K103N, V106M and Y188L Significantly Reduce HIV-1 Subtype C Phenotypic Susceptibility to Doravirine
Abstract
1. Introduction
2. Materials and Methods
2.1. Vectors
2.2. Laboratory-Adapted Strains
2.3. HEK293T Cell Culture
2.4. Antiretroviral Drugs
2.5. Generation of Laboratory-Adapted Strain-Derived PSVs
2.6. Selection of NNRTI DRMs
2.7. Generation of NNRTI-Resistance Mutations
2.8. Production of HIV-1-like Pseudoviruses
2.9. In Vitro Phenotypic DOR Susceptibility Testing
2.10. Statistics
3. Results
3.1. Selection of NNRTI DRMs
3.2. In Vitro Phenotypic DOR Susceptibility Testing
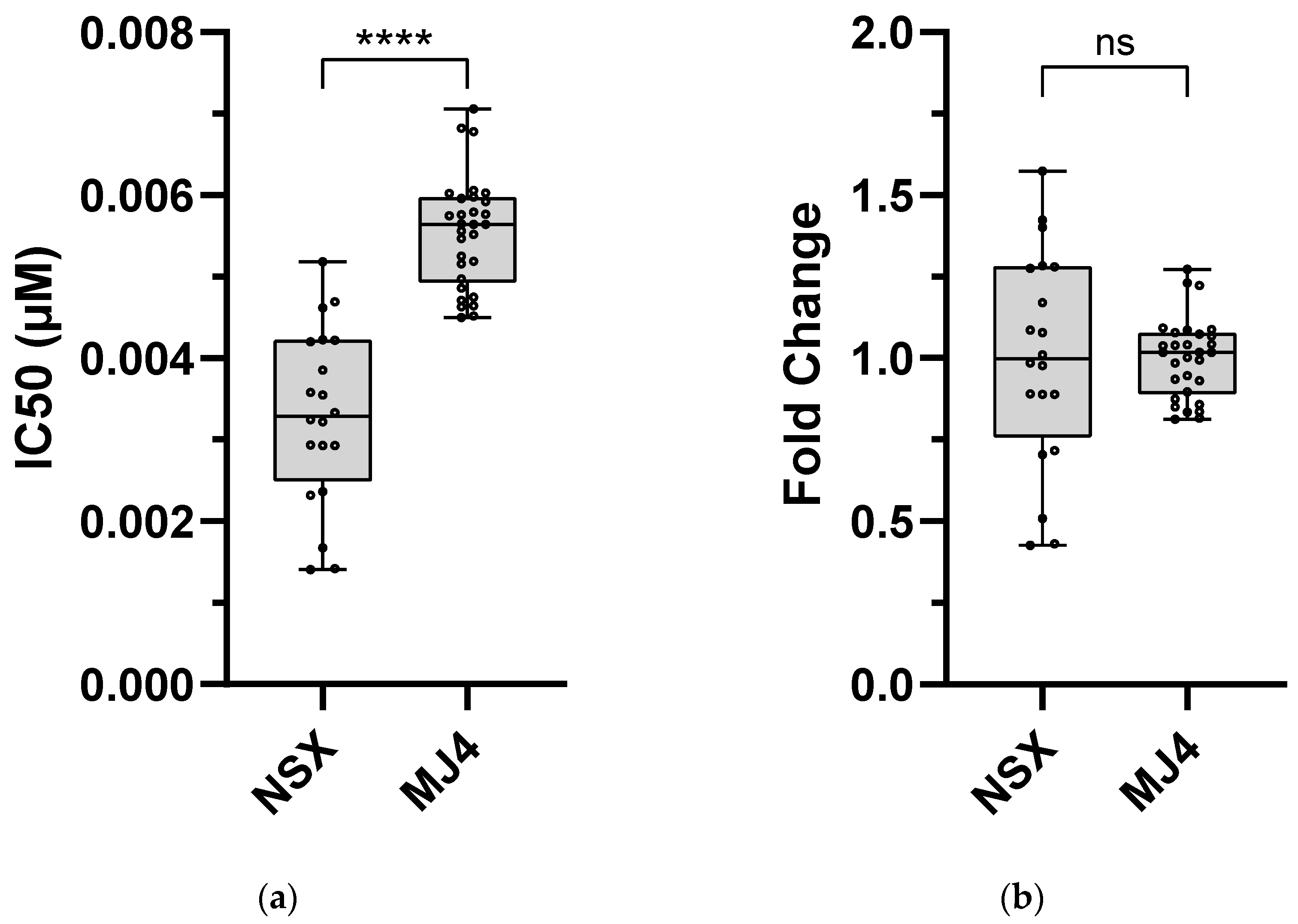
3.2.1. DOR Technical Cut-Off and Assay-Defined Classifications
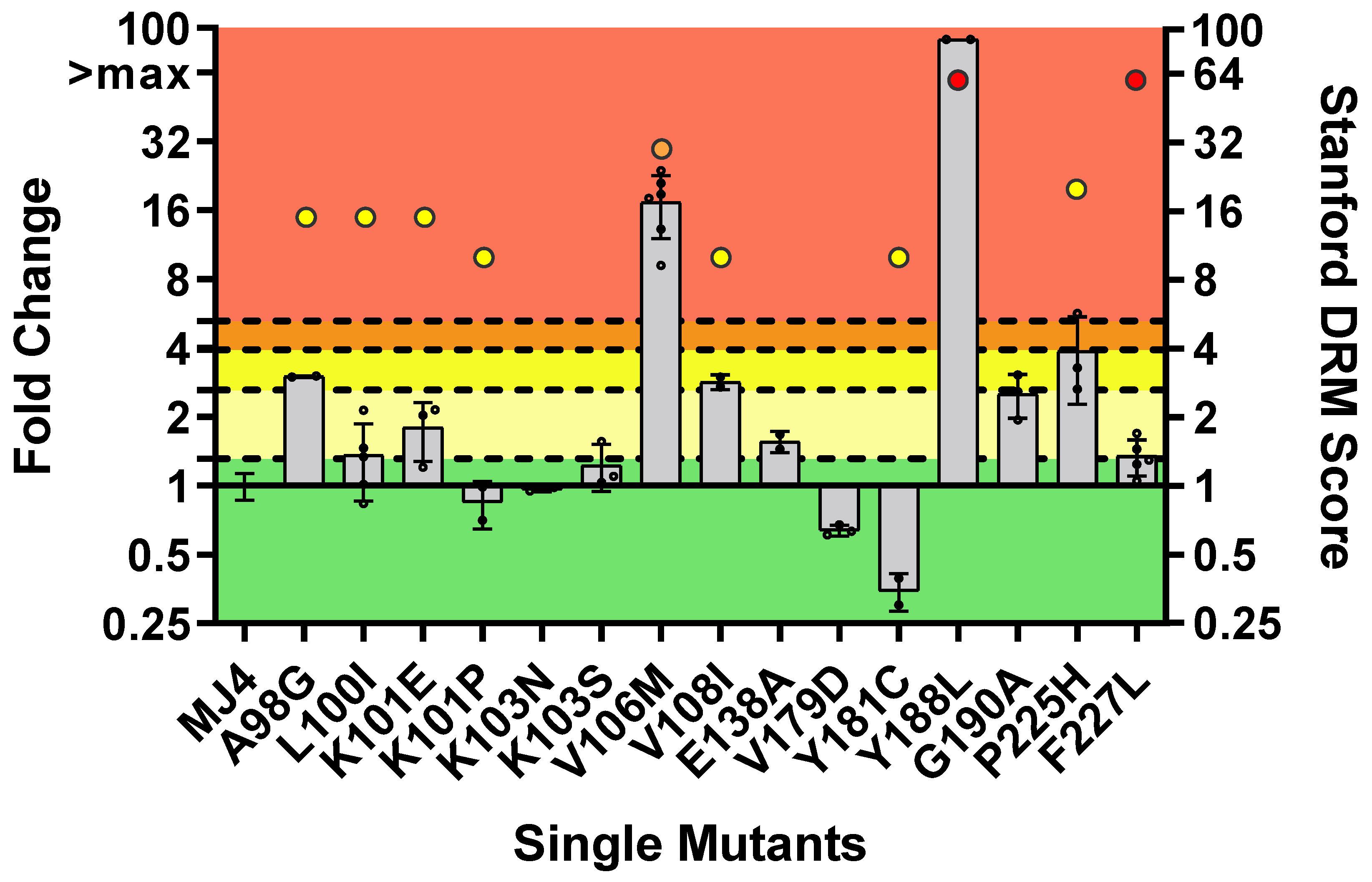
3.2.2. DOR Susceptibility of Single NNRTI DRMs
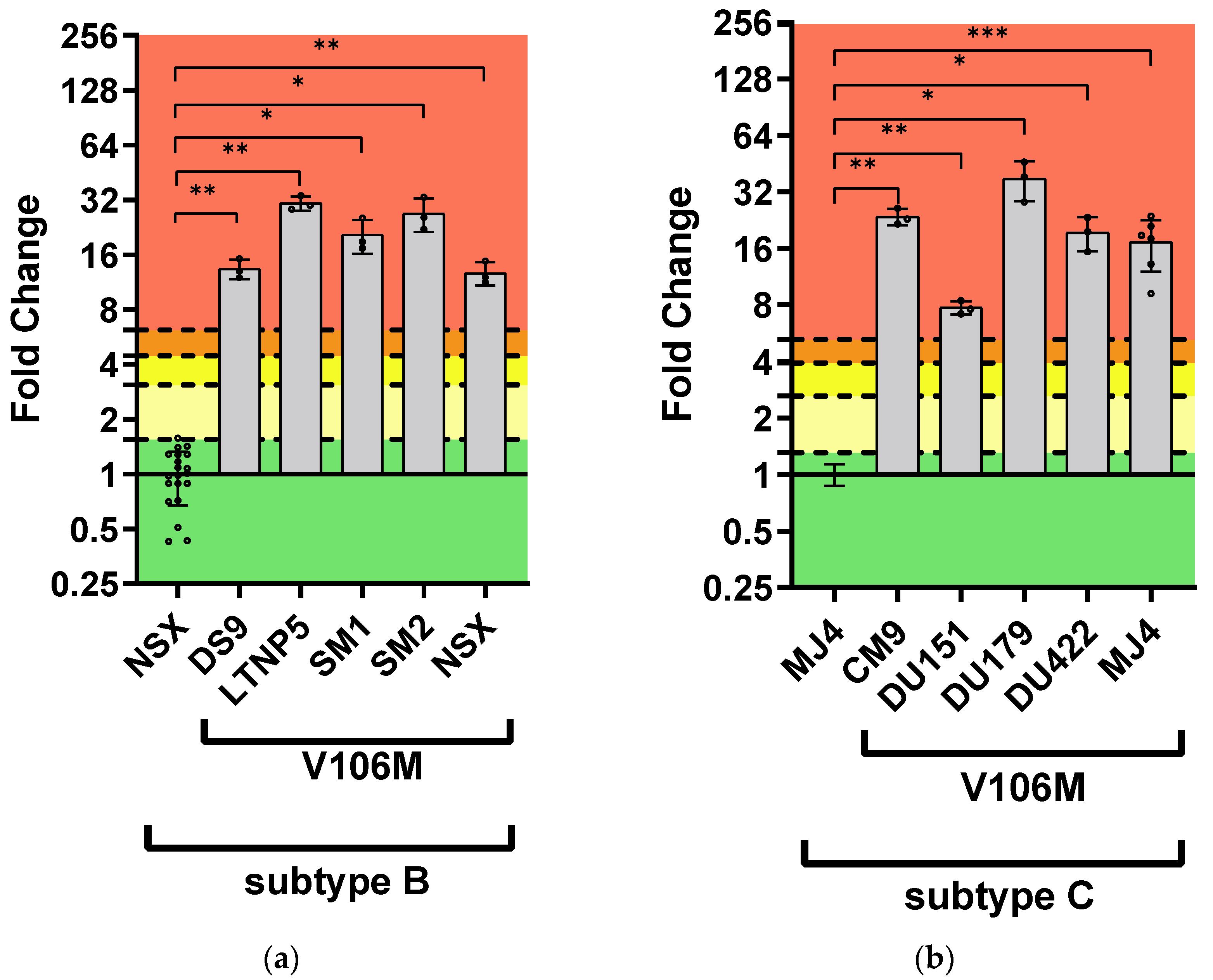
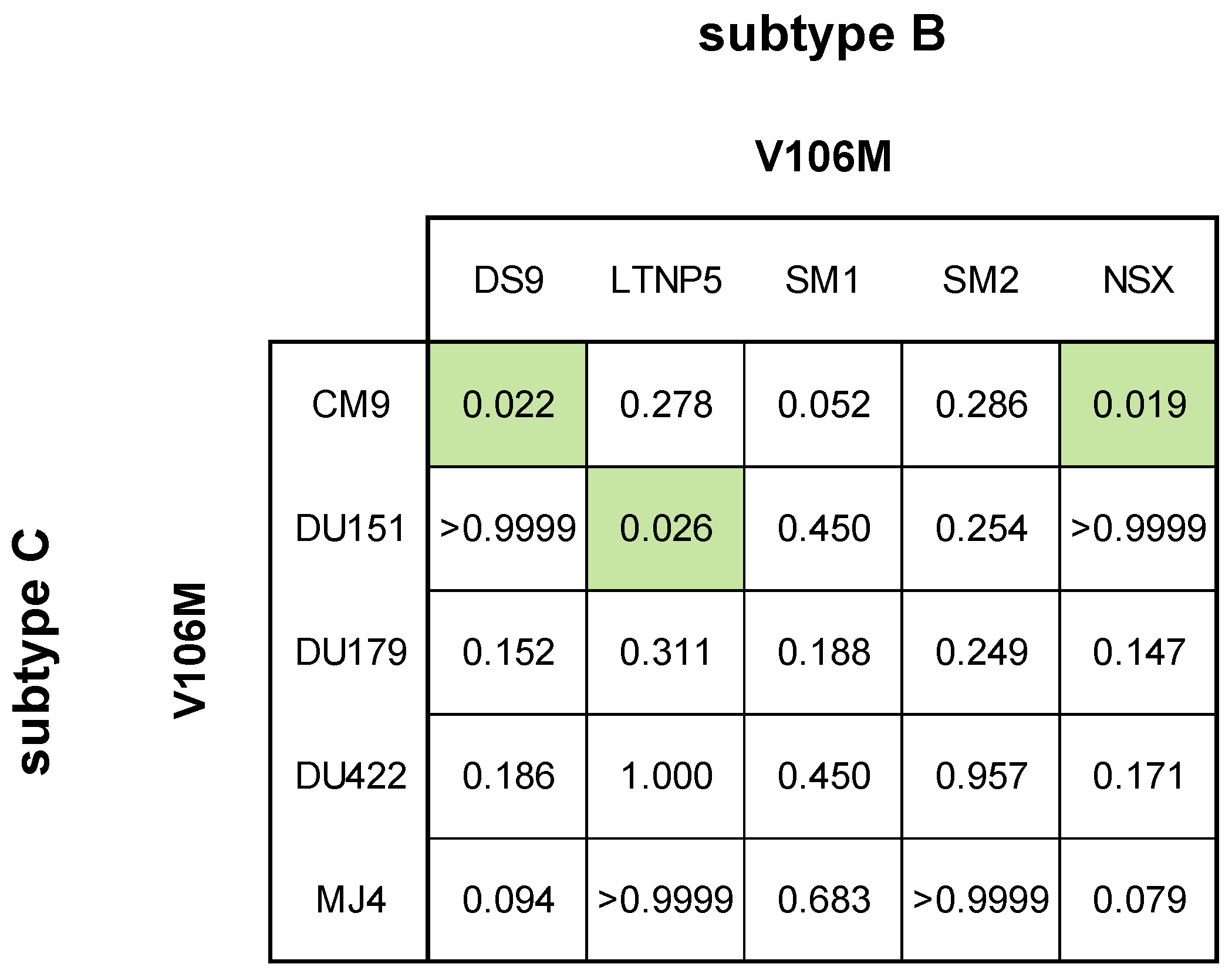
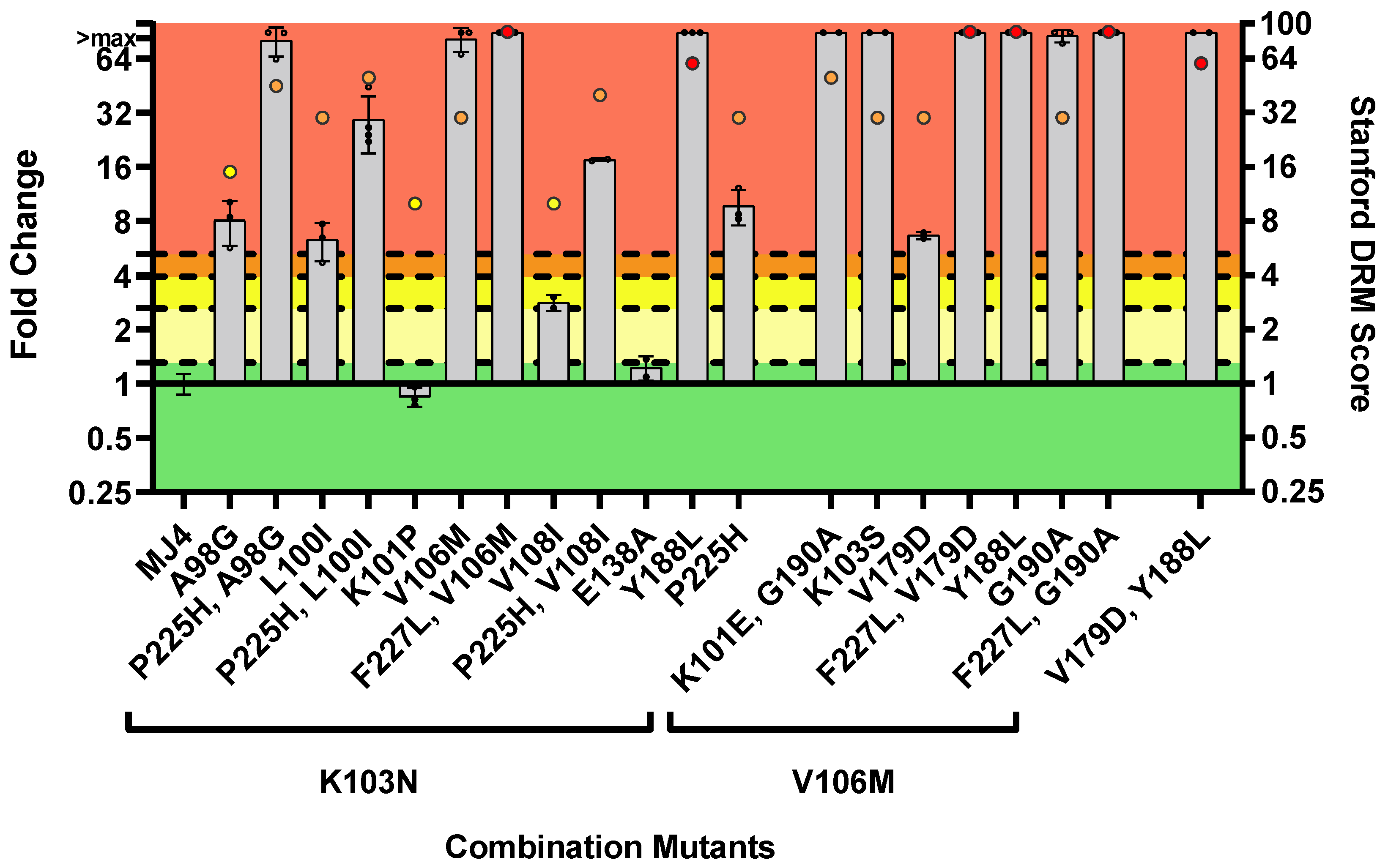
3.2.3. DOR Susceptibility of Combined NNRTI DRMs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hymes, K.B.; Cheung, T.; Greene, J.B.; Prose, N.S.; Marcus, A.; Ballard, H.; William, D.C.; Laubenstein, L.J. Kaposi’s Sarcoma in Homosexual Men-a Report of Eight Cases. Lancet 1981, 2, 598–600. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS. Global HIV & AIDS Statistics—Fact Sheet, 2023 Estimates; Joint United Nations Programme on HIV/AIDS: Geneva, Switzerland, 2023; pp. 365–367. [Google Scholar]
- WHO. Epidemiological Fact Sheet—HIV Statistics, Globally and by WHO Region, 2023; World Health Organ: Geneva, Switzerland, 2023. [Google Scholar]
- UNAIDS. Global HIV & AIDS Statistics—Country Fact Sheet—South Africa, 2023 Estimates; Joint United Nations Programme on HIV/AIDS: Geneva, Switzerland, 2023. [Google Scholar]
- Elangovan, R.; Jenks, M.; Yun, J.; Dickson-Tetteh, L.; Kirtley, S.; Hemelaar, J.; WHO-UNAIDS Network for HIV Isolation and Characterisation; Abimiku, A.G.; Agwale, S.; Archibald, C.; et al. Global and Regional Estimates for Subtype-Specific Therapeutic and Prophylactic HIV-1 Vaccines: A Modeling Study. Front. Microbiol. 2021, 12, 690647. [Google Scholar] [CrossRef] [PubMed]
