Evaluation of Different Formulations on the Viability of Phages for Use in Agriculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria, Phages and Cultures
2.2. Viability of Phages in Different Excipients
2.3. Viability of Phages under Different Environmental Conditions and Protective Effect of Different Excipients
2.4. Viability of Phages on Kiwi Plants under Field Conditions
2.5. Evaluation of Different Storage Conditions
2.6. Effectiveness of Phages with Different Excipients in Kiwifruit Leaf Discs
2.7. Artificial Inoculation of Phages and Detection inside of Kiwi Plants
2.8. Statistical Analysis
3. Results
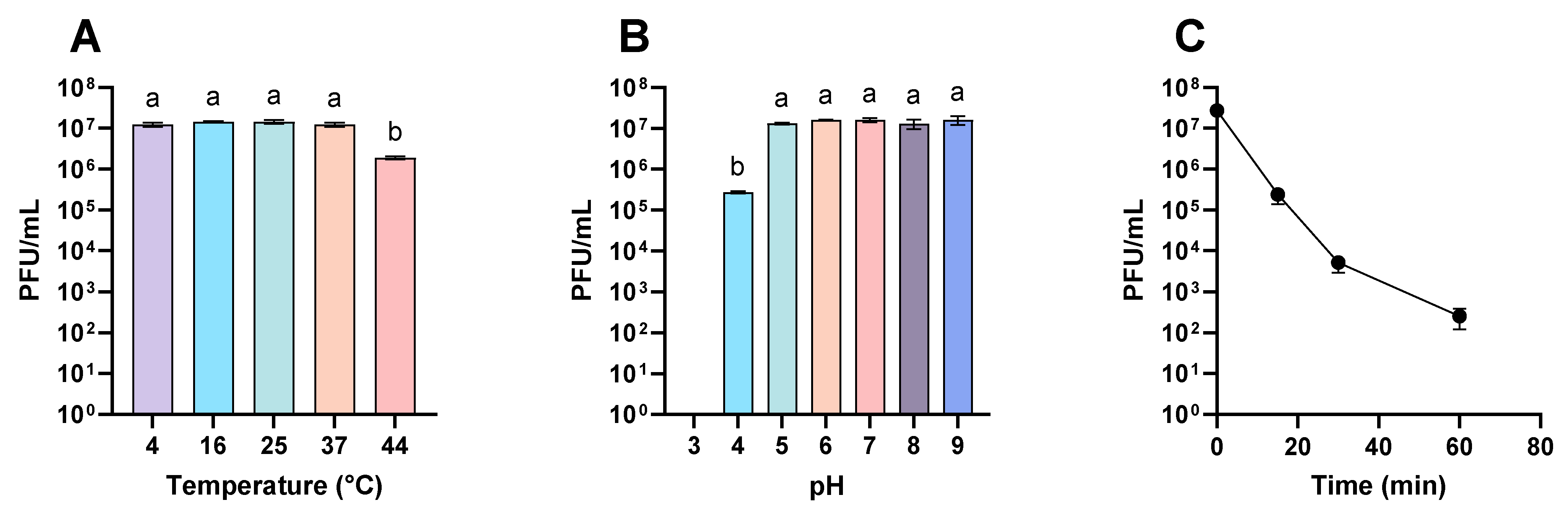
3.1. Viability of Phages under Different Environmental Conditions
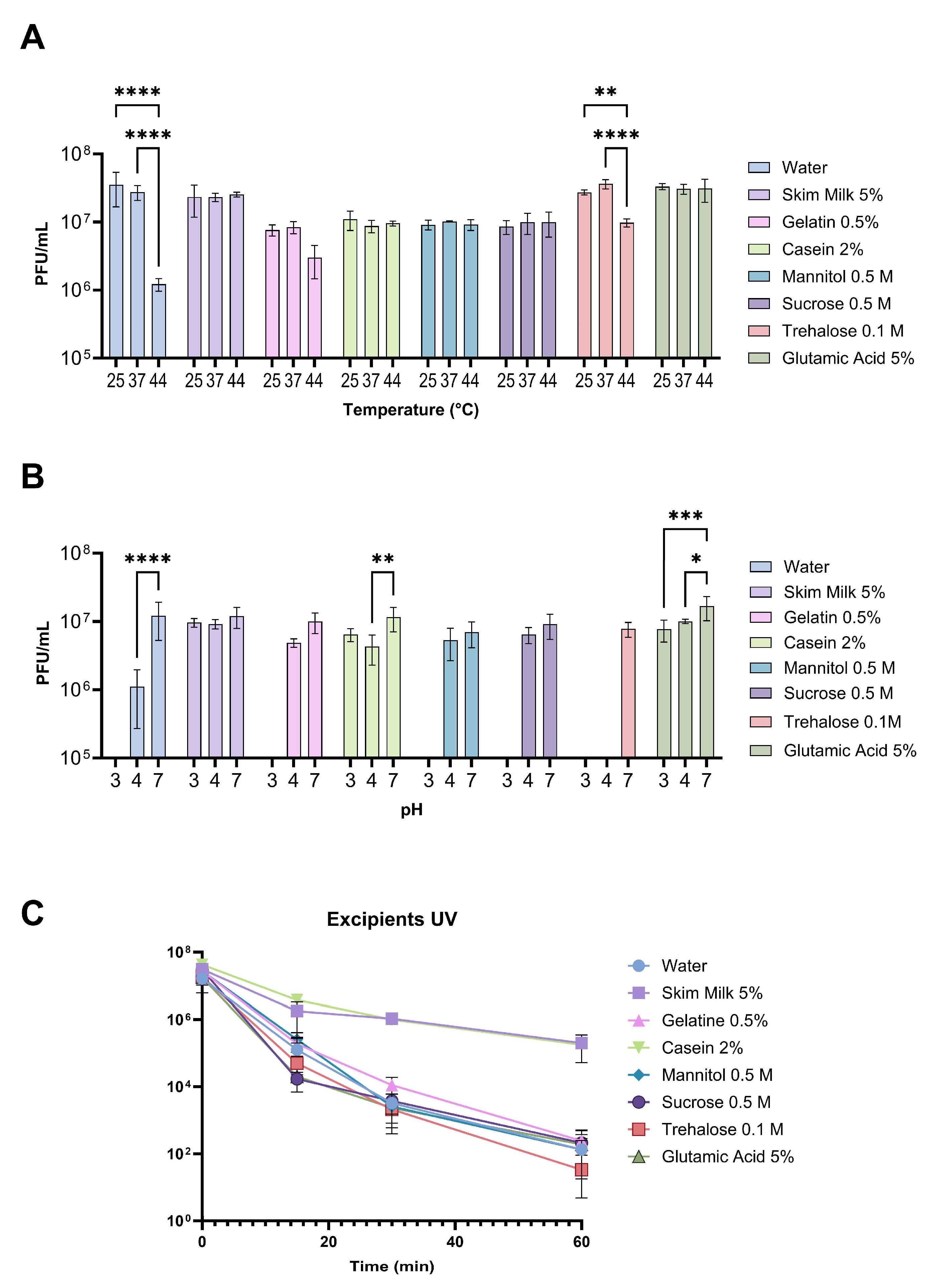
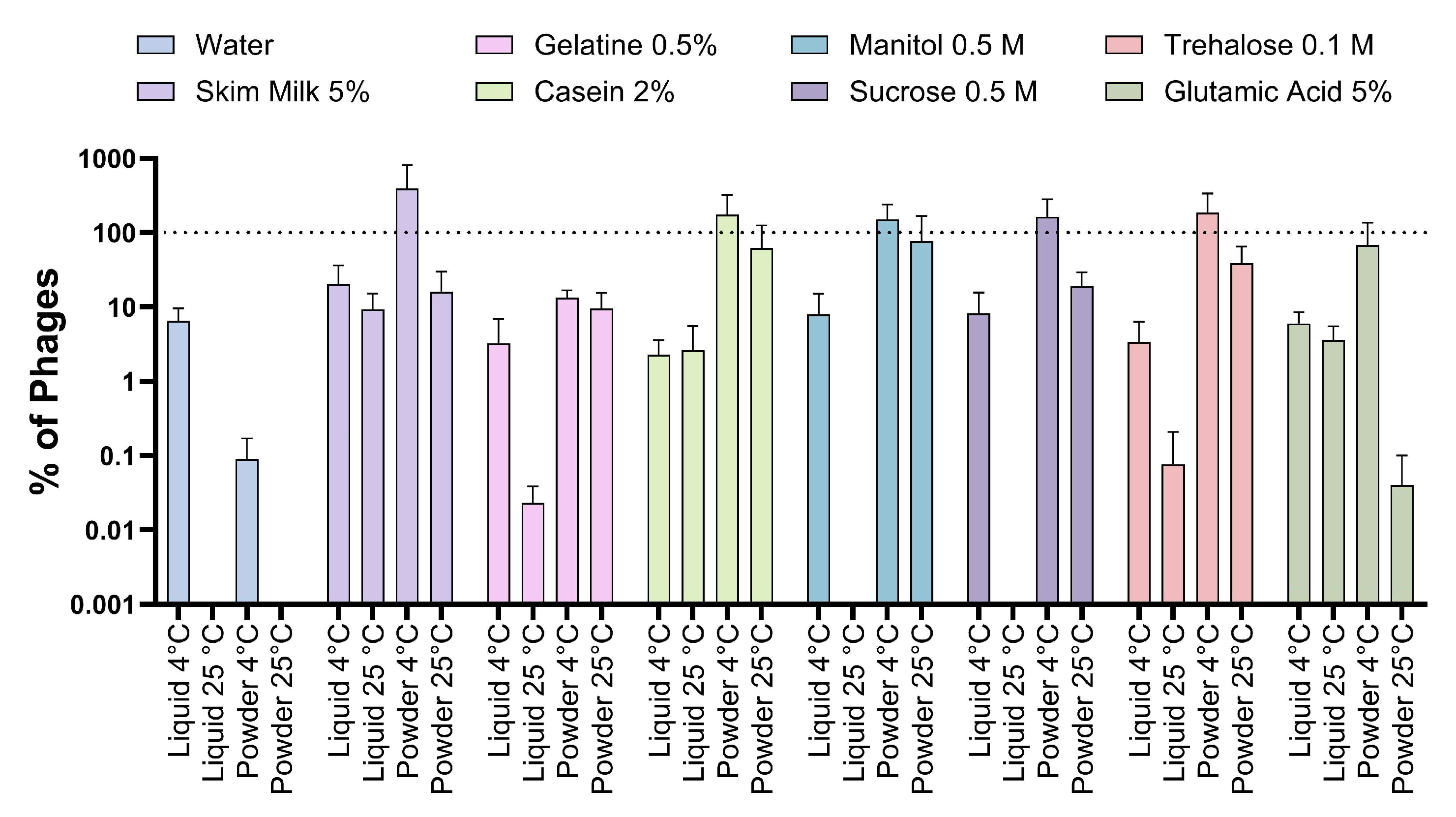
3.2. Viability of Phages Suspended with Different Excipients under Different Environmental Conditions
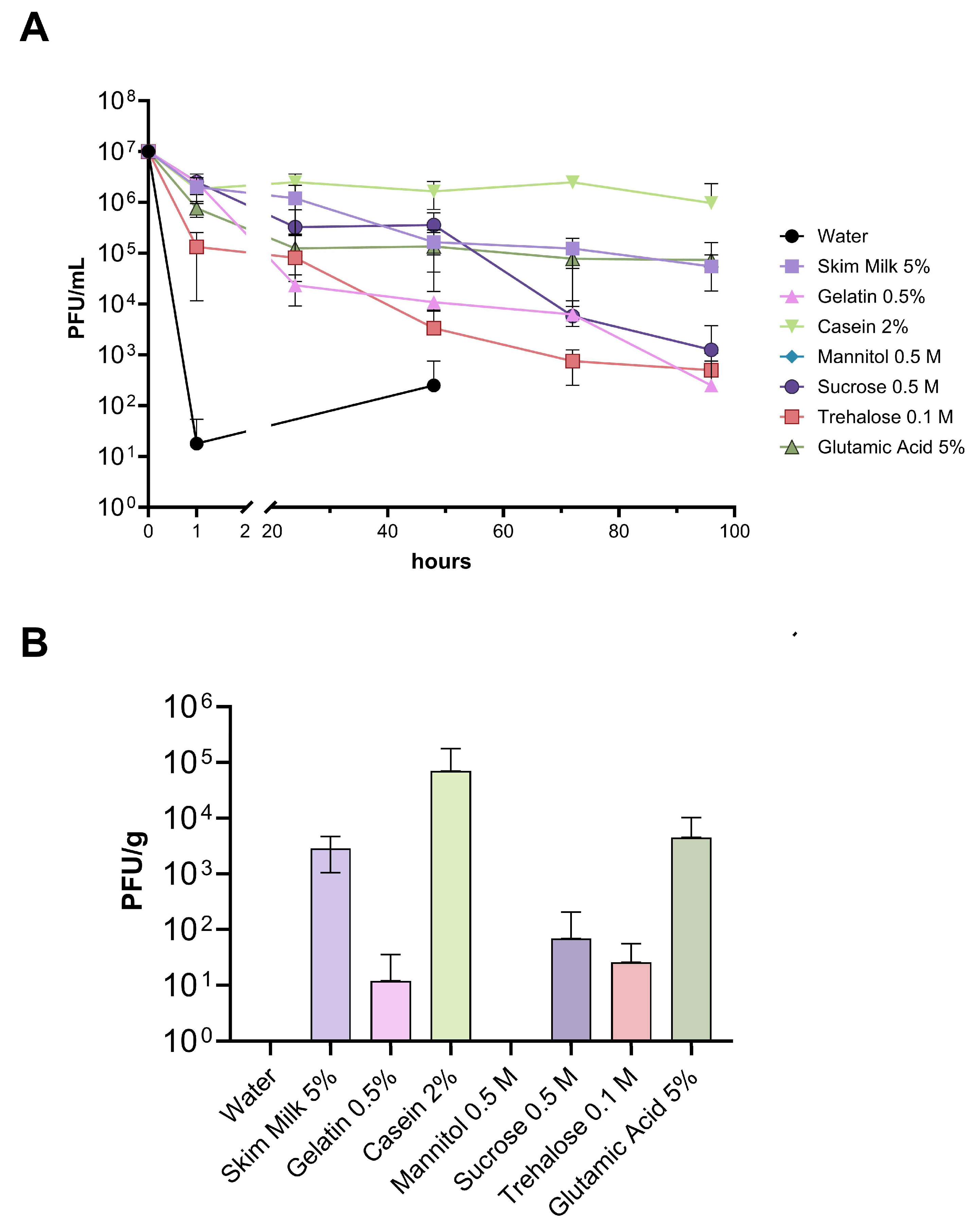
3.3. Viability of Phages on Kiwi Plant Surface under Field Conditions
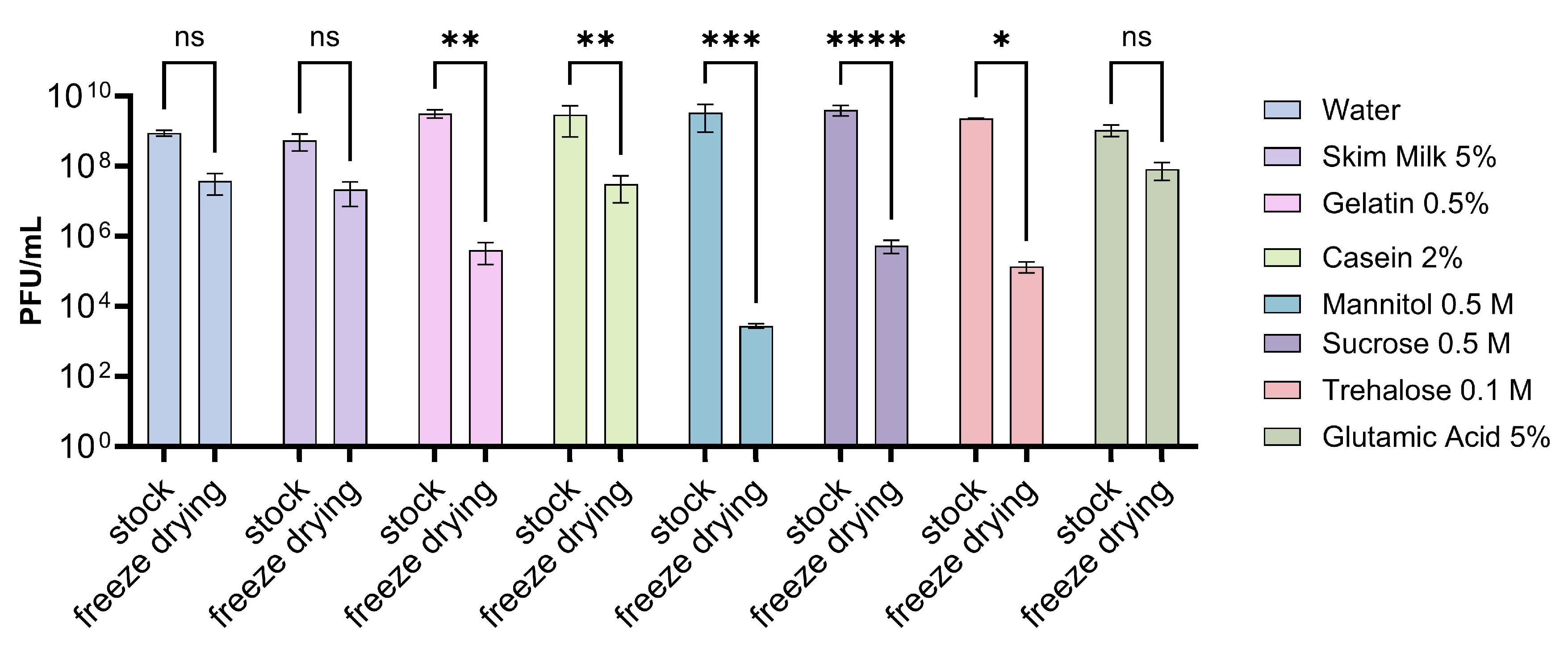
3.4. Evaluation of Different Storage Conditions
3.5. Effectiveness of Phages with Different Excipients to Control Psa and Persistence in Kiwi Plants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pirnay, J.-P.; Ferry, T.; Resch, G. Recent Progress toward the Implementation of Phage Therapy in Western Medicine. FEMS Microbiol. Rev. 2022, 46, fuab040. [Google Scholar] [CrossRef] [PubMed]
- Moye, Z.; Woolston, J.; Sulakvelidze, A. Bacteriophage Applications for Food Production and Processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Nokhwal, A.; Anand, T.; Ravikant; Vaid, R.K. Bacteriophage Therapy: An Emerging Paradigm in Fish Disease Management. Aquac. Int. 2023, 31, 777–805. [Google Scholar] [CrossRef]
- Buttimer, C.; McAuliffe, O.; Ross, R.P.; Hill, C.; O’Mahony, J.; Coffey, A. Bacteriophages and Bacterial Plant Diseases. Front. Microbiol. 2017, 8, 34. [Google Scholar] [CrossRef]
- Svircev, A.; Roach, D.; Castle, A. Framing the Future with Bacteriophages in Agriculture. Viruses 2018, 10, 218. [Google Scholar] [CrossRef]
