The Prevalence and Molecular Characterization of Bovine Leukemia Virus among Dairy Cattle in Henan Province, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Sample Collection and DNA Extraction
2.3. Amplification of the env Gene
2.4. Phylogenetic Analysis of the env Gene
2.5. Ancestral State Reconstruction of G1 and G7 in Henan
2.6. Rates of Substitution
2.7. Positive Selection Pressure Analysis
2.8. Statistical Analysis
3. Results
3.1. Prevalence of BLV
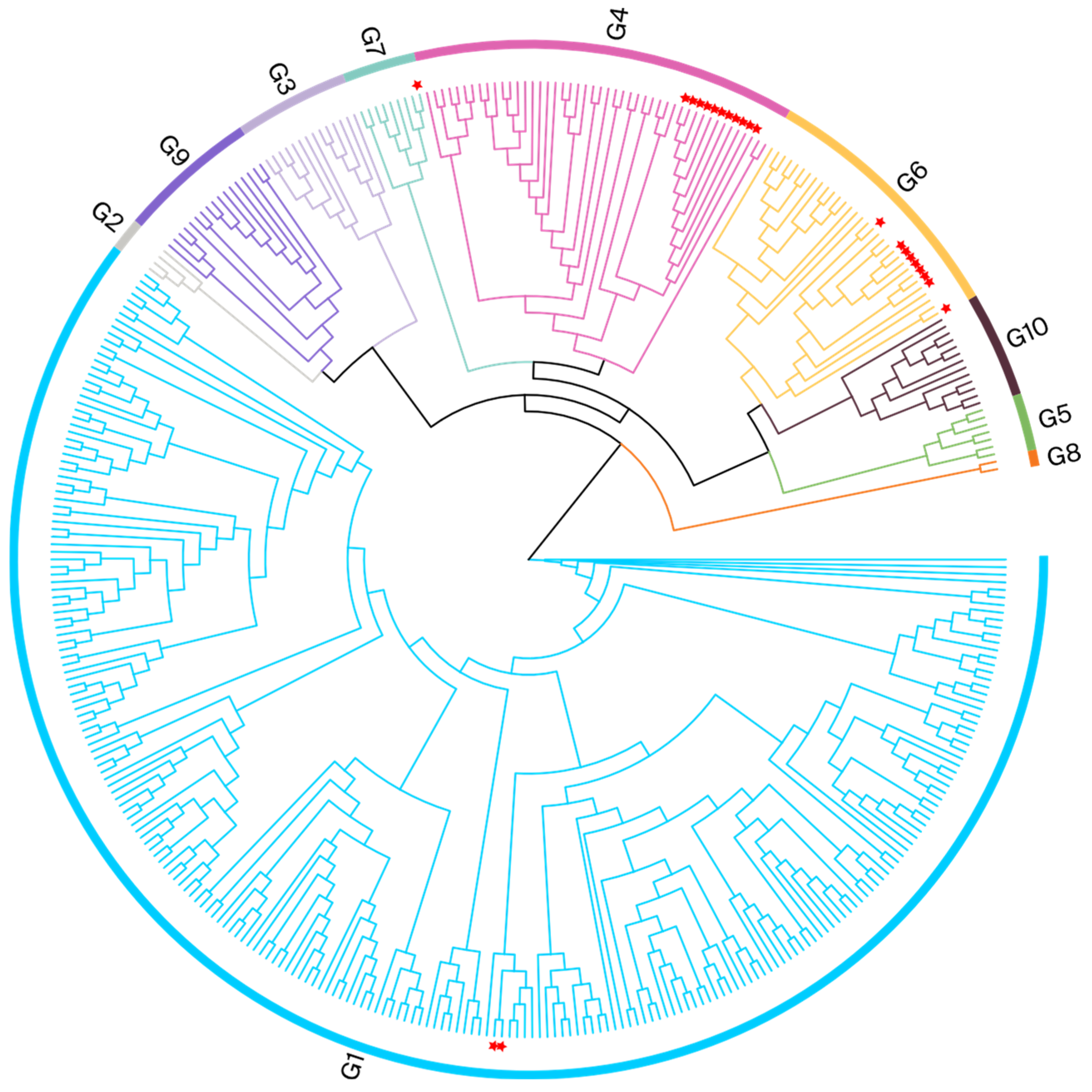
3.2. ML-Based Phylogenetic Analysis
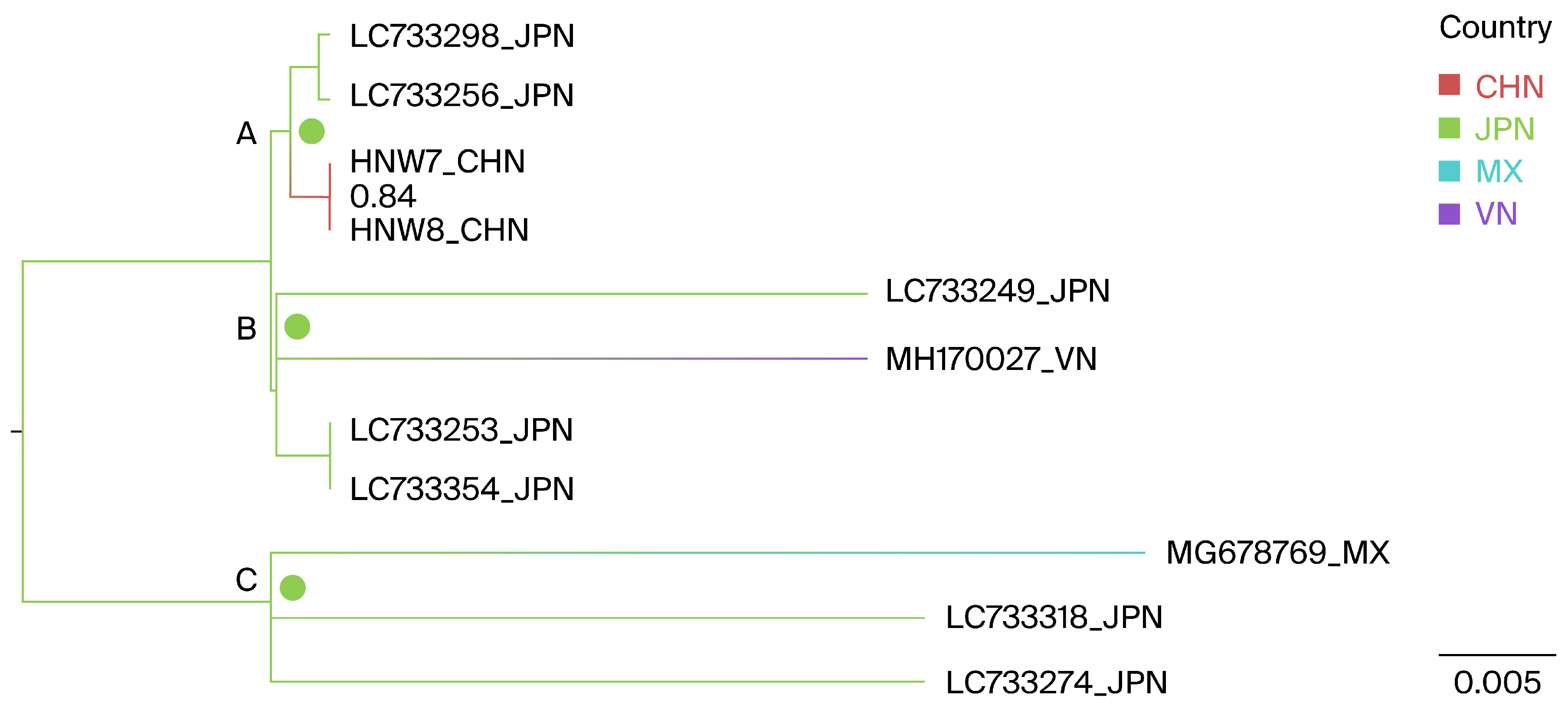
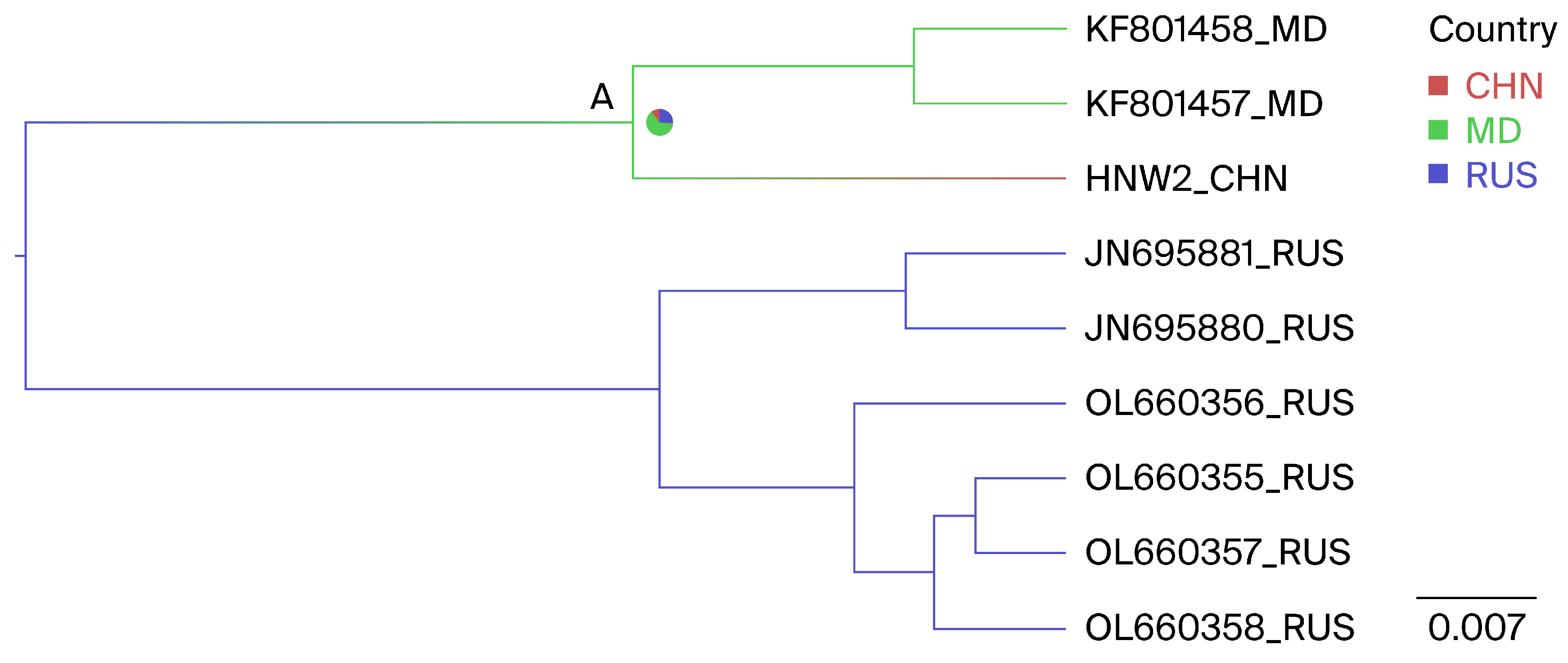
3.3. Phylogeographic Reconstruction of Henan G1
3.4. Phylogeographic Reconstruction of Henan G7
3.5. Nucleotide Substitution Rate Estimation by Bayesian Analysis
3.6. Positive Selection Sites and Amino Acid Variations in the env Gene
4. Discussion
4.1. Prevalence of BLV among Dairy Cattle in Henan
4.2. Phylogenetic Insights into the BLV env Gene
4.3. Phylogeographic Reconstruction of Henan G1 and G7
4.4. BLV env Gene Substitutions and Positive Selection
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marawan, M.A.; Alouffi, A.; El Tokhy, S.; Badawy, S.; Shirani, I.; Dawood, A.; Guo, A.; Almutairi, M.M.; Alshammari, F.A.; Selim, A. Bovine leukaemia virus: Current epidemiological circumstance and future prospective. Viruses 2021, 13, 2167. [Google Scholar] [CrossRef] [PubMed]
- Polat, M.; Ohno, A.; Takeshima, S.-n.; Kim, J.; Kikuya, M.; Matsumoto, Y.; Mingala, C.N.; Onuma, M.; Aida, Y. Detection and molecular characterization of bovine leukemia virus in Philippine cattle. Arch. Virol. 2015, 160, 285–296. [Google Scholar] [CrossRef]
- Ruiz, V.; Porta, N.; Lomónaco, M.; Trono, K.; Alvarez, I. Bovine Leukemia Virus Infection in Neonatal Calves. Risk Factors and Control Measures. Front. Vet. Sci. 2018, 5, 267. [Google Scholar] [CrossRef]
- Gillet, N.; Florins, A.; Boxus, M.; Burteau, C.; Nigro, A.; Vandermeers, F.; Balon, H.; Bouzar, A.; Defoiche, J.; Burny, A.; et al. Mechanisms of leukemogenesis induced by bovine leukemia virus: Prospects for novel anti-retroviral therapies in human. Retrovirology 2007, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Polat, M.; Moe, H.; Shimogiri, T.; Moe, K.; Takeshima, S.; Aida, Y. The molecular epidemiological study of bovine leukemia virus infection in Myanmar cattle. Arch. Virol. 2017, 162, 425–437. [Google Scholar] [CrossRef]
- Ohnuki, N.; Kobayashi, T.; Matsuo, M.; Nishikaku, K.; Kusama, K.; Torii, Y.; Inagaki, Y.; Hori, M.; Imakawa, K.; Satou, Y. A target enrichment high throughput sequencing system for characterization of BLV whole genome sequence, integration sites, clonality and host SNP. Sci. Rep. 2021, 11, 4521. [Google Scholar] [CrossRef]
- Polat, M.; Takeshima, S.; Aida, Y. Epidemiology and genetic diversity of bovine leukemia virus. Virol. J. 2017, 14, 209. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Chapman, R.; Douglass, N.; Carulei, O.; van Rensburg, J.; Williamson, A.-L. Phylogenetic analysis of South African bovine leukaemia virus (BLV) isolates. Viruses 2020, 12, 898. [Google Scholar] [CrossRef]
