Demonstration of Insect Vector-Mediated Transfer of a Betasatellite between Two Helper Viruses
Abstract
:1. Introduction
2. Material and Methods
2.1. Viruses and Betasatellite
2.2. Plant Material, Virus, and Betasatellite Inoculation
2.3. Transmission by the Vector Bemisia tabaci
2.4. Plant DNA Extraction
2.5. PCR Detection and Quantitative PCR
3. Results
3.1. Success of Source Plant Inoculation
3.2. Transfer of a Betasatellite between Two Helper Viruses Infecting Different Hosts
3.3. Efficient Vector Transmission of Different Virus–Betasatellite Associations
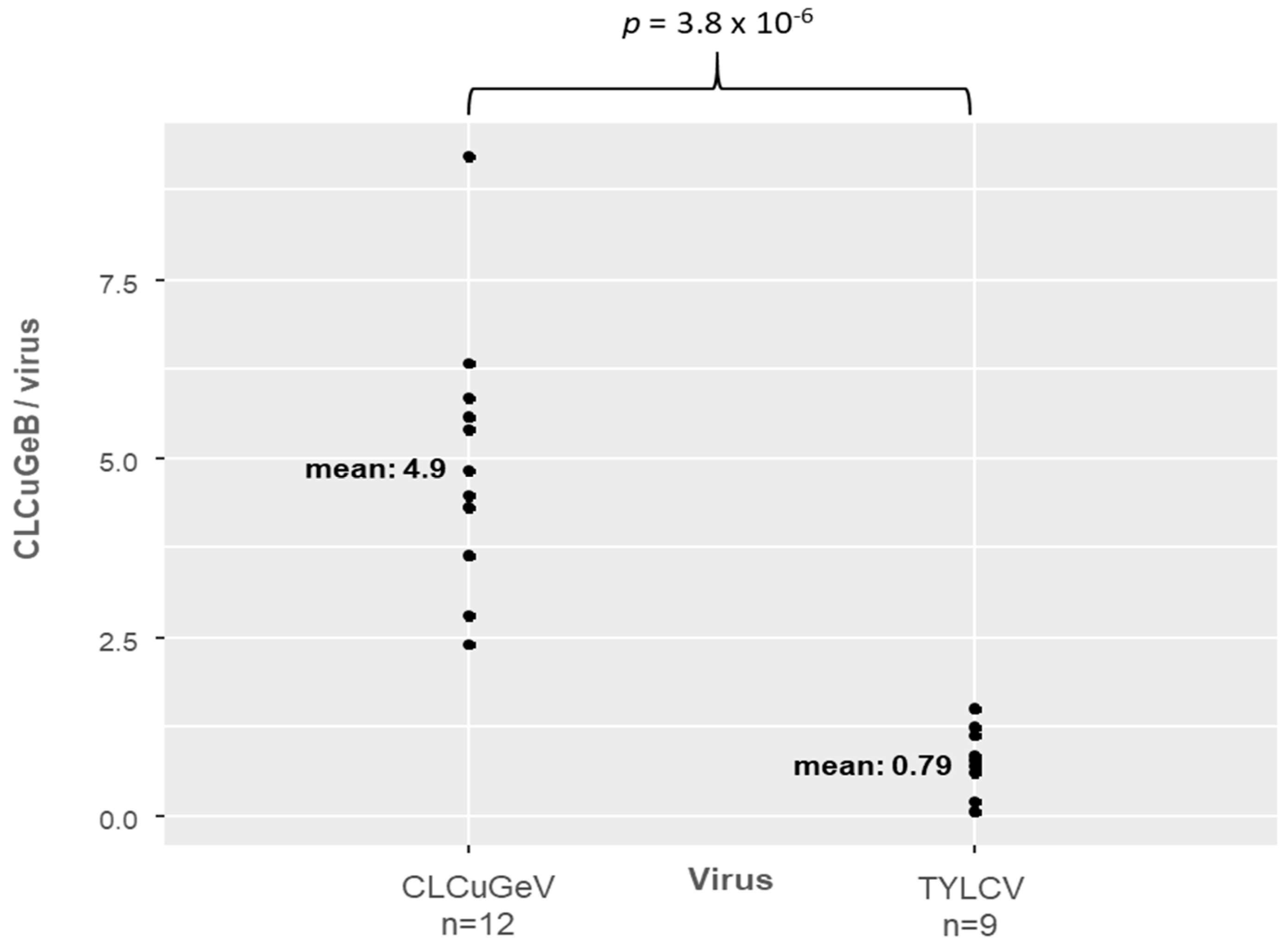
3.4. Efficient Replication of the Betasatellite by Different Helper Viruses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zerbini, F.M.; Briddon, R.W.; Idris, A.; Martin, D.P.; Moriones, E.; Navas-Castillo, J.; Rivera-Bustamante, R.; Roumagnac, P.; Varsani, A.; ICTV Report Consortium. ICTV virus taxonomy profile: Geminiviridae. J. Gen. Virol. 2017, 98, 131–133. [Google Scholar] [CrossRef]
- Ghanim, M.; Morin, S.; Czosnek, H. Rate of tomato yellow leaf curl virus translocation in the circulative transmission pathway of its vector, the whitefly Bemisia tabaci. Phytopathology 2001, 91, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.C.; Torres-Jerez, I.; Brown, J.K. Tracing the geminivirus-whitefly transmission pathway by polymerase chain reaction in whitefly extracts, saliva, hemolymph, and honeydew. Phytopathology 1999, 89, 239–246. [Google Scholar] [CrossRef]
- Zeidan, M.; Czosnek, H. Acquisition of tomato yellow leaf curl virus by the whitefly Bemisia tabaci. J. Gen. Virol. 1991, 72, 2607–2614. [Google Scholar] [CrossRef] [PubMed]
- Fiallo-Olivé, E.; Navas-Castillo, J. Begomoviruses: What is the secret(s) of their success? Trends Plant Sci. 2023, 28, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Kanakala, S.; Ghanim, M. Global genetic diversity and geographical distribution of Bemisia tabaci and its bacterial endosymbionts. PLoS ONE 2019, 14, e0213946. [Google Scholar] [CrossRef]
- Gilbertson, R.L.; Batuman, O.; Webster, C.G.; Adkins, S. Role of the insect supervectors Bemisia tabaci and Frankliniella occidentalis in the emergence and global spread of plant viruses. Annu. Rev. Virol. 2015, 2, 67–93. [Google Scholar] [CrossRef]
- Briddon, R.W.; Mansoor, S.; Bedford, I.D.; Pinner, M.S.; Saunders, K.; Stanley, J.; Zafar, Y.; Malik, K.A.; Markham, P.G. Identification of DNA components required for induction of cotton leaf curl disease. Virology 2001, 285, 234–243. [Google Scholar] [CrossRef]
