Changes in the HIV Epidemic in Lower Silesia, Poland, Between 2010 and 2020: The Characteristics of the Key Populations
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Characteristics of Patients at the First Visit
3.2. Route of Infection
3.3. CD4+ and the Route of Infection
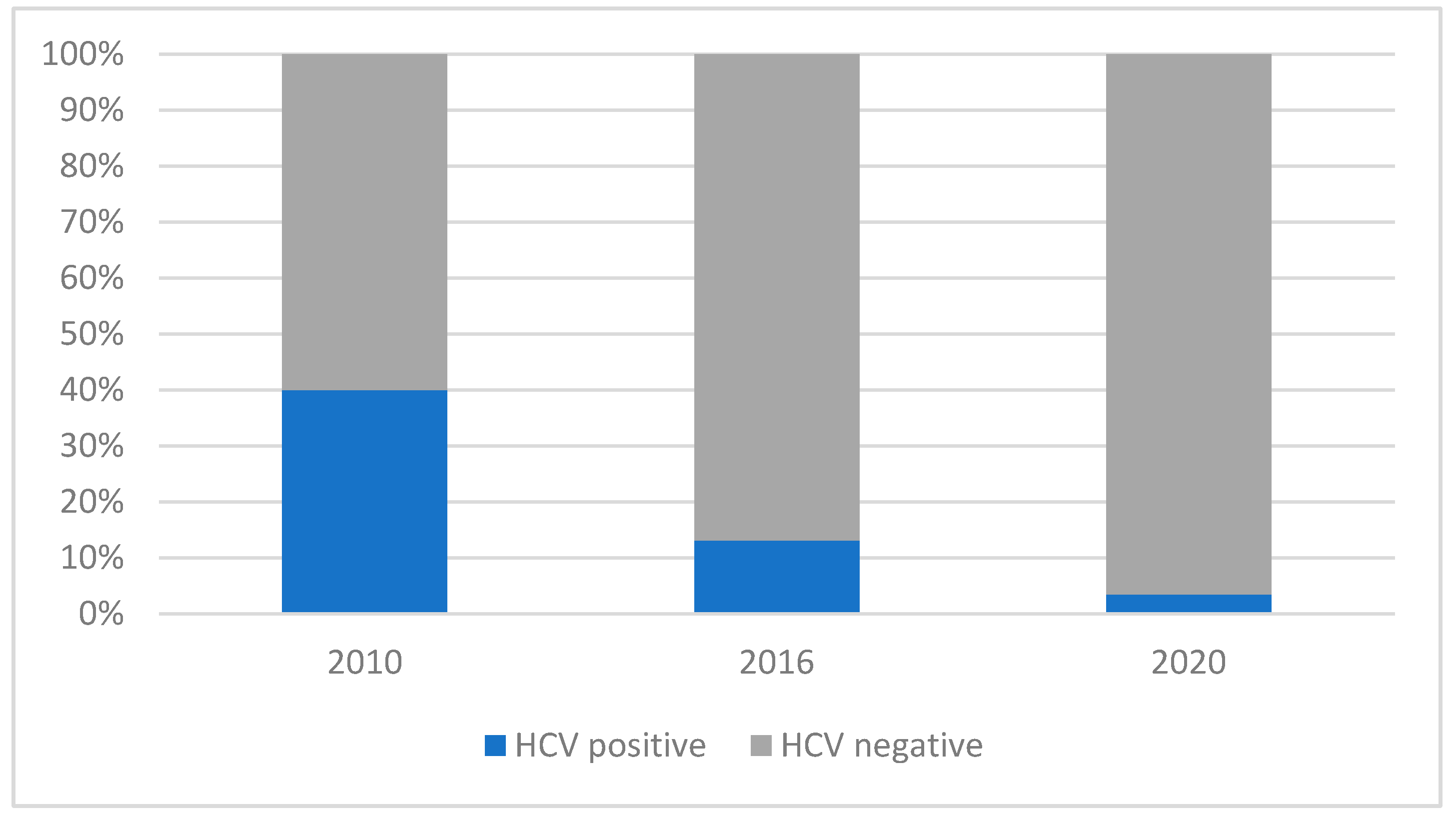
3.4. Hepatitis C Infection in the Three Periods
3.5. Syphilis
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organisation. HIV and AIDS. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed on 19 August 2023).
- Wojewódzka Stacja Sanitarno-Epidemiologiczna w Poznaniu HIV i AIDS. Available online: https://www.gov.pl/web/wsse-poznan/hiv-i-aids (accessed on 19 August 2023).