- Sieiro, C.; Areal-Hermida, L.; Pichardo-Gallardo, Á.; Almuiña-González, R.; De Miguel, T.; Sánchez, S.; Sánchez-Pérez, Á.; Villa, T.G. A Hundred Years of Bacteriophages: Can Phages Replace Antibiotics in Agriculture and Aquaculture? Antibiotics 2020, 9, 493. [Google Scholar] [CrossRef] [PubMed]
- Green, S.I.; Clark, J.R.; Santos, H.H.; Weesner, K.E.; Salazar, K.C.; Aslam, S.; Campbell, J.W.; Doernberg, S.B.; Blodget, E.; Morris, M.I.; et al. A Retrospective, Observational Study of 12 Cases of Expanded-Access Customized Phage Therapy: Production, Characteristics, and Clinical Outcomes. Clin. Infect. Dis. 2023, 77, 1079–1091. [Google Scholar] [CrossRef]
- Bumunang, E.W.; Zaheer, R.; Niu, D.; Narvaez-Bravo, C.; Alexander, T.; McAllister, T.A.; Stanford, K. Bacteriophages for the Targeted Control of Foodborne Pathogens. Foods 2023, 12, 2734. [Google Scholar] [CrossRef]
- Córdova, P.; Rivera-González, J.P.; Rojas-Martínez, V.; Fiore, N.; Bastías, R.; Zamorano, A.; Vera, F.; Barrueto, J.; Díaz, B.; Ilabaca-Díaz, C.; et al. Phytopathogenic Pseudomonas Syringae as a Threat to Agriculture: Perspectives of a Promising Biological Control Using Bacteriophages and Microorganisms. Horticulturae 2023, 9, 712. [Google Scholar] [CrossRef]
- Halawa, E.M. Challenges of Bacteriophages Application in Controlling Bacterial Plant Diseases and How to Overcome Them. J. Genet. Eng. Biotechnol. 2023, 21, 98. [Google Scholar] [CrossRef]
- Korniienko, N.; Kharina, A.; Budzanivska, I.; Burketová, L.; Kalachova, T. Phages of Phytopathogenic Bacteria: High Potential, but Challenging Application. Plant Prot. Sci. 2022, 58, 81–91. [Google Scholar] [CrossRef]
- Lomelí-Ortega, C.O.; Balcázar, J.L.; Quiroz-Guzmán, E. Phage Therapy and Aquaculture: Progress and Challenges. Int. Microbiol. 2023, 26, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Fabijan, A.P.; Iredell, J.; Danis-Wlodarczyk, K.; Kebriaei, R.; Abedon, S.T. Translating Phage Therapy into the Clinic: Recent Accomplishments but Continuing Challenges. PLoS Biol. 2023, 21, e3002119. [Google Scholar] [CrossRef]
- Álvarez, B.; Gadea-Pallás, L.; Rodríguez, A.; Vicedo, B.; Figàs-Segura, À.; Biosca, E.G. Viability, Stability and Biocontrol Activity in Planta of Specific Ralstonia solanacearum Bacteriophages after Their Conservation Prior to Commercialization and Use. Viruses 2022, 14, 183. [Google Scholar] [CrossRef]