- Fiallo-Olivé, E.; Tovar, R.; Navas-Castillo, J. Deciphering the biology of deltasatellites from the New World: Maintenance by New World begomoviruses and whitefly transmission. New Phytol. 2016, 212, 680–692. [Google Scholar] [CrossRef]
- Mansoor, S.; Khan, S.H.; Bashir, A.; Saeed, M.; Zafar, Y.; Malik, K.A.; Briddon, R.; Stanley, J.; Markham, P.G. Identification of a novel circular single-stranded DNA associated with cotton leaf curl disease in Pakistan. Virology 1999, 259, 190–199. [Google Scholar] [CrossRef]
- Mar, T.B.; Mendes, I.R.; Lau, D.; Fiallo-Olivé, E.; Navas-Castillo, J.; Alves, M.S.; Murilo Zerbini, F. Interaction between the New World begomovirus euphorbia yellow mosaic virus and its associated alphasatellite: Effects on infection and transmission by the whitefly Bemisia tabaci. J. Gen. Virol. 2017, 98, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Saunders, K.; Stanley, J. A nanovirus-like DNA component associated with yellow vein disease of Ageratum conyzoides: Evidence for interfamilial recombination between plant DNA Viruses. Virology 1999, 264, 142–152. [Google Scholar] [CrossRef]
- Zhou, X. Advances in understanding begomovirus satellites. Annu. Rev. Phytopathol. 2013, 51, 357–381. [Google Scholar] [CrossRef]
- Briddon, R.W.; Stanley, J. Subviral agents associated with plant single-stranded DNA viruses. Virology 2006, 344, 198–210. [Google Scholar] [CrossRef]
- Cui, X.; Tao, X.; Xie, Y.; Fauquet, C.M.; Zhou, X. A DNAβ associated with tomato yellow leaf curl China virus is required for symptom induction. J. Virol. 2004, 78, 13966–13974. [Google Scholar] [CrossRef]
- Jose, J.; Usha, R. Bhendi yellow vein mosaic disease in India is caused by association of a DNA β satellite with a begomovirus. Virology 2003, 305, 310–317. [Google Scholar] [CrossRef]
- Saunders, K.; Bedford, I.D.; Briddon, R.W.; Markham, P.G.; Wong, S.M.; Stanley, J. A unique virus complex causes ageratum yellow vein disease. Proc. Natl. Acad. Sci. USA 2000, 97, 6890–6895. [Google Scholar] [CrossRef]
- Azzam, O.; Frazer, J.; De La Rosa, D.; Beaver, J.S.; Ahlquist, P.; Maxwell, D.P. Whitefly transmission and efficient ssDNA accumulation of bean golden mosaic geminivirus require functional coat protein. Virology 1994, 204, 289–296. [Google Scholar] [CrossRef]
- Briddon, R.W.; Pinner, M.S.; Stanley, J.; Markham, P.G. Geminivirus coat protein gene replacement alters insect specificity. Virology 1990, 177, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Qazi, J.; Amin, I.; Mansoor, S.; Iqbal, M.J.; Briddon, R.W. Contribution of the satellite encoded gene βC1 to cotton leaf curl disease symptoms. Virus Res. 2007, 128, 135–139. [Google Scholar] [CrossRef]
