ddPCR for the Detection and Absolute Quantification of Oropouche Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Line and Virus
2.2. Viral Titration
2.3. Clinical Samples and in House PCRs
2.4. RNA Extraction
2.5. ddPCR
2.6. Analytical Sensitivity (Limit of Detection, LoD) Analysis
2.7. Statistics
3. Results
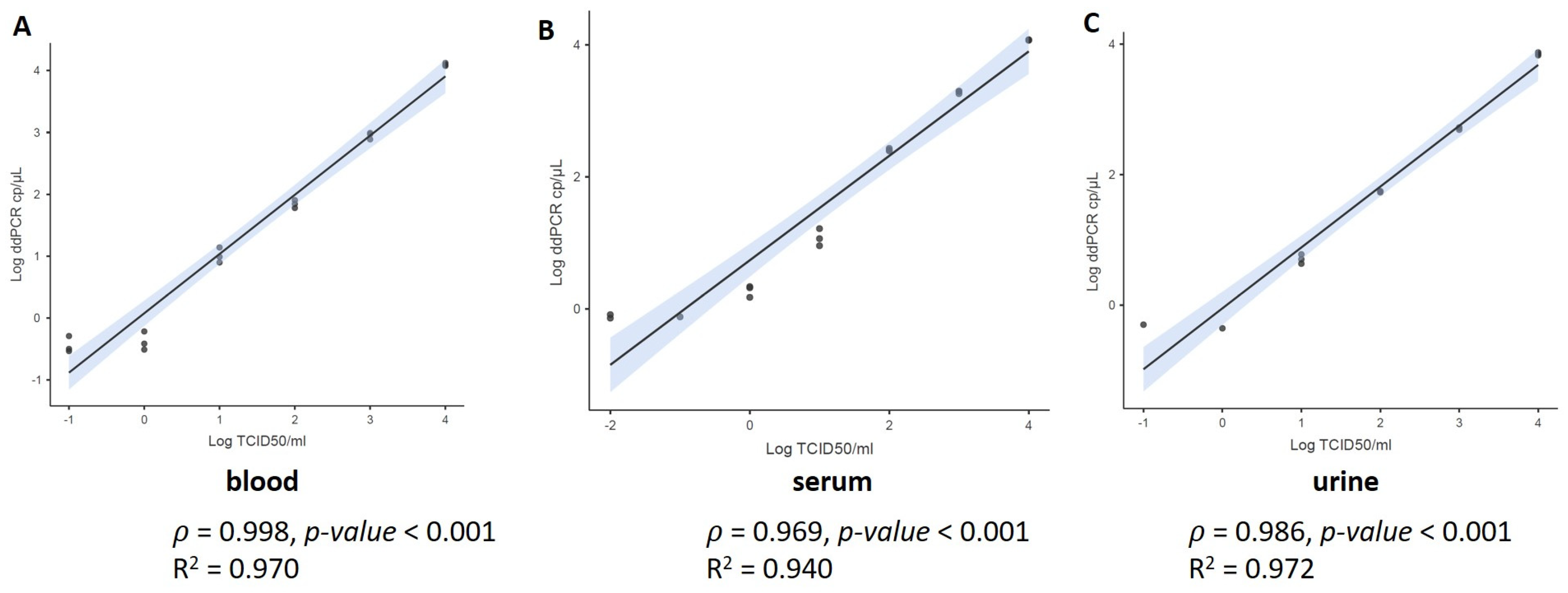
3.1. Limit of Detection Analysis (LoD)
3.2. Clinical Samples
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Da Rosa, J.F.T.; De Souza, W.M.; de Paula Pinheiro, F.; Figueiredo, M.L.; Cardoso, J.F.; Acrani, G.O.; Nunes, M.R.T. Oropouche Virus: Clinical, Epidemiological, and Molecular Aspects of a Neglected Orthobunyavirus. Am. J. Trop. Med. Hyg. 2017, 96, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- WHO. Disease Outbreak News Oropouche Virus Disease—Cuba. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON521 (accessed on 12 August 2024).