- Pluta, A.; Albritton, L.; Rola-Łuszczak, M.; Kuźmak, J. Computational analysis of envelope glycoproteins from diverse geographical isolates of bovine leukemia virus identifies highly conserved peptide motifs. Retrovirology 2018, 15, 2. [Google Scholar] [CrossRef]
- Bai, L.; Takeshima, S.; Sato, M.; Davis, W.; Wada, S.; Kohara, J.; Aida, Y. Mapping of CD4 T-cell epitopes in bovine leukemia virus from five cattle with differential susceptibilities to bovine leukemia virus disease progression. Virol. J. 2019, 16, 157. [Google Scholar] [CrossRef]
- Gatot, J.; Callebaut, I.; Van Lint, C.; Demonté, D.; Kerkhofs, P.; Portetelle, D.; Burny, A.; Willems, L.; Kettmann, R. Bovine leukemia virus SU protein interacts with zinc, and mutations within two interacting regions differently affect viral fusion and infectivity in vivo. J. Virol. 2002, 76, 7956–7967. [Google Scholar] [CrossRef] [PubMed]
- Lorin, A.; Lins, L.; Stroobant, V.; Brasseur, R.; Charloteaux, B. Determination of the minimal fusion peptide of bovine leukemia virus gp30. Biochem. Biophys. Res. Commun. 2007, 355, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Reichert, M.; Winnicka, A.; Willems, L.; Kettmann, R.; Cantor, G. Role of the proline-rich motif of bovine leukemia virus transmembrane protein gp30 in viral load and pathogenicity in sheep. J. Virol. 2001, 75, 8082–8089. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oie, A.H.S. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; Bulletin—Office International des Épizootie: Paris, France, 2015; pp. 1092–1106. [Google Scholar]
- Asfaw, Y.; Tsuduku, S.; Konishi, M.; Murakami, K.; Tsuboi, T.; Wu, D.; Sentsui, H. Distribution and superinfection of bovine leukemia virus genotypes in Japan. Arch. Virol. 2005, 150, 493–505. [Google Scholar] [CrossRef]
- Polat, M.; Takeshima, S.; Hosomichi, K.; Kim, J.; Miyasaka, T.; Yamada, K.; Arainga, M.; Murakami, T.; Matsumoto, Y.; de la Barra Diaz, V.; et al. A new genotype of bovine leukemia virus in South America identified by NGS-based whole genome sequencing and molecular evolutionary genetic analysis. Retrovirology 2016, 13, 4. [Google Scholar] [CrossRef]
- Ochirkhuu, N.; Konnai, S.; Odbileg, R.; Nishimori, A.; Okagawa, T.; Murata, S.; Ohashi, K. Detection of bovine leukemia virus and identification of its genotype in Mongolian cattle. Arch. Virol. 2016, 161, 985–991. [Google Scholar] [CrossRef]
- Moratorio, G.; Obal, G.; Dubra, A.; Correa, A.; Bianchi, S.; Buschiazzo, A.; Cristina, J.; Pritsch, O. Phylogenetic analysis of bovine leukemia viruses isolated in South America reveals diversification in seven distinct genotypes. Arch. Virol. 2010, 155, 481–489. [Google Scholar] [CrossRef]
- Yu, C.; Wang, X.; Zhou, Y.; Wang, Y.; Zhang, X.; Zheng, Y. Genotyping bovine leukemia virus in dairy cattle of Heilongjiang, northeastern China. BMC Vet. Res. 2019, 15, 179. [Google Scholar] [CrossRef]
