Unveiling the Connection: Viral Infections and Genes in dNTP Metabolism
Abstract
1. Introduction
2. Interactions between Various DNA Viruses and Genes Involved in dNTP Metabolism
2.1. Herpesviruses
2.1.1. DHFR
2.1.2. RNRs
2.1.3. SAMHD1
2.2. Poxvirus and African Swine Fever Virus
2.2.1. RNR
2.2.2. SAMHD1
2.3. Adenoviruses
2.3.1. DHFR
2.3.2. RNRs
2.4. Human Papillomavirus (HPV) and Polyomavirus
2.4.1. RNRs
2.4.2. SAMHD1
2.5. Parvovirus
RNRs
3. Interactions between Various Viruses Whose Replication Is through Reverse Transcription and Genes Involved in dNTP Metabolism
3.1. Retrovirus
3.1.1. RNRs
3.1.2. SAMHD1
3.2. Hepatitis B Virus (HBV)
3.2.1. RNRs
3.2.2. SAMHD1
4. Interactions between Various RNA Viruses and Genes Involved in dNTP Metabolism
4.1. Coronaviruses
DHFR
4.2. Influenza Viruses
DHFR
4.3. Zika Virus
4.3.1. DHFR
4.3.2. SAMHD1
4.4. HCV
RNRs
4.5. Chikungunya Virus
SAMHD1
4.6. Respiratory Syncytial Virus
DHFR
5. Regulation of Viral Infections by Genes Involved in dNTP Metabolism Independent from dNTP Level
5.1. RNR
5.2. SAMHD1
6. Application of Oncolytic Viruses without Viral RNRs
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Buj, R.; Aird, K.M. Deoxyribonucleotide Triphosphate Metabolism in Cancer and Metabolic Disease. Front. Endocrinol. 2018, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Herrick, J.; Sclavi, B. Ribonucleotide reductase and the regulation of DNA replication: An old story and an ancient heritage. Mol. Microbiol. 2007, 63, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Coggins, S.A.; Mahboubi, B.; Schinazi, R.F.; Kim, B. SAMHD1 Functions and Human Diseases. Viruses 2020, 12, 382. [Google Scholar] [CrossRef]
- Kohnken, R.; Kodigepalli, K.M.; Wu, L. Regulation of deoxynucleotide metabolism in cancer: Novel mechanisms and therapeutic implications. Mol. Cancer 2015, 14, 176. [Google Scholar] [CrossRef]
- Bhagat, K.; Kumar, N.; Kaur Gulati, H.; Sharma, A.; Kaur, A.; Singh, J.V.; Singh, H.; Bedi, P.M.S. Dihydrofolate reductase inhibitors: Patent landscape and phases of clinical development (2001–2021). Expert. Opin. Ther. Pat. 2022, 32, 1079–1095. [Google Scholar] [CrossRef]
- Raimondi, M.V.; Randazzo, O.; La Franca, M.; Barone, G.; Vignoni, E.; Rossi, D.; Collina, S. DHFR Inhibitors: Reading the Past for Discovering Novel Anticancer Agents. Molecules 2019, 24, 1140. [Google Scholar] [CrossRef]
- Shamshad, H.; Bakri, R.; Mirza, A.Z. Dihydrofolate reductase, thymidylate synthase, and serine hydroxy methyltransferase: Successful targets against some infectious diseases. Mol. Biol. Rep. 2022, 49, 6659–6691. [Google Scholar] [CrossRef]
- Blakley, R.L. Eukaryotic dihydrofolate reductase. Adv. Enzymol. Relat. Areas. Mol. Biol. 1995, 70, 23–102. [Google Scholar]
- Nordlund, P.; Reichard, P. Ribonucleotide reductases. Annu. Rev. Biochem. 2006, 75, 681–706. [Google Scholar] [CrossRef]
- Burnim, A.A.; Spence, M.A.; Xu, D.; Jackson, C.J.; Ando, N. Comprehensive phylogenetic analysis of the ribonucleotide reductase family reveals an ancestral clade. eLife 2022, 11, e79790. [Google Scholar] [CrossRef] [PubMed]
- Long, M.J.C.; Ly, P.; Aye, Y. Still no Rest for the Reductases: Ribonucleotide Reductase (RNR) Structure and Function: An Update. Subcell. Biochem. 2022, 99, 155–197. [Google Scholar] [PubMed]
- D’Angiolella, V.; Donato, V.; Forrester, F.M.; Jeong, Y.T.; Pellacani, C.; Kudo, Y.; Saraf, A.; Florens, L.; Washburn, M.P.; Pagano, M. Cyclin F-mediated degradation of ribonucleotide reductase M2 controls genome integrity and DNA repair. Cell 2012, 149, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.C.; Yen, Y. Novel regulators and molecular mechanisms of p53R2 and its disease relevance. Biochimie 2016, 123, 81–84. [Google Scholar] [CrossRef]
- Ballana, E.; Este, J.A. SAMHD1: At the crossroads of cell proliferation, immune responses, and virus restriction. Trends Microbiol. 2015, 23, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.R.; Caswell, S.J.; Kunzelmann, S.; Arnold, L.H.; Purkiss, A.G.; Kelly, G.; Taylor, I.A. Crystal structures of SAMHD1 inhibitor complexes reveal the mechanism of water-mediated dNTP hydrolysis. Nat. Commun. 2020, 11, 3165. [Google Scholar] [CrossRef]
- Deutschmann, J.; Gramberg, T. SAMHD1… and Viral Ways around It. Viruses 2021, 13, 395. [Google Scholar] [CrossRef]
- Lembo, D.; Gribaudo, G.; Cavallo, R.; Riera, L.; Angeretti, A.; Hertel, L.; Landolfo, S. Human cytomegalovirus stimulates cellular dihydrofolate reductase activity in quiescent cells. Intervirology 1999, 42, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Cinquina, C.C.; Grogan, E.; Sun, R.; Lin, S.F.; Beardsley, G.P.; Miller, G. Dihydrofolate reductase from Kaposi’s sarcoma-associated herpesvirus. Virology 2000, 268, 201–217. [Google Scholar] [CrossRef][Green Version]
- Gaspar, G.; De Clercq, E.; Neyts, J. Gammaherpesviruses encode functional dihydrofolate reductase activity. Biochem. Biophys. Res. Commun. 2002, 297, 756–759. [Google Scholar] [CrossRef]
- Trimble, J.J.; Murthy, S.C.; Bakker, A.; Grassmann, R.; Desrosiers, R.C. A gene for dihydrofolate reductase in a herpesvirus. Science 1988, 239, 1145–1147. [Google Scholar] [CrossRef]
