The Effect of a TLR3 Agonist on Airway Allergic Inflammation and Viral Infection in Immunoproteasome-Deficient Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Rhinovirus 1B (RV-A1B) Preparation
2.2. Mice
2.3. Mouse Model of House Dust Mite (HDM) Challenge and Viral Infection
2.4. Poly I:C Treatment in Mice with HDM Challenge and RV-A1B Infection
2.5. ELISAs
2.6. Reverse Transcription and Quantitative Real-Time PCR (RT-qPCR)
2.7. Statistical Analysis
3. Results
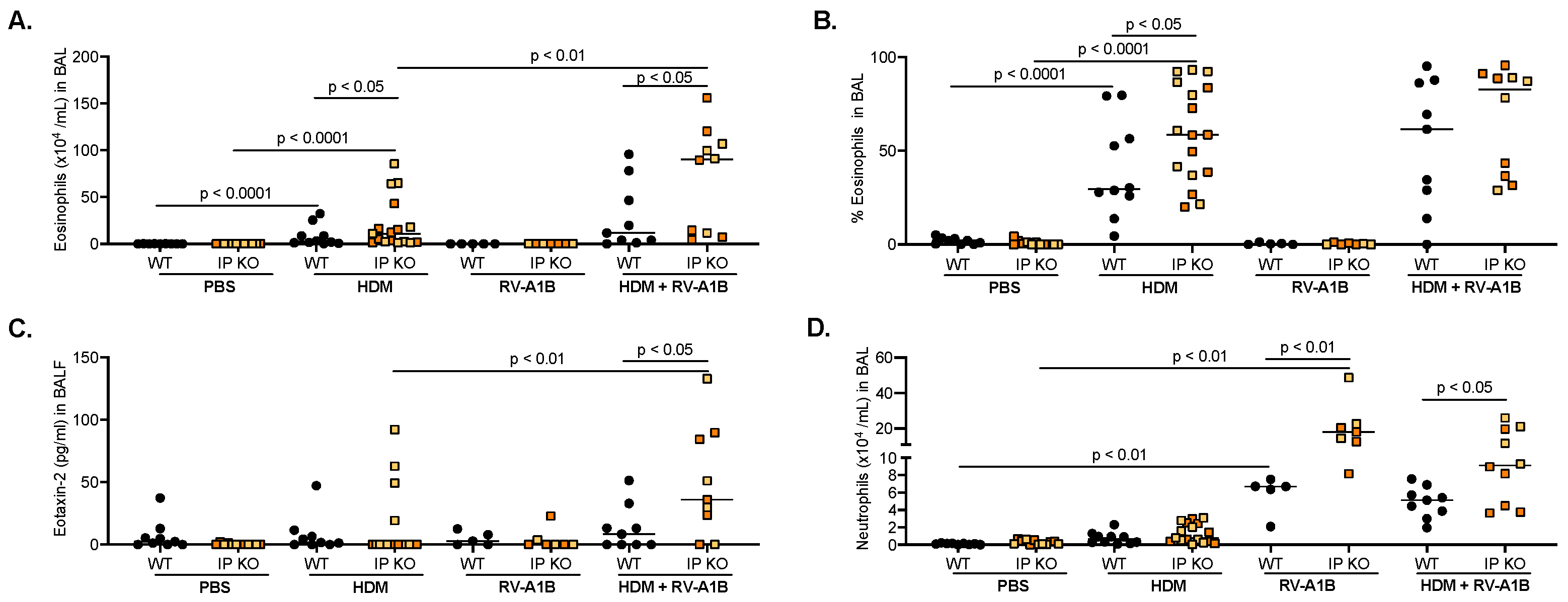
3.1. IP Deficiency Exacerbates Allergic Airway Inflammation after RV-A1B Infection
3.2. IP Deficiency Increases Viral Load and Inhibits Antiviral Gene Expression
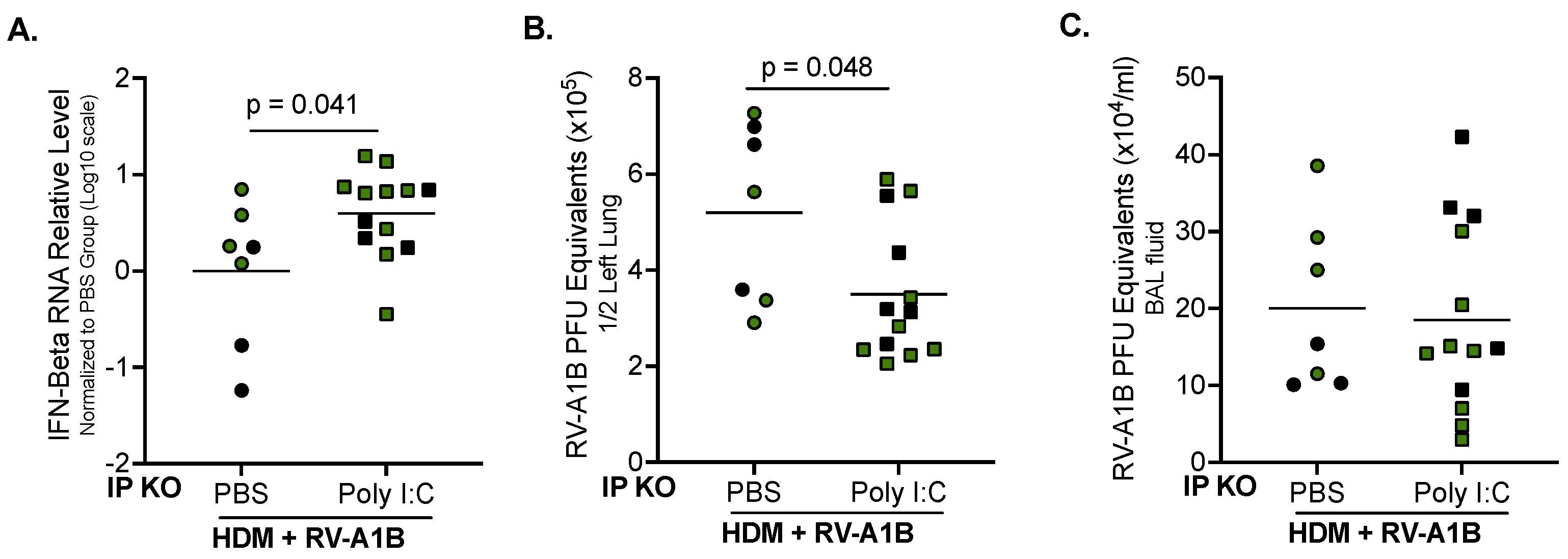
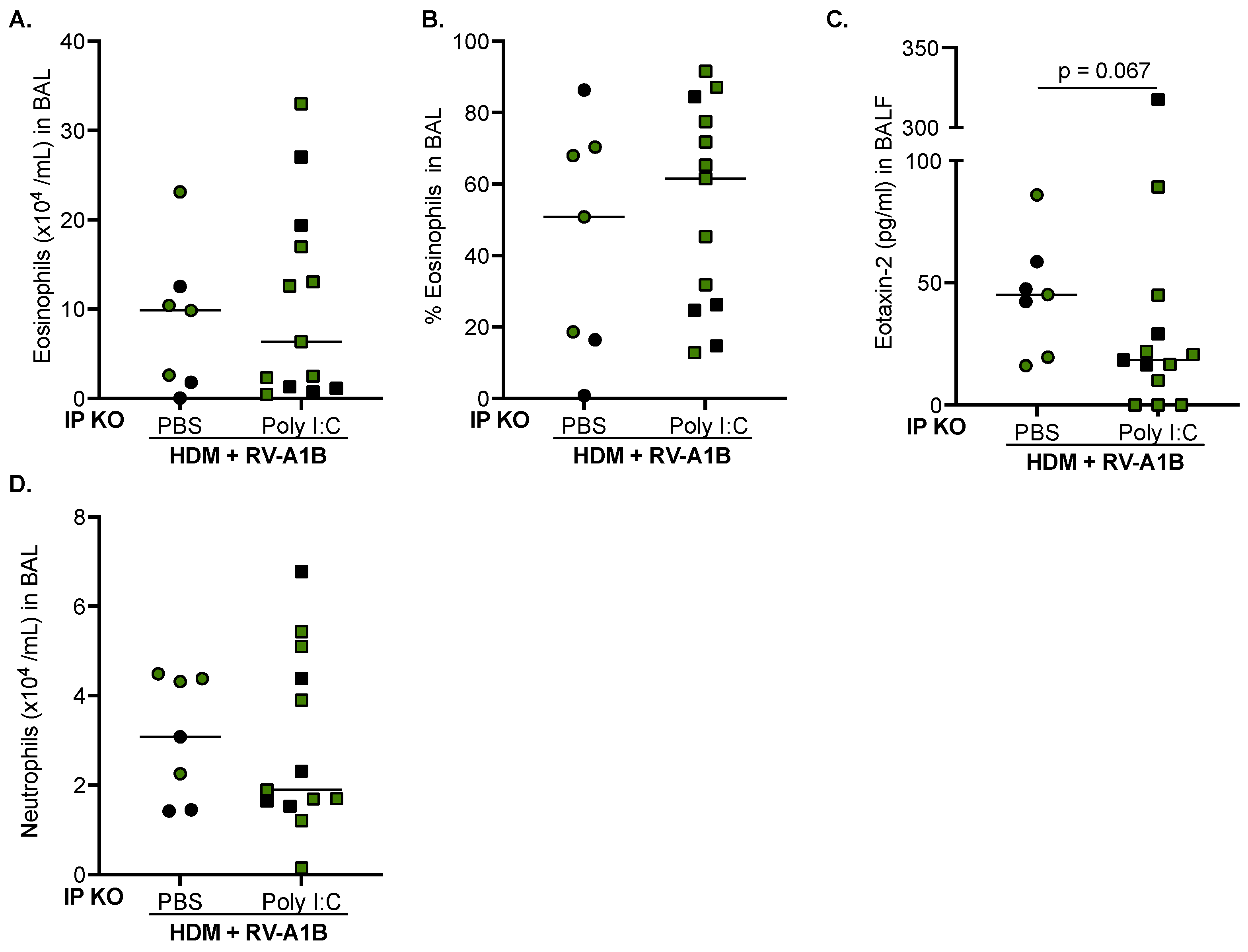
3.3. Poly I:C Increases IFN-Beta Expression and Decreases Viral Load in IP KO Mice Treated with HDM
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonzalez-Uribe, V.; Romero-Tapia, S.J.; Castro-Rodriguez, J.A. Asthma Phenotypes in the Era of Personalized Medicine. J. Clin. Med. 2023, 12, 6207. [Google Scholar] [CrossRef] [PubMed]
- Schatz, M.; Rosenwasser, L. The allergic asthma phenotype. J. Allergy Clin. Immunol. Pract. 2014, 2, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Akar-Ghibril, N.; Casale, T.; Custovic, A.; Phipatanakul, W. Allergic Endotypes and Phenotypes of Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Johansson, S.G.; Hourihane, J.O.; Bousquet, J.; Bruijnzeel-Koomen, C.; Dreborg, S.; Haahtela, T.; Kowalski, M.L.; Mygind, N.; Ring, J.; van Cauwenberge, P.; et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy 2001, 56, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Oddy, W.H.; de Klerk, N.H.; Sly, P.D.; Holt, P.G. The effects of respiratory infections, atopy, and breastfeeding on childhood asthma. Eur. Respir. J. 2002, 19, 899–905. [Google Scholar] [CrossRef]