- Sultanov, A.; Rola-Łuszczak, M.; Mamanova, S.; Ryło, A.; Osiński, Z.; Saduakassova, M.A.; Bashenova, E.; Kuźmak, J. Molecular characterization of bovine leukemia virus with the evidence of a new genotype circulating in cattle from Kazakhstan. Pathogens 2022, 11, 180. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.; Kang, C.S.; Cho, D.H.; Shin, D.H.; Yum, Y.N.; Oh, J.H.; Kim, S.H.; Hwang, M.S.; Lim, C.J. Investigation of the bovine leukemia virus proviral DNA in human leukemias and lung cancers in Korea. J. Korean Med. Sci. 2005, 20, 603–606. [Google Scholar] [CrossRef]
- Corredor-Figueroa, A.P.; Salas, S.; Olaya-Galán, N.N.; Quintero, J.S.; Fajardo, Á.; Soñora, M.; Moreno, P.; Cristina, J.; Sánchez, A.; Tobón, J. Prevalence and molecular epidemiology of bovine leukemia virus in Colombian cattle. Infect. Genet. Evol. 2020, 80, 104171. [Google Scholar] [CrossRef]
- Gao, A.; Kouznetsova, V.; Tsigelny, I. Bovine leukemia virus relation to human breast cancer: Meta-analysis. Microb. Pathog. 2020, 149, 104417. [Google Scholar] [CrossRef] [PubMed]
- Delarmelina, E.; Buzelin, M.A.; Souza, B.S.D.; Souto, F.M.; Reis, J.K.P.D. High positivity values for bovine leukemia virus in human breast cancer cases from Minas Gerais, Brazil. PLoS ONE 2020, 15, e0239745. [Google Scholar] [CrossRef]
- Ma, B.; Gong, Q.; Sheng, C.; Liu, Y.; Ge, G.; Li, D.; Diao, N.; Shi, K.; Li, J.; Sun, Z.; et al. Prevalence of bovine leukemia in 1983-2019 in China: A systematic review and meta-analysis. Microb. Pathog. 2021, 150, 104681. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chu, S.; Shang, S.; Yang, Z.; Wang, C. Short communication: Genotyping and single nucleotide polymorphism analysis of bovine leukemia virus in Chinese dairy cattle. J. Dairy Sci. 2019, 102, 3469–3473. [Google Scholar] [CrossRef]
- Hubrecht, R. Revised Australian Code for the care and use of animals for scientific purposes. Anim. Welf. 2013, 22, 491. [Google Scholar] [CrossRef]
- Department of Agriculture and Rural Affairs of Hainan Province. Henan Animal Husbandry Development Achieves a Good Start in 2021. Available online: https://www.henan.gov.cn/2021/05-25/2151291.html (accessed on 25 May 2021).
- Heller, D.; Hoppe, A.; Restrepo, S.; Gatti, L.; Tournier, A.L.; Tapon, N.; Basler, K.; Mao, Y. EpiTools: An open-source image analysis toolkit for quantifying epithelial growth dynamics. Dev. Cell 2016, 36, 103–116. [Google Scholar] [CrossRef]
- Sayers, E.W.; Beck, J.; Bolton, E.E.; Bourexis, D.; Brister, J.R.; Canese, K.; Comeau, D.C.; Funk, K.; Kim, S.; Klimke, W. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2021, 49, D10–D17. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.; Wang, G. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef]
- Bielejec, F.; Baele, G.; Vrancken, B.; Suchard, M.A.; Rambaut, A.; Lemey, P. SpreaD3: Interactive visualization of spatiotemporal history and trait evolutionary processes. Mol. Biol. Evol. 2016, 33, 2167–2169. [Google Scholar] [CrossRef]
- Zhao, X.; Buehring, G. Natural genetic variations in bovine leukemia virus envelope gene: Possible effects of selection and escape. Virology 2007, 366, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Delport, W.; Poon, A.F.; Frost, S.D.; Kosakovsky Pond, S.L. Datamonkey 2010: A suite of phylogenetic analysis tools for evolutionary biology. Bioinformatics 2010, 26, 2455–2457. [Google Scholar] [CrossRef] [PubMed]