- Umrao, P.D.; Kumar, V.; Kaistha, S.D. Biocontrol Potential of Bacteriophage ɸsp1 against Bacterial Wilt-Causing Ralstonia solanacearum in Solanaceae Crops. Egypt. J. Biol. Pest Control. 2021, 31, 61. [Google Scholar] [CrossRef]
- Huang, B.; Ge, L.; Xiang, D.; Tan, G.; Liu, L.; Yang, L.; Jing, Y.; Liu, Q.; Chen, W.; Li, Y.; et al. Isolation, Characterization, and Genomic Analysis of a Lytic Bacteriophage, PQ43W, with the Potential of Controlling Bacterial Wilt. Front. Microbiol. 2024, 15, 1396213. [Google Scholar] [CrossRef]
- Yang, K.; Wang, X.; Hou, R.; Lu, C.; Fan, Z.; Li, J.; Wang, S.; Xu, Y.; Shen, Q.; Friman, V.-P.; et al. Rhizosphere Phage Communities Drive Soil Suppressiveness to Bacterial Wilt Disease. Microbiome 2023, 11, 16. [Google Scholar] [CrossRef]
- Retamales, J.; Núñez, P.; Alvarado, R.; Campan, E.D.M.; Otto, T.; Segovia, C.; Vasquez, I.; Santander, J. Characterization of Xanthomonas arboricola Pv. Juglandis Bacteriophages against Bacterial Walnut Blight and Field Evaluation. Viruses 2022, 14, 1380. [Google Scholar] [CrossRef]
- Kizheva, Y.; Urshev, Z.; Dimitrova, M.; Bogatzevska, N.; Moncheva, P.; Hristova, P. Phenotypic and Genotypic Characterization of Newly Isolated Xanthomonas euvesicatoria-Specific Bacteriophages and Evaluation of Their Biocontrol Potential. Plants 2023, 12, 947. [Google Scholar] [CrossRef]
- Jain, L.; Kumar, V.; Jain, S.K.; Kaushal, P.; Ghosh, P.K. Isolation of Bacteriophages Infecting Xanthomonas Oryzae Pv. Oryzae and Genomic Characterization of Novel Phage vB_XooS_NR08 for Biocontrol of Bacterial Leaf Blight of Rice. Front. Microbiol. 2023, 14, 1084025. [Google Scholar] [CrossRef]
- Stonier, T.; McSharry, J.; Speitel, T. Agrobacterium tumefaciens Conn. IV. Bacteriophage PB21 and Its Inhibitory Effect on Tumor Induction. J. Virol. 1967, 1, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Rabiey, M.; Roy, S.R.; Holtappels, D.; Franceschetti, L.; Quilty, B.J.; Creeth, R.; Sundin, G.W.; Wagemans, J.; Lavigne, R.; Jackson, R.W. Phage Biocontrol to Combat Pseudomonas syringae Pathogens Causing Disease in Cherry. Microb. Biotechnol. 2020, 13, 1428–1445. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, S.; Volckaert, A.; Venneman, S.; Declercq, B.; Vandenheuvel, D.; Allonsius, C.N.; Van Malderghem, C.; Jang, H.B.; Briers, Y.; Noben, J.P.; et al. Characterization of Novel Bacteriophages for Biocontrol of Bacterial Blight in Leek Caused by Pseudomonas syringae Pv. Porri. Front. Microbiol. 2016, 7, 279. [Google Scholar] [CrossRef]
- Flores, O.; Retamales, J.; Núñez, M.; León, M.; Salinas, P.; Besoain, X.; Yañez, C.; Bastías, R. Characterization of Bacteriophages against Pseudomonas syringae Pv. Actinidiae with Potential Use as Natural Antimicrobials in Kiwifruit Plants. Microorganisms 2020, 8, 974. [Google Scholar] [CrossRef]
- Jones, J.B.; Vallad, G.E.; Iriarte, F.B.; Obradović, A.; Wernsing, M.H.; Jackson, L.E.; Balogh, B.; Hong, J.C.; Momol, M.T. Considerations for Using Bacteriophages for Plant Disease Control. Bacteriophage 2012, 2, e23857. [Google Scholar] [CrossRef]
- Liu, S.; Quek, S.-Y.; Huang, K. Advanced Strategies to Overcome the Challenges of Bacteriophage-Based Antimicrobial Treatments in Food and Agricultural Systems. Crit. Rev. Food Sci. Nutr. 2023, 0, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Frampton, R.A.; Pitman, A.R.; Fineran, P.C. Advances in Bacteriophage-Mediated Control of Plant Pathogens. Int. J. Microbiol. 2012, 2012, 326452. [Google Scholar] [CrossRef]
- Qin, C.; Tao, J.; Liu, T.; Liu, Y.; Xiao, N.; Li, T.; Gu, Y.; Yin, H.; Meng, D. Responses of Phyllosphere Microbiota and Plant Health to Application of Two Different Biocontrol Agents. AMB Express 2019, 9, 42. [Google Scholar] [CrossRef]
- Jo, S.J.; Kim, S.G.; Park, J.; Lee, Y.M.; Giri, S.S.; Lee, S.B.; Jung, W.J.; Hwang, M.H.; Park, J.H.; Roh, E.; et al. Optimizing the Formulation of Erwinia Bacteriophages for Improved UV Stability and Adsorption on Apple Leaves. Heliyon 2023, 9, e22034. [Google Scholar] [CrossRef]
- Iriarte, F.B.; Balogh, B.; Momol, M.T.; Smith, L.M.; Wilson, M.; Jones, J.B. Factors Affecting Survival of Bacteriophage on Tomato Leaf Surfaces. Appl. Environ. Microbiol. 2007, 73, 1704–1711. [Google Scholar] [CrossRef]