- Xepapadaki, P.; Bachert, C.; Finotto, S.; Jartti, T.; Konstantinou, G.N.; Kiefer, A.; Kowalski, M.; Lewandowska-Polak, A.; Lukkarinen, H.; Roumpedaki, E.; et al. Contribution of repeated infections in asthma persistence from preschool to school age: Design and characteristics of the PreDicta cohort. Pediatr. Allergy Immunol. 2018, 29, 383–393. [Google Scholar] [CrossRef]
- Kennedy, J.L.; Pham, S.; Borish, L. Rhinovirus and Asthma Exacerbations. Immunol. Allergy Clin. N. Am. 2019, 39, 335–344. [Google Scholar] [CrossRef]
- Lee, W.M.; Lemanske, R.F., Jr.; Evans, M.D.; Vang, F.; Pappas, T.; Gangnon, R.; Jackson, D.J.; Gern, J.E. Human rhinovirus species and season of infection determine illness severity. Am. J. Respir. Crit. Care Med. 2012, 186, 886–891. [Google Scholar] [CrossRef]
- Ritchie, A.I.; Farne, H.A.; Singanayagam, A.; Jackson, D.J.; Mallia, P.; Johnston, S.L. Pathogenesis of Viral Infection in Exacerbations of Airway Disease. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. 2), S115–S132. [Google Scholar] [CrossRef]
- Baker, T.A.; Bach, H.H., IV; Gamelli, R.L.; Love, R.B.; Majetschak, M. Proteasomes in lungs from organ donors and patients with end-stage pulmonary diseases. Physiol. Res. 2014, 63, 311–319. [Google Scholar] [CrossRef]
- Groettrup, M.; Soza, A.; Kuckelkorn, U.; Kloetzel, P.M. Peptide antigen production by the proteasome: Complexity provides efficiency. Immunol. Today 1996, 17, 429–435. [Google Scholar] [CrossRef]
- van den Eshof, B.L.; Medfai, L.; Nolfi, E.; Wawrzyniuk, M.; Sijts, A. The Function of Immunoproteasomes-An Immunologists’ Perspective. Cells 2021, 10, 3360. [Google Scholar] [CrossRef]
- Griffin, T.A.; Nandi, D.; Cruz, M.; Fehling, H.J.; Kaer, L.V.; Monaco, J.J.; Colbert, R.A. Immunoproteasome assembly: Cooperative incorporation of interferon gamma (IFN-gamma)-inducible subunits. J. Exp. Med. 1998, 187, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Batra, S. Emerging role of immunoproteasomes in pathophysiology. Immunol. Cell Biol. 2016, 94, 812–820. [Google Scholar] [CrossRef]