- Giovanetti, M.; Ciccozzi, M.; Parolin, C.; Borsetti, A. Molecular Epidemiology of HIV-1 in African Countries: A Comprehensive Overview. Pathogens 2020, 9, 1072. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Menon, S.; Crowe, M.; Agarwal, N.; Biccler, J.; Bbosa, N.; Ssemwanga, D.; Adungo, F.; Moecklinghoff, C.; Macartney, M.; et al. Geographic and Population Distributions of Human Immunodeficiency Virus (HIV)–1 and HIV-2 Circulating Subtypes: A Systematic Literature Review and Meta-Analysis (2010–2021). J. Infect. Dis. 2023, 228, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, Z.; Wang, X.; Liu, H.; Ding, G.; Su, Y.; Zhu, L.; Wang, N. A Systematic Review of Cohort Studies of the Quality of Life in HIV/AIDS Patients after Antiretroviral Therapy. Int. J. STD AIDS 2014, 25, 771–777. [Google Scholar] [CrossRef]
- HIV-CAUSAL Collaboration; Ray, M.; Logan, R.; Sterne, J.A.C.; Hernández-Díaz, S.; Robins, J.M.; Sabin, C.; Bansi, L.; van Sighem, A.; de Wolf, F.; et al. The Effect of Combined Antiretroviral Therapy on the Overall Mortality of HIV-Infected Individuals. AIDS 2010, 24, 123–137. [Google Scholar] [CrossRef]
- Attia, S.; Egger, M.; Müller, M.; Zwahlen, M.; Low, N. Sexual Transmission of HIV According to Viral Load and Antiretroviral Therapy: Systematic Review and Meta-Analysis. AIDS 2009, 23, 1397. [Google Scholar] [CrossRef]
- Legesse, T.A.; Reta, M.A. Adherence to Antiretroviral Therapy and Associated Factors among People Living with HIV/AIDS in Hara Town and Its Surroundings, North-Eastern Ethiopia: A Cross-Sectional Study. Ethiop. J. Health Sci. 2019, 29, 299–308. [Google Scholar] [CrossRef]
- Bertagnolio, S.; Hermans, L.; Jordan, M.R.; Avila-Rios, S.; Iwuji, C.; Derache, A.; Delaporte, E.; Wensing, A.; Aves, T.; Borhan, A.S.M.; et al. Clinical Impact of Pretreatment Human Immunodeficiency Virus Drug Resistance in People Initiating Nonnucleoside Reverse Transcriptase Inhibitor–Containing Antiretroviral Therapy: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2021, 224, 377–388. [Google Scholar] [CrossRef]
- Wensing, A.M.; Calvez, V.; Ceccherini, F.; Charpentier, C.; Günthard, H.F.; Paredes, R.; Shafer, R.W.; Richman, D.D. 2022 Update of the Drug Resistance Mutations in HIV-1. Top. Antivir. Med. 2022, 30, 559–574. [Google Scholar]
