Unleashing Nature’s Allies: Comparing the Vertical Transmission Dynamics of Insect-Specific and Vertebrate-Infecting Flaviviruses in Mosquitoes
Abstract
:1. Introduction
2. Insect-Specific Flaviviruses (ISFs)
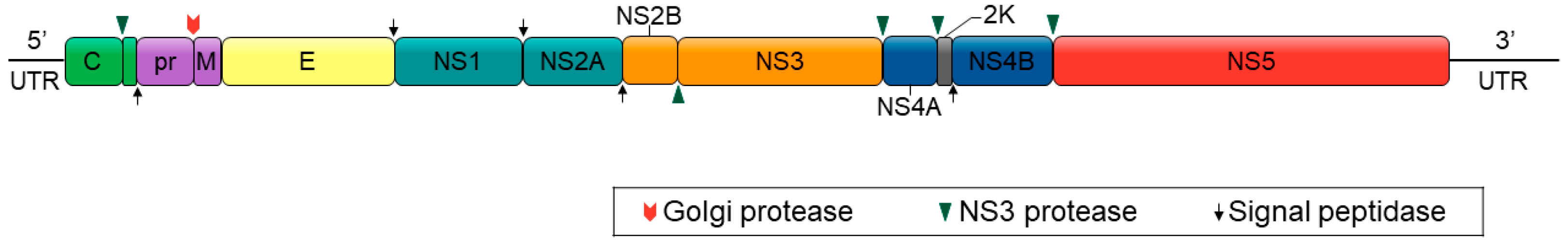
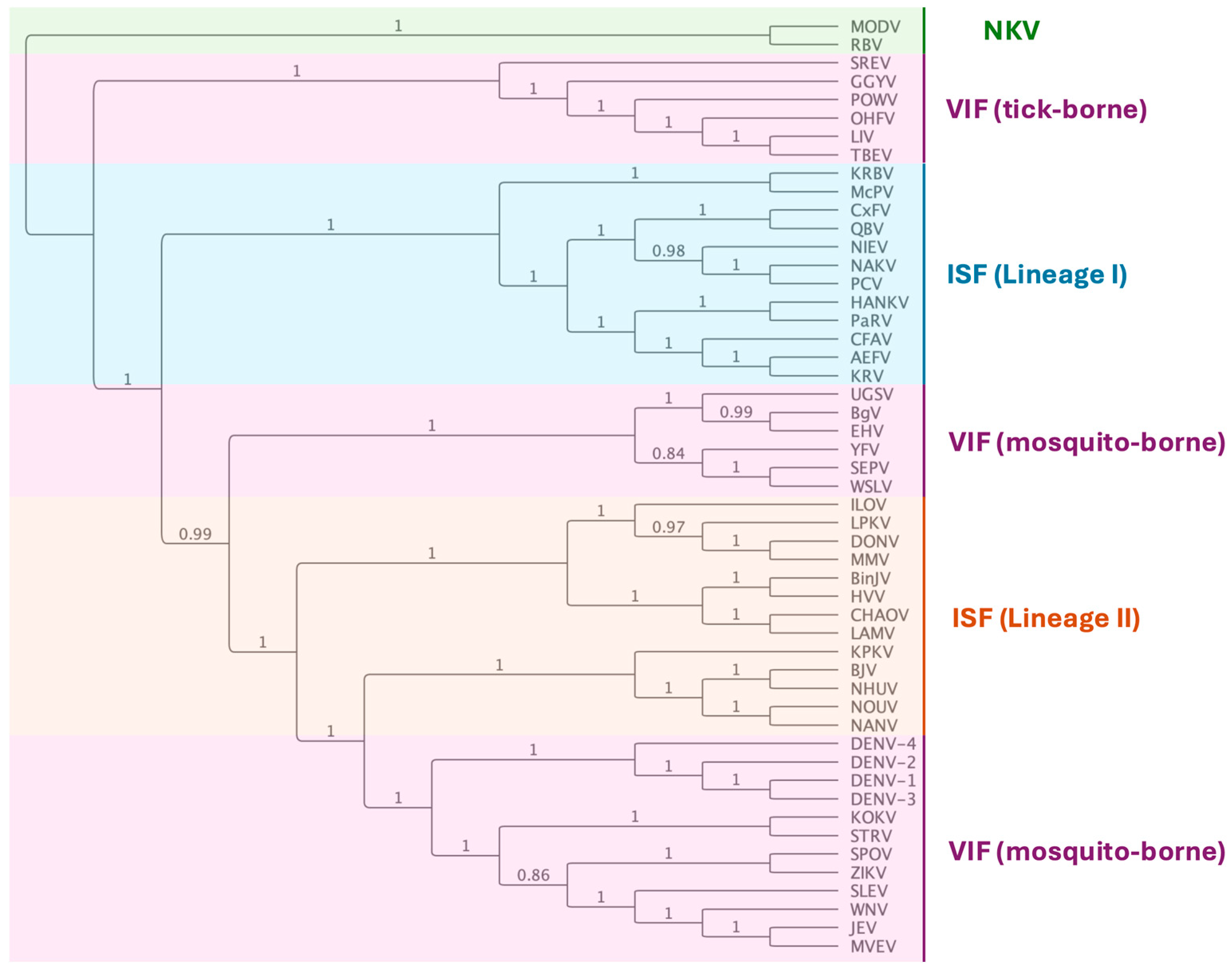
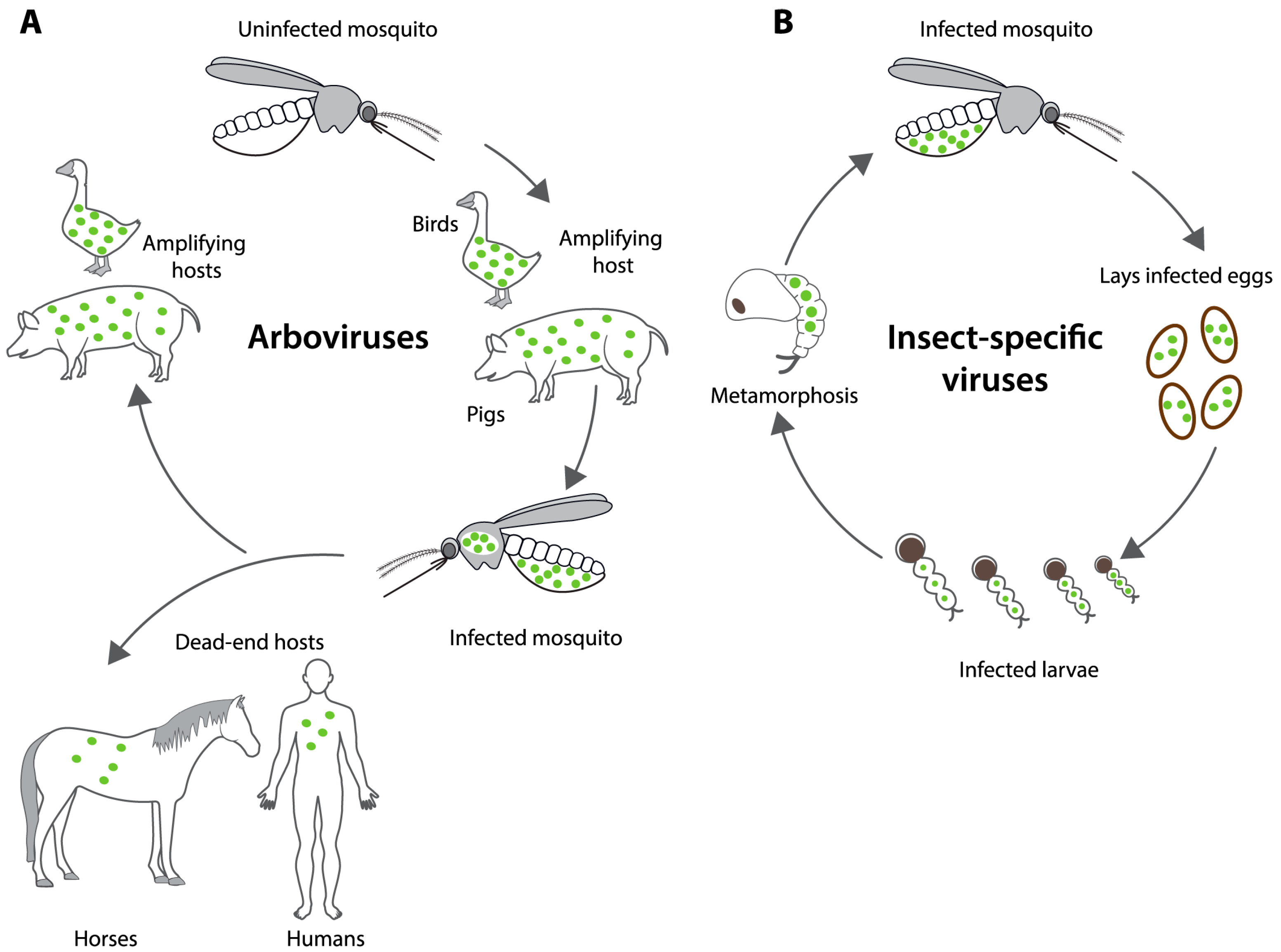
3. Vertical Transmission of Flaviviruses in Mosquitoes
3.1. Natural Occurrence of VIF and ISF Vertical Transmission in Mosquitoes
| Virus a | Mosquito | Strain | Design b | Detected c | FIR d /100 | MFIR /1000 | N | Reference |
|---|---|---|---|---|---|---|---|---|
| Vertebrate-infecting flaviviruses | ||||||||
| DENV(1–4) | Ae. aegypti | Havana, Cuba | L | qRT | 9.0 | 4102 | [70] | |
| DENV-1 | Ae. aegypti | Sao Paulo, Brazil | L | qRT | 0.0 | 910 | [67] | |
| DENV-1 | Ae. aegypti | Mexico | A | nRT | 0.1 | 2 390 | [71] | |
| DENV-2 | Ae. aegypti | Sinaloa, Mexico | L | RT | 6.5 | 308 | [72] | |
| DENV-2 | Ae. aegypti | Mexico | A | nRT | 1.9 | 2 390 | [71] | |
| DENV-3 | Ae. aegypti | Mexico | A | nRT | 2.5 | 2 390 | [71] | |
| DENV-3 | Ae. albopictus | Sao Paulo, Brazil | A | qRT | 0.6 | 3270 | [64] | |
| DENV-4 | Ae. aegypti | Mato Grosso, Brazil | L | qRT | 2.1 | 4490 | [68] | |
| DENV-4 | Ae. albopictus | Mato Grosso, Brazil | L | qRT | 7.0 | 296 | [68] | |
| DENV-4 | Ae. aegypti | Sinaloa, Mexico | A | RT | 22.3 | 672 | [73] | |
| DENV | Ae. aegypti | Yucatan, Mexico | A | qRT | 0.9 | 1278 | [74] | |
| ZIKV | Ae. aegypti | Amazonas, Brazil | L | qRT | 0.5 | 2057 | [58] | |
| ZIKV | Ae. aegypti | Mato Grosso, Brazil | L | qRT | 1.8 | 4490 | [68] | |
| ZIKV | Ae. albopictus | Mato Grosso, Brazil | L | qRT | 7.2 | 296 | [68] | |
| ZIKV | Ae. aegypti | Morelos, Mexico | L | qRT | 3.9 | 4300 | [75] | |
| ZIKV | Ae. aegypti | Yucatan, Mexico | A | qRT | 0.1 | 1278 | [74] | |
| Lineage I Insect-specific flaviviruses | ||||||||
| AeFV | Ae. luteocephalus | West Kenya | A | qRT | 44.1 | 68 | [76] | |