- Ferrington, D.A.; Gregerson, D.S. Immunoproteasomes: Structure, function, and antigen presentation. Prog. Mol. Biol. Transl. Sci. 2012, 109, 75–112. [Google Scholar] [CrossRef]
- Aki, M.; Shimbara, N.; Takashina, M.; Akiyama, K.; Kagawa, S.; Tamura, T.; Tanahashi, N.; Yoshimura, T.; Tanaka, K.; Ichihara, A. Interferon-gamma induces different subunit organizations and functional diversity of proteasomes. J. Biochem. 1994, 115, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Keller, I.E.; Vosyka, O.; Takenaka, S.; Kloss, A.; Dahlmann, B.; Willems, L.I.; Verdoes, M.; Overkleeft, H.S.; Marcos, E.; Adnot, S.; et al. Regulation of immunoproteasome function in the lung. Sci. Rep. 2015, 5, 10230. [Google Scholar] [CrossRef] [PubMed]
- Kammerl, I.E.; Dann, A.; Mossina, A.; Brech, D.; Lukas, C.; Vosyka, O.; Nathan, P.; Conlon, T.M.; Wagner, D.E.; Overkleeft, H.S.; et al. Impairment of Immunoproteasome Function by Cigarette Smoke and in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2016, 193, 1230–1241. [Google Scholar] [CrossRef] [PubMed]
- Kammerl, I.E.; Meiners, S. Proteasome function shapes innate and adaptive immune responses. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 311, L328–L336. [Google Scholar] [CrossRef]
- Basler, M.; Beck, U.; Kirk, C.J.; Groettrup, M. The antiviral immune response in mice devoid of immunoproteasome activity. J. Immunol. 2011, 187, 5548–5557. [Google Scholar] [CrossRef]
- Kast, J. Immunoproteasome deficiency in non-small cell lung cancer and its relevance to immunotherapy. J. Thorac. Dis. 2016, 8, E1082–E1086. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McCarthy, M.K.; Weinberg, J.B. The immunoproteasome and viral infection: A complex regulator of inflammation. Front. Microbiol. 2015, 6, 21. [Google Scholar] [CrossRef]
- Dimasuay, K.G.; Schaunaman, N.; Berg, B.; Cervantes, D.; Kruger, E.; Heppner, F.L.; Ferrington, D.A.; Chu, H.W. Airway epithelial immunoproteasome subunit LMP7 protects against rhinovirus infection. Sci. Rep. 2022, 12, 14507. [Google Scholar] [CrossRef] [PubMed]
