM5025780 | Female | 56 | Healthy | -- | PBMC | PMID: 35281000 [11] |
| GSM7512193 | Female | 61 | Healthy | -- | PBMC | PMID: 38259488 [13] |
| GSM8123915 | Female | 25–50 | Healthy | -- | PBMC | PMID: 38509155 [14] |
| GSM8123910 | Female | 25–50 | Healthy | -- | PBMC | PMID: 38509155 [14] |
| GSM8123895 | Female | 25–50 | Healthy | -- | PBMC | PMID: 38509155 [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Qin, X. Type I Interferonopathy among Non-Elderly Female Patients with Post-Acute Sequelae of COVID-19. Viruses 2024, 16, 1369. https://doi.org/10.3390/v16091369
Xu D, Qin X. Type I Interferonopathy among Non-Elderly Female Patients with Post-Acute Sequelae of COVID-19. Viruses. 2024; 16(9):1369. https://doi.org/10.3390/v16091369
Chicago/Turabian StyleXu, Donghua, and Xuebin Qin. 2024. "Type I Interferonopathy among Non-Elderly Female Patients with Post-Acute Sequelae of COVID-19" Viruses 16, no. 9: 1369. https://doi.org/10.3390/v16091369