- Wade, M.; Kowalik, T.F.; Mudryj, M.; Huang, E.S.; Azizkhan, J.C. E2F mediates dihydrofolate reductase promoter activation and multiprotein complex formation in human cytomegalovirus infection. Mol. Cell. Biol. 1992, 12, 4364–4374. [Google Scholar]
- Lembo, D.; Angeretti, A.; Gariglio, M.; Landolfo, S. Murine cytomegalovirus induces expression and enzyme activity of cellular dihydrofolate reductase in quiescent cells. J. Gen. Virol. 1998, 79, 2803–2807. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lembo, D.; Cavallo, R.; Cornaglia, M.; Mondo, A.; Hertel, L.; Angeretti, A.; Landolfo, S. Overexpression of cellular dihydrofolate reductase abolishes the anticytomegaloviral activity of methotrexate. Arch. Virol. 1999, 144, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, J.G.; Leib, D.A.; Goldstein, D.J.; Bogard, C.L.; Schaffer, P.A.; Weller, S.K.; Coen, D.M. A herpes simplex virus ribonucleotide reductase deletion mutant is defective for productive acute and reactivatable latent infections of mice and for replication in mouse cells. Virology 1989, 173, 276–283. [Google Scholar] [CrossRef]
- Spector, T.; Averett, D.R.; Nelson, D.J.; Lambe, C.U.; Morrison, R.W., Jr.; St Clair, M.H.; Furman, P.A. Potentiation of antiherpetic activity of acyclovir by ribonucleotide reductase inhibition. Proc. Natl. Acad. Sci. USA 1985, 82, 4254–4257. [Google Scholar] [CrossRef] [PubMed]
- Coen, D.M.; Goldstein, D.J.; Weller, S.K. Herpes simplex virus ribonucleotide reductase mutants are hypersensitive to acyclovir. Antimicrob. Agents. Chemother. 1989, 33, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Averett, D.R.; Furman, P.A.; Spector, T. Ribonucleotide reductase of herpes simplex virus type 2 resembles that of herpes simplex virus type 1. J. Virol. 1984, 52, 981–983. [Google Scholar] [CrossRef] [PubMed]
- Spector, T. Inhibition of ribonucleotide reductases encoded by herpes simplex viruses. Pharmacol. Ther. 1985, 31, 295–302. [Google Scholar] [CrossRef]
- Spector, T.; Jones, T.E. Herpes simplex type 1 ribonucleotide reductase. Mechanism studies with inhibitors. J. Biol. Chem. 1985, 260, 8694–8697. [Google Scholar] [CrossRef]
- Aurelian, L. Herpes simplex virus type 2: Unique biological properties include neoplastic potential mediated by the PK domain of the large subunit of ribonucleotide reductase. Front. Biosci. 1998, 3, d237–d249. [Google Scholar] [CrossRef][Green Version]
- Smith, C.C.; Peng, T.; Kulka, M.; Aurelian, L. The PK domain of the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10) is required for immediate-early gene expression and virus growth. J. Virol. 1998, 72, 9131–9141. [Google Scholar] [CrossRef] [PubMed]
- Heineman, T.C.; Cohen, J.I. Deletion of the varicella-zoster virus large subunit of ribonucleotide reductase impairs growth of virus in vitro. J. Virol. 1994, 68, 3317–3323. [Google Scholar] [CrossRef]
- Lankinen, H.; Graslund, A.; Thelander, L. Induction of a new ribonucleotide reductase after infection of mouse L cells with pseudorabies virus. J. Virol. 1982, 41, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Lyu, C.; Li, W.D.; Peng, J.M.; Cai, X.H. Identification of interaction domains in the pseudorabies virus ribonucleotide reductase large and small subunits. Vet. Microbiol. 2020, 246, 108740. [Google Scholar] [CrossRef]
- Bhave, S.; Elford, H.; McVoy, M.A. Ribonucleotide reductase inhibitors hydroxyurea, didox, and trimidox inhibit human cytomegalovirus replication in vitro and synergize with ganciclovir. Antiviral. Res. 2013, 100, 151–158. [Google Scholar] [CrossRef]
- Gibson, T.; Stockwell, P.; Ginsburg, M.; Barrell, B. Homology between two EBV early genes and HSV ribonucleotide reductase and 38K genes. Nucleic Acids Res. 1984, 12, 5087–5099. [Google Scholar] [CrossRef]
- Wang, S.S.; Chen, L.W.; Chen, L.Y.; Tsai, H.H.; Shih, Y.C.; Yang, C.T.; Chang, P.J. Transcriptional regulation of the ORF61 and ORF60 genes of Kaposi’s sarcoma-associated herpesvirus. Virology 2010, 397, 311–321. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, W.; Chen, Q.; Ren, Y.; Zhang, Y.; Lu, L.; Gui, L.; Xu, D. The identification of viral ribonucleotide reductase encoded by ORF23 and ORF141 genes and effect on CyHV-2 replication. Front. Microbiol. 2023, 14, 1154840. [Google Scholar] [CrossRef]
- Hollenbaugh, J.A.; Gee, P.; Baker, J.; Daly, M.B.; Amie, S.M.; Tate, J.; Kasai, N.; Kanemura, Y.; Kim, D.H.; Ward, B.M.; et al. Host factor SAMHD1 restricts DNA viruses in non-dividing myeloid cells. PLoS Pathog. 2013, 9, e1003481. [Google Scholar] [CrossRef]
- Kim, E.T.; Roche, K.L.; Kulej, K.; Spruce, L.A.; Seeholzer, S.H.; Coen, D.M.; Diaz-Griffero, F.; Murphy, E.A.; Weitzman, M.D. SAMHD1 Modulates Early Steps during Human Cytomegalovirus Infection by Limiting NF-kappaB Activation. Cell Rep. 2019, 28, 434–448.e6. [Google Scholar] [CrossRef]
- Deutschmann, J.; Schneider, A.; Gruska, I.; Vetter, B.; Thomas, D.; Kiessling, M.; Wittmann, S.; Herrmann, A.; Schindler, M.; Milbradt, J.; et al. A viral kinase counteracts in vivo restriction of murine cytomegalovirus by SAMHD1. Nat. Microbiol. 2019, 4, 2273–2284. [Google Scholar] [CrossRef]