- Saeed, M.; Behjatnia, S.A.A.; Mansoor, S.; Zafar, Y.; Hasnain, S.; Rezaian, M.A. A Single complementary-sense transcript of a geminiviral DNA β satellite is determinant of pathogenicity. Mol. Plant-Microbe Interact. 2005, 18, 7–14. [Google Scholar] [CrossRef]
- Saunders, K.; Norman, A.; Gucciardo, S.; Stanley, J. The DNA β satellite component associated with ageratum yellow vein disease encodes an essential pathogenicity protein (βC1). Virology 2004, 324, 37–47. [Google Scholar] [CrossRef]
- Cui, X.; Li, G.; Wang, D.; Hu, D.; Zhou, X. A begomovirus DNA beta-encoded protein binds DNA, functions as a suppressor of RNA silencing, and targets the cell nucleus. J. Virol. 2005, 79, 10764–10775. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yang, X.; Bisaro, D.M.; Zhou, X. The βC1 Protein of geminivirus–betasatellite complexes: A target and repressor of host defenses. Mol. Plant 2018, 11, 1424–1426. [Google Scholar] [CrossRef]
- Kumar, J.; Gunapati, S.; Singh, S.P.; Kumar, A.; Lalit, A.; Sharma, N.C.; Puranik, R.; Tuli, R. A new betasatellite associated with cotton leaf curl Burewala virus infecting tomato in India: Influence on symptoms and viral accumulation. Arch. Virol. 2013, 158, 1349–1353. [Google Scholar] [CrossRef]
- Sivalingam, P.N.; Varma, A. Role of betasatellite in the pathogenesis of a bipartite begomovirus affecting tomato in India. Arch. Virol. 2012, 157, 1081–1092. [Google Scholar] [CrossRef]
- Saunders, K.; Briddon, R.W.; Stanley, J. Replication promiscuity of DNA-β satellites associated with monopartite begomoviruses; deletion mutagenesis of the ageratum yellow vein virus DNA-β satellite localizes sequences involved in replication. J. Gen. Virol. 2008, 89, 3165–3172. [Google Scholar] [CrossRef]
- Kon, T.; Rojas, M.R.; Abdourhamane, I.K.; Gilbertson, R.L.Y. 2009. Roles and interactions of begomoviruses and satellite DNAs associated with okra leaf curl disease in Mali, West Africa. J. Gen. Virol. 2009, 90, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Nawaz-ul-Rehman, M.S.; Fauquet, C.M. Evolution of geminiviruses and their satellites. FEBS Lett. 2009, 583, 1825–1832. [Google Scholar] [CrossRef]
- Ueda, S.; Onuki, M.; Yamashita, M.; Yamato, Y. Pathogenicity and insect transmission of a begomovirus complex between tomato yellow leaf curl virus and ageratum yellow vein betasatellite. Virus Genes 2012, 44, 338–344. [Google Scholar] [CrossRef]