- Kuczewski, A.; Orsel, K.; Barkema, H.; Mason, S.; Erskine, R.; van der Meer, F. Invited review: Bovine leukemia virus-Transmission, control, and eradication. J. Dairy Sci. 2021, 104, 6358–6375. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fan, W.; Mao, Y.; Yang, Z.; Lu, G.; Zhang, R.; Zhang, H.; Szeto, C.; Wang, C. Bovine leukemia virus infection in cattle of China: Association with reduced milk production and increased somatic cell score. J. Dairy Sci. 2016, 99, 3688–3697. [Google Scholar] [CrossRef]
- Wang, C. Bovine leukemia virus infection in Taiwan: Epidemiological study. J. Vet. Med. Sci. 1991, 53, 395–398. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Baloch, A.; Pan, Y.; Xu, F.; Tian, L.; Zeng, Q. Molecular epidemiology and characterization of bovine leukemia virus in domestic yaks (Bos grunniens) on the Qinghai-Tibet Plateau, China. Arch. Virol. 2018, 163, 659–670. [Google Scholar] [CrossRef]
- Mousavi, S.; Haghparast, A.; Mohammadi, G.; Tabatabaeizadeh, S. Prevalence of bovine leukemia virus (BLV) infection in the northeast of Iran. Vet. Res. Forum Int. Q. J. 2014, 5, 135–139. [Google Scholar]
- Murakami, K.; Kobayashi, S.; Konishi, M.; Kameyama, K.; Tsutsui, T. Nationwide survey of bovine leukemia virus infection among dairy and beef breeding cattle in Japan from 2009–2011. J. Vet. Med. Sci. 2013, 75, 1123–1126. [Google Scholar] [CrossRef]
- Lee, E.; Kim, E.-J.; Ratthanophart, J.; Vitoonpong, R.; Kim, B.-H.; Cho, I.-S.; Song, J.-Y.; Lee, K.-K.; Shin, Y.-K. Molecular epidemiological and serological studies of bovine leukemia virus (BLV) infection in Thailand cattle. Infect. Genet. Evol. 2016, 41, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Hamada, R.; Metwally, S.; Polat, M.; Borjigin, L.; Ali, A.; Abdel-Hady, A.; Mohamed, A.; Wada, S.; Aida, Y. Detection and Molecular Characterization of Bovine Leukemia Virus in Egyptian Dairy Cattle. Front. Vet. Sci. 2020, 7, 608. [Google Scholar] [CrossRef]
- John, E.; Keefe, G.; Cameron, M.; Stryhn, H.; McClure, J. Development and implementation of a risk assessment and management program for enzootic bovine leukosis in Atlantic Canada. J. Dairy Sci. 2020, 103, 8398–8406. [Google Scholar] [CrossRef] [PubMed]
- Abbasian, B. Molecular Cloning and Sequencing of Bovine Rhinotracheitis Virus GB Gene in Iran. In Proceedings of the 3rd Congress of European Microbiological Societies (FEMS 2009), Guteborg, Sweden, 28 June–2 July 2009. [Google Scholar]
- Rodriguez, S.M.; Golemba, M.D.; Campos, R.H.; Trono, K.; Jones, L.R. Bovine leukemia virus can be classified into seven genotypes: Evidence for the existence of two novel clades. J. Gen. Virol. 2009, 90 Pt 11, 2788–2797. [Google Scholar] [CrossRef]
- Sherr, C.; Fedele, L.; Donner, L.; Turek, L. Restriction endonuclease mapping of unintegrated proviral DNA of Snyder-Theilen feline sarcoma virus: Localization of sarcoma-specific sequences. J. Virol. 1979, 32, 860–875. [Google Scholar] [CrossRef]