- Gupta, R.; Gregson, J.; Parkin, N.; Haile-Selassie, H.; Tanuri, A.; Andrade Forero, L.; Kaleebu, P.; Watera, C.; Aghokeng, A.; Mutenda, N.; et al. HIV-1 Drug Resistance before Initiation or Re-Initiation of First-Line Antiretroviral Therapy in Low-Income and Middle-Income Countries: A Systematic Review and Meta-Regression Analysis. Lancet Infect. Dis. 2018, 18, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Moyo, S.; Hunt, G.; Zuma, K.; Zungu, M.; Marinda, E.; Mabaso, M.; Kana, V.; Kalimashe, M.; Ledwaba, J.; Naidoo, I.; et al. HIV Drug Resistance Profile in South Africa: Findings and Implications from the 2017 National HIV Household Survey. PLoS ONE 2020, 15, e0241071. [Google Scholar] [CrossRef] [PubMed]
- Tatz, G.; Mouton, H.; Chisholm, B.; Swart, A.; Uys, A.; Cohen, K. Adverse Reactions to Dolutegravir Reported to the National HIV & TB Health Care Worker Hotline in South Africa. Depression 2021, 1, 100. [Google Scholar]
- Bourgi, K.; Jenkins, C.A.; Rebeiro, P.F.; Palella, F.; Moore, R.D.; Altoff, K.N.; Gill, J.; Rabkin, C.S.; Gange, S.J.; Horberg, M.A.; et al. Weight Gain among Treatment-Naïve Persons with HIV Starting Integrase Inhibitors Compared to Non-Nucleoside Reverse Transcriptase Inhibitors or Protease Inhibitors in a Large Observational Cohort in the United States and Canada. J. Int. AIDS Soc. 2020, 23, e25484. [Google Scholar] [CrossRef] [PubMed]
- Sax, P.E.; Erlandson, K.M.; Lake, J.E.; Mccomsey, G.A.; Orkin, C.; Esser, S.; Brown, T.T.; Rockstroh, J.K.; Wei, X.; Carter, C.C.; et al. Weight Gain Following Initiation of Antiretroviral Therapy: Risk Factors in Randomized Comparative Clinical Trials. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 71, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Sachs, N.A.; Xu, M.; Grobler, J.; Blair, W.; Hazuda, D.J.; Miller, M.D.; Lai, M.-T. Doravirine Suppresses Common Nonnucleoside Reverse Transcriptase Inhibitor-Associated Mutants at Clinically Relevant Concentrations. Antimicrob. Agents Chemother. 2016, 60, 2241–2247. [Google Scholar] [CrossRef]
- Fulco, P.P.; Herity, L.B. Doravirine Use in Treatment-Experienced HIV-Positive Patients. Ann. Pharmacother. 2021, 55, 127–130. [Google Scholar] [CrossRef]
- Wong, A.; Goldstein, D.; Mallolas, J.; DeJesus, E.; Johnson, M.; Molina, J.-M.; Pozniak, A.; Rodgers, A.; Teal, V.; Hepler, D.; et al. Efficacy and Safety of Doravirine/Lamivudine/Tenofovir Disoproxil Fumarate (DOR/3TC/TDF) in Treatment-Naive Adults With HIV-1 and Transmitted Nonnucleoside Reverse Transcriptase Inhibitor Resistance Mutations. J. Acquir. Immune Defic. Syndr. 2019, 82, e47–e49. [Google Scholar] [CrossRef]