| AeFV | Ae. albopictus | Bangkok | C | RT | 100 | 130 | [77] | |
| An(g)FV | An. gambiae | West Kenya | A | qRT | 85.7 | 35 | [76] | |
| CFAV | Ae. aegypti | West Kenya | A | qRT | 4.1 | 729 | [76] | |
| CFAV | Ae. aegypti | Galveston | C | RT | 100 | 56 | [78] | |
| CFAV | Ae. aegypti | Galveston ♀ × Iquitos ♂ | C | RT | 92.6 | 68 | [78] | |
| CFAV | Ae. aegypti | Galveston ♂ × Iquitos ♀ | C | RT | 75.9 | 104 | [78] | |
| CxFV | Cx. pipiens | Colorado | C | qRT | 90.3 | 52 | [79] | |
| CxFV | Cx. pipiens | Iowa | A | qRT | 97.4 | 540 | [80] | |
| PARV | Ae. vigilax | Sydney | A | TC | 141.9 | [39] | ||
| PARV | Ae. vigilax | Brisbane | A | TC | 29.64 | [39] | ||
3.2. Experimentally Induced VIF and ISF Vertical Transmission Rates in Mosquitoes
| Virus a | Mosquito | Strain | Design b | Tested c | Detection d | FIR e /100 | MFIR e /1000 | n | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Vertebrate-infecting flaviviruses | |||||||||
| DENV-1 | Ae. albopictus | Guangdong | Or | L | qRT | 17.6 | 154 | [88] | |
| DENV-2 | Ae. aegypti | Mexico | Or (6) | L (GC1) | TC | 0 | 500 | [89] | |
| DENV-2 | Ae. aegypti | Mexico | Or (6) | L (GC2, 10 d) | TC | 27.7 | 980 | [89] | |
| DENV-2 | Ae. aegypti | Mexico | Or (6) | L (GC2, 21 d) | TC | 34.4 | 1180 | [89] | |
| DENV-2 | Ae. aegypti | Hano | Or | A | qRT | 0.2 | 222 | [92] | |
| WNV | Ae. vexans | Connecticut | Or (5) | A | qRT | 21.7 | 1389 | [93] | |
| ZIKV | Ae. aegypti | Manaus | Or (5) | A | qRT | 43.3 | 600 | [94] | |
| ZIKV | Ae. aegypti | UGAL | Or | A | qRT | 14.3 | 15 | [90] | |
| ZIKV | Ae. aegypti | Florida | Or (6) | A | qRT | 10 | 56 | [95] | |
| ZIKV | Ae. aegypti | Florida | Or (6) | A | qRT | 6.4 | 20 | [95] | |
| ZIKV | Ae. albopictus | Guangdong | Or (5) | L | RT | 53.3 | 75 | [96] | |
| ZIKV | Ae. albopictus | Beijing | Or (8) | A | qRT | 32.6 | 120 | [97] | |
| ZIKV | Ae. albopictus | GUA, GT | Or (6) | A | qRT | ≈5–6 | ≈15 | [91] | |