- Rola-Łuszczak, M.; Pluta, A.; Olech, M.; Donnik, I.; Petropavlovskiy, M.; Gerilovych, A.; Vinogradova, I.; Choudhury, B.; Kuźmak, J. The molecular characterization of bovine leukaemia virus isolates from Eastern Europe and Siberia and its impact on phylogeny. PLoS ONE 2013, 8, e58705. [Google Scholar] [CrossRef] [PubMed]
- Inabe, K.; Ikuta, K.; Aida, Y. Transmission and propagation in cell culture of virus produced by cells transfected with an infectious molecular clone of bovine leukemia virus. Virology 1998, 245, 53–64. [Google Scholar] [CrossRef]
- Matsumura, K.; Inoue, E.; Osawa, Y.; Okazaki, K. Molecular epidemiology of bovine leukemia virus associated with enzootic bovine leukosis in Japan. Virus Res. 2011, 155, 343–348. [Google Scholar] [CrossRef]
- Inoue, E.; Matsumura, K.; Maekawa, K.; Nagatsuka, K.; Nobuta, M.; Hirata, M.; Minagawa, A.; Osawa, Y.; Okazaki, K. Genetic heterogeneity among bovine leukemia viruses in Japan and their relationship to leukemogenicity. Arch. Virol. 2011, 156, 1137–1141. [Google Scholar] [CrossRef]
- Burgu, I.; Alkan, F.; Karaoglu, T.; Bilge-Dagalp, S.; Can-Sahna, K.; Güngör, B.; Demir, B. Control and eradication programme of enzootic bovine leucosis (EBL) from selected dairy herds in Turkey. DTW. Dtsch. Tierarztl. Wochenschr. 2005, 112, 271–274. [Google Scholar] [PubMed]
- Hemmatzadeh, F. Sequencing and phylogenetic analysis of gp51 gene of bovine leukaemia virus in Iranian isolates. Vet. Res. Commun. 2007, 31, 783–789. [Google Scholar] [CrossRef]
- Moe, K.; Polat, M.; Borjigin, L.; Matsuura, R.; Hein, S.; Moe, H.; Aida, Y. New evidence of bovine leukemia virus circulating in Myanmar cattle through epidemiological and molecular characterization. PLoS ONE 2020, 15, e0229126. [Google Scholar] [CrossRef]
- Dube, S.; Abbott, L.; Dube, D.K.; Dolcini, G.; Gutierrez, S.; Ceriani, C.; Juliarena, M.; Ferrer, J.; Perzova, R.; Poiesz, B.J. The complete genomic sequence of an in vivo low replicating BLV strain. Virol. J. 2009, 6, 120. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Kim, E.-J.; Joung, H.-K.; Kim, B.-H.; Song, J.-Y.; Cho, I.-S.; Lee, K.-K.; Shin, Y.-K. Sequencing and phylogenetic analysis of the gp51 gene from Korean bovine leukemia virus isolates. Virol. J. 2015, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Licursi, M.; Inoshima, Y.; Wu, D.; Yokoyama, T.; González, E.T.; Sentsui, H. Genetic heterogeneity among bovine leukemia virus genotypes and its relation to humoral responses in hosts. Virus Res. 2002, 86, 101–110. [Google Scholar] [CrossRef]
- Ababneh, M.M.; Al-Rukibat, R.K.; Hananeh, W.M.; Nasar, A.T.; Al-Zghoul, M.B. Detection and molecular characterization of bovine leukemia viruses from Jordan. Arch. Virol. 2012, 157, 2343–2348. [Google Scholar] [CrossRef]
- Yi, S.; Niu, J.; Wang, H.; Dong, G.; Zhao, Y.; Dong, H.; Guo, Y.; Wang, K.; Hu, G. Detection and genetic characterization of feline bocavirus in Northeast China. Virol. J. 2018, 15, 125. [Google Scholar] [CrossRef]
- Sabala, E.D.; Eric, C. The Impact of Japan’s Trade Agreements and Safeguard Renegotiation on U.S. Access to Japan’s Beef Market; Economic Research Service; U.S. Department of Agriculture: Washington, DC, USA, 2023; pp. 29–30.