- Feng, M.; Wang, D.; Grobler, J.A.; Hazuda, D.J.; Miller, M.D.; Lai, M.-T. In vitro Resistance Selection with Doravirine (MK-1439), a Novel Nonnucleoside Reverse Transcriptase Inhibitor with Distinct Mutation Development Pathways. Antimicrob. Agents Chemother. 2014, 59, 590–598. [Google Scholar] [CrossRef]
- Lai, M.-T.; Feng, M.; Falgueyret, J.-P.; Tawa, P.; Witmer, M.; DiStefano, D.; Li, Y.; Burch, J.; Sachs, N.; Lu, M.; et al. In vitro Characterization of MK-1439, a Novel HIV-1 Nonnucleoside Reverse Transcriptase Inhibitor. Antimicrob. Agents Chemother. 2014, 58, 1652–1663. [Google Scholar] [CrossRef]
- Orkin, C.; Squires, K.E.; Molina, J.-M.; Sax, P.E.; Wong, W.-W.; Sussmann, O.; Kaplan, R.; Lupinacci, L.; Rodgers, A.; Xu, X.; et al. Doravirine/Lamivudine/Tenofovir Disoproxil Fumarate Is Non-Inferior to Efavirenz/Emtricitabine/Tenofovir Disoproxil Fumarate in Treatment-Naive Adults With Human Immunodeficiency Virus–1 Infection: Week 48 Results of the DRIVE-AHEAD Trial. Clin. Infect. Dis. 2019, 68, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.G.; Oliveira, M.; Ibanescu, R.-I.; Routy, J.-P.; Thomas, R. Cell Culture Selections Reveal Favourable Drug Resistance Profiles for Doravirine and Islatravir. J. Antimicrob. Chemother. 2021, 76, 2137–2142. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.J.; Pauly, G.T.; Akram, A.; Melody, K.; Ambrose, Z.; Schneider, J.P.; Hughes, S.H. Rilpivirine and Doravirine Have Complementary Efficacies Against NNRTI-Resistant HIV-1 Mutants. JAIDS J. Acquir. Immune Defic. Syndr. 2016, 72, 485. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.S.; Gilmartin, J.; Cilissen, C.; De Lepeleire, I.; Van Bortel, L.; Dockendorf, M.F.; Tetteh, E.; Ancona, J.K.; Liu, R.; Guo, Y.; et al. Safety, Tolerability and Pharmacokinetics of Doravirine, a Novel HIV Non-Nucleoside Reverse Transcriptase Inhibitor, after Single and Multiple Doses in Healthy Subjects. Antivir. Ther. 2015, 20, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Boyle, A.; Moss, C.E.; Marzolini, C.; Khoo, S. Clinical Pharmacodynamics, Pharmacokinetics, and Drug Interaction Profile of Doravirine. Clin. Pharmacokinet. 2019, 58, 1553–1565. [Google Scholar] [CrossRef]