| ZIKV | Ae. albopictus | HC | Or (6) | A | qRT | 0 | ≈15 | [91] | |
| ZIKV | Ae. albopictus | Florida | Or (6) | A | qRT | 6.4 | 20 | [95] | |
| ZIKV | Ae. albopictus | Catalonia | IT (8) | A | TC | 50.25 | 559 | [98] | |
| Lineage I Insect-specific flaviviruses | |||||||||
| CFAV | Ae. aegypti | Bangkok | IT | A (F1) | RT | 78.3 | 60 | [86] | |
| CFAV | Ae. aegypti | Bangkok | IT | A (F2) | RT | 100.0 | 34 | [86] | |
| CFAV | Ae. aegypti | Mengei | IT (5) | A | qRT | 0 | 60 | [84] | |
| CFAV | Ae. aegypti | Haikou | IT (7) | A | qRT | 0 | 120 | [84] | |
| CFAV | Ae. aegypti | Poza Rico, Florida, Bangkok | IT (3) | L | qRT | 0.05 | 1962 | [85] | |
| CxFV | Cx. pipiens | Iowa | IT | A | RT | 0 | 950 | [80] | |
| KRV | Ae. aegypti | Nairobi | Or (7) | A | TC | 3.9 | 410 | [83] | |
| PCV | Cx. annulirostris | Brisbane | IT (5) | A | RT | 0 | 1038 | [40] | |
| Lineage II Insect-specific flaviviruses | |||||||||
| CHAOV | Ae. aegypti | UGAL | IT (2) | A | qRT | 100 | 27 | [87] | |
| CHAOV-ZIKV | Ae. aegypti | UGAL | IT (2) | A | qRT | ≈15 | 27 | [87] | |
| NHUV | Cx. pipiens | Florida | IT (9) | A | RT | 66 | 3 | [57] | |
4. Potential Determinants of ISF Vertical Transmission
4.1. Host Factors
4.2. Viral Factors
4.3. Microbiome
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Messina, J.P.; Brady, O.J.; Golding, N.; Kraemer, M.U.G.; Wint, G.R.W.; Ray, S.E.; Pigott, D.M.; Shearer, F.M.; Johnson, K.; Earl, L.; et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019, 4, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J. Dengue, urbanization and globalization: The unholy trinity of the 21(st) century. Trop. Med. Health 2011, 39, 3–11. [Google Scholar] [CrossRef]
- Kramer, L.D.; Styer, L.M.; Ebel, G.D. A global perspective on the epidemiology of West Nile virus. Annu. Rev. Entomol. 2008, 53, 61–81. [Google Scholar] [CrossRef] [PubMed]