- Gotoh, T.; Nishimura, T.; Kuchida, K.; Mannen, H. The Japanese Wagyu beef industry: Current situation and future prospects—A review. Asian-Australas. J. Anim. Sci. 2018, 31, 933–950. [Google Scholar] [CrossRef]
- Schwingel, D.; Andreolla, A.P.; Erpen, L.M.; Frandoloso, R.; Kreutz, L.C. Bovine leukemia virus DNA associated with breast cancer in women from South Brazil. Sci. Rep. 2019, 9, 2949. [Google Scholar] [CrossRef]
- Pakbin, B.; Rossen, J.W.; Brück, W.M.; Montazeri, N.; Allahyari, S.; Dibazar, S.P.; Abdolvahabi, R.; Mahmoudi, R.; Peymani, A.; Samimi, R. Prevalence of foodborne and zoonotic viral pathogens in raw cow milk samples. FEMS Microbiol. Lett. 2022, 369, fnac108. [Google Scholar] [CrossRef]
- Van Dooren, S.; Pybus, O.G.; Salemi, M.; Liu, H.-F.; Goubau, P.; Remondegui, C.; Talarmin, A.; Gotuzzo, E.; Alcantara, L.C.J.; Galvão-Castro, B. The low evolutionary rate of human T-cell lymphotropic virus type-1 confirmed by analysis of vertical transmission chains. Mol. Biol. Evol. 2004, 21, 603–611. [Google Scholar] [CrossRef]
- Silva, G.; Marques, N.; Nolasco, G. The evolutionary rate of citrus tristeza virus ranks among the rates of the slowest RNA viruses. J. Gen. Virol. 2012, 93, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Sanjuán, R. From molecular genetics to phylodynamics: Evolutionary relevance of mutation rates across viruses. PLoS Pathog. 2012, 8, e1002685. [Google Scholar] [CrossRef]
- Schuh, A.J.; Ward, M.J.; Leigh Brown, A.J.; Barrett, A.D. Dynamics of the emergence and establishment of a newly dominant genotype of Japanese encephalitis virus throughout Asia. J. Virol. 2014, 88, 4522–4532. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, J.; Yu, J.; Meng, F.; Zhao, Y.; Li, J.; Sun, P.; Sun, S.; Zhang, Z.; Liu, C. Alignment of Rutaceae genomes reveals lower genome fractionation level than Eudicot genomes affected by extra Polyploidization. Front. Plant Sci. 2019, 10, 986. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, H.; Li, L.; Wang, E.; Zhou, X.; Gu, Y.; Wu, X.; Shen, L.; Zeng, W. Whole genome sequencing and comparative genomic analyses of Lysinibacillus pakistanensis LZH-9, a halotolerant strain with excellent COD removal capability. Microorganisms 2020, 8, 716. [Google Scholar] [CrossRef] [PubMed]
- Renzette, N.; Gibson, L.; Bhattacharjee, B.; Fisher, D.; Schleiss, M.R.; Jensen, J.D.; Kowalik, T.F. Rapid intrahost evolution of human cytomegalovirus is shaped by demography and positive selection. PLoS Genet. 2013, 9, e1003735. [Google Scholar] [CrossRef]
- Camargos, M.F.; Pereda, A.; Stancek, D.; Rocha, M.A.; Reis, J.K.P.d.; Greiser-Wilke, I.; Leite, R.C. Molecular characterization of the env gene from Brazilian field isolates of Bovine leukemia virus. Virus Genes 2007, 34, 343–350. [Google Scholar] [CrossRef]
- Maresova, L.; Pasieka, T.J.; Grose, C. Varicella-zoster Virus gB and gE coexpression, but not gB or gE alone, leads to abundant fusion and syncytium formation equivalent to those from gH and gL coexpression. J. Virol. 2001, 75, 9483–9492. [Google Scholar] [CrossRef] [PubMed]