- Hadapad, A.B.; Hire, R.S.; Vijayalakshmi, N.; Dongre, T.K. UV Protectants for the Biopesticide Based on Bacillus sphaericus Neide and Their Role in Protecting the Binary Toxins from UV Radiation. J. Invertebr. Pathol. 2009, 100, 147–152. [Google Scholar] [CrossRef]
- Skliros, D.; Papazoglou, P.; Gkizi, D.; Paraskevopoulou, E.; Katharios, P.; Goumas, D.E.; Tjamos, S.; Flemetakis, E. In Planta Interactions of a Novel Bacteriophage against Pseudomonas syringae pv. tomato. Appl. Microbiol. Biotechnol. 2023, 107, 3801–3815. [Google Scholar] [CrossRef] [PubMed]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, Stabilisation and Encapsulation of Bacteriophage for Phage Therapy. Adv. Colloid Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef]
- Balogh, B.; Jones, J.; Iriarte, F.; Momol, M. Phage Therapy for Plant Disease Control. CPB 2010, 11, 48–57. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Ghosh, D. The Stabilizing Excipients in Dry State Therapeutic Phage Formulations. AAPS PharmSciTech 2020, 21, 133. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Chia, S.L.; Tan, G.H. Screening and Formulation of Novel Carriers for Xanthomonas Bacteriophage to Control Bacterial Leaf Blight Disease. MJM 2022, 18, 490–504. [Google Scholar] [CrossRef]
- Balogh, B.; Jones, J.B.; Momol, M.T.; Olson, S.M.; Obradovic, A.; King, P.; Jackson, L.E. Improved Efficacy of Newly Formulated Bacteriophages for Management of Bacterial Spot on Tomato. Plant Dis. 2003, 87, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Gašić, K.; Kuzmanović, N.; Ivanović, M.; Prokić, A.; Šević, M.; Obradović, A. Complete Genome of the Xanthomonas euvesicatoria Specific Bacteriophage KΦ1, Its Survival and Potential in Control of Pepper Bacterial Spot. Front. Microbiol. 2018, 9, 2021. [Google Scholar] [CrossRef] [PubMed]
- Engel, H.W.B.; Smith, L.; Berwald, L.G. The Preservation of Mycobacteriophages by Means of Freeze Drying. Am. Rev. Respir. Dis. 1974, 109, 561–566. [Google Scholar]
- Davies, J.D.; Kelly, M.J. The Preservation of Bacteriophage H1 of Corynebacterium Ulcerans U 103 by Freeze-Drying. Epidemiol. Infect. 1969, 67, 573–583. [Google Scholar] [CrossRef]
- Merabishvili, M.; Vervaet, C.; Pirnay, J.-P.; Vos, D.D.; Verbeken, G.; Mast, J.; Chanishvili, N.; Vaneechoutte, M. Stability of Staphylococcus aureus Phage ISP after Freeze-Drying (Lyophilization). PLoS ONE 2013, 8, e68797. [Google Scholar] [CrossRef] [PubMed]