- Mourad, O.; Makhani, L.; Chen, L.H. Chikungunya: An emerging public health concern. Curr. Infect. Dis. Rep. 2022, 24, 217–228. [Google Scholar] [CrossRef]
- Simonin, Y. Circulation of West Nile virus and Usutu virus in Europe: Overview and challenges. Viruses 2024, 16, 599. [Google Scholar] [CrossRef]
- Yakob, L.; Hu, W.; Frentiu, F.D.; Gyawali, N.; Hugo, L.E.; Johnson, B.; Lau, C.; Furuya-Kanamori, L.; Magalhaes, R.S.; Devine, G. Japanese encephalitis emergence in Australia: The potential population at risk. Clin. Infect. Dis. 2023, 76, 335–337. [Google Scholar] [CrossRef]
- de Souza, W.M.; Weaver, S.C. Effects of climate change and human activities on vector-borne diseases. Nat. Rev. Microbiol. 2024, 22, 476–491. [Google Scholar] [CrossRef]
- Mullard, A. FDA approves first chikungunya vaccine. Nat. Rev. Drug Discov. 2024, 23, 8. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Y.; Li, H.; Zhu, J.; Song, W.; Chen, K.; Zhang, Y.; Lou, Y. Vaccine development for mosquito-borne viral diseases. Front. Immunol. 2023, 14, 1161149. [Google Scholar] [CrossRef]
- Thomas, S.J. Is new dengue vaccine efficacy data a relief or cause for concern? NPJ Vaccines 2023, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- WHO. Launch of the WHO Global Arbovirus Initiative. Meeting Report; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Corbel, V.; Achee, N.L.; Chandre, F.; Coulibaly, M.B.; Dusfour, I.; Fonseca, D.M.; Grieco, J.; Juntarajumnong, W.; Lenhart, A.; Martins, A.J.; et al. Tracking insecticide resistance in mosquito vectors of arboviruses: The worldwide insecticide resistance network (WIN). PLoS Negl. Trop. Dis. 2016, 10, e0005054. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Q.; Zhang, L.; Liu, N. Chlorpyrifos resistance in mosquito Culex quinquefasciatus. J. Med. Entomol. 2005, 42, 815–820. [Google Scholar] [CrossRef]