- Gatot, J.-S.; Callebaut, I.; Mornon, J.-P.; Portetelle, D.; Burny, A.; Kerkhofs, P.; Kettmann, R.; Willems, L. Conservative mutations in the immunosuppressive region of the bovine leukemia virus transmembrane protein affect fusion but not infectivity in vivo. J. Biol. Chem. 1998, 273, 12870–12880. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, S.; Sawai, E.T.; Radke, K. Dileucine and YXXL motifs in the cytoplasmic tail of the bovine leukemia virus transmembrane envelope protein affect protein expression on the cell surface. J. Virol. 2004, 78, 8301–8311. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Willems, L.; Gatot, J.-S.; Mammerickx, M.; Portetelle, D.; Burny, A.; Kerkhofs, P.; Kettmann, R. The YXXL signalling motifs of the bovine leukemia virus transmembrane protein are required for in vivo infection and maintenance of high viral loads. J. Virol. 1995, 69, 4137–4141. [Google Scholar] [CrossRef] [PubMed]
- Inabe, K.; Nishizawa, M.; Tajima, S.; Ikuta, K.; Aida, Y. The YXXL sequences of a transmembrane protein of bovine leukemia virus are required for viral entry and incorporation of viral envelope protein into virions. J. Virol. 1999, 73, 1293–1301. [Google Scholar] [CrossRef]
- Abdala, A.; Alvarez, I.; Brossel, H.; Calvinho, L.; Carignano, H.; Franco, L.; Gazon, H.; Gillissen, C.; Hamaidia, M.; Hoyos, C.; et al. BLV: Lessons on vaccine development. Retrovirology 2019, 16, 26. [Google Scholar] [CrossRef]
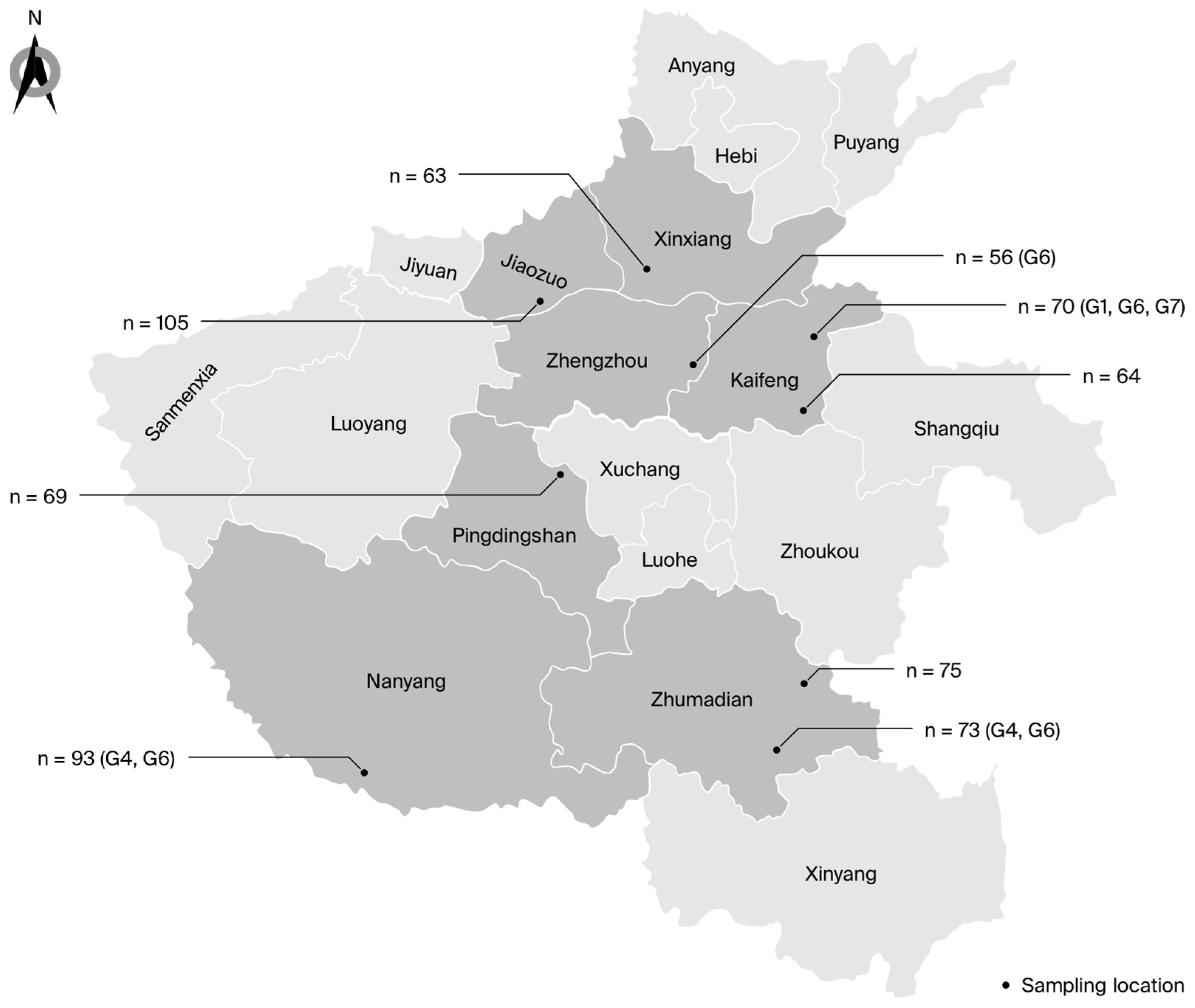

| Category | Cities | Herd Size | Samples | nPCR | Animal-Level Prevalence (%) | 95% CI (Lower, Upper) | OR | 95% CI (Lower, Upper) | p * |
|---|---|---|---|---|---|---|---|---|---|
| Farm G | Jiaozuo | 827 | 105 | 0 | 0 | 0–3.5 | - | - | - |
| Farm C | Zhumadian | 581 | 75 | 0 | 0 | 0–4.8 | - | - | - |
| Farm E | Pingdingshan | 563 | 69 | 0 | 0 | 0–5.2 | - | - | - |
| Farm B | Kaifeng | 506 | 64 | 0 | 0 | 0–5.6 | - | - | - |
| Farm H | Xinxiang | 498 | 63 | 0 | 0 | 0–5.7 | - | - | - |
| Farm I | Zhengzhou | 440 | 56 | 1 | 1.8 | 0–9.6 | 1.0 | - | - |
| Farm D | Zhumadian | 574 | 73 | 3 | 4.1 | 0.9–11.5 | 2.4 | 0.2–23.3 | 0.6 |
| Farm A | Kaifeng | 554 | 70 | 8 | 11.4 | 5.1–21.3 | 7.1 | 0.9–58.6 | 0.04 |
| Farm F | Nanyang | 738 | 93 | 11 | 11.8 | 6.1–20.2 | 7.4 | 0.9–58.8 | 0.03 |
| Total | - | 5281 | 668 | 23 | 3.4 | 2.2–5.1 | - | - | - |
| Cities | Samples | nPCR | Animal-Level Prevalence (%) | 95% CI (Lower, Upper) | ORs | 95% CI (Lower, Upper) | p * |
|---|---|---|---|---|---|---|---|
| Jiaozuo | 105 | 0 | 0 | 0–3.5 | - | - | - |
| Pingdingshan | 69 | 0 | 0 | 0–5.2 | - | - | - |
| Xinxiang | 63 | 0 | 0 | 0–5.7 | - | - | - |
| Zhengzhou | 56 | 1 | 1.8 | 0–9.6 | 1 | - | - |
| Zhumadian | 148 | 3 | 2 | 0.4–5.8 | 1.1 | 0.1–11.2 | 1 |
| Kaifeng | 134 | 8 | 6 | 2.6–11.4 | 3.5 | 0.4–28.6 | 0.3 |
| Nanyang | 93 | 11 | 11.8 | 6.1–20.1 | 7.4 | 0.9–58.8 | 0.03 |
| Cities | Farm | Partial env | Full-Length env |
|---|---|---|---|
| Kaifeng | A | G1/G6/G7 | G1/G6/G7 |
| Zhumadian | D | G4/G6 | G4/G6 |