- Orkin, C.; Squires, K.E.; Molina, J.-M.; Sax, P.E.; Sussmann, O.; Lin, G.; Kumar, S.; Hanna, G.J.; Hwang, C.; Martin, E.; et al. Doravirine/Lamivudine/Tenofovir Disoproxil Fumarate (TDF) Versus Efavirenz/Emtricitabine/TDF in Treatment-Naive Adults With Human Immunodeficiency Virus Type 1 Infection: Week 96 Results of the Randomized, Double-Blind, Phase 3 DRIVE-AHEAD Noninferiority Trial. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 73, 33–42. [Google Scholar] [CrossRef]
- Molina, J.-M.; Squires, K.; Sax, P.E.; Cahn, P.; Lombaard, J.; DeJesus, E.; Lai, M.-T.; Rodgers, A.; Lupinacci, L.; Kumar, S.; et al. Doravirine versus Ritonavir-Boosted Darunavir in Antiretroviral-Naive Adults with HIV-1 (DRIVE-FORWARD): 96-Week Results of a Randomised, Double-Blind, Non-Inferiority, Phase 3 Trial. Lancet HIV 2020, 7, e16–e26. [Google Scholar] [CrossRef]
- Orkin, C.; Molina, J.-M.; Cahn, P.; Lombaard, J.; Supparatpinyo, K.; Kumar, S.; Campbell, H.; Wan, H.; Teal, V.; Xu, Z.J.; et al. Safety and Efficacy of Doravirine as First-Line Therapy in Adults with HIV-1: Week 192 Results from the Open-Label Extensions of the DRIVE-FORWARD and DRIVE-AHEAD Phase 3 Trials. Lancet HIV 2024, 11, e75–e85. [Google Scholar] [CrossRef]
- Kumar, P.; Johnson, M.; Molina, J.-M.; Rizzardini, G.; Cahn, P.; Bickel, M.; Wan, H.; Xu, Z.J.; Morais, C.; Sklar, P.; et al. Brief Report: Switching to DOR/3TC/TDF Maintains HIV-1 Virologic Suppression Through Week 144 in the DRIVE-SHIFT Trial. J. Acquir. Immune Defic. Syndr. 2021, 87, 801–805. [Google Scholar] [CrossRef]
- EACS Guidelines Version 10.0 November 2019; European AIDS Clinical Society: Brussels, Belgium, 2019.
- FDA Approves First Two-Drug Complete Regimen for HIV-Infected Patients Who Have Never Received Antiretroviral Treatment; U.S. Department of Health and Human Services: Rockville, ML, USA, 2019.
- SAHPRA’s Registered Health Products Database; South African Health Products Regulatory Authority: Pretoria, South Africa, 2024; Available online: https://medapps.sahpra.org.za:6006/ (accessed on 16 September 2024).
- NDH. 2023 ART Clinical Guidelines for the Management of HIV in Adults, Pregnancy and Breastfeeding, Adolescents, Children, Infants and Neonates; Republic of South Africa National Department of Health: Pretoria, South Africa, 2023.
- Parry, C.M.; Kohli, A.; Boinett, C.J.; Towers, G.J.; McCormick, A.L.; Pillay, D. Gag Determinants of Fitness and Drug Susceptibility in Protease Inhibitor-Resistant Human Immunodeficiency Virus Type 1. J. Virol. 2009, 83, 9094–9101. [Google Scholar] [CrossRef]