- Dimasuay, K.G.; Sanchez, A.; Schaefer, N.; Polanco, J.; Ferrington, D.A.; Chu, H.W. Immunoproteasomes as a novel antiviral mechanism in rhinovirus-infected airways. Clin. Sci. 2018, 132, 1711–1723. [Google Scholar] [CrossRef]
- Tamir, H.; Melamed, S.; Erez, N.; Politi, B.; Yahalom-Ronen, Y.; Achdout, H.; Lazar, S.; Gutman, H.; Avraham, R.; Weiss, S.; et al. Induction of Innate Immune Response by TLR3 Agonist Protects Mice against SARS-CoV-2 Infection. Viruses 2022, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Zhang, J.; Yu, F.S. Toll-like receptor 3 agonist poly(I:C)-induced antiviral response in human corneal epithelial cells. Immunology 2006, 117, 11–21. [Google Scholar] [CrossRef]
- Wu, Q.; van Dyk, L.F.; Jiang, D.; Dakhama, A.; Li, L.; White, S.R.; Gross, A.; Chu, H.W. Interleukin-1 receptor-associated kinase M (IRAK-M) promotes human rhinovirus infection in lung epithelial cells via the autophagic pathway. Virology 2013, 446, 199–206. [Google Scholar] [CrossRef]
- Caudill, C.M.; Jayarapu, K.; Elenich, L.; Monaco, J.J.; Colbert, R.A.; Griffin, T.A. T cells lacking immunoproteasome subunits MECL-1 and LMP7 hyperproliferate in response to polyclonal mitogens. J. Immunol. 2006, 176, 4075–4082. [Google Scholar] [CrossRef]
- Fehling, H.J.; Swat, W.; Laplace, C.; Kuhn, R.; Rajewsky, K.; Muller, U.; von Boehmer, H. MHC class I expression in mice lacking the proteasome subunit LMP-7. Science 1994, 265, 1234–1237. [Google Scholar] [CrossRef]
- Dimasuay, K.G.; Schaunaman, N.; Martin, R.J.; Pavelka, N.; Kolakowski, C.; Gottlieb, R.A.; Holguin, F.; Chu, H.W. Parkin, an E3 ubiquitin ligase, enhances airway mitochondrial DNA release and inflammation. Thorax 2020, 75, 717–724. [Google Scholar] [CrossRef]
- Nouri, H.R.; Schaunaman, N.; Kraft, M.; Li, L.; Numata, M.; Chu, H.W. Tollip deficiency exaggerates airway type 2 inflammation in mice exposed to allergen and influenza A virus: Role of the ATP/IL-33 signaling axis. Front. Immunol. 2023, 14, 1304758. [Google Scholar] [CrossRef]