- Briddon, R.W.; Bull, S.E.; Amin, I.; Idris, A.M.; Mansoor, S.; Bedford, I.D.; Dhawan, P.; Rishi, N.; Siwatch, S.S.; Abdel-Salam, A.M.; et al. Diversity of DNA β, a satellite molecule associated with some monopartite begomoviruses. Virology 2003, 312, 106–121. [Google Scholar] [CrossRef]
- Idris, A.M.; Brown, J.K. Identification of a new monopartite begomovirus associated with leaf curl disease of cotton in Gezira, Sudan. Plant Dis. 2000, 84, 809. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Amin, I.; Hussain, M.; Zafar, Y.; Bull, S.; Briddon, R.W.; Markham, P.G. Association of a disease complex involving a begomovirus, DNA 1 and a distinct DNA beta with leaf curl disease of okra in Pakistan. Plant Dis. 2001, 85, 922. [Google Scholar] [CrossRef] [PubMed]
- Sohrab, S.S.; Mirza, Z.; Karim, S.; Rana, D.; Abuzenadah, A.M.; Chaudhary, A.G.; Bhattacharya, P.S. Detection of begomovirus associated with okra leaf curl disease. Arch. Phytopathol. Plant Prot. 2013, 46, 1047–1053. [Google Scholar] [CrossRef]
- Idris, A.; Al-Saleh, M.; Piatek, M.J.; Al-Shahwan, I.; Ali, S.; Brown, J.K. Viral metagenomics: Analysis of begomoviruses by Illumina High-Throughput Sequencing. Viruses 2014, 6, 1219–1236. [Google Scholar] [CrossRef] [PubMed]
- Salem, N.M.; Shahid, M.S.; Idris, A.; Hattab, D.; Abdeen, A. Complete nucleotide sequence of a begomovirus associated with an alphasatellite and a betasatellite naturally infecting okra in Jordan. Arch. Virol. 2021, 166, 2033–2036. [Google Scholar] [CrossRef]
- Idris, A.M.; Brown, J.K. Molecular analysis of cotton leaf curl virus-Sudan reveals an evolutionary history of recombination. Virus Genes 2002, 24, 249–256. [Google Scholar] [CrossRef]
- Shih, S.L.; Kumar, S.; Tsai, W.S.; Lee, L.M.; Green, S.K. Complete nucleotide sequences of okra isolates of Cotton leaf curl Gezira virus and their associated DNA-β from Niger. Arch. Virol. 2009, 154, 369–372. [Google Scholar] [CrossRef]
- Tiendrébéogo, F.; Lefeuvre, P.; Hoareau, M.; Villemot, J.; Konaté, G.; Traoré, A.S.; Barro, N.; Traoré, V.S.; Reynaud, B.; Traoré, O.; et al. Molecular diversity of Cotton leaf curl Gezira virus isolates and their satellite DNAs associated with okra leaf curl disease in Burkina Faso. Virol. J. 2010, 7, 48. [Google Scholar] [CrossRef]
- Tiendrébéogo, F.; Traoré, V.S.E.; Lett, J.-M.; Barro, N.; Konaté, G.; Traoré, A.S.; Traoré, O. Impact of okra leaf curl disease on morphology and yield of okra. Crop Prot. 2010, 29, 712–716. [Google Scholar] [CrossRef]
- Anfoka, G.; Ahmad, F.H.; Altaleb, M.; Al Shhab, M. Detection of satellite DNA beta in tomato plants with tomato yellow leaf curl disease in Jordan. Plant Dis. 2014, 98, 1017. [Google Scholar] [CrossRef]