- Badia, R.; Angulo, G.; Riveira-Munoz, E.; Pujantell, M.; Puig, T.; Ramirez, C.; Torres-Torronteras, J.; Marti, R.; Pauls, E.; Clotet, B.; et al. Inhibition of herpes simplex virus type 1 by the CDK6 inhibitor PD-0332991 (palbociclib) through the control of SAMHD1. J. Antimicrob. Chemother. 2016, 71, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Lv, D.W.; Li, R. Conserved Herpesvirus Protein Kinases Target SAMHD1 to Facilitate Virus Replication. Cell Rep. 2019, 28, 449–459.e5. [Google Scholar] [CrossRef] [PubMed]
- Businger, R.; Deutschmann, J.; Gruska, I.; Milbradt, J.; Wiebusch, L.; Gramberg, T.; Schindler, M. Human cytomegalovirus overcomes SAMHD1 restriction in macrophages via pUL97. Nat. Microbiol. 2019, 4, 2260–2272. [Google Scholar] [CrossRef] [PubMed]
- De Meo, S.; Dell’Oste, V.; Molfetta, R.; Tassinari, V.; Lotti, L.V.; Vespa, S.; Pignoloni, B.; Covino, D.A.; Fantuzzi, L.; Bona, R.; et al. SAMHD1 phosphorylation and cytoplasmic relocalization after human cytomegalovirus infection limits its antiviral activity. PLoS Pathog. 2020, 16, e1008855. [Google Scholar] [CrossRef]
- Hyeon, S.; Lee, M.K.; Kim, Y.E.; Lee, G.M.; Ahn, J.H. Degradation of SAMHD1 Restriction Factor Through Cullin-Ring E3 Ligase Complexes During Human Cytomegalovirus Infection. Front. Cell. Infect. Microbiol. 2020, 10, 391. [Google Scholar] [CrossRef]
- Greseth, M.D.; Traktman, P. The Life Cycle of the Vaccinia Virus Genome. Annu. Rev. Virol. 2022, 9, 239–259. [Google Scholar] [CrossRef]
- Li, Z.; Chen, W.; Qiu, Z.; Li, Y.; Fan, J.; Wu, K.; Li, X.; Zhao, M.; Ding, H.; Fan, S.; et al. African Swine Fever Virus: A Review. Life 2022, 12, 1255. [Google Scholar] [CrossRef]
- Slabaugh, M.; Roseman, N.; Davis, R.; Mathews, C. Vaccinia virus-encoded ribonucleotide reductase: Sequence conservation of the gene for the small subunit and its amplification in hydroxyurea-resistant mutants. J. Virol. 1988, 62, 519–527. [Google Scholar] [CrossRef]
- Howell, M.L.; Roseman, N.A.; Slabaugh, M.B.; Mathews, C.K. Vaccinia virus ribonucleotide reductase. Correlation between deoxyribonucleotide supply and demand. J. Biol. Chem. 1993, 268, 7155–7162. [Google Scholar] [CrossRef]
- Slabaugh, M.B.; Davis, R.E.; Roseman, N.A.; Mathews, C.K. Vaccinia virus ribonucleotide reductase expression and isolation of the recombinant large subunit. J. Biol. Chem. 1993, 268, 17803–17810. [Google Scholar] [CrossRef]
- Hendricks, S.P.; Mathews, C.K. Allosteric regulation of vaccinia virus ribonucleotide reductase, analyzed by simultaneous monitoring of its four activities. J. Biol. Chem. 1998, 273, 29512–29518. [Google Scholar] [CrossRef]
- Romeo, A.M.; Christen, L.; Niles, E.G.; Kosman, D.J. Intracellular chelation of iron by bipyridyl inhibits DNA virus replication: Ribonucleotide reductase maturation as a probe of intracellular iron pools. J. Biol. Chem. 2001, 276, 24301–24308. [Google Scholar] [CrossRef]
- Hendricks, S.P.; Mathews, C.K. Differential effects of hydroxyurea upon deoxyribonucleoside triphosphate pools, analyzed with vaccinia virus ribonucleotide reductase. J. Biol. Chem. 1998, 273, 29519–29523. [Google Scholar] [CrossRef]
- Slabaugh, M.B.; Howell, M.L.; Wang, Y.; Mathews, C.K. Deoxyadenosine reverses hydroxyurea inhibition of vaccinia virus growth. J. Virol. 1991, 65, 2290–2298. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zheng, J.; Huang, T.; Wang, X.; Li, J.; Jin, F.; Wei, W.; Chen, X.; Liu, C.; Bao, M.; et al. Identification of several African swine fever virus replication inhibitors by screening of a library of FDA-approved drugs. Virology 2024, 593, 110014. [Google Scholar] [CrossRef] [PubMed]
- Sliva, K.; Martin, J.; von Rhein, C.; Herrmann, T.; Weyrich, A.; Toda, M.; Schnierle, B.S. Interference with SAMHD1 Restores Late Gene Expression of Modified Vaccinia Virus Ankara in Human Dendritic Cells and Abrogates Type I Interferon Expression. J. Virol. 2019, 93, e01097-19. [Google Scholar]
- Feng, W.; Zhou, L.; Du, H.; Okoth, E.; Mrode, R.; Jin, W.; Hu, Z.; Liu, J.F. Transcriptome analysis reveals gene expression changes of pigs infected with non-lethal African swine fever virus. Genet. Mol. Biol. 2023, 46, e20230037. [Google Scholar] [CrossRef] [PubMed]
- Yoder, S.S.; Robberson, B.L.; Leys, E.J.; Hook, A.G.; Al-Ubaidi, M.; Yeung, C.Y.; Kellems, R.E.; Berget, S.M. Control of cellular gene expression during adenovirus infection: Induction and shut-off of dihydrofolate reductase gene expression by adenovirus type 2. Mol. Cell Biol. 1983, 3, 819–828. [Google Scholar]
- Yoder, S.S.; Berget, S.M. Posttranscriptional control of DHFR gene expression during adenovirus 2 infection. J. Virol. 1985, 54, 72–77. [Google Scholar] [CrossRef]
- Dai, Y.; Gold, B.; Vishwanatha, J.K.; Rhode, S.L. Mimosine inhibits viral DNA synthesis through ribonucleotide reductase. Virology 1994, 205, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Anacker, D.C.; Aloor, H.L.; Shepard, C.N.; Lenzi, G.M.; Johnson, B.A.; Kim, B.; Moody, C.A. HPV31 utilizes the ATR-Chk1 pathway to maintain elevated RRM2 levels and a replication-competent environment in differentiating Keratinocytes. Virology 2016, 499, 383–396. [Google Scholar] [CrossRef]