- Rosinska, M. Current Trends in HIV/AIDS Epidemiology in Poland, 1999–2004. Eurosurveillance 2006, 11, 11–12. [Google Scholar] [CrossRef]
- Niedźwiedzka-Stadnik, M.; Rosińska, M. HIV and AIDS in Poland in 2010. Prz. Epidemiol. 2012, 66, 315–323. [Google Scholar]
- European Centre for Disease Prevention and Control; WHO Regional Office for Europe. HIV/AIDS Surveillance in Europe 2021: 2020 Data; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2021. [Google Scholar]
- Departament Analiz i Innowacji. NFZ o Zdrowiu HIV AIDS; Centrala Narodowego Funduszu Zdrowia; Departament Analiz i Innowacji: Warsaw, Poland, 2022; ISBN 978-83-956980-9-5. [Google Scholar]
- R Core Team. R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 7 September 2024).
- Schauberger, P.; Walker, A. Read, Write and Edit Xlsx Files; Openxlsx. 2021. Available online: https://ycphs.github.io/openxlsx/ (accessed on 7 September 2024).
- Antinori, A.; Coenen, T.; Costagiola, D.; Dedes, N.; Ellefson, M.; Gatell, J.; Girardi, E.; Johnson, M.; Kirk, O.; Lundgren, J.; et al. Late Presentation of HIV Infection: A Consensus Definition. HIV Med. 2011, 12, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Niedźwiedzka-Stadnik Marta; Nowakowska-Radziwonka Ewa. Zakażenia HIV i Zachorowania Na AIDS w Polsce w Latach 1986–2021; Narodowy Instytut Zdrowia Publicznego—Państwowy Zakład Higieny: Warsaw, Poland, 2021. [Google Scholar]
- Krajowe Centrum do Spraw AIDS. Sprawozdanie z Realizacji w 2010 Roku Krajowego Programu Zwalczania AIDS i Zapobiegania Zakazėniom HIV Na Lata 2007–2011; Department of Health: Warsaw, Poland, 2011. [Google Scholar]
- Krajowe Centrum do Spraw AIDS. Sprawozdanie z Realizacji Krajowego Programu Zapobiegania Zakażeniom HIV i Zwalczania AIDS w Latach 2012–2016; Department of Health, Warsaw: Poland, 2017. [Google Scholar]
- Krajowe Centrum do Spraw AIDS. Sprawozdanie z Realizacji Krajowego Programu Zapobiegania Zakażeniom HIV i Zwalczania AIDS w 2020 Roku; Department of Health: Warsaw, Poland, 2021. [Google Scholar]
- European Centre for Disease Prevention and Control; WHO Regional Office for Europe. HIV/AIDS Surveillance in Europe 2022—2021 Data; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Cotter, T.G.; Stier, M.W.; Aronsohn, A. PRO: Needle Exchange Programs Should Be Instituted to Reduce Hepatitis C Virus Transmission. Clin. Liver Dis. 2018, 12, 170–172. [Google Scholar] [CrossRef]
- Malczewski, A.; Jablonski, P.; Kidawa, M.; Dalmata, M.; Bevz, M.; Jędruszak, Ł. Raport o Stanie Narkomanii w Polsce 2020; Krajowe Biuro do Spraw Przeciwdziałania Narkomanii: Warsaw, Poland, 2020; ISBN 978-83-959842-6-6. [Google Scholar]
- Siwak, E.; Horban, A.; Witak-Jędra, M.; Cielniak, I.; Firląg-Burkacka, E.; Leszczyszyn-Pynka, M.; Witor, A.; Muller, K.; Bociąga-Jasik, M.; Kalinowska-Nowak, A.; et al. Long-term Trends in HIV Care Entry: Over 15 Years of Clinical Experience from Poland. HIV Med. 2019, 20, 581–590. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control; WHO Regional Office for Europe. HIV/AIDS Surveillance in Europe 2019: 2018 Data; European Centre for Disease Prevention and Control: Stockholm, Sweden; WHO Regional Office for Europe: Copenhagen, Denmark, 2019. [Google Scholar]
