N6-Methyladenosine Positively Regulates Coxsackievirus B3 Replication
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Western Blot
2.3. Indirect Immunofluorescence Assay (IFA)
2.4. Quantitative Reverse-Transcription PCR (qRT–PCR)
2.5. Generation of Stable Cell Lines
2.6. MeRIP-qPCR
2.7. Cell Counting Kit-8 (CCK-8)
2.8. Statistical Analysis
3. Results
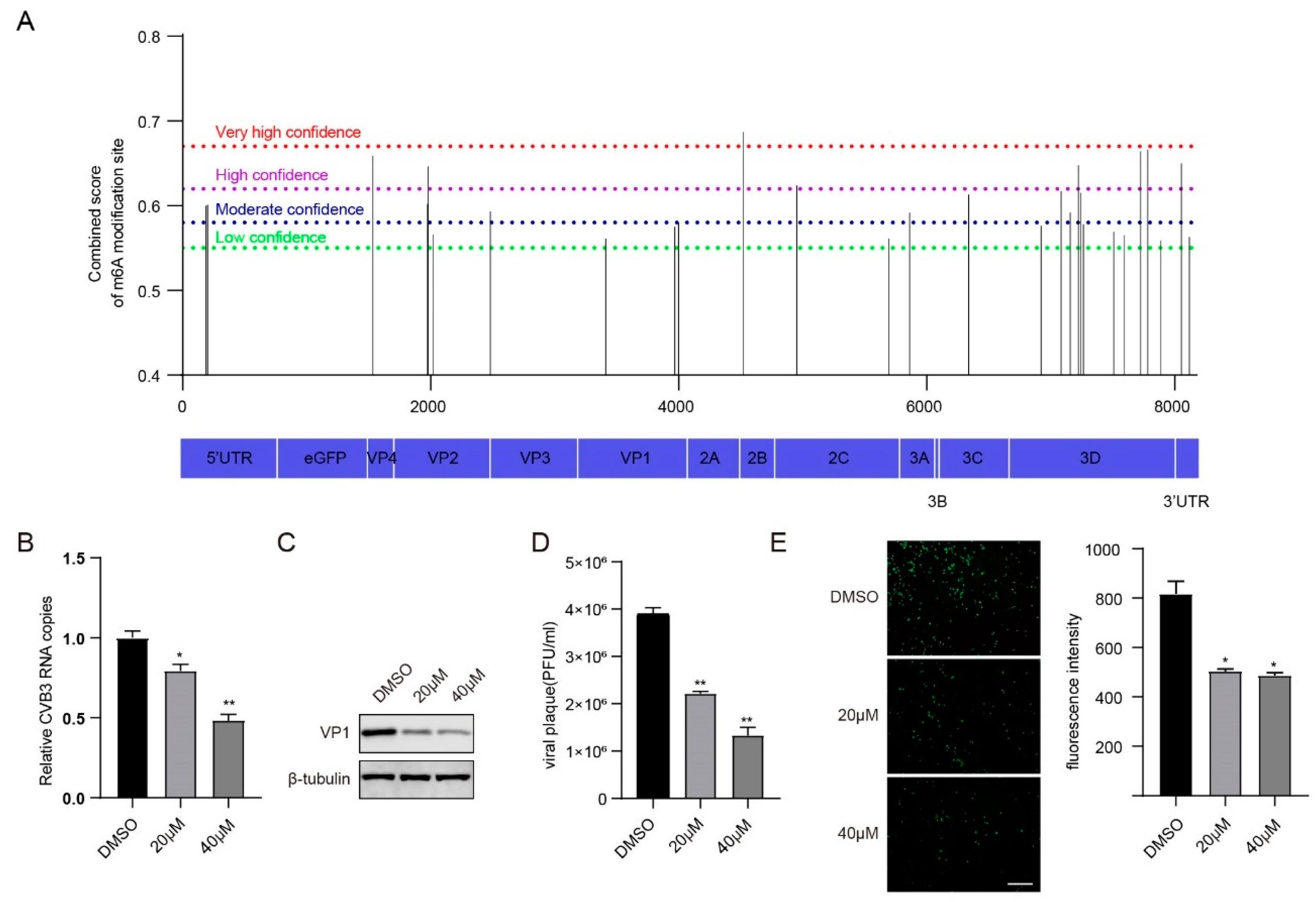
3.1. m6A Modification Regulates CVB3 Replication
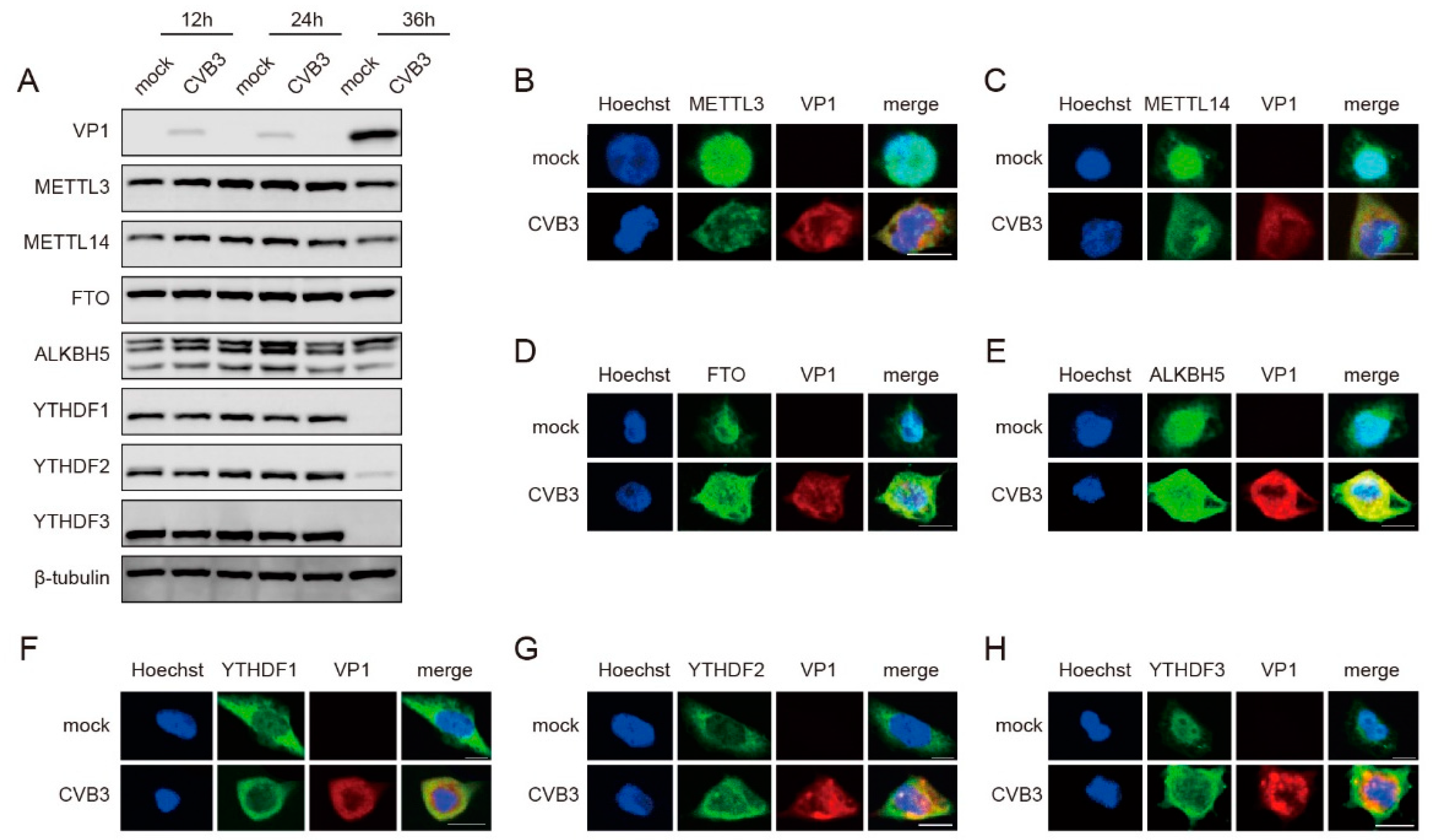
3.2. CVB3 Infection Modulates the Expression Patterns of m6A-Related Proteins
3.3. METTL3 and METTL14 Enhance CVB3 Replication in HeLa Cells
3.4. FTO and ALKBH5 Inhibit CVB3 Replication in HeLa Cells
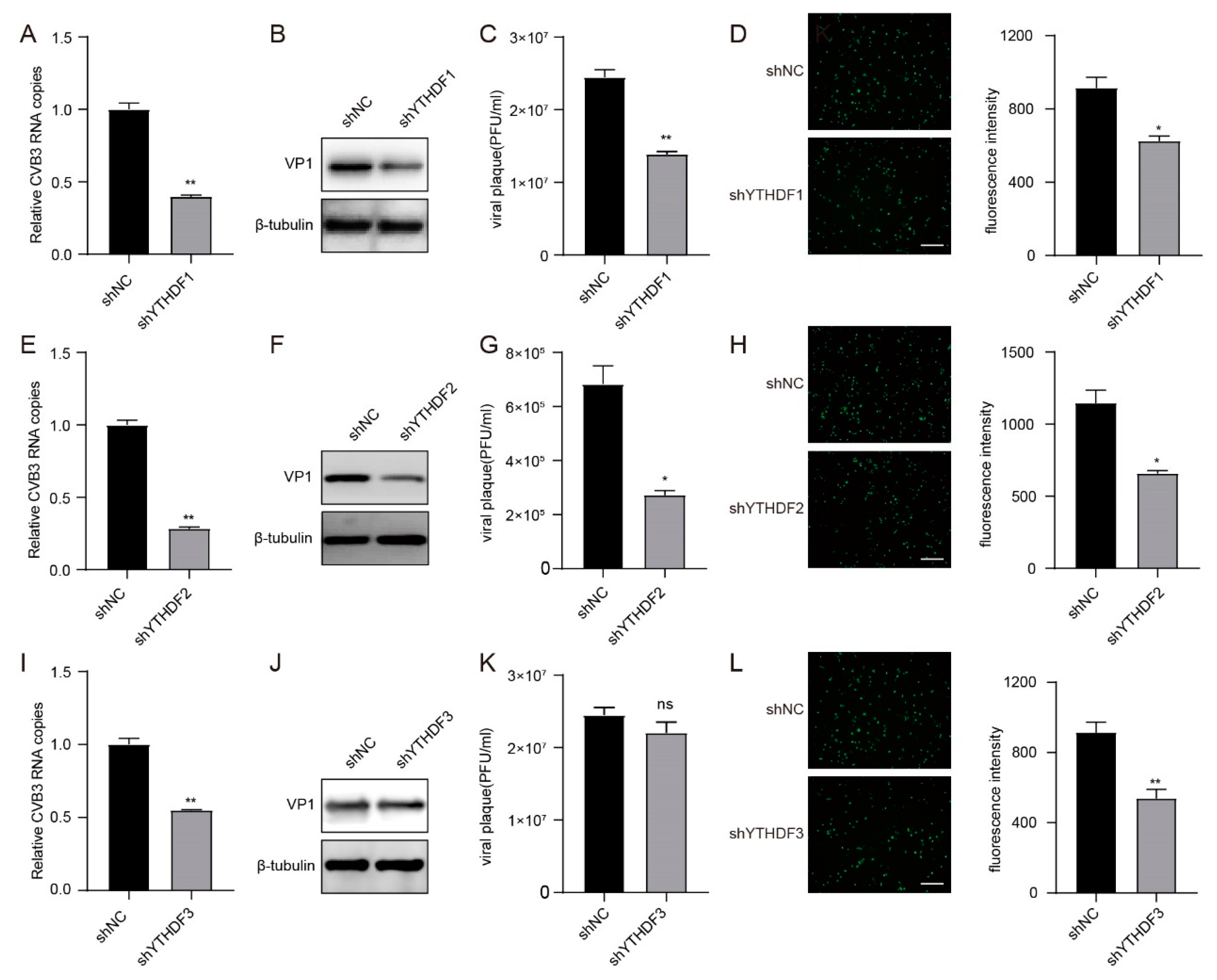
3.5. YTHDF1, YTHDF2 and YTHDF3 Interference with the Expression of CVB3 RNA
3.6. Defects in the Replication of m6A-Abrogating CVB3 Mutants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garmaroudi, F.S.; Marchant, D.; Hendry, R.; Luo, H.; Yang, D.; Ye, X.; Shi, J.; McManus, B.M. Coxsackievirus B3 replication and pathogenesis. Future Microbiol. 2015, 10, 629–653. [Google Scholar] [CrossRef]
- Yip, F.; Lai, B.; Yang, D. Role of Coxsackievirus B3-Induced Immune Responses in the Transition from Myocarditis to Dilated Cardiomyopathy and Heart Failure. Int. J. Mol. Sci. 2023, 24, 7717. [Google Scholar] [CrossRef] [PubMed]
- Peischard, S.; Ho, H.T.; Theiss, C.; Strutz-Seebohm, N.; Seebohm, G. A Kidnapping Story: How Coxsackievirus B3 and Its Host Cell Interact. Cell. Physiol. Biochem. 2019, 53, 121–140. [Google Scholar] [PubMed]
- Zaccara, S.; Ries, R.; Jaffrey, S. Reading, writing and erasing mRNA methylation. Nat. Rev. Mol. Cell Biol. 2019, 20, 608–624. [Google Scholar] [CrossRef]
- Xiao, W.; Adhikari, S.; Dahal, U.; Chen, Y.; Hao, Y.; Sun, B.; Sun, H.; Li, A.; Ping, X.; Lai, W.; et al. Nuclear m(6)A Reader YTHDC1 Regulates mRNA Splicing. Mol. Cell 2016, 61, 507–519. [Google Scholar] [CrossRef]