- Wang, N.; Zhan, T.; Ke, T.; Huang, X.; Ke, D.; Wang, Q.; Li, H. Increased expression of RRM2 by human papillomavirus E7 oncoprotein promotes angiogenesis in cervical cancer. Br. J. Cancer 2014, 110, 1034–1044. [Google Scholar] [CrossRef]
- Bjursell, G.; Magnusson, G. Replication of polyoma DNA accumlation of early replicative intermediates during hydroxyurea inhibition. Virology 1976, 74, 249–251. [Google Scholar] [CrossRef]
- James, C.D.; Prabhakar, A.T.; Otoa, R.; Evans, M.R.; Wang, X.; Bristol, M.L.; Zhang, K.; Li, R.; Morgan, I.M. SAMHD1 Regulates Human Papillomavirus 16-Induced Cell Proliferation and Viral Replication during Differentiation of Keratinocytes. mSphere 2019, 4, 00448-19. [Google Scholar] [CrossRef] [PubMed]
- James, C.D.; Youssef, A.; Prabhakar, A.T.; Otoa, R.; Witt, A.; Lewis, R.L.; Bristol, M.L.; Wang, X.; Zhang, K.; Li, R.; et al. Human Papillomavirus 16 replication converts SAMHD1 into a homologous recombination factor and promotes its recruitment to replicating viral DNA. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Li, H.C.; Yang, C.H.; Lo, S.Y. Cellular factors involved in the hepatitis C virus life cycle. World J. Gastroenterol. 2021, 27, 4555–4581. [Google Scholar] [CrossRef]
- Allouch, A.; David, A.; Amie, S.M.; Lahouassa, H.; Chartier, L.; Margottin-Goguet, F.; Barre-Sinoussi, F.; Kim, B.; Saez-Cirion, A.; Pancino, G. p21-mediated RNR2 repression restricts HIV-1 replication in macrophages by inhibiting dNTP biosynthesis pathway. Proc. Natl. Acad. Sci. USA 2013, 110, E3997–E4006. [Google Scholar] [CrossRef]
- Loaiza, J.D.; Chvatal-Medina, M.; Hernandez, J.C.; Rugeles, M.T. Integrase inhibitors: Current protagonists in antiretroviral therapy. Immunotherapy 2023, 15, 1477–1495. [Google Scholar] [CrossRef]
- Committee, P.S.; Welch, S.; Sharland, M.; Lyall, E.G.; Tudor-Williams, G.; Niehues, T.; Wintergerst, U.; Bunupuradah, T.; Hainaut, M.; Della Negra, M.; et al. PENTA 2009 guidelines for the use of antiretroviral therapy in paediatric HIV-1 infection. HIV Med. 2009, 10, 591–613. [Google Scholar] [CrossRef]
- Clouser, C.L.; Chauhan, J.; Bess, M.A.; van Oploo, J.L.; Zhou, D.; Dimick-Gray, S.; Mansky, L.M.; Patterson, S.E. Anti-HIV-1 activity of resveratrol derivatives and synergistic inhibition of HIV-1 by the combination of resveratrol and decitabine. Bioorg. Med. Chem. Lett. 2012, 22, 6642–6646. [Google Scholar] [CrossRef] [PubMed]
- Clouser, C.L.; Holtz, C.M.; Mullett, M.; Crankshaw, D.L.; Briggs, J.E.; Chauhan, J.; VanHoutan, I.M.; Patterson, S.E.; Mansky, L.M. Analysis of the ex vivo and in vivo antiretroviral activity of gemcitabine. PLoS ONE 2011, 6, e15840. [Google Scholar] [CrossRef] [PubMed]
- Clouser, C.L.; Patterson, S.E.; Mansky, L.M. Exploiting drug repositioning for discovery of a novel HIV combination therapy. J. Virol. 2010, 84, 9301–9309. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.B.; Roth, M.E.; Bonnac, L.; Maldonado, J.O.; Xie, J.; Clouser, C.L.; Patterson, S.E.; Kim, B.; Mansky, L.M. Dual anti-HIV mechanism of clofarabine. Retrovirology 2016, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.Y.; Johns, D.G.; Chokekuchai, S.; Mitsuya, H. Disparate actions of hydroxyurea in potentiation of purine and pyrimidine 2′,3′-dideoxynucleoside activities against replication of human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 1995, 92, 8333–8337. [Google Scholar] [CrossRef]
- Rawson, J.M.O.; Roth, M.E.; Xie, J.; Daly, M.B.; Clouser, C.L.; Landman, S.R.; Reilly, C.S.; Bonnac, L.; Kim, B.; Patterson, S.E.; et al. Synergistic reduction of HIV-1 infectivity by 5-azacytidine and inhibitors of ribonucleotide reductase. Bioorg. Med. Chem. 2016, 24, 2410–2422. [Google Scholar] [CrossRef]
- Pauls, E.; Ruiz, A.; Badia, R.; Permanyer, M.; Gubern, A.; Riveira-Munoz, E.; Torres-Torronteras, J.; Alvarez, M.; Mothe, B.; Brander, C.; et al. Cell cycle control and HIV-1 susceptibility are linked by CDK6-dependent CDK2 phosphorylation of SAMHD1 in myeloid and lymphoid cells. J. Immunol. 2014, 193, 1988–1997. [Google Scholar] [CrossRef]
- Pauls, E.; Badia, R.; Torres-Torronteras, J.; Ruiz, A.; Permanyer, M.; Riveira-Munoz, E.; Clotet, B.; Marti, R.; Ballana, E.; Este, J.A. Palbociclib, a selective inhibitor of cyclin-dependent kinase4/6, blocks HIV-1 reverse transcription through the control of sterile alpha motif and HD domain-containing protein-1 (SAMHD1) activity. AIDS 2014, 28, 2213–2222. [Google Scholar] [CrossRef]
- Pauls, E.; Ruiz, A.; Riveira-Munoz, E.; Permanyer, M.; Badia, R.; Clotet, B.; Keppler, O.T.; Ballana, E.; Este, J.A. p21 regulates the HIV-1 restriction factor SAMHD1. Proc. Natl. Acad. Sci. USA 2014, 111, E1322–E1324. [Google Scholar] [CrossRef]
- Bowen, N.E.; Oo, A.; Kim, B. Mechanistic Interplay between HIV-1 Reverse Transcriptase Enzyme Kinetics and Host SAMHD1 Protein: Viral Myeloid-Cell Tropism and Genomic Mutagenesis. Viruses 2022, 14, 1622. [Google Scholar] [CrossRef]