- James, A.; Dixit, N.M. Transmitted HIV-1 Is More Virulent in Heterosexual Individuals than Men-Who-Have-Sex-with-Men. PLoS Pathog. 2022, 18, e1010319. [Google Scholar] [CrossRef]
- Wójcik-Cichy, K.; Jabłonowska, O.; Piekarska, A.; Jabłonowska, E. The High Incidence of Late Presenters for HIV/AIDS Infection in the Lodz Province, Poland in the Years 2009–2016: We Are Still Far from the UNAIDS 90% Target. AIDS Care 2018, 30, 1538–1541. [Google Scholar] [CrossRef]
- Jabłonowska, E.; Szetela, B.; Bielecki, M.; Horban, A.; Bociąga-Jasik, M.; Mularska, E.; Hlebowicz, M.; Olczak, A.; Parczewski, M.; Grzeszczuk, A.; et al. Acquired Immune Deficiency Syndrome (AIDS) and Late Presentation in Poland—Data from Test and Keep in Care (TAK) Polska Project. HIV Med. 2021, 22, 387–396. [Google Scholar] [CrossRef]
- Manavi, K.; McMillan, A.; Ogilvie, M.; Scott, G. Heterosexual Men and Women with HIV Test Positive at a Later Stage of Infection than Homo- or Bisexual Men. Int. J. STD AIDS 2004, 15, 811–814. [Google Scholar] [CrossRef]
- Buchacz, K.; Armon, C.; Palella, F.J.; Baker, R.K.; Tedaldi, E.; Durham, M.D.; Brooks, J.T. CD4 Cell Counts at HIV Diagnosis among HIV Outpatient Study Participants, 2000–2009. AIDS Res. Treat. 2012, 2012, 869841. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krajowe Centrum do Spraw AIDS. Raport 1: Diagnoza Stanu Wiedzy Polaków Na Temat HIV/AIDS i Zakażeń Przenoszonych Drogą Płciową (ZPDP) Oraz Zachowania Seksualne; Department of Health: Warsaw, Poland, 2014. [Google Scholar]
- Public Health Scotland. HIV in Scotland: Update to 31 December 2022; Public Health Scotland: Edinburgh, Scotland, 2023. [Google Scholar]
- Boesecke, C.; Schellberg, S.; Schneider, J.; Schuettfort, G.; Stocker, H. Prevalence, Characteristics and Challenges of Late HIV Diagnosis in Germany: An Expert Narrative Review. Infection 2023, 51, 1223–1239. [Google Scholar] [CrossRef] [PubMed]
- van Wijhe, M.; Fischer, T.K.; Fonager, J. Identification of Risk Factors Associated with National Transmission and Late Presentation of HIV-1, Denmark, 2009 to 2017. Eurosurveillance 2021, 26, 2002008. [Google Scholar] [CrossRef] [PubMed]
- Kunkiewicz, M.; Kańtoch, A.; Reich, A. Renesanse of Syphilis: About the Raising Prevalence of Treponema Pallidum Infection. Forum Dermatol. 2017, 3, 41–43. [Google Scholar]
- Kojima, N.; Klausner, J.D. An Update on the Global Epidemiology of Syphilis. Curr. Epidemiol. Rep. 2018, 5, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Dashwood, T.; Walmsley, S. The Intersection of HIV and Syphilis: Update on the Key Considerations in Testing and Management. Curr. HIV/AIDS Rep. 2021, 18, 280–288. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Syphilis—Annual Epidemiological Report for 2021; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2023. [Google Scholar]
- Bartosz, S.; Jasyk, Z.; Krzywicka, I.; Giniewicz, K.; Ankiersztejn-Bartczak, M. HIV Home Testing in Poland—Underappreciated Approach despite Huge Potential; Poster presented at European AIDS Clinical Society (EACS): Warsaw, Poland, 2023. [Google Scholar]
- Brian, R.; Wood, M. Acute and Recent HIV Infection. Available online: https://www.hiv.uw.edu/go/screening-diagnosis/acute-recent-early-hiv/core-concept/all#page-title (accessed on 28 December 2023).