- Roundtree, I.; Luo, G.; Zhang, Z.; Wang, X.; Zhou, T.; Cui, Y.; Sha, J.; Huang, X.; Guerrero, L.; Xie, P.; et al. YTHDC1 mediates nuclear export of N-methyladenosine methylated mRNAs. eLife 2017, 6, e31311. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, Z.; Gomez, A.; Hon, G.; Yue, Y.; Han, D.; Fu, Y.; Parisien, M.; Dai, Q.; Jia, G.; et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 2014, 505, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhao, Y.; He, J.; Zhang, Y.; Xi, H.; Liu, M.; Ma, J.; Wu, L. YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat. Commun. 2016, 7, 12626. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, B.; Roundtree, I.; Lu, Z.; Han, D.; Ma, H.; Weng, X.; Chen, K.; Shi, H.; He, C. N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell 2015, 161, 1388–1399. [Google Scholar] [CrossRef]
- Dominissini, D.; Moshitch-Moshkovitz, S.; Schwartz, S.; Salmon-Divon, M.; Ungar, L.; Osenberg, S.; Cesarkas, K.; Jacob-Hirsch, J.; Amariglio, N.; Kupiec, M.; et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012, 485, 201–206. [Google Scholar] [CrossRef]
- Meyer, K.; Saletore, Y.; Zumbo, P.; Elemento, O.; Mason, C.; Jaffrey, S. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 2012, 149, 1635–1646. [Google Scholar] [CrossRef]
- Meyer, K.; Jaffrey, S. The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat. Rev. Mol. Cell Biol. 2014, 15, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Narayan, P.; Rottman, F. An in vitro system for accurate methylation of internal adenosine residues in messenger RNA. Science 1988, 242, 1159–1162. [Google Scholar] [CrossRef] [PubMed]
- Csepany, T.; Lin, A.; Baldick, C.; Beemon, K. Sequence specificity of mRNA N6-adenosine methyltransferase. J. Biol. Chem. 1990, 265, 20117–20122. [Google Scholar] [CrossRef] [PubMed]