- Riveira-Munoz, E.; Ruiz, A.; Pauls, E.; Permanyer, M.; Badia, R.; Mothe, B.; Crespo, M.; Clotet, B.; Brander, C.; Ballana, E.; et al. Increased expression of SAMHD1 in a subset of HIV-1 elite controllers. J. Antimicrob. Chemother. 2014, 69, 3057–3060. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharifi, H.J.; Paine, D.N.; Fazzari, V.A.; Tipple, A.F.; Patterson, E.; de Noronha, C.M.C. Sulforaphane Reduces SAMHD1 Phosphorylation To Protect Macrophages from HIV-1 Infection. J. Virol. 2022, 96, e0118722. [Google Scholar] [CrossRef] [PubMed]
- Spragg, C.J.; Emerman, M. Antagonism of SAMHD1 is actively maintained in natural infections of simian immunodeficiency virus. Proc. Natl. Acad. Sci. USA 2013, 110, 21136–21141. [Google Scholar] [CrossRef]
- Fregoso, O.I.; Ahn, J.; Wang, C.; Mehrens, J.; Skowronski, J.; Emerman, M. Evolutionary toggling of Vpx/Vpr specificity results in divergent recognition of the restriction factor SAMHD1. PLoS Pathog. 2013, 9, e1003496. [Google Scholar] [CrossRef]
- Bowen, N.E.; Tao, S.; Cho, Y.J.; Schinazi, R.F.; Kim, B. Vpx requires active cellular dNTP biosynthesis to effectively counteract the anti-lentivirus activity of SAMHD1 in macrophages. J. Biol. Chem. 2023, 299, 104984. [Google Scholar] [CrossRef]
- Mereby, S.A.; Maehigashi, T.; Holler, J.M.; Kim, D.H.; Schinazi, R.F.; Kim, B. Interplay of ancestral non-primate lentiviruses with the virus-restricting SAMHD1 proteins of their hosts. J. Biol. Chem. 2018, 293, 16402–16412. [Google Scholar] [CrossRef]
- Coggins, S.A.; Kim, D.H.; Schinazi, R.F.; Desrosier, R.C.; Kim, B. Enhanced enzyme kinetics of reverse transcriptase variants cloned from animals infected with SIVmac239 lacking viral protein X. J. Biol. Chem. 2020, 295, 16975–16986. [Google Scholar] [CrossRef] [PubMed]
- Plitnik, T.; Sharkey, M.E.; Mahboubi, B.; Kim, B.; Stevenson, M. Incomplete Suppression of HIV-1 by SAMHD1 Permits Efficient Macrophage Infection. Pathog. Immun. 2018, 3, 197–223. [Google Scholar] [CrossRef]
- Gramberg, T.; Kahle, T.; Bloch, N.; Wittmann, S.; Mullers, E.; Daddacha, W.; Hofmann, H.; Kim, B.; Lindemann, D.; Landau, N.R. Restriction of diverse retroviruses by SAMHD1. Retrovirology 2013, 10, 26. [Google Scholar] [CrossRef]
- Ballana, E.; Badia, R.; Terradas, G.; Torres-Torronteras, J.; Ruiz, A.; Pauls, E.; Riveira-Munoz, E.; Clotet, B.; Marti, R.; Este, J.A. SAMHD1 specifically affects the antiviral potency of thymidine analog HIV reverse transcriptase inhibitors. Antimicrob. Agents Chemother. 2014, 58, 4804–4813. [Google Scholar] [CrossRef][Green Version]
- Tsukuda, S.; Watashi, K. Hepatitis B virus biology and life cycle. Antiviral Res. 2020, 182, 104925. [Google Scholar] [CrossRef]
- Cohen, D.; Adamovich, Y.; Reuven, N.; Shaul, Y. Hepatitis B virus activates deoxynucleotide synthesis in nondividing hepatocytes by targeting the R2 gene. Hepatology 2010, 51, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Ricardo-Lax, I.; Ramanan, V.; Michailidis, E.; Shamia, T.; Reuven, N.; Rice, C.M.; Shlomai, A.; Shaul, Y. Hepatitis B virus induces RNR-R2 expression via DNA damage response activation. J. Hepatol. 2015, 63, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, Z.; Tian, J.; Liu, Q.; Dong, J.; Guo, L.; Hai, B.; Liu, X.; Yao, H.; Chen, Z.; et al. Pterostilbene inhibits hepatocellular carcinoma proliferation and HBV replication by targeting ribonucleotide reductase M2 protein. Am. J. Cancer Res. 2021, 11, 2975–2989. [Google Scholar] [PubMed]
- Broennimann, K.; Ricardo-Lax, I.; Adler, J.; Michailidis, E.; de Jong, Y.P.; Reuven, N.; Shaul, Y. RNR-R2 Upregulation by a Short Non-Coding Viral Transcript. Biomolecules 2021, 11, 1822. [Google Scholar] [CrossRef]
- Gearhart, T.L.; Bouchard, M.J. Replication of the hepatitis B virus requires a calcium-dependent HBx-induced G1 phase arrest of hepatocytes. Virology 2010, 407, 14–25. [Google Scholar] [CrossRef]
- Broennimann, K.; Ricardo-Lax, I.; Adler, J.; Shaul, Y. Evidence for a Hepatitis B Virus Short RNA Fragment Directly Targeting the Cellular RRM2 Gene. Cells 2022, 11, 2248. [Google Scholar] [CrossRef]
- Jin, X.; Yu, W.; Wang, A.; Qiu, Y. Serum Ribonucleotide Reductase Subunit M2 in Patients with Chronic Liver Diseases and Hepatocellular Carcinoma. Lab. Med. 2023, 54, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, Z.; Hou, C.; Wang, M.; Chen, X.; Lin, Q.; Song, R.; Lou, M.; Zhu, L.; Qiu, Y.; et al. Inhibition of hepatitis B virus replication by targeting ribonucleotide reductase M2 protein. Biochem. Pharmacol. 2016, 103, 118–128. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, M.; Pan, X.; Zhu, Y.; Yan, H.; Jiang, T.; Shen, Y.; Dong, X.; Zheng, N.; Lu, J.; et al. Inhibition of Hepatitis B virus replication by SAMHD1. Biochem. Biophys. Res. Commun. 2014, 450, 1462–1468. [Google Scholar] [CrossRef]
- Sommer, A.F.; Riviere, L.; Qu, B.; Schott, K.; Riess, M.; Ni, Y.; Shepard, C.; Schnellbacher, E.; Finkernagel, M.; Himmelsbach, K.; et al. Restrictive influence of SAMHD1 on Hepatitis B Virus life cycle. Sci. Rep. 2016, 6, 26616. [Google Scholar]
- Wing, P.A.; Davenne, T.; Wettengel, J.; Lai, A.G.; Zhuang, X.; Chakraborty, A.; D’Arienzo, V.; Kramer, C.; Ko, C.; Harris, J.M.; et al. A dual role for SAMHD1 in regulating HBV cccDNA and RT-dependent particle genesis. Life Sci. Alliance 2019, 2, e201900355. [Google Scholar] [CrossRef]