- Dakhama, A.; Al Mubarak, R.; Pavelka, N.; Voelker, D.; Seibold, M.; Ledford, J.G.; Kraft, M.; Li, L.; Chu, H.W. Tollip Inhibits ST2 Signaling in Airway Epithelial Cells Exposed to Type 2 Cytokines and Rhinovirus. J. Innate Immun. 2020, 12, 103–115. [Google Scholar] [CrossRef]
- Schaunaman, N.; Sanchez, A.; Dimasuay, K.G.; Pavelka, N.; Numata, M.; Alam, R.; Martin, R.J.; Chu, H.W. Interleukin 1 Receptor-Like 1 (IL1RL1) Promotes Airway Bacterial and Viral Infection and Inflammation. Infect. Immun. 2019, 87, e00319–e00340. [Google Scholar] [CrossRef]
- Schaunaman, N.; Dimasuay, K.G.; Cervantes, D.; Li, L.; Numata, M.; Kraft, M.; Chu, H.W. Tollip Inhibits IL-33 Release and Inflammation in Influenza A Virus-Infected Mouse Airways. J. Innate Immun. 2023, 15, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, F.; Basler, M.; Rao, T.N.; Fehling, H.J.; Groettrup, M. Immunoproteasome Inhibition Reduces the T Helper 2 Response in Mouse Models of Allergic Airway Inflammation. Front. Immunol. 2022, 13, 870720. [Google Scholar] [CrossRef]
- Volkov, A.; Hagner, S.; Loser, S.; Alnahas, S.; Raifer, H.; Hellhund, A.; Garn, H.; Steinhoff, U. beta5i subunit deficiency of the immunoproteasome leads to reduced Th2 response in OVA induced acute asthma. PLoS ONE 2013, 8, e60565. [Google Scholar] [CrossRef]
- Chen, S.; Kammerl, I.E.; Vosyka, O.; Baumann, T.; Yu, Y.; Wu, Y.; Irmler, M.; Overkleeft, H.S.; Beckers, J.; Eickelberg, O.; et al. Immunoproteasome dysfunction augments alternative polarization of alveolar macrophages. Cell Death Differ. 2016, 23, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Yashiroda, H.; Ishihara, S.; Lo, M.; Murata, S. The Molecular Mechanisms Governing the Assembly of the Immuno- and Thymoproteasomes in the Presence of Constitutive Proteasomes. Cells 2022, 11, 1580. [Google Scholar] [CrossRef] [PubMed]
- Groettrup, M.; Standera, S.; Stohwasser, R.; Kloetzel, P.M. The subunits MECL-1 and LMP2 are mutually required for incorporation into the 20S proteasome. Proc. Natl. Acad. Sci. USA 1997, 94, 8970–8975. [Google Scholar] [CrossRef]