- González-Menéndez, E.; Fernández, L.; Gutiérrez, D.; Rodríguez, A.; Martínez, B.; García, P. Comparative Analysis of Different Preservation Techniques for the Storage of Staphylococcus Phages Aimed for the Industrial Development of Phage-Based Antimicrobial Products. PLoS ONE 2018, 13, e0205728. [Google Scholar] [CrossRef]
- Flores, O.; Prince, C.; Nuñez, M.; Vallejos, A.; Mardones, C.; Yañez, C.; Besoain, X.; Bastías, R. Genetic and Phenotypic Characterization of Indole-Producing Isolates of Pseudomonas syringae Pv. Actinidiae Obtained from Chilean Kiwifruit Orchards. Front. Microbiol. 2018, 9, 1907. [Google Scholar] [CrossRef]
- Kutter, E. Phage Host Range and Efficiency of Plating. In Bacteriophages: Methods and Protocols, Volume 1: Isolation, Characterization, and Interactions; Clokie, M.R.J., Kropinski, A.M., Eds.; Methods in Molecular BiologyTM; Humana Press: Totowa, NJ, USA, 2009; pp. 141–149. ISBN 978-1-60327-164-6. [Google Scholar]
- Manohar, P.; Ramesh, N. Improved Lyophilization Conditions for Long-Term Storage of Bacteriophages. Sci. Rep. 2019, 9, 15242. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.A. Comparison of Several Methods for Preserving Bacteriophages. Appl. Microbiol. 1962, 10, 466–471. [Google Scholar] [CrossRef]
- Brown, T.L.; Thomas, T.; Odgers, J.; Petrovski, S.; Spark, M.J.; Tucci, J. Bacteriophage Formulated into a Range of Semisolid and Solid Dosage Forms Maintain Lytic Capacity against Isolated Cutaneous and Opportunistic Oral Bacteria. J. Pharm. Pharmacol. 2017, 69, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Puapermpoonsiri, U.; Ford, S.J.; van der Walle, C.F. Stabilization of Bacteriophage during Freeze Drying. Int. J. Pharm. 2010, 389, 168–175. [Google Scholar] [CrossRef]
- Prencipe, S.; Gullino, M.L.; Spadaro, D. Pseudomonas syringae Pv. Actinidiae Isolated from Actinidia chinensis Var. Deliciosa in Northern Italy: Genetic Diversity and Virulence. Eur. J. Plant Pathol. 2018, 150, 191–204. [Google Scholar] [CrossRef]