- Hu, J.; Qiao, M.; Chen, Y.; Tang, H.; Zhang, W.; Tang, D.; Pi, S.; Dai, J.; Tang, N.; Huang, A.; et al. Cyclin E2-CDK2 mediates SAMHD1 phosphorylation to abrogate its restriction of HBV replication in hepatoma cells. FEBS Lett. 2018, 592, 1893–1904. [Google Scholar] [CrossRef]
- Decosterd, L.A.; Cottin, E.; Chen, X.; Lejeune, F.; Mirimanoff, R.O.; Biollaz, J.; Coucke, P.A. Simultaneous determination of deoxyribonucleoside in the presence of ribonucleoside triphosphates in human carcinoma cells by high-performance liquid chromatography. Anal. Biochem. 1999, 270, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Harmenberg, J.; Cox, S.; Akesson-Johansson, A. Improved sample preparation method for high-performance liquid chromatography of deoxyribonucleoside triphosphates from cell culture extracts. J. Chromatogr. 1990, 508, 75–79. [Google Scholar] [CrossRef]
- Chen, Y.T.; Chang, Y.H.; Pathak, N.; Tzou, S.C.; Luo, Y.C.; Hsu, Y.C.; Li, T.N.; Lee, J.Y.; Chen, Y.C.; Huang, Y.W.; et al. Methotrexate inhibition of SARS-CoV-2 entry, infection and inflammation revealed by bioinformatics approach and a hamster model. Front. Immunol. 2022, 13, 1080897. [Google Scholar] [CrossRef] [PubMed]
- Iaconis, D.; Caccuri, F.; Manelfi, C.; Talarico, C.; Bugatti, A.; Filippini, F.; Zani, A.; Novelli, R.; Kuzikov, M.; Ellinger, B.; et al. DHFR Inhibitors Display a Pleiotropic Anti-Viral Activity against SARS-CoV-2: Insights into the Mechanisms of Action. Viruses 2023, 15, 1128. [Google Scholar] [CrossRef]
- Francesconi, V.; Giovannini, L.; Santucci, M.; Cichero, E.; Costi, M.P.; Naesens, L.; Giordanetto, F.; Tonelli, M. Synthesis, biological evaluation and molecular modeling of novel azaspiro dihydrotriazines as influenza virus inhibitors targeting the host factor dihydrofolate reductase (DHFR). Eur. J. Med. Chem. 2018, 155, 229–243. [Google Scholar] [CrossRef]
- Tonelli, M.; Naesens, L.; Gazzarrini, S.; Santucci, M.; Cichero, E.; Tasso, B.; Moroni, A.; Costi, M.P.; Loddo, R. Host dihydrofolate reductase (DHFR)-directed cycloguanil analogues endowed with activity against influenza virus and respiratory syncytial virus. Eur. J. Med. Chem. 2017, 135, 467–478. [Google Scholar] [CrossRef]
- Beck, S.; Zhu, Z.; Oliveira, M.F.; Smith, D.M.; Rich, J.N.; Bernatchez, J.A.; Siqueira-Neto, J.L. Mechanism of Action of Methotrexate Against Zika Virus. Viruses 2019, 11, 338. [Google Scholar] [CrossRef]
- Wichit, S.; Hamel, R.; Zanzoni, A.; Diop, F.; Cribier, A.; Talignani, L.; Diack, A.; Ferraris, P.; Liegeois, F.; Urbach, S.; et al. SAMHD1 Enhances Chikungunya and Zika Virus Replication in Human Skin Fibroblasts. Int. J. Mol. Sci. 2019, 20, 1695. [Google Scholar] [CrossRef]
- Kitab, B.; Satoh, M.; Ohmori, Y.; Munakata, T.; Sudoh, M.; Kohara, M.; Tsukiyama-Kohara, K. Ribonucleotide reductase M2 promotes RNA replication of hepatitis C virus by protecting NS5B protein from hPLIC1-dependent proteasomal degradation. J. Biol. Chem. 2019, 294, 5759–5773. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Wu, C.H.; Lo, S.Y.; Lua, A.C.; Chan, Y.R.; Li, H.C. Hepatitis C Virus Down-Regulates the Expression of Ribonucleotide Reductases to Promote Its Replication. Pathogens 2023, 12, 892. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.Z.; Moraes, S.N.; Shaban, N.M.; Fanunza, E.; Bierle, C.J.; Southern, P.J.; Bresnahan, W.A.; Rice, S.A.; Harris, R.S. APOBECs and Herpesviruses. Viruses 2021, 13, 390. [Google Scholar] [CrossRef]
- Chen, S.; Bonifati, S.; Qin, Z.; St Gelais, C.; Wu, L. SAMHD1 Suppression of Antiviral Immune Responses. Trends Microbiol. 2019, 27, 254–267. [Google Scholar] [CrossRef]
- Thientosapol, E.S.; Bosnjak, D.; Durack, T.; Stevanovski, I.; van Geldermalsen, M.; Holst, J.; Jahan, Z.; Shepard, C.; Weninger, W.; Kim, B.; et al. SAMHD1 enhances immunoglobulin hypermutation by promoting transversion mutation. Proc. Natl. Acad. Sci. USA 2018, 115, 4921–4926. [Google Scholar] [CrossRef] [PubMed]
- Chabaud, S.; Lambert, H.; Sasseville, A.M.; Lavoie, H.; Guilbault, C.; Massie, B.; Landry, J.; Langelier, Y. The R1 subunit of herpes simplex virus ribonucleotide reductase has chaperone-like activity similar to Hsp27. FEBS Lett. 2003, 545, 213–218. [Google Scholar] [CrossRef]
- Cheng, A.Z.; Yockteng-Melgar, J.; Jarvis, M.C.; Malik-Soni, N.; Borozan, I.; Carpenter, M.A.; McCann, J.L.; Ebrahimi, D.; Shaban, N.M.; Marcon, E.; et al. Epstein-Barr virus BORF2 inhibits cellular APOBEC3B to preserve viral genome integrity. Nat. Microbiol. 2019, 4, 78–88. [Google Scholar] [CrossRef]
- Moraes, S.N.; Becker, J.T.; Moghadasi, S.A.; Shaban, N.M.; Auerbach, A.A.; Cheng, A.Z.; Harris, R.S. Evidence linking APOBEC3B genesis and evolution of innate immune antagonism by gamma-herpesvirus ribonucleotide reductases. eLlife 2022, 11, e83893. [Google Scholar] [CrossRef]
- Shaban, N.M.; Yan, R.; Shi, K.; Moraes, S.N.; Cheng, A.Z.; Carpenter, M.A.; McLellan, J.S.; Yu, Z.; Harris, R.S. Cryo-EM structure of the EBV ribonucleotide reductase BORF2 and mechanism of APOBEC3B inhibition. Sci. Adv. 2022, 8, eabm2827. [Google Scholar] [CrossRef]