- Basler, M.; Moebius, J.; Elenich, L.; Groettrup, M.; Monaco, J.J. An altered T cell repertoire in MECL-1-deficient mice. J. Immunol. 2006, 176, 6665–6672. [Google Scholar] [CrossRef]
- Van Kaer, L.; Ashton-Rickardt, P.G.; Eichelberger, M.; Gaczynska, M.; Nagashima, K.; Rock, K.L.; Goldberg, A.L.; Doherty, P.C.; Tonegawa, S. Altered peptidase and viral-specific T cell response in LMP2 mutant mice. Immunity 1994, 1, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Norbury, C.C.; Cho, Y.; Yewdell, J.W.; Bennink, J.R. Immunoproteasomes shape immunodominance hierarchies of antiviral CD8(+) T cells at the levels of T cell repertoire and presentation of viral antigens. J. Exp. Med. 2001, 193, 1319–1326. [Google Scholar] [CrossRef]
- Looi, K.; Larcombe, A.N.; Perks, K.L.; Berry, L.J.; Zosky, G.R.; Rigby, P.; Knight, D.A.; Kicic, A.; Stick, S.M. Previous Influenza Infection Exacerbates Allergen Specific Response and Impairs Airway Barrier Integrity in Pre-Sensitized Mice. Int. J. Mol. Sci. 2021, 22, 8790. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, N.G.; Psarras, S.; Manoussakis, E.; Saxoni-Papageorgiou, P. The role of respiratory viruses in the origin and exacerbations of asthma. Curr. Opin. Allergy Clin. Immunol. 2003, 3, 39–44. [Google Scholar] [CrossRef]
- Sigurs, N.; Gustafsson, P.M.; Bjarnason, R.; Lundberg, F.; Schmidt, S.; Sigurbergsson, F.; Kjellman, B. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am. J. Respir. Crit. Care Med. 2005, 171, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.K.; Croft, M. Rhinovirus Infection Promotes Eosinophilic Airway Inflammation after Prior Exposure to House Dust Mite Allergen. Immunohorizons 2020, 4, 498–507. [Google Scholar] [CrossRef]
- Siegle, J.S.; Hansbro, N.; Herbert, C.; Rosenberg, H.F.; Domachowske, J.B.; Asquith, K.L.; Foster, P.S.; Kumar, R.K. Early-life viral infection and allergen exposure interact to induce an asthmatic phenotype in mice. Respir. Res. 2010, 11, 14. [Google Scholar] [CrossRef]