- Gupta, R.K.; Kohli, A.; Mccormick, A.L.; Towers, G.J.; Pillay, D.; Parry, C.M. Full Length HIV-1 Gag Determines Protease Inhibitor Susceptibility within in vitro Assays. AIDS 2010, 24, 1651–1655. [Google Scholar] [CrossRef] [PubMed]
- Naldini, L.; Blömer, U.; Gallay, P.; Ory, D.; Mulligan, R.; Gage, F.H.; Verma, I.M.; Trono, D. In Vivo Gene Delivery and Stable Transduction of Nondividing Cells by a Lentiviral Vector. Science 1996, 272, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, Z.; Zhang, Y. A Simple and Economical Site-Directed Mutagenesis Method for Large Plasmids by Direct Transformation of Two Overlapping PCR Fragments. BioTechniques 2022, 73, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.F.; Shafer, R.W. Web Resources for HIV Type 1 Genotypic-Resistance Test Interpretation. Clin. Infect. Dis. 2006, 42, 1608–1618. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.-Y.; Gonzales, M.J.; Kantor, R.; Betts, B.J.; Ravela, J.; Shafer, R.W. Human Immunodeficiency Virus Reverse Transcriptase and Protease Sequence Database. Nucleic Acids Res. 2003, 31, 298–303. [Google Scholar] [CrossRef]
- Shafer, R.W. Rationale and Uses of a Public HIV Drug-Resistance Database. J. Infect. Dis. 2006, 194 (Suppl. S1), S51–S58. [Google Scholar] [CrossRef]
- Melikian, G.L.; Rhee, S.-Y.; Varghese, V.; Porter, D.; White, K.; Taylor, J.; Towner, W.; Troia, P.; Burack, J.; DeJesus, E.; et al. Non-Nucleoside Reverse Transcriptase Inhibitor (NNRTI) Cross-Resistance: Implications for Preclinical Evaluation of Novel NNRTIs and Clinical Genotypic Resistance Testing. J. Antimicrob. Chemother. 2014, 69, 12–20. [Google Scholar] [CrossRef]
- Rimsky, L.; Vingerhoets, J.; Van Eygen, V.; Eron, J.; Clotet, B.; Hoogstoel, A.; Boven, K.; Picchio, G. Genotypic and Phenotypic Characterization of HIV-1 Isolates Obtained From Patients on Rilpivirine Therapy Experiencing Virologic Failure in the Phase 3 ECHO and THRIVE Studies: 48-Week Analysis. JAIDS J. Acquir. Immune Defic. Syndr. 2012, 59, 39. [Google Scholar] [CrossRef]
- Tambuyzer, L.; Vingerhoets, J.; Azijn, H.; Daems, B.; Nijs, S.; de Béthune, M.-P.; Picchio, G. Characterization of Genotypic and Phenotypic Changes in HIV-1-Infected Patients with Virologic Failure on an Etravirine-Containing Regimen in the DUET-1 and DUET-2 Clinical Studies. Available online: https://www.liebertpub.com/doi/10.1089/aid.2009.0302 (accessed on 21 March 2024).