- Yockteng-Melgar, J.; Shire, K.; Cheng, A.Z.; Malik-Soni, N.; Harris, R.S.; Frappier, L. G1/S Cell Cycle Induction by Epstein-Barr Virus BORF2 Is Mediated by P53 and APOBEC3B. J. Virol. 2022, 96, e0066022. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.Z.; Moraes, S.N.; Attarian, C.; Yockteng-Melgar, J.; Jarvis, M.C.; Biolatti, M.; Galitska, G.; Dell’Oste, V.; Frappier, L.; Bierle, C.J.; et al. A Conserved Mechanism of APOBEC3 Relocalization by Herpesviral Ribonucleotide Reductase Large Subunits. J. Virol. 2019, 93, e01539-19. [Google Scholar] [CrossRef]
- Stewart, J.A.; Holland, T.C.; Bhagwat, A.S. Human Herpes Simplex Virus-1 depletes APOBEC3A from nuclei. Virology 2019, 537, 104–109. [Google Scholar] [CrossRef]
- Fanunza, E.; Cheng, A.Z.; Auerbach, A.A.; Stefanovska, B.; Moraes, S.N.; Lokensgard, J.R.; Biolatti, M.; Dell’Oste, V.; Bierle, C.J.; Bresnahan, W.A.; et al. Human cytomegalovirus mediates APOBEC3B relocalization early during infection through a ribonucleotide reductase-independent mechanism. J. Virol. 2023, 97, e0078123. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Leng, M.; Tan, C.; Zhu, L.; Pang, Y.; Zhang, X.; Chang, Y.F.; Lin, W. Critical role for ribonucleoside-diphosphate reductase subunit M2 in ALV-J-induced activation of Wnt/beta-catenin signaling via interaction with P27. J. Virol. 2023, 97, e0026723. [Google Scholar] [CrossRef] [PubMed]
- Oo, A.; Zandi, K.; Shepard, C.; Bassit, L.C.; Musall, K.; Goh, S.L.; Cho, Y.J.; Kim, D.H.; Schinazi, R.F.; Kim, B. Elimination of Aicardi-Goutieres syndrome protein SAMHD1 activates cellular innate immunity and suppresses SARS-CoV-2 replication. J. Biol. Chem. 2022, 298, 101635. [Google Scholar] [CrossRef]
- Sze, A.; Belgnaoui, S.M.; Olagnier, D.; Lin, R.; Hiscott, J.; van Grevenynghe, J. Host restriction factor SAMHD1 limits human T cell leukemia virus type 1 infection of monocytes via STING-mediated apoptosis. Cell Host Microbe 2013, 14, 422–434. [Google Scholar] [CrossRef]
- Silva, T.; Temerozo, J.R.; do Vale, G.; Ferreira, A.C.; Soares, V.C.; Dias, S.S.G.; Sardella, G.; Bou-Habib, D.C.; Siqueira, M.; Souza, T.M.L.; et al. The Chemokine CCL5 Inhibits the Replication of Influenza A Virus Through SAMHD1 Modulation. Front. Cell. Infect. Microbiol. 2021, 11, 549020. [Google Scholar] [CrossRef]
- Zhao, Z.; Han, S.; Zhang, Q.; Wang, Y.; Yue, K.; Abbas, S.; He, H. Impaired influenza A virus replication by the host restriction factor SAMHD1 which inhibited by PA-mediated dephosphorylation of the host transcription factor IRF3. Virol. J. 2024, 21, 33. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Z.; Huan, C.; Liu, X.; Zhang, W. SAMHD1 Inhibits Multiple Enteroviruses by Interfering with the Interaction between VP1 and VP2 Proteins. J. Virol. 2021, 95, e0062021. [Google Scholar] [CrossRef]
- Choi, J.; Ryoo, J.; Oh, C.; Hwang, S.; Ahn, K. SAMHD1 specifically restricts retroviruses through its RNase activity. Retrovirology 2015, 12, 46. [Google Scholar] [CrossRef]
- Ryoo, J.; Choi, J.; Oh, C.; Kim, S.; Seo, M.; Kim, S.Y.; Seo, D.; Kim, J.; White, T.E.; Brandariz-Nunez, A.; et al. The ribonuclease activity of SAMHD1 is required for HIV-1 restriction. Nat. Med. 2014, 20, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, J.M.; St Gelais, C.; de Silva, S.; Yount, J.S.; Tang, C.; Ji, X.; Shepard, C.; Xiong, Y.; Kim, B.; Wu, L. SAMHD1-mediated HIV-1 restriction in cells does not involve ribonuclease activity. Nat. Med. 2016, 22, 1072–1074. [Google Scholar] [CrossRef]
- Seamon, K.J.; Sun, Z.; Shlyakhtenko, L.S.; Lyubchenko, Y.L.; Stivers, J.T. SAMHD1 is a single-stranded nucleic acid binding protein with no active site-associated nuclease activity. Nucleic Acids Res. 2015, 43, 6486–6499. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, M.; Li, D.; Jiang, Z.; Liu, C.; Shi, X.; Wu, C.; Chen, X.; Lin, G.; Hu, C. Identification of the SAMHD1 gene in grass carp and its roles in inducing apoptosis and inhibiting GCRV proliferation. Fish. Shellfish. Immunol. 2019, 88, 606–618. [Google Scholar] [CrossRef]
- Irwin, C.R.; Hitt, M.M.; Evans, D.H. Targeting Nucleotide Biosynthesis: A Strategy for Improving the Oncolytic Potential of DNA Viruses. Front. Oncol. 2017, 7, 229. [Google Scholar] [CrossRef]
- Woo, Y.; Warner, S.G.; Geha, R.; Stanford, M.M.; Decarolis, P.; Rahman, M.M.; Singer, S.; McFadden, G.; Fong, Y. The Oncolytic Activity of Myxoma Virus against Soft Tissue Sarcoma Is Mediated by the Overexpression of Ribonucleotide Reductase. Clin. Med. Insights Oncol. 2021, 15, 1179554921993069. [Google Scholar] [CrossRef]
- Foloppe, J.; Kempf, J.; Futin, N.; Kintz, J.; Cordier, P.; Pichon, C.; Findeli, A.; Vorburger, F.; Quemeneur, E.; Erbs, P. The Enhanced Tumor Specificity of TG6002, an Armed Oncolytic Vaccinia Virus Deleted in Two Genes Involved in Nucleotide Metabolism. Mol. Ther. Oncolytics 2019, 14, 1–14. [Google Scholar] [CrossRef]