- Uller, L.; Leino, M.; Bedke, N.; Sammut, D.; Green, B.; Lau, L.; Howarth, P.H.; Holgate, S.T.; Davies, D.E. Double-stranded RNA induces disproportionate expression of thymic stromal lymphopoietin versus interferon-beta in bronchial epithelial cells from donors with asthma. Thorax 2010, 65, 626–632. [Google Scholar] [CrossRef]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef]
- Yan, N.; Chen, Z.J. Intrinsic antiviral immunity. Nat. Immunol. 2012, 13, 214–222. [Google Scholar] [CrossRef]
- Paludan, S.R.; Bowie, A.G. Immune sensing of DNA. Immunity 2013, 38, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Cook, L.E.; Locke, M.C.; Young, A.R.; Monte, K.; Hedberg, M.L.; Shimak, R.M.; Sheehan, K.C.F.; Veis, D.J.; Diamond, M.S.; Lenschow, D.J. Distinct Roles of Interferon Alpha and Beta in Controlling Chikungunya Virus Replication and Modulating Neutrophil-Mediated Inflammation. J. Virol. 2019, 94, e00819–e00840. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Yang, Y.; Hu, F.; Chen, X.; Zhou, J.; Li, Y.; Xu, Y.; Wang, H.; Chen, Y.; Zhang, M. TLR3 Regulated Poly I:C-Induced Neutrophil Extracellular Traps and Acute Lung Injury Partly through p38 MAP Kinase. Front. Microbiol. 2018, 9, 3174. [Google Scholar] [CrossRef]
- Stowell, N.C.; Seideman, J.; Raymond, H.A.; Smalley, K.A.; Lamb, R.J.; Egenolf, D.D.; Bugelski, P.J.; Murray, L.A.; Marsters, P.A.; Bunting, R.A.; et al. Long-term activation of TLR3 by poly(I:C) induces inflammation and impairs lung function in mice. Respir. Res. 2009, 10, 43. [Google Scholar] [CrossRef]
- Harris, P.; Sridhar, S.; Peng, R.; Phillips, J.E.; Cohn, R.G.; Burns, L.; Woods, J.; Ramanujam, M.; Loubeau, M.; Tyagi, G.; et al. Double-stranded RNA induces molecular and inflammatory signatures that are directly relevant to COPD. Mucosal Immunol. 2013, 6, 474–484. [Google Scholar] [CrossRef]