| Nanyang | F | G4/G6 | G4/G6 |
| Zhengzhou | I | G6 | G6 |
| From | To | BFs | Posterior Probability |
|---|---|---|---|
| Japan | Mexico | 24.6 | 0.92 |
| Japan | Vietnam | 24.7 | 0.92 |
| Japan | China | 111.6 | 0.98 |
| From | To | BFs | Posterior Probability |
|---|---|---|---|
| Moldova | China | 7.8 | 0.86 |
| Russia | China | 2.0 | 0.62 |
| Russia | Moldova | 3.9 | 0.76 |
| Name | aa | Nucleotide | Substitution Rate (Subs/Site/Year) | 95% HPD (Subs/Site/Year) |
|---|---|---|---|---|
| Overall | 1–515 | 1–1545 | 3.15 × 10−4 | 2.18 × 10−4–4.30 × 10−4 |
| Leader | 1–33 | 1–99 | 2.38 × 10−3 | 7.58 × 10−4–4.70 × 10−3 |
| gp51 | 34–301 | 100–903 | 4.39 × 10−4 | 3.18 × 10−4–5.73 × 10−4 |
| gp30 | 302–515 | 904–1545 | 5.44 × 10−4 | 3.55 × 10−4–7.59 × 10−4 |
| China | 1–515 | 1–1545 | 3.93 × 10−3 | 6.67 × 10−4–8.49 × 10−4 |
| Others | 1–515 | 1–1545 | 2.25 × 10−4 | 1.62 × 10−4–2.96 × 10−4 |
| Codon | MEME | FUBAR | SLAC | FEL | ||||
|---|---|---|---|---|---|---|---|---|
| β+ | p | β-α | Post.Pr | dN-dS | p | α = β | p | |
| 115 | 3.08 | 0.07 | 0.789 | 0.83 | 2.41 | 0.198 | 0.852 | 0.0504 |
| 290 | 15.68 | 0.05 | 0.653 | 0.688 | 1.65 | 0.390 | 1.067 | 0.2935 |
| 291 | 3.15 | 0.05 | 4.513 | 0.925 | 5.412 | 0.054 | 2.269 | 0.0379 |
| 326 | 1.96 | 0.05 | 2.019 | 0.921 | 4.209 | 0.059 | 1.965 | 0.0373 |
| 385 | 2.08 | 0.05 | 1.838 | 0.927 | 3.603 | 0.089 | 1.436 | 0.0349 |
| 479 | 10,000 | 0.02 | 0.071 | 0.13 | 0.76 | 0.527 | 2.687 | 0.9392 |
| 480 | 1.81 | 0.07 | 1.64 | 0.898 | 3.61 | 0.088 | 1.281 | 0.0485 |
| 504 | 25.06 | 0.04 | 0.073 | 0.166 | 1.201 | 0.473 | 2.315 | 0.9978 |
| 505 | 1062.6 | 0.01 | −0.997 | 0.131 | −2.001 | 0.872 | 0.489 | 0.4844 |
| Site | 291 | 326 | 385 | 480 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Majority | A | A | P | T | ||||||||||
| Substitution | T | V | G | T | V | S | L | R | H | S | A | P | S | I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhu, X.; Zhang, Z.; Chen, J.; Chen, Y.; Hu, C.; Chen, X.; Robertson, I.D.; Guo, A. The Prevalence and Molecular Characterization of Bovine Leukemia Virus among Dairy Cattle in Henan Province, China. Viruses 2024, 16, 1399. https://doi.org/10.3390/v16091399
Zhao Y, Zhu X, Zhang Z, Chen J, Chen Y, Hu C, Chen X, Robertson ID, Guo A. The Prevalence and Molecular Characterization of Bovine Leukemia Virus among Dairy Cattle in Henan Province, China. Viruses. 2024; 16(9):1399. https://doi.org/10.3390/v16091399
Chicago/Turabian StyleZhao, Yuxi, Xiaojie Zhu, Zhen Zhang, Jianguo Chen, Yingyu Chen, Changmin Hu, Xi Chen, Ian D. Robertson, and Aizhen Guo. 2024. "The Prevalence and Molecular Characterization of Bovine Leukemia Virus among Dairy Cattle in Henan Province, China" Viruses 16, no. 9: 1399. https://doi.org/10.3390/v16091399
APA StyleZhao, Y., Zhu, X., Zhang, Z., Chen, J., Chen, Y., Hu, C., Chen, X., Robertson, I. D., & Guo, A. (2024). The Prevalence and Molecular Characterization of Bovine Leukemia Virus among Dairy Cattle in Henan Province, China. Viruses, 16(9), 1399. https://doi.org/10.3390/v16091399