- Gelbart, D.; Chen, L.; Alon, T.; Dobrinin, S.; Levin, I.; Lapidot, M. The recent association of a DNA betasatellite with tomato yellow leaf curl virus in Israel–A new threat to tomato production. Crop Prot. 2020, 128, 104995. [Google Scholar] [CrossRef]
- Abass, M.O.; Lahuf, A.A. First report of the satellite DNA beta associated with tomato yellow leaf curl virus–Mild on tomato in Iraq. Plant Health Prog. 2022, 23, 480–481. [Google Scholar] [CrossRef]
- Anfoka, G.; Altaleb, M.; Haj Ahmad, F.; Abu Obaida, M. Charlock mustard (Sinapis arvensis): A weed reservoir for begomoviruses and associated betasatellite in Jordan. Can. J. Plant Pathol. 2017, 39, 325–333. [Google Scholar] [CrossRef]
- Rosario, K.; Marr, C.; Varsani, A.; Kraberger, S.; Stainton, D.; Moriones, E.; Polston, J.E.; Breitbart, M. Begomovirus-associated satellite DNA diversity captured through Vector-Enabled Metagenomic (VEM) surveys using whiteflies (Aleyrodidae). Viruses 2016, 8, 36. [Google Scholar] [CrossRef]
- Kumar, J.; Kumar, J.; Singh, S.P.; Tuli, R. Association of satellites with a mastrevirus in natural infection: Complexity of wheat dwarf India virus disease. J. Virol. 2014, 88, 7093–7104. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.; Tahir, M.N.; Mustafa, R.; Kamal, H.; Khan, M.Z.; Mansoor, S.; Briddon, R.W.; Amin, I. Identification of a dicot infecting mastrevirus along with alpha- and betasatellite associated with leaf curl disease of spinach (Spinacia oleracea) in Pakistan. Virus Res. 2018, 256, 174–182. [Google Scholar] [CrossRef]
- Sangeeta; Kumar, R.V.; Yadav, B.K.; Bhatt, B.S.; Krishna, R.; Krishnan, N.; Karkute, S.G.; Kumar, S.; Singh, B.; Singh, A.K. Diverse begomovirus-betasatellite complexes cause tomato leaf curl disease in the western India. Virus Res. 2023, 328, 199079. [Google Scholar] [CrossRef] [PubMed]
- Delatte, H.; Holota, H.; Naze, F.; Peterschmitt, M.; Reynaud, B.; Lett, J.M. The presence of both recombinant and nonrecombinant strains of tomato yellow leaf curl virus on tomato in Réunion Island. Plant Pathol. 2005, 54, 262. [Google Scholar] [CrossRef]
- Belabess, Z.; Peterschmitt, M.; Granier, M.; Tahiri, A.; Blenzar, A.; Urbino, C. The non-canonical tomato yellow leaf curl virus recombinant that displaced its parental viruses in southern Morocco exhibits a high selective advantage in experimental conditions. J. Gen. Virol. 2016, 97, 3433–3445. [Google Scholar] [CrossRef]