- Balestra, G.M.; Taratufolo, M.C.; Vinatzer, B.A.; Mazzaglia, A. A Multiplex PCR Assay for Detection of Pseudomonas syringae Pv. Actinidiae and Differentiation of Populations with Different Geographic Origin. Plant Dis. 2013, 97, 472–478. [Google Scholar] [CrossRef]
- Rahimi-Midani, A.; Choi, T.-J. Transport of Phage in Melon Plants and Inhibition of Progression of Bacterial Fruit Blotch. Viruses 2020, 12, 477. [Google Scholar] [CrossRef]
- Orynbayev, A.T.; Dzhalilov, F.S.U.; Ignatov, A.N. Improved Efficacy of Formulated Bacteriophage in Control of Black Rot Caused by Xanthomonas campestris Pv. Campestris on Cabbage Seedlings. Arch. Phytopathol. Plant Prot. 2020, 53, 379–394. [Google Scholar] [CrossRef]
- Matinkhoo, S.; Lynch, K.H.; Dennis, J.J.; Finlay, W.H.; Vehring, R. Spray-Dried Respirable Powders Containing Bacteriophages for the Treatment of Pulmonary Infections. J. Pharm. Sci. 2011, 100, 5197–5205. [Google Scholar] [CrossRef]
- Vandenheuvel, D.; Meeus, J.; Lavigne, R.; Van den Mooter, G. Instability of Bacteriophages in Spray-Dried Trehalose Powders Is Caused by Crystallization of the Matrix. Int. J. Pharm. 2014, 472, 202–205. [Google Scholar] [CrossRef]
- Yan, W.; He, R.; Tang, X.; Tian, B.; Liu, Y.; Tong, Y.; To, K.K.W.; Leung, S.S.Y. The Influence of Formulation Components and Environmental Humidity on Spray-Dried Phage Powders for Treatment of Respiratory Infections Caused by Acinetobacter baumannii. Pharmaceutics 2021, 13, 1162. [Google Scholar] [CrossRef] [PubMed]
- Mehta, B.M. Chemical Composition of Milk and Milk Products. In Handbook of Food Chemistry; Cheung, P.C.K., Mehta, B.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 511–553. ISBN 978-3-642-36605-5. [Google Scholar]
- Wdowiak, M.; Paczesny, J.; Raza, S. Enhancing the Stability of Bacteriophages Using Physical, Chemical, and Nano-Based Approaches: A Review. Pharmaceutics 2022, 14, 1936. [Google Scholar] [CrossRef]
- Sitohy, M.; Chobert, J.-M.; Karwowska, U.; Gozdzicka-Jozefiak, A.; Haertlé, T. Inhibition of Bacteriophage M13 Replication with Esterified Milk Proteins. J. Agric. Food Chem. 2006, 54, 3800–3806. [Google Scholar] [CrossRef] [PubMed]
- García-Anaya, M.C.; Sepúlveda, D.R.; Rios-Velasco, C.; Zamudio-Flores, P.B.; Sáenz-Mendoza, A.I.; Acosta-Muñiz, C.H. The Role of Food Compounds and Emerging Technologies on Phage Stability. Innov. Food Sci. Emerg. Technol. 2020, 64, 102436. [Google Scholar] [CrossRef]