- Narayan, P.; Ludwiczak, R.; Goodwin, E.; Rottman, F. Context effects on N6-adenosine methylation sites in prolactin mRNA. Nucleic Acids Res. 1994, 22, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yue, Y.; Han, D.; Wang, X.; Fu, Y.; Zhang, L.; Jia, G.; Yu, M.; Lu, Z.; Deng, X.; et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 2014, 10, 93–95. [Google Scholar] [CrossRef]
- Ping, X.; Sun, B.; Wang, L.; Xiao, W.; Yang, X.; Wang, W.; Adhikari, S.; Shi, Y.; Lv, Y.; Chen, Y.; et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014, 24, 177–189. [Google Scholar] [CrossRef]
- Zheng, G.; Dahl, J.; Niu, Y.; Fedorcsak, P.; Huang, C.; Li, C.; Vågbø, C.; Shi, Y.; Wang, W.; Song, S.; et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell 2013, 49, 18–29. [Google Scholar] [CrossRef]
- Jia, G.; Fu, Y.; Zhao, X.; Dai, Q.; Zheng, G.; Yang, Y.; Yi, C.; Lindahl, T.; Pan, T.; Yang, Y.; et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011, 7, 885–887. [Google Scholar] [CrossRef]
- Williams, G.; Gokhale, N.; Horner, S. N6Regulation of Viral Infection by the RNA Modification -Methyladenosine. Annu. Rev. Virol. 2019, 6, 235–253. [Google Scholar] [CrossRef]
- Wu, F.; Cheng, W.; Zhao, F.; Tang, M.; Diao, Y.; Xu, R. Association of N6-methyladenosine with viruses and related diseases. Virol. J. 2019, 16, 133. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Hao, S.; Chen, H.; Chen, Z.; Zhang, Y.; Wang, J.; Wang, H.; Zhang, B.; Qiu, J.; Deng, F.; et al. N6-methyladenosine modification and METTL3 modulate enterovirus 71 replication. Nucleic Acids Res. 2019, 47, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, Z.; Shu, B.; Meng, J.; Zhang, Y.; Zheng, C.; Ke, X.; Gong, P.; Hu, Q.; Wang, H. SUMO Modification Stabilizes Enterovirus 71 Polymerase 3D To Facilitate Viral Replication. J. Virol. 2016, 90, 10472–10485. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, N.; McIntyre, A.; McFadden, M.; Roder, A.; Kennedy, E.; Gandara, J.; Hopcraft, S.; Quicke, K.; Vazquez, C.; Willer, J.; et al. N6-Methyladenosine in Flaviviridae Viral RNA Genomes Regulates Infection. Cell Host Microbe 2016, 20, 654–665. [Google Scholar] [CrossRef]
- Knowlton, K.; Jeon, E.; Berkley, N.; Wessely, R.; Huber, S. A mutation in the puff region of VP2 attenuates the myocarditic phenotype of an infectious cDNA of the Woodruff variant of coxsackievirus B3. J. Virol. 1996, 70, 7811–7818. [Google Scholar] [CrossRef]
- Feuer, R.; Mena, I.; Pagarigan, R.; Slifka, M.; Whitton, J. Cell cycle status affects coxsackievirus replication, persistence, and reactivation in vitro. J. Virol. 2002, 76, 4430–4440. [Google Scholar] [CrossRef]
- Bader, J.; Brown, N.; Chiang, P.; Cantoni, G. 3-Deazaadenosine, an inhibitor of adenosylhomocysteine hydrolase, inhibits reproduction of Rous sarcoma virus and transformation of chick embryo cells. Virology 1978, 89, 494–505. [Google Scholar] [CrossRef]
- Fischer, A.; Müller, K.; Scholtissek, C. Specific inhibition of the synthesis of influenza virus late proteins and stimulation of early, M2, and NS2 protein synthesis by 3-deazaadenosine. Virology 1990, 177, 523–531. [Google Scholar] [CrossRef]
- Fustin, J.; Doi, M.; Yamaguchi, Y.; Hida, H.; Nishimura, S.; Yoshida, M.; Isagawa, T.; Morioka, M.; Kakeya, H.; Manabe, I.; et al. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell 2013, 155, 793–806. [Google Scholar] [CrossRef]
- Courtney, D.; Kennedy, E.; Dumm, R.; Bogerd, H.; Tsai, K.; Heaton, N.; Cullen, B. Epitranscriptomic Enhancement of Influenza A Virus Gene Expression and Replication. Cell Host Microbe 2017, 22, 377–386.e5. [Google Scholar] [CrossRef]