- Kimura, H.; Usui, F.; Karasawa, T.; Kawashima, A.; Shirasuna, K.; Inoue, Y.; Komada, T.; Kobayashi, M.; Mizushina, Y.; Kasahara, T.; et al. Immunoproteasome subunit LMP7 Deficiency Improves Obesity and Metabolic Disorders. Sci. Rep. 2015, 5, 15883. [Google Scholar] [CrossRef]
- Gualdoni, G.A.; Mayer, K.A.; Kapsch, A.M.; Kreuzberg, K.; Puck, A.; Kienzl, P.; Oberndorfer, F.; Fruhwirth, K.; Winkler, S.; Blaas, D.; et al. Rhinovirus induces an anabolic reprogramming in host cell metabolism essential for viral replication. Proc. Natl. Acad. Sci. USA 2018, 115, E7158–E7165. [Google Scholar] [CrossRef] [PubMed]
- Phan, J.A.; Kicic, A.; Berry, L.J.; Fernandes, L.B.; Zosky, G.R.; Sly, P.D.; Larcombe, A.N. Rhinovirus exacerbates house-dust-mite induced lung disease in adult mice. PLoS ONE 2014, 9, e92163. [Google Scholar] [CrossRef] [PubMed]
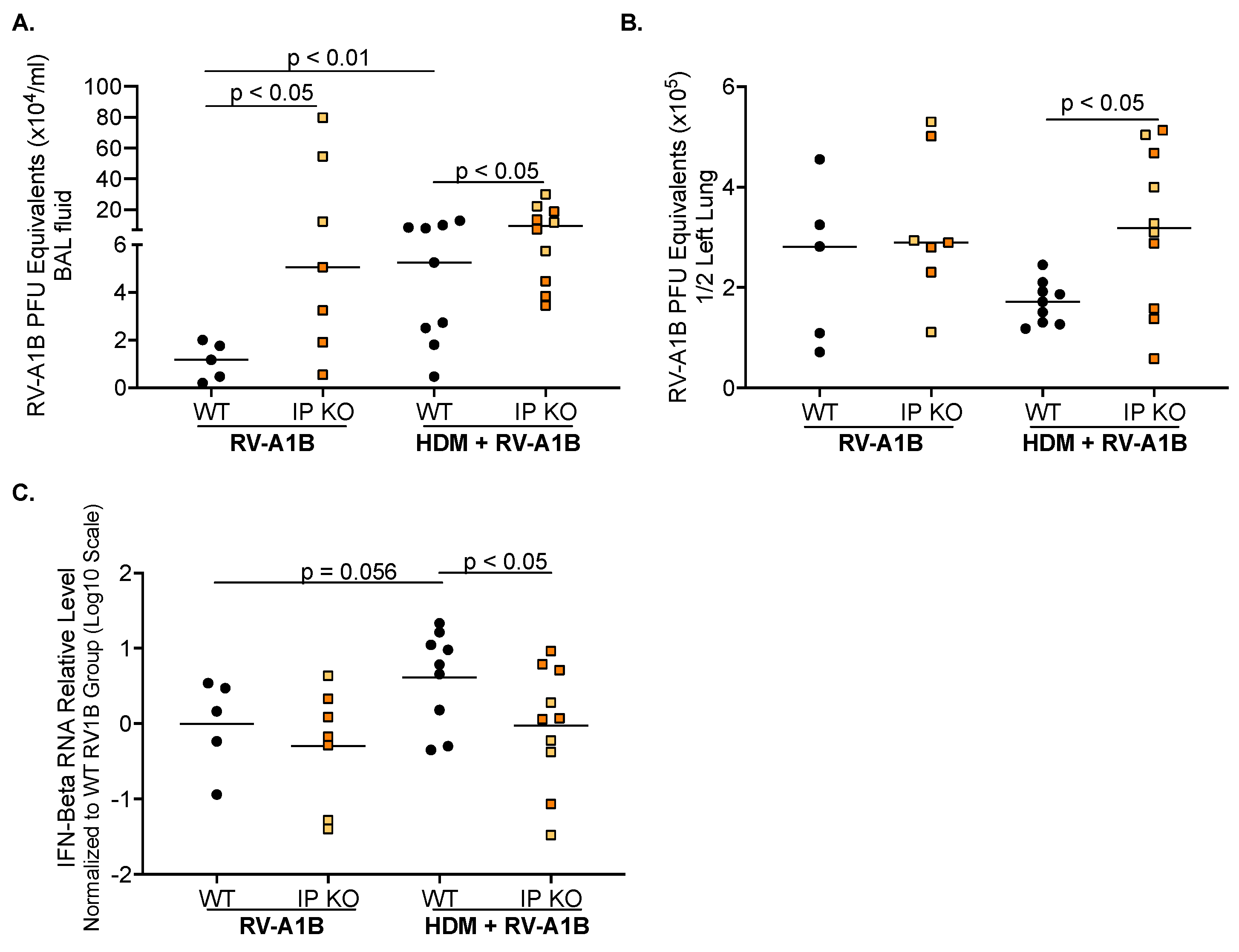
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaunaman, N.; Nichols, T.; Cervantes, D.; Hartsoe, P.; Ferrington, D.A.; Chu, H.W. The Effect of a TLR3 Agonist on Airway Allergic Inflammation and Viral Infection in Immunoproteasome-Deficient Mice. Viruses 2024, 16, 1384. https://doi.org/10.3390/v16091384
Schaunaman N, Nichols T, Cervantes D, Hartsoe P, Ferrington DA, Chu HW. The Effect of a TLR3 Agonist on Airway Allergic Inflammation and Viral Infection in Immunoproteasome-Deficient Mice. Viruses. 2024; 16(9):1384. https://doi.org/10.3390/v16091384
Chicago/Turabian StyleSchaunaman, Niccolette, Taylor Nichols, Diana Cervantes, Paige Hartsoe, Deborah A. Ferrington, and Hong Wei Chu. 2024. "The Effect of a TLR3 Agonist on Airway Allergic Inflammation and Viral Infection in Immunoproteasome-Deficient Mice" Viruses 16, no. 9: 1384. https://doi.org/10.3390/v16091384
APA StyleSchaunaman, N., Nichols, T., Cervantes, D., Hartsoe, P., Ferrington, D. A., & Chu, H. W. (2024). The Effect of a TLR3 Agonist on Airway Allergic Inflammation and Viral Infection in Immunoproteasome-Deficient Mice. Viruses, 16(9), 1384. https://doi.org/10.3390/v16091384