- Ward, R.L.; Mahler, R.J. Uptake of Bacteriophage F2 through Plant Roots. Appl. Environ. Microbiol. 1982, 43, 1098–1103. [Google Scholar] [CrossRef]
- Iriarte, F.B.; Obradović, A.; Wernsing, M.H.; Jackson, L.E.; Balogh, B.; Hong, J.A.; Momol, M.T.; Jones, J.B.; Vallad, G.E. Soil-Based Systemic Delivery and Phyllosphere in Vivo Propagation of Bacteriophages: Two Possible Strategies for Improving Bacteriophage Persistence for Plant Disease Control. Bacteriophage 2012, 2, e23530. [Google Scholar] [CrossRef]

| Excipient | Concentration | Reference |
|---|---|---|
| Polyethylene glycol (PEG 600) | 5% (w/v) | [41,45] |
| Glutamic acid | 5% (w/v) | [39] |
| Skim milk | 5% (w/v) | [42] |
| Casein | 2% (w/v) | [23] |
| Gelatin | 0.5% (w/v) | [39,45] |
| para-aminobenzoic acid (PABA) | 0.1% (w/v) | [31] |
| Glycerol | 50–30% (w/v) | [46] |
| Polyvinylpyrrolidone (PVP) | 0.5 M | [41] |
| Ethylenediaminetetraacetic acid (EDTA) | 1–10 mM | [47] |
| Mannitol | 0.5 M | [41,45] |
| Sucrose | 0.1 M | [41,42,45] |
| Trehalose | 0.1 M | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
León, M.; Araya, J.; Nuñez, M.; Arce, M.; Guzmán, F.; Yáñez, C.; Besoain, X.; Bastías, R. Evaluation of Different Formulations on the Viability of Phages for Use in Agriculture. Viruses 2024, 16, 1430. https://doi.org/10.3390/v16091430
León M, Araya J, Nuñez M, Arce M, Guzmán F, Yáñez C, Besoain X, Bastías R. Evaluation of Different Formulations on the Viability of Phages for Use in Agriculture. Viruses. 2024; 16(9):1430. https://doi.org/10.3390/v16091430
Chicago/Turabian StyleLeón, Marcela, Jorge Araya, Mauricio Nuñez, Manuel Arce, Fanny Guzmán, Carolina Yáñez, Ximena Besoain, and Roberto Bastías. 2024. "Evaluation of Different Formulations on the Viability of Phages for Use in Agriculture" Viruses 16, no. 9: 1430. https://doi.org/10.3390/v16091430
APA StyleLeón, M., Araya, J., Nuñez, M., Arce, M., Guzmán, F., Yáñez, C., Besoain, X., & Bastías, R. (2024). Evaluation of Different Formulations on the Viability of Phages for Use in Agriculture. Viruses, 16(9), 1430. https://doi.org/10.3390/v16091430