- Feng, Z.; Zhou, F.; Tan, M.; Wang, T.; Chen, Y.; Xu, W.; Li, B.; Wang, X.; Deng, X.; He, M. Targeting m6A modification inhibits herpes virus 1 infection. Genes Dis. 2022, 9, 1114–1128. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Chen, E.; Nilsen, T. Kaposi’s Sarcoma-Associated Herpesvirus Utilizes and Manipulates RNA N-Adenosine Methylation To Promote Lytic Replication. J. Virol. 2017, 91, e00466-17. [Google Scholar] [CrossRef]
- Gonzales-van Horn, S.; Sarnow, P. Making the Mark: The Role of Adenosine Modifications in the Life Cycle of RNA Viruses. Cell Host Microbe 2017, 21, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shi, Y.; Shen, H.; Xie, W. mA-binding proteins: The emerging crucial performers in epigenetics. J. Hematol. Oncol. 2020, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Lichinchi, G.; Gao, S.; Saletore, Y.; Gonzalez, G.; Bansal, V.; Wang, Y.; Mason, C.; Rana, T. Dynamics of the human and viral m(6)A RNA methylomes during HIV-1 infection of T cells. Nat. Microbiol. 2016, 1, 16011. [Google Scholar] [CrossRef]
- Narayan, P.; Ayers, D.; Rottman, F.; Maroney, P.; Nilsen, T. Unequal distribution of N6-methyladenosine in influenza virus mRNAs. Mol. Cell. Biol. 1987, 7, 1572–1575. [Google Scholar]
- Lichinchi, G.; Zhao, B.; Wu, Y.; Lu, Z.; Qin, Y.; He, C.; Rana, T. Dynamics of Human and Viral RNA Methylation during Zika Virus Infection. Cell Host Microbe 2016, 20, 666–673. [Google Scholar]
- Zhang, X.; Hao, H.; Ma, L.; Zhang, Y.; Hu, X.; Chen, Z.; Liu, D.; Yuan, J.; Hu, Z.; Guan, W. Methyltransferase-like 3 Modulates Severe Acute Respiratory Syndrome Coronavirus-2 RNA N6-Methyladenosine Modification and Replication. mBio 2021, 12, e0106721. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, Z.; Xue, M.; Zhao, B.; Harder, O.; Li, A.; Liang, X.; Gao, T.; Xu, Y.; Zhou, J.; et al. N-methyladenosine modification enables viral RNA to escape recognition by RNA sensor RIG-I. Nat. Microbiol. 2020, 5, 584–598. [Google Scholar] [CrossRef]