- Conflon, D.; Granier, M.; Tiendrébéogo, F.; Gentit, P.; Peterschmitt, M.; Urbino, C. Accumulation and transmission of alphasatellite, betasatellite and tomato yellow leaf curl virus in susceptible and Ty-1-resistant tomato plants. Virus Res. 2018, 253, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.G.; Kerr, A.; Tempé, J.; Petit, A. Arginine catabolism: A new function of both octopine and nopaline Ti-plasmids of Agrobacterium. Mol. Gen. Genet. 1979, 173, 263–269. [Google Scholar] [CrossRef]
- Koncz, C.; Schell, J. The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol. Gen. Genet. 1986, 204, 383–396. [Google Scholar] [CrossRef]
- Dellaporta, S.L.; Wood, J.; Hicks, J.B. A plant DNA minipreparation: Version II. Plant Mol. Biol. Rep. 1983, 1, 19–21. [Google Scholar] [CrossRef]
- Ruijter, J.M.; Ramakers, C.; Hoogaars, W.M.H.; Karlen, Y.; Bakker, O.; van den Hoff, M.J.B.; Moorman, A.F.M. Amplification efficiency: Linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009, 37, e45. [Google Scholar] [CrossRef]
- Martin, D.P.; Biagini, P.; Lefeuvre, P.; Golden, M.; Roumagnac, P.; Varsani, A. Recombination in eukaryotic single stranded DNA viruses. Viruses 2011, 3, 1699–1738. [Google Scholar] [CrossRef]
- Ber, R.; Navot, N.; Zamir, D.; Antignus, Y.; Cohen, S.; Czosnek, H. Infection of tomato by the tomato yellow leaf curl virus: Susceptibility to infection, symptom development, and accumulation of viral DNA. Arch. Virol. 1990, 112, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Michelson, I.; Zamir, D.; Czosnek, H. Accumulation and translocation of tomato yellow leaf curl virus (TYLCV) in a Lycopersicon esculentum breeding line containing the L. chilense TYLCV tolerance gene Ty-1. Phytopathology 1994, 84, 928–933. [Google Scholar] [CrossRef]
- Bedford, I.D.; Briddon, R.W.; Brown, J.K.; Rosell, R.C.; Markham, P.G. Geminivirus transmission and biological characterisation of Bemisia tabaci (Gennadius) biotypes from different geographic regions. Ann. Appl. Biol. 1994, 125, 311–325. [Google Scholar] [CrossRef]
- Roy, B.; Chakraborty, P.; Ghosh, A. How many begomovirus copies are acquired and inoculated by its vector, whitefly (Bemisia tabaci) during feeding? PLoS ONE 2021, 16, e0258933. [Google Scholar] [CrossRef]
- Péréfarres, F.; Thébaud, G.; Lefeuvre, P.; Chiroleu, F.; Rimbaud, L.; Hoareau, M.; Reynaud, B.; Lett, J.M. Frequency-dependent assistance as a way out of competitive exclusion between two strains of an emerging virus. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133374. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Gadhave, K.R.; Buck, J.W.; Dutta, B.; Coolong, T.; Adkins, S.; Simmons, A.M.; Srinivasan, R. Effects of host plants and their infection status on acquisition and inoculation of a plant virus by its hemipteran vector. Pathogens 2023, 12, 1119. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, M.; Friedmann, M.; Pilowsky, M.; Ben-Joseph, R.; Cohen, S. Effect of host plant resistance to tomato yellow leaf curl virus (TYLCV) on virus acquisition and transmission by its whitefly vector. Phytopathology 2001, 91, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Al Shihi, A.A.; Al Sadi, A.M.; Deadman, M.; Briddon, R.W.; Shahid, M.S. Identification of a distinct strain of Cotton leaf curl Gezira virus infecting tomato in Oman. J. Phytopathol. 2018, 166, 199–205. [Google Scholar] [CrossRef]
- AlHudaib, K.A.; Almaghasla, M.I.; El-Ganainy, S.M.; Arshad, M.; Drou, N.; Sattar, M.N. High-throughput sequencing identified distinct bipartite and monopartite begomovirus variants associated with DNA-satellites from tomato and muskmelon plants in Saudi Arabia. Plants 2023, 12, 6. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Ding, T.; Chu, D. Synergistic effects of a tomato chlorosis virus and tomato yellow leaf curl virus mixed infection on host tomato plants and the whitefly vector. Front. Plant Sci. 2021, 12, 672400. [Google Scholar] [CrossRef]
- Ontiveros, I.; López-Moya, J.J.; Díaz-Pendón, J.A. Coinfection of tomato plants with tomato yellow leaf curl virus and tomato chlorosis virus affects the interaction with host and whiteflies. Phytopathology 2022, 112, 944–952. [Google Scholar] [CrossRef]
- Vo, T.; Troiano, E.; Lal, A.; Hoang, P.; Kil, E.-J.; Lee, S.; Parrella, G. ToLCNDV-ES infection in tomato is enhanced by TYLCV: Evidence from field survey and agroinoculation. Front. Microbiol. 2022, 13, 954460. [Google Scholar] [CrossRef]
- Lefeuvre, P.; Lett, J.-M.; Reynaud, B.; Martin, D.P. Avoidance of protein fold disruption in natural virus recombinants. PLoS Pathog. 2007, 3, e181. [Google Scholar] [CrossRef]
- Padidam, M.; Sawyer, S.; Fauquet, C.M. Possible emergence of new geminiviruses by frequent recombination. Virology 1999, 265, 218–225. [Google Scholar] [CrossRef]
- Voorburg, C.M.; Yan, Z.; Bergua-Vidal, M.; Wolters, A.-M.A.; Bai, Y.; Kormelink, R. Ty-1, a universal resistance gene against geminiviruses that is compromised by co-replication of a betasatellite. Mol. Plant Pathol. 2020, 21, 160–172. [Google Scholar] [CrossRef] [PubMed]

| Targeted Viral Clone (Accession No.) | Primer Name a | Primer Sequence (All in the 5′-to-3′ Direction) | Annealing Conditions | Extension Conditions | Primer Final Molarity |
|---|---|---|---|---|---|
| Tandem construct in pCAMBIA 2300 CLCuGeV (FN554531) CLCuGeB (FN55457) | M13-8436-F M13-8333-R | GTAAAACGACGGCCAG CAGGAAACAGCTATGAC | 50 °C–50 s | 69 °C 6 min for CLCuGeV 3 min for CLCuGeB | 400 nM |