- Pan American Health Organization; World Health Organization. Public Health Risk Assessment related to Oropouche Virus (OROV) in the Region of the Americas General Risk Statement, 3 August 2024. Washington, D.C. 2024. Available online: https://www.paho.org/en/documents/public-health-risk-assessment-related-oropouche-virus-orov-region-americas-3-august-2024 (accessed on 12 August 2024).
- Fujita, D.M.; Salvador, F.S.; da Silva Nali, L.H.; de Andrade Júnior, H.F. Oropouche in Brazil in 2024. J. Travel Med. 2024, 31, taae075. [Google Scholar] [CrossRef] [PubMed]
- Situation Report No 28—Dengue Epidemiological Situation in the Region of the Americas—Epidemiological Week 28. 2024. Available online: https://www.paho.org/en/documents/situation-report-no-32-dengue-epidemiological-situation-region-americas-epidemiological (accessed on 12 August 2024).
- Castilletti, C.; Mori, A.; Matucci, A.; Ronzoni, N.; Van Duffel, L.; Rossini, G.; Sponga, P.; D’Errico, M.L.; Rodari, P.; Cristini, F.; et al. Oropouche fever cases diagnosed in Italy in two epidemiologically non-related travellers from Cuba, late May to early June 2024. Eurosurveillance 2024, 29, 2400362. [Google Scholar] [CrossRef] [PubMed]
- ECDC, Threat Assessment Brief: Oropouche Virus Disease Cases Imported to the European Union. Available online: https://www.ecdc.europa.eu/en/publications-data/threat-assessment-brief-oropouche-virus-disease-cases-imported-european-union (accessed on 12 August 2024).
- Pan American Health Organisation Epidemiological Alert—Oropouche in the Region of the Americas—2 February 2024. Available online: https://www.paho.org/en/documents/epidemiological-alert-oropouche-region-americas-2-february-2024 (accessed on 16 July 2024).
- Castilletti, C.; Mori, A.; Pomari, E.; Matucci, A.; Martelli, G.; Curiale, S.; Gobbi, F.G. First diagnoses of Oropouche virus in Europe: How can we strengthen communication and preparedness globally? Lancet Infect. Dis. 2024. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Stittleburg, V.; Cardozo, F.; Bopp, N.; Cantero, C.; López, S.; Bernal, C.; Mendoza, L.; Aguilar, P.; Pinsky, B.A.; et al. Real-time RT-PCR for the detection and quantitation of Oropouche virus. Diagn. Microbiol. Infect. Dis. 2020, 96, 114894. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.J.; Lanciotti, R.S. Consensus amplification and novel multiplex sequencing method for S segment species identification of 47 viruses of the Orthobunyavirus, Phlebovirus, and Nairovirus genera of the family Bunyaviridae. J. Clin. Microbiol. 2009, 47, 2398–2404. [Google Scholar] [CrossRef] [PubMed]
- Naveca, F.G.; do Nascimento, V.A.; de Souza, V.C.; Nunes, B.T.D.; Rodrigues, D.S.G.; da Costa Vasconcelos, P.F. Multiplexed reverse transcription real-time polymerase chain reaction for simultaneous detection of Mayaro, Oropouche, and oropouche-like viruses. Mem. Inst. Oswaldo Cruz 2017, 112, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Kojabad, A.A.; Farzanehpour, M.; Galeh, H.E.G.; Dorostkar, R.; Jafarpour, A.; Bolandian, M.; Nodooshan, M.M. Droplet digital PCR of viral DNA/RNA, current progress, challenges, and future perspectives. J. Med. Virol. 2021, 93, 4182–4197. [Google Scholar] [CrossRef] [PubMed]
- Rutsaert, S.; Bosman, K.; Trypsteen, W.; Nijhuis, M.; Vandekerckhove, L. Digital PCR as a tool to measure HIV persistence. Retrovirology 2018, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A Simple Method Of Estimating Fifty Per Cent Endpoints12. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Mori, A.; Matucci, A.; Pomari, E.; Accordini, S.; Piubelli, C.; Donini, A.; Nicolini, L.; Castilletti, C. Urine: A Pitfall for Molecular Detection of Toscana Virus? An Analytical Proof-of-Concept Study. Viruses 2024, 16, 98. [Google Scholar] [CrossRef] [PubMed]
- Biel, S.S.; Held, T.K.; Landt, O.; Niedrig, M.; Gelderblom, H.R.; Siegert, W.; Nitsche, A. Rapid quantification and differentiation of human polyomavirus DNA in undiluted urine from patients after bone marrow transplantation. J. Clin. Microbiol. 2000, 38, 3689–3695. [Google Scholar] [CrossRef] [PubMed]
- BioRad, Droplet Digital PCR Applications Guide. Available online: https://www.bio-rad.com/webroot/web/pdf/lsr/literature/Bulletin_6407.pdf (accessed on 12 July 2024).