- Basson, A.E.; Charalambous, S.; Hoffmann, C.J.; Morris, L. HIV-1 Re-Suppression on a First-Line Regimen despite the Presence of Phenotypic Drug Resistance. PLoS ONE 2020, 15, e0234937. [Google Scholar] [CrossRef]
- Basson, A.E.; Rhee, S.-Y.; Parry, C.M.; El-Khatib, Z.; Charalambous, S.; De Oliveira, T.; Pillay, D.; Hoffmann, C.; Katzenstein, D.; Shafer, R.W.; et al. Impact of Drug Resistance-Associated Amino Acid Changes in HIV-1 Subtype C on Susceptibility to Newer Nonnucleoside Reverse Transcriptase Inhibitors. Antimicrob. Agents Chemother. 2015, 59, 960–971. [Google Scholar] [CrossRef]
- Steegen, K.; Bronze, M.; Papathanasopoulos, M.A.; van Zyl, G.; Goedhals, D.; Variava, E.; MacLeod, W.; Sanne, I.; Stevens, W.S.; Carmona, S. HIV-1 Antiretroviral Drug Resistance Patterns in Patients Failing NNRTI-Based Treatment: Results from a National Survey in South Africa. J. Antimicrob. Chemother. 2017, 72, 210–219. [Google Scholar] [CrossRef] [PubMed]
- NDH. National Consolidated Guidelines for the Prevention of Mother-to-Child Transmission of HIV and the Management of HIV in Children, Adolescents and Adults; National Department of Health: Pretoria, South Africa, 2015.
- NDH. 2019 ART Clinical Guidelines for the Management of HIV in Adults, Pregnancy and Breastfeeding, Adolescents, Children, Infants and Neonates; National Department of Health: Pretoria, South Africa, 2019.
- Jenny-Avital, E.R.; Stein, D.K. Persistence of HIV Drug Resistance Mutations: More Clues from Clinical Observations. Clin. Infect. Dis. 2004, 38, 1507–1508. [Google Scholar] [CrossRef] [PubMed]
- Little, S.J.; Frost, S.D.W.; Wong, J.K.; Smith, D.M.; Pond, S.L.K.; Ignacio, C.C.; Parkin, N.T.; Petropoulos, C.J.; Richman, D.D. Persistence of Transmitted Drug Resistance among Subjects with Primary Human Immunodeficiency Virus Infection. J. Virol. 2008, 82, 5510–5518. [Google Scholar] [CrossRef] [PubMed]
- Pao, D.; Andrady, U.; Clarke, J.; Dean, G.; Drake, S.; Fisher, M.; Green, T.; Kumar, S.; Murphy, M.; Tang, A.; et al. Long-Term Persistence of Primary Genotypic Resistance after HIV-1 Seroconversion. J. Acquir. Immune Defic. Syndr. 2004, 37, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Machnowska, P.; Meixenberger, K.; Schmidt, D.; Jessen, H.; Hillenbrand, H.; Gunsenheimer-Bartmeyer, B.; Hamouda, O.; Kücherer, C.; Bannert, N. Prevalence and Persistence of Transmitted Drug Resistance Mutations in the German HIV-1 Seroconverter Study Cohort. PLoS ONE 2019, 14, e0209605. [Google Scholar] [CrossRef]
- Van Zyl, G.U.; Liu, T.F.; Claassen, M.; Engelbrecht, S.; de Oliveira, T.; Preiser, W.; Wood, N.T.; Travers, S.; Shafer, R.W. Trends in Genotypic HIV-1 Antiretroviral Resistance between 2006 and 2012 in South African Patients Receiving First- and Second-Line Antiretroviral Treatment Regimens. PLoS ONE 2013, 8, e67188. [Google Scholar] [CrossRef]
- Kassaye, S.; Johnston, E.; McColgan, B.; Kantor, R.; Zijenah, L.; Katzenstein, D. Envelope Coreceptor Tropism, Drug Resistance, and Viral Evolution among Subtype C HIV-1-Infected Individuals Receiving Nonsuppressive Antiretroviral Therapy. J. Acquir. Immune Defic. Syndr. 2009, 50, 9–18. [Google Scholar] [CrossRef]
- Grossman, Z.; Istomin, V.; Averbuch, D.; Lorber, M.; Risenberg, K.; Levi, I.; Chowers, M.; Burke, M.; Yaacov, N.B.; Schapiro, J.M.; et al. Genetic Variation at NNRTI Resistance-Associated Positions in Patients Infected with HIV-1 Subtype C. AIDS 2004, 18, 909. [Google Scholar] [CrossRef]
- Brenner, B.; Turner, D.; Oliveira, M.; Moisi, D.; Detorio, M.; Carobene, M.; Marlink, R.G.; Schapiro, J.; Roger, M.; Wainberg, M.A. A V106M Mutation in HIV-1 Clade C Viruses Exposed to Efavirenz Confers Cross-Resistance to Non-Nucleoside Reverse Transcriptase Inhibitors. AIDS 2003, 17, F1–F5. [Google Scholar] [CrossRef]
- Bacheler, L.T.; Anton, E.D.; Kudish, P.; Baker, D.; Bunville, J.; Krakowski, K.; Bolling, L.; Aujay, M.; Wang, X.V.; Ellis, D.; et al. Human Immunodeficiency Virus Type 1 Mutations Selected in Patients Failing Efavirenz Combination Therapy. Antimicrob. Agents Chemother. 2000, 44, 2475–2484. [Google Scholar] [CrossRef]