- Kennedy, E.; Bogerd, H.; Kornepati, A.; Kang, D.; Ghoshal, D.; Marshall, J.; Poling, B.; Tsai, K.; Gokhale, N.; Horner, S.; et al. Posttranscriptional m(6)A Editing of HIV-1 mRNAs Enhances Viral Gene Expression. Cell Host Microbe 2016, 19, 675–685. [Google Scholar] [CrossRef]
- Tirumuru, N.; Zhao, B.; Lu, W.; Lu, Z.; He, C.; Wu, L. N(6)-methyladenosine of HIV-1 RNA regulates viral infection and HIV-1 Gag protein expression. eLife 2016, 5, e15528. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Zhao, B.; Zhang, Z.; Lu, M.; Harder, O.; Chen, P.; Lu, Z.; Li, A.; Ma, Y.; Xu, Y.; et al. Viral N-methyladenosine upregulates replication and pathogenesis of human respiratory syncytial virus. Nat. Commun. 2019, 10, 4595. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, F.; Vega-Gibson, A.; Catrileo, J.; Gaete-Argel, A.; Riquelme-Barrios, S.; Alonso-Palomares, L.; Tapia, L.; Valiente-Echeverría, F.; Soto-Rifo, R.; Acevedo, M.L. N6-Methyladenosine Negatively Regulates Human Respiratory Syncytial Virus Replication. Front. Cell Dev. Biol. 2021, 9, 739445. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Gao, Z.; Sun, J.; Qiao, H.; Zhao, Y.; Cui, Y.; Zhao, B.; Wang, W.; Chiu, S.; Chuai, X. N6-Methyladenosine Positively Regulates Coxsackievirus B3 Replication. Viruses 2024, 16, 1448. https://doi.org/10.3390/v16091448
Zhao H, Gao Z, Sun J, Qiao H, Zhao Y, Cui Y, Zhao B, Wang W, Chiu S, Chuai X. N6-Methyladenosine Positively Regulates Coxsackievirus B3 Replication. Viruses. 2024; 16(9):1448. https://doi.org/10.3390/v16091448
Chicago/Turabian StyleZhao, Hainian, Zhiyun Gao, Jiawen Sun, Hongxiu Qiao, Yan Zhao, Yan Cui, Baoxin Zhao, Weijie Wang, Sandra Chiu, and Xia Chuai. 2024. "N6-Methyladenosine Positively Regulates Coxsackievirus B3 Replication" Viruses 16, no. 9: 1448. https://doi.org/10.3390/v16091448