- Nayak, S.; Acharjya, B. VDRL Test and Its Interpretation. Indian J. Dermatol. 2012, 57, 3. [Google Scholar] [CrossRef] [PubMed]

| Year | ||||
|---|---|---|---|---|
| Variables | 2010 | 2016 | 2020 | Total |
| n | 43 (21.29) | 80 (39.60) | 79 (39.11) | 202 (100) |
| Gender: | ||||
| Women | 9 (20.93) | 7 (8.75) | 11 (13.92) | 27 (13.37) |
| (Pregnancy) | (1 (11.11)) | (0 (0.00)) | (5 (45.45)) | (6 (22.22)) |
| Men | 34 (79.07) | 73 (91.25) | 68 (86.08) | 175 (86.63) |
| Route of infection: | ||||
| MSM | 18 (41.86) | 59 (73.75) | 51 (64.56) | 128 (63.36) |
| HTXs | 6 (13.95) | 10 (12.50) | 18 (22.78) | 34 (16.83) |
| IDUs | 13 (30.23) | 4 (5.00) | 3 (3.79) | 20 (9.90) |
| Unknown | 6 (13.95) | 7 (8.75) | 7 (8.86) | 20 (9.90) |
| Diseases: | ||||
| Acute retroviral disease | 7 (16.27) | 21 (26.25) | 24 (30.38) | 52 (25.74) |
| AIDS | 6 (13.95) | 16 (20.00) | 17 (21.52) | 39 (19.31) |
| Anti-HCV-positive | 14 (32.56) | 10 (12.50) | 2 (2.53) | 26 (12.87) |
| HBsAg-positive | 0 (0.00) | 1 (1.25) | 3 (3.80) | 4 (1.98) |
| VDRL-positive | 7 (16.27) | 10 (12.50) | 14 (17.72) | 31 (15.35) |
| Year | |||
|---|---|---|---|
| Variables | 2010 | 2016 | 2020 |
| Age | 43, 33.72 | 80, 31.35 | 79, 33.06 |
| (28.59–38.67) | (26.77–37.16) | (26.97–38.25) | |
| Viral load (copies/mL) | 36, 29350.00 | 77, 27900.00 | 79, 28900.00 |
| (7960.00–58600.00) | (15200.00–83000.00) | (8670.00–133500.00) | |
| CD4+ T cell count | 43, 359.00 | 80, 444.50 | 79, 380.00 |
| (273.00–481.00) | (246.75–557.25) | (235.50–518.00) |
| Year | ||||
|---|---|---|---|---|
| Route of Infection | 2010 | 2016 | 2020 | Total |
| Total | 37 (100.00) | 73 (100.00) | 72 (100.00) | 182 (100.00) |
| MSM | 18 (48.65) | 59 (80.82) | 51 (70.83) | 128 (70.33) |
| HTXs | 6 (16.22) | 10 (13.70) | 18 (25.00) | 34 (18.69) |
| IDUs | 13 (35.14) | 4 (5.48) | 3 (4.17) | 20 (10.99) |
| Year | ||||
|---|---|---|---|---|
| Route of Infection | 2010 | 2016 | 2020 | Total |
| MSM | 18, 481.0 (337.25–584.75) | 59, 488.00 (405.00–571.50) | 51, 416.00 (293.00–524.50) | 128, 464.50 (330.75–565.50) |
| HTXs | 6, 267.00 * (4.00–414.00) | 10, 224.00 (111.75–270.75) | 18, 286.50 (127.00–537.25) | 34, 250.00 (118.00–413.75) |
| IDUs | 13, 353.00 (274.00–417.00) | 4, 157.50 * (48.00–395.00) | 3, 93.00 * (60.00–336.00) | 20, 327.00 (156.75–386.75) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozieł, A.; Cieślik, A.; Janek, Ł.; Szymczak, A.; Domański, I.; Knysz, B.; Szetela, B. Changes in the HIV Epidemic in Lower Silesia, Poland, Between 2010 and 2020: The Characteristics of the Key Populations. Viruses 2024, 16, 1445. https://doi.org/10.3390/v16091445
Kozieł A, Cieślik A, Janek Ł, Szymczak A, Domański I, Knysz B, Szetela B. Changes in the HIV Epidemic in Lower Silesia, Poland, Between 2010 and 2020: The Characteristics of the Key Populations. Viruses. 2024; 16(9):1445. https://doi.org/10.3390/v16091445
Chicago/Turabian StyleKozieł, Aleksandra, Aleksandra Cieślik, Łucja Janek, Aleksandra Szymczak, Igor Domański, Brygida Knysz, and Bartosz Szetela. 2024. "Changes in the HIV Epidemic in Lower Silesia, Poland, Between 2010 and 2020: The Characteristics of the Key Populations" Viruses 16, no. 9: 1445. https://doi.org/10.3390/v16091445
APA StyleKozieł, A., Cieślik, A., Janek, Ł., Szymczak, A., Domański, I., Knysz, B., & Szetela, B. (2024). Changes in the HIV Epidemic in Lower Silesia, Poland, Between 2010 and 2020: The Characteristics of the Key Populations. Viruses, 16(9), 1445. https://doi.org/10.3390/v16091445