- Potts, K.G.; Irwin, C.R.; Favis, N.A.; Pink, D.B.; Vincent, K.M.; Lewis, J.D.; Moore, R.B.; Hitt, M.M.; Evans, D.H. Deletion of F4L (ribonucleotide reductase) in vaccinia virus produces a selective oncolytic virus and promotes anti-tumor immunity with superior safety in bladder cancer models. EMBO Mol. Med. 2017, 9, 638–654. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Makvandi, M.; Abbasi, S.; Azadmanesh, K.; Teimoori, A. Developing oncolytic Herpes simplex virus type 1 through UL39 knockout by CRISPR-Cas9. Iran. J. Basic. Med. Sci. 2020, 23, 937–944. [Google Scholar] [PubMed]
- Sehrawat, R.; Rathee, P.; Khatkar, S.; Akkol, E.; Khayatkashani, M.; Nabavi, S.M.; Khatkar, A. DihydrofolateReductase (DHFR) Inhibitors: A Comprehensive Review. Curr. Med. Chem. 2024, 31, 799–824. [Google Scholar] [CrossRef] [PubMed]
- Schott, K.; Majer, C.; Bulashevska, A.; Childs, L.; Schmidt, M.H.H.; Rajalingam, K.; Munder, M.; Konig, R. SAMHD1 in cancer: Curse or cure? J. Mol. Med. 2022, 100, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.I.; Wang, P.; Wong, A.Y.L.; Petrova, B.; Persaud, R.; Soukhtehzari, S.; Lopez McDonald, M.; Hanke, D.; Christensen, J.; Iliev, P.; et al. Cycloguanil and Analogues Potently Target DHFR in Cancer Cells to Elicit Anti-Cancer Activity. Metabolites 2023, 13, 151. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wu, L.; Wang, M.; Cheng, A.; Yang, Q.; Wu, Y.; Jia, R.; Zhu, D.; Zhao, X.; Chen, S.; et al. Alpha-Herpesvirus Thymidine Kinase Genes Mediate Viral Virulence and Are Potential Therapeutic Targets. Front. Microbiol. 2019, 10, 941. [Google Scholar] [CrossRef]
- Angus, S.P.; Wheeler, L.J.; Ranmal, S.A.; Zhang, X.; Markey, M.P.; Mathews, C.K.; Knudsen, E.S. Retinoblastoma tumor suppressor targets dNTP metabolism to regulate DNA replication. J. Biol. Chem. 2002, 277, 44376–44384. [Google Scholar] [CrossRef]
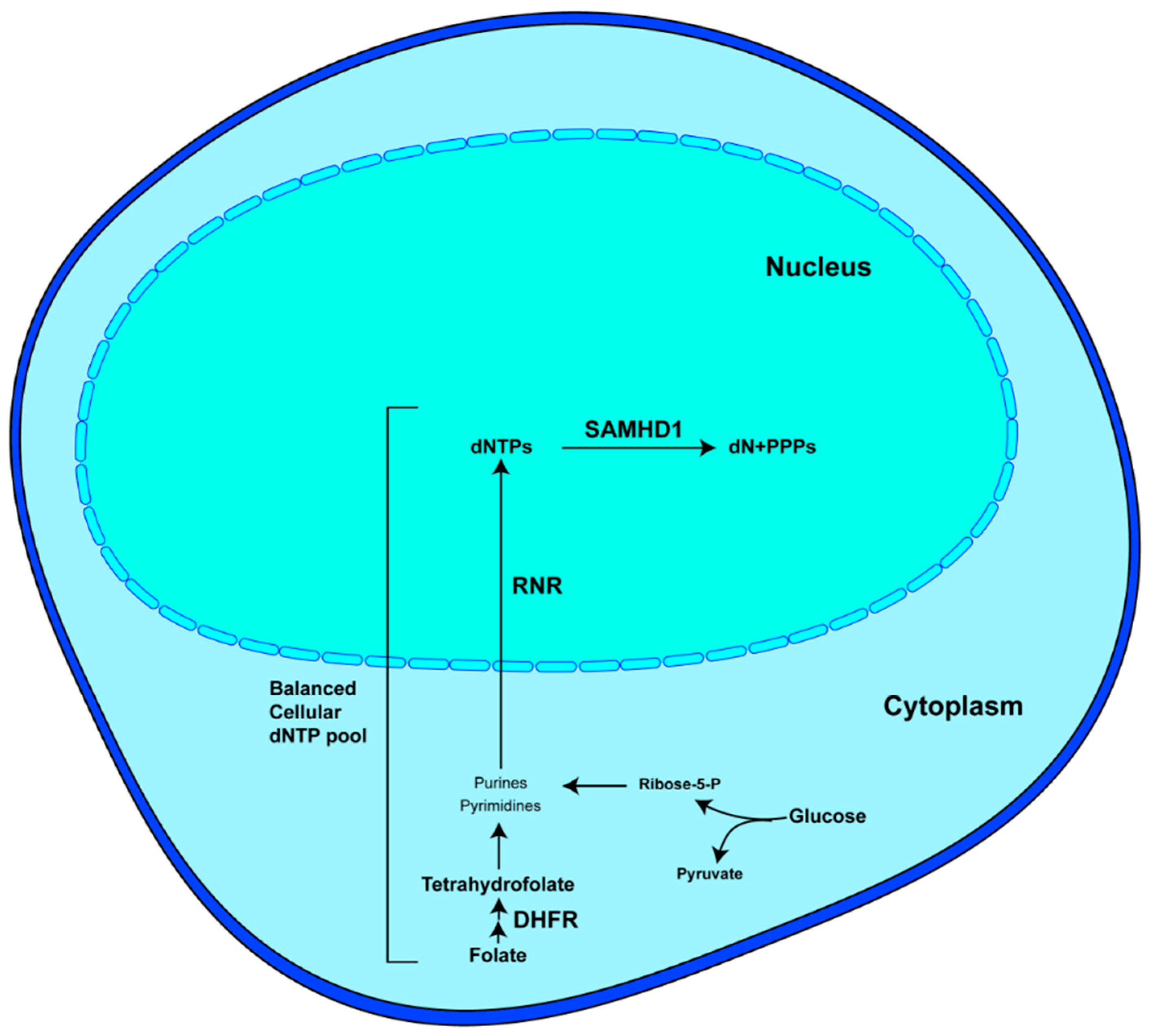
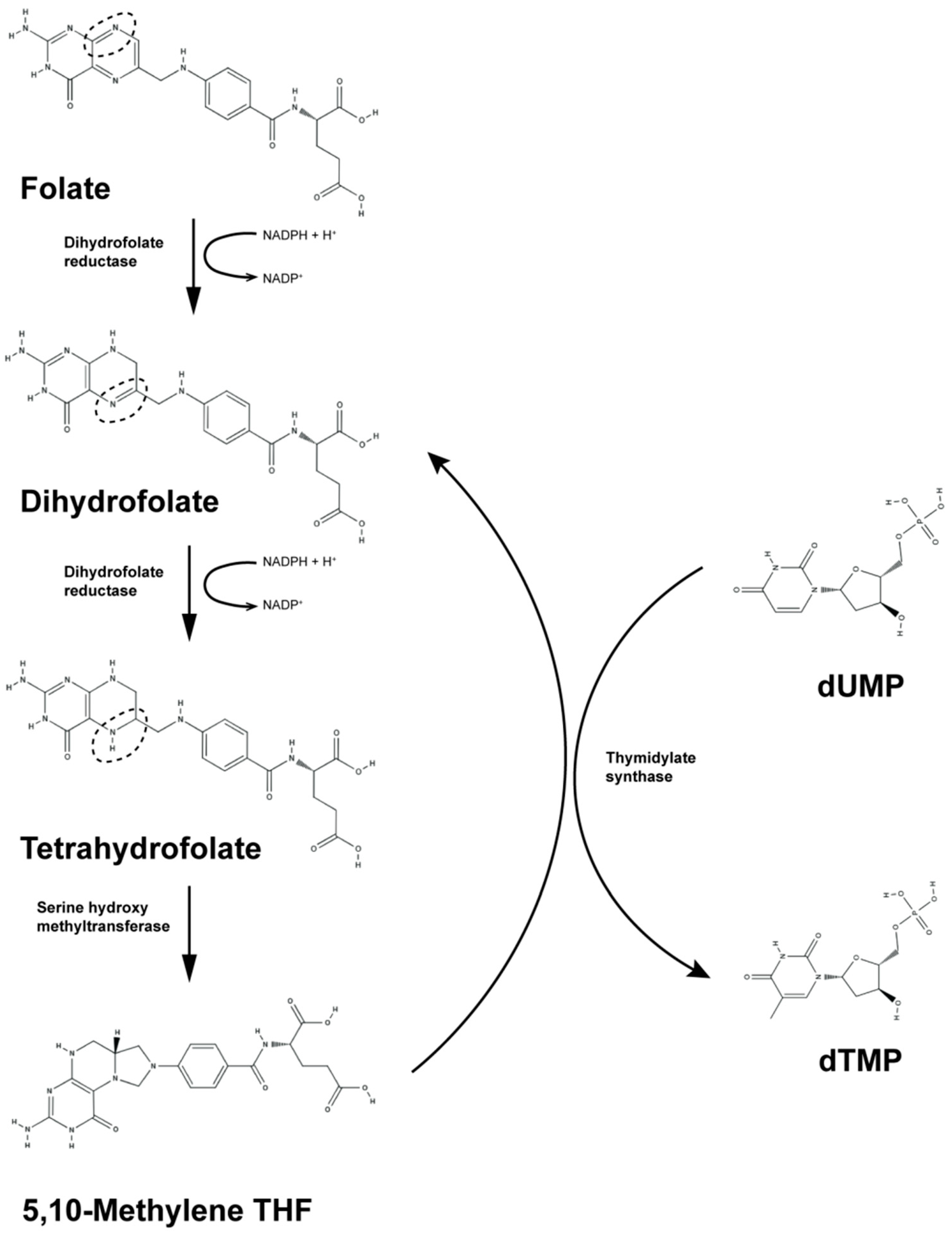
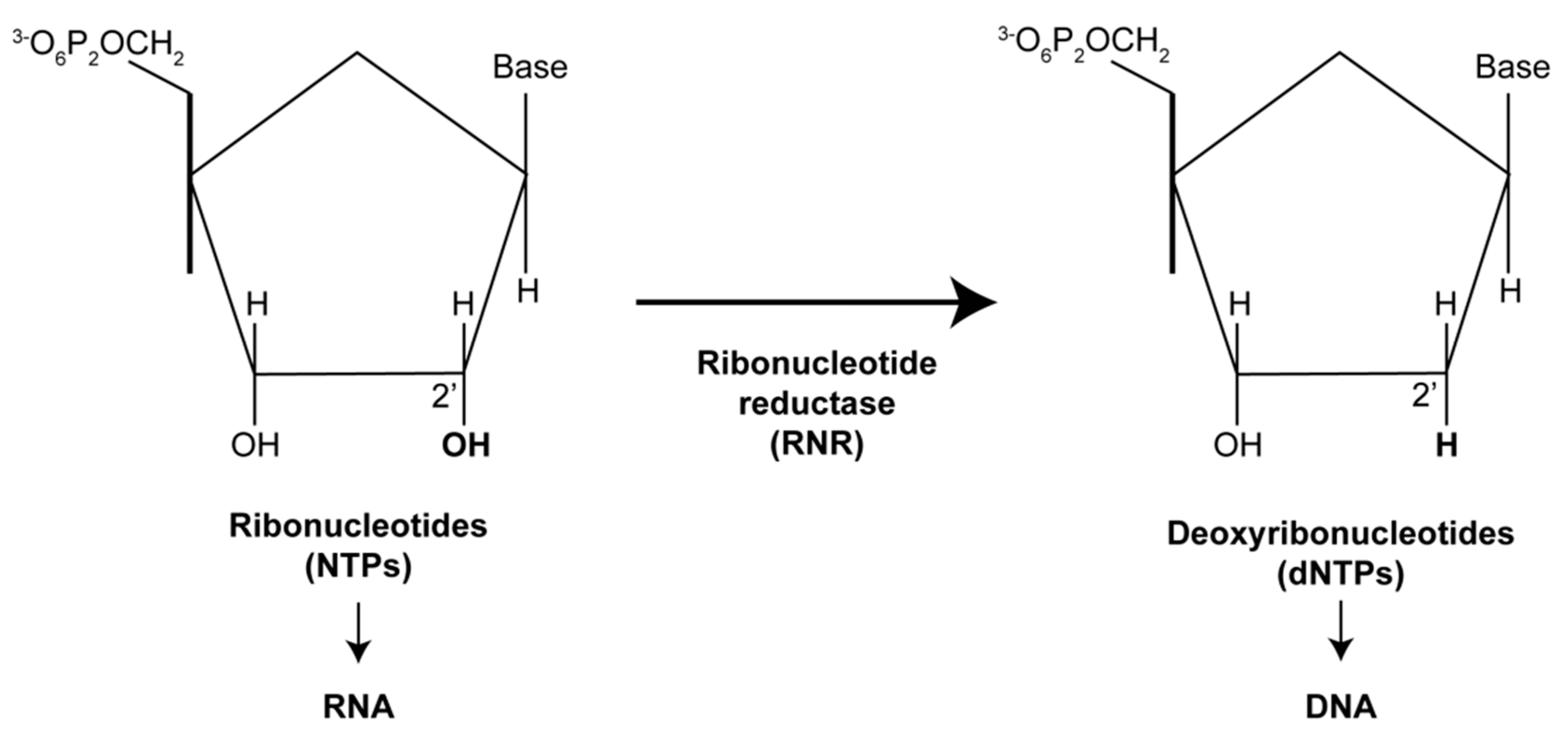
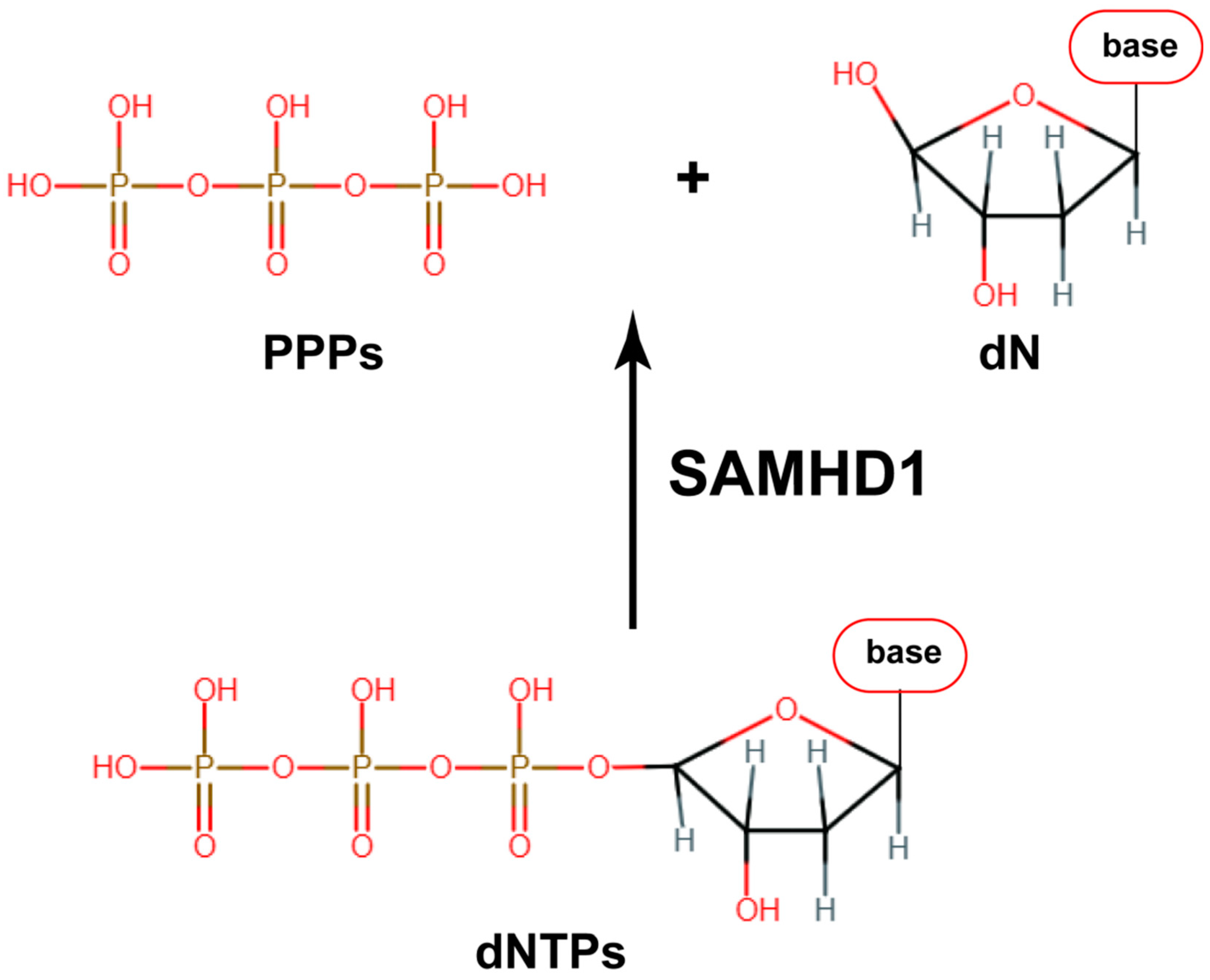
| Virus | Genes | References |
|---|---|---|
| HSV | HSVs encode viral RRM1 and RRM2. | [24,25] |
| HSVs encode a viral protein kinase to phosphorylate cellular SAMHD1 protein and inhibit its dNTPase activity. | [43,44] | |
| VZV | VZV encodes viral RRM1 and RRM2. | [32] |
| VZV encodes a viral protein kinase to phosphorylate cellular SAMHD1 protein and inhibit its dNTPase activity. | [43,44] | |
| EBV | EBV encodes viral RRM1 and RRM2. | [36,37] |
| EBV encodes a viral protein kinase to phosphorylate cellular SAMHD1 protein and inhibit its dNTPase activity. | [43,44] | |
| CMV | CMV activates the cellular DHFR gene expression. | [17,21] |
| CMV encodes a viral protein kinase to phosphorylate cellular SAMHD1 protein and inhibit its dNTPase activity; CMV could also downregulate or relocalize cellular SAMHD1. | [43,44] | |
| HHV-6/7 | HHV-6/7 encode a viral protein kinase to phosphorylate cellular SAMHD1 protein and inhibit its dNTPase activity. | [43,44] |
| HHV-8 | HHV-8 contains a viral DHFR gene. | [18] |
| HHV-8 encodes viral RRM1 and RRM2. | [36,37] | |
| HHV-8 encodes a viral protein kinase to phosphorylate cellular SAMHD1 protein and inhibit its dNTPase activity. | [43,44] | |
| Vaccinia virus (VV) | VV encodes viral RRM1 and RRM2. | [49] |
| Cellular SAMHD1 protein significantly inhibits VV infection. | [39] | |
| Adenovirus | Adenovirus activates the cellular DHFR gene expression. | [59,60] |
| HPV | HPV31 upregulates cellular RRM2 expression. | [62] |
| HPV16 replication is enhanced in the absence of cellular SAMHD1 protein. | [65] | |
| HIV | Reduction of RRM2 expression suppresses HIV-1 replication. | [68] |
| The cellular SAMHD1 protein significantly inhibits HIV-1 infection. | [15] | |
| HIV-2 and several SIV strains antagonize the cellular SAMHD1 restriction using Vpx. | [16,84,86] | |
| HBV | HBV X gene upregulates cellular RRM2 expression. | |
| Cellular SAMHD1 protein inhibits HBV infection. | [93,94,95,96] | |
| SARS-CoV-2 | The cellular DHFR activity is required for SARS-CoV-2 infection. | [106,107] |
| Influenza Viruses | The cellular DHFR activity is essential for the infections of influenza viruses | [108,109] |
| Zika Virus | The cellular DHFR activity is essential for Zika virus infections. | [110] |
| Zika virus upregulates the cellular SAMHD1 expression. | [111] | |
| HCV | The cellular RRM2 expression is upregulated by HCV infection in quiescent hepatocytes. | [112] |
| The expression of cellular RRMs was downregulated in HCV-infected Huh7.5 cells. | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, S.-Y.; Lai, M.-J.; Yang, C.-H.; Li, H.-C. Unveiling the Connection: Viral Infections and Genes in dNTP Metabolism. Viruses 2024, 16, 1412. https://doi.org/10.3390/v16091412
Lo S-Y, Lai M-J, Yang C-H, Li H-C. Unveiling the Connection: Viral Infections and Genes in dNTP Metabolism. Viruses. 2024; 16(9):1412. https://doi.org/10.3390/v16091412
Chicago/Turabian StyleLo, Shih-Yen, Meng-Jiun Lai, Chee-Hing Yang, and Hui-Chun Li. 2024. "Unveiling the Connection: Viral Infections and Genes in dNTP Metabolism" Viruses 16, no. 9: 1412. https://doi.org/10.3390/v16091412
APA StyleLo, S.-Y., Lai, M.-J., Yang, C.-H., & Li, H.-C. (2024). Unveiling the Connection: Viral Infections and Genes in dNTP Metabolism. Viruses, 16(9), 1412. https://doi.org/10.3390/v16091412