| CLCuGeV (FN554531) | CLCuGeV-84-F CLCuGeV-507-R | CTGTCCAATCAGAACGCGC TACATTCGGTACATCCTCGG | 50 °C–45 s | 72 °C–30 s | 200 nM |
| CLCuGeB (FN55457) | CLCuGeB-282-F CLCuGeB-699-R | CACTTCGACTAACTCCTCCG TCGTATGAGCCTGTATGACG | 54 °C–45 s | 72 °C–30 s | 200 nM |
| TYLCV (AM409201) | TY-451-F TY-1029-R | GCCCATGTAYCGRAAGCC GGRTTAGARGCATGMGTAC | 57 °C–60 s | 72 °C–30 s | 200 nM |
| TYLCV (AM409201) | TY-451-F TY-1846-R | GCCCATGTAYCGRAAGCC TCATTGATGACGTAGACCC | 54 °C–90 s | 72 °C–60 s | 200 nM |
| TYLCV (AM409201) | TY-1431-F TY-1576-R | AAACGCCATTCTCTGCC CACAAGATAGCCAAGAAGAAACC | 60 °C–20 s | 72 °C–20 s | 300 nM |
| CLCuGeB (FN55457) | CLCuGeB-343-F CLCuGeB-424-R | AACCCATTCATTATTTC CGTTCATCATACCATA | 52 °C–30 s | 72 °C–20 s | 300 nM |
| CLCuGeV (FN554531) | CLCuGeV-238-F CLCuGeV-338-R | TACCTTCAGGCTGTTCGAG GCTTCGACATAGTTAGTACGGCGG | 62 °C–15 s | 72 °C–17 s | 600 nM |
| Tomato 25S rRNA gene (X13557) | 25SRNA-1137-F 25SRNA-1297-R | AGAACTGGCGATGCGGGATG GTTGATTCGGCAGGTGAGTTGT | 62 °C–20 s | 72 °C–10 s | 300 nM |
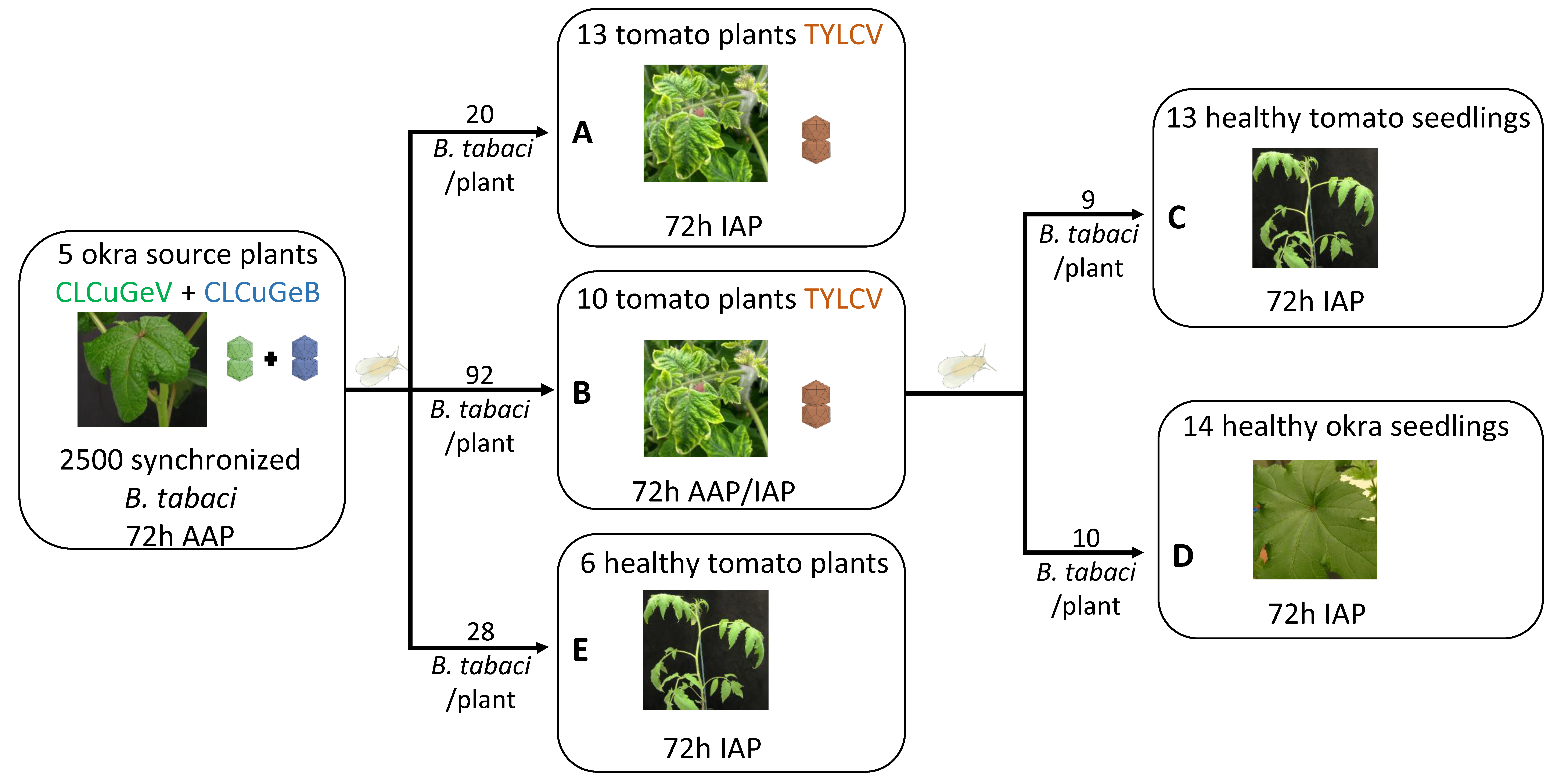
| Source Plant a | Recipient Plant a (Experiment) | Average Number of Insects/ Plant | Number of Recipient Plants Analyzed b | Infection Type | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No Infection | TYLCV | GeV | GeB | TYLCV + GeB | GeV + GeB | TYLCV + GeV | TYLCV + GeV + GeB | ||||
| Okra GeV + GeB | Tomato TYLCV (A) | 20 | 13 | 0 | 6 | 0 | 0 | 7 | 0 | 0 | 0 |
| Okra GeV + GeB | Tomato TYLCV (B) | 92 | 10 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 6 |
| Okra GeV + GeB then Tomato TYLCV | Tomato Healthy (C) | 9 | 13 | 0 | 3 | 0 | 0 | 9 | 0 | 0 | 1 |
| Okra Healthy (D) | 10 | 14 | 2 | 0 | 0 | 0 | 0 | 12 | 0 | 0 | |
| Okra GeV + GeB | Tomato Healthy (E) | 28 | 6 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fouad, N.; Granier, M.; Blanc, S.; Thébaud, G.; Urbino, C. Demonstration of Insect Vector-Mediated Transfer of a Betasatellite between Two Helper Viruses. Viruses 2024, 16, 1420. https://doi.org/10.3390/v16091420
Fouad N, Granier M, Blanc S, Thébaud G, Urbino C. Demonstration of Insect Vector-Mediated Transfer of a Betasatellite between Two Helper Viruses. Viruses. 2024; 16(9):1420. https://doi.org/10.3390/v16091420
Chicago/Turabian StyleFouad, Noun, Martine Granier, Stéphane Blanc, Gaël Thébaud, and Cica Urbino. 2024. "Demonstration of Insect Vector-Mediated Transfer of a Betasatellite between Two Helper Viruses" Viruses 16, no. 9: 1420. https://doi.org/10.3390/v16091420