- Reuman, E.C.; Rhee, S.-Y.; Holmes, S.P.; Shafer, R.W. Constrained Patterns of Covariation and Clustering of HIV-1 Non-Nucleoside Reverse Transcriptase Inhibitor Resistance Mutations. J. Antimicrob. Chemother. 2010, 65, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Asante-Appiah, E.; Lai, J.; Wan, H.; Yang, D.; Martin, E.A.; Sklar, P.; Hazuda, D.; Petropoulos, C.J.; Walworth, C.; Grobler, J.A. Impact of HIV-1 Resistance-Associated Mutations on Susceptibility to Doravirine: Analysis of Real-World Clinical Isolates. Antimicrob. Agents Chemother. 2021, 65, 10.1128/aac.01216-21. [Google Scholar] [CrossRef] [PubMed]
- Sterrantino, G.; Borghi, V.; Callegaro, A.P.; Bruzzone, B.; Saladini, F.; Maggiolo, F.; Maffongelli, G.; Andreoni, M.; De Gennaro, M.; Gianotti, N.; et al. Prevalence of Predicted Resistance to Doravirine in HIV-1-Positive Patients after Exposure to Non-Nucleoside Reverse Transcriptase Inhibitors. Int. J. Antimicrob. Agents 2019, 53, 515–519. [Google Scholar] [CrossRef]
- Rhee, S.-Y.; Schapiro, J.M.; Saladini, F.; Zazzi, M.; Khoo, S.; Shafer, R.W. Potential Role of Doravirine for the Treatment of HIV-1-Infected Persons with Transmitted Drug Resistance. AIDS Res. Ther. 2023, 20, 8. [Google Scholar] [CrossRef]
- Obasa, A.E.; Engelbrecht, S.; Jacobs, G.B. Near Full-Length HIV-1 Subtype B Sequences from the Early South African Epidemic, Detecting a BD Unique Recombinant Form (URF) from a Sample in 1985. Sci. Rep. 2019, 9, 6227. [Google Scholar] [CrossRef] [PubMed]
- Mikhail, M.; Wang, B.; Lemey, P.; Beckholdt, B.; Vandamme, A.-M.; Gill, M.J.; Saksena, N.K. Full-Length HIV Type 1 Genome Analysis Showing Evidence for HIV Type 1 Transmission from a Nonprogressor to Two Recipients Who Progressed to AIDS. AIDS Res. Hum. Retroviruses 2005, 21, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Ledwaba, J.; Sayed, Y.; Pillay, V.; Morris, L.; Hunt, G. Low Frequency of Protease Inhibitor Resistance Mutations and Insertions in HIV-1 Subtype C Protease Inhibitor-Naïve Sequences. AIDS Res. Hum. Retroviruses 2019, 35, 673–678. [Google Scholar] [CrossRef]
- Maldarelli, F.; Kearney, M.; Palmer, S.; Stephens, R.; Mican, J.; Polis, M.A.; Davey, R.T.; Kovacs, J.; Shao, W.; Rock-Kress, D.; et al. HIV Populations Are Large and Accumulate High Genetic Diversity in a Nonlinear Fashion. J. Virol. 2013, 87, 10313–10323. [Google Scholar] [CrossRef]
- Papathanasopoulos, M.A.; Cilliers, T.; Morris, L.; Mokili, J.L.; Dowling, W.; Birx, D.L.; McCutchan, F.E. Full-Length Genome Analysis of HIV-1 Subtype C Utilizing CXCR4 and Intersubtype Recombinants Isolated in South Africa. AIDS Res. Hum. Retroviruses 2002, 18, 879–886. [Google Scholar] [CrossRef]
- Williamson, C.; Morris, L.; Maughan, M.F.; Ping, L.-H.; Dryga, S.A.; Thomas, R.; Reap, E.A.; Cilliers, T.; van Harmelen, J.; Pascual, A.; et al. Characterization and Selection of HIV-1 Subtype C Isolates for Use in Vaccine Development. AIDS Res. Hum. Retroviruses 2003, 19, 133–144. [Google Scholar] [CrossRef]
- van Harmelen, J.; Williamson, C.; Kim, B.; Morris, L.; Carr, J.; Karim, S.S.; McCutchan, F. Characterization of Full-Length HIV Type 1 Subtype C Sequences from South Africa. AIDS Res. Hum. Retroviruses 2001, 17, 1527–1531. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reddy, N.; Papathanasopoulos, M.; Steegen, K.; Basson, A.E. K103N, V106M and Y188L Significantly Reduce HIV-1 Subtype C Phenotypic Susceptibility to Doravirine. Viruses 2024, 16, 1493. https://doi.org/10.3390/v16091493
Reddy N, Papathanasopoulos M, Steegen K, Basson AE. K103N, V106M and Y188L Significantly Reduce HIV-1 Subtype C Phenotypic Susceptibility to Doravirine. Viruses. 2024; 16(9):1493. https://doi.org/10.3390/v16091493
Chicago/Turabian StyleReddy, Nikita, Maria Papathanasopoulos, Kim Steegen, and Adriaan Erasmus Basson. 2024. "K103N, V106M and Y188L Significantly Reduce HIV-1 Subtype C Phenotypic Susceptibility to Doravirine" Viruses 16, no. 9: 1493. https://doi.org/10.3390/v16091493
APA StyleReddy, N., Papathanasopoulos, M., Steegen, K., & Basson, A. E. (2024). K103N, V106M and Y188L Significantly Reduce HIV-1 Subtype C Phenotypic Susceptibility to Doravirine. Viruses, 16(9), 1493. https://doi.org/10.3390/v16091493