- Huggett, J.F. The Digital MIQE Guidelines Update: Minimum Information for Publication of Quantitative Digital PCR Experiments for 2020. Clin. Chem. 2020, 66, 1012–1029. [Google Scholar] [PubMed]
- do Nascimento, V.A.; Santos, J.H.A.; Monteiro, D.C.d.S.; Pessoa, K.P.; Cardoso, A.J.L.; de Souza, V.C.; Abdalla, L.F.; Naveca, F.G. Oropouche virus detection in saliva and urine. Mem. Inst. Oswaldo Cruz 2020, 115, e190338. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, L.M.D.S.; Carvalho, R.H.; Bandeira, A.C.; Sardi, S.I.; Campos, G.S. Oropouche Virus Detection in Febrile Patients’ Saliva and Urine Samples in Salvador, Bahia, Brazil. Jpn. J. Infect. Dis. 2020, 73, 164–165. [Google Scholar] [CrossRef] [PubMed]
- de Kock, R.; Baselmans, M.; Scharnhorst, V.; Deiman, B. Sensitive detection and quantification of SARS-CoV-2 by multiplex droplet digital RT-PCR. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 807–813. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.F.; Gianella, S.; Letendre, S.; Scheffler, K.; Kosakovsky Pond, S.L.; Smith, D.M.; Strain, M.; Ellis, R.J. Comparative Analysis of Cell-Associated HIV DNA Levels in Cerebrospinal Fluid and Peripheral Blood by Droplet Digital PCR. PLoS ONE 2015, 10, e0139510. [Google Scholar] [CrossRef] [PubMed]
- Pomari, E.; Mori, A.; Accordini, S.; Donini, A.; Cordioli, M.; Tacconelli, E.; Castilletti, C. Evaluation of a ddPCR Commercial Assay for the Absolute Quantification of the Monkeypox Virus West Africa in Clinical Samples. Diagnostics 2023, 13, 1349. [Google Scholar] [CrossRef] [PubMed]
| In House rt-PCR (Reference) | |||||
|---|---|---|---|---|---|
| Specimen | ddPCR | Positive | Negative | SE [95% CI] | SP [95% CI] |
| Whole blood | Positive | 7 | 1 | 100% [59.04 to 100] | 50% [1.26 to 98.74] |
| Negative | 0 | 1 | |||
| Serum | Positive | 5 | 0 | 100% [47.82 to 100.00] | 100% [54.07 to 100.00] |
| Negative | 0 | 6 | |||
| Urine | Positive | 3 | 1 | 75% [19.41 to 99.37] | 75% [19.41 to 99.37] |
| Negative | 1 | 3 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pomari, E.; Matucci, A.; Accordini, S.; Mantovani, R.P.; Gianesini, N.; Mori, A.; Castilletti, C. ddPCR for the Detection and Absolute Quantification of Oropouche Virus. Viruses 2024, 16, 1426. https://doi.org/10.3390/v16091426
Pomari E, Matucci A, Accordini S, Mantovani RP, Gianesini N, Mori A, Castilletti C. ddPCR for the Detection and Absolute Quantification of Oropouche Virus. Viruses. 2024; 16(9):1426. https://doi.org/10.3390/v16091426
Chicago/Turabian StylePomari, Elena, Andrea Matucci, Silvia Accordini, Rebeca Passarelli Mantovani, Natasha Gianesini, Antonio Mori, and Concetta Castilletti. 2024. "ddPCR for the Detection and Absolute Quantification of Oropouche Virus" Viruses 16, no. 9: 1426. https://doi.org/10.3390/v16091426