- Silva, J.V.J., Jr.; Lopes, T.R.R.; Oliveira-Filho, E.F.; Oliveira, R.A.S.; Duraes-Carvalho, R.; Gil, L. Current status, challenges and perspectives in the development of vaccines against yellow fever, dengue, Zika and chikungunya viruses. Acta Trop. 2018, 182, 257–263. [Google Scholar] [CrossRef]
- Walker, T.; Johnson, P.H.; Moreira, L.A.; Iturbe-Ormaetxe, I.; Frentiu, F.D.; McMeniman, C.J.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P.; et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011, 476, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef]
- van den Hurk, A.F.; Hall-Mendelin, S.; Pyke, A.T.; Frentiu, F.D.; McElroy, K.; Day, A.; Higgs, S.; O’Neill, S.L. Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl. Trop. Dis. 2012, 6, e1892. [Google Scholar] [CrossRef]
- O’Neill, S.L. World Mosquito Program Annual Review 2022; The World Mosquito Program: Clayton, Australia, 2022. [Google Scholar]
- Utarini, A.; Indriani, C.; Ahmad, R.A.; Tantowijoyo, W.; Arguni, E.; Ansari, M.R.; Supriyati, E.; Wardana, D.S.; Meitika, Y.; Ernesia, I.; et al. Efficacy of Wolbachia-infected mosquito deployments for the control of dengue. N. Engl. J. Med. 2021, 384, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.B.; Riback, T.I.S.; Sylvestre, G.; Costa, G.; Peixoto, J.; Dias, F.B.S.; Tanamas, S.K.; Simmons, C.P.; Dufault, S.M.; Ryan, P.A.; et al. Effectiveness of Wolbachia-infected mosquito deployments in reducing the incidence of dengue and other Aedes-borne diseases in Niteroi, Brazil: A quasi-experimental study. PLoS Negl. Trop. Dis. 2021, 15, e0009556. [Google Scholar] [CrossRef]
- Ross, P.A.; Wiwatanaratanabutr, I.; Axford, J.K.; White, V.L.; Endersby-Harshman, N.M.; Hoffmann, A.A. Wolbachia Infections in Aedes aegypti differ markedly in their response to cyclical heat stress. PLoS Pathog. 2017, 13, e1006006. [Google Scholar] [CrossRef]
- Blitvich, B.J.; Firth, A.E. Insect-specific flaviviruses: A systematic review of their discovery, host range, mode of transmission, superinfection exclusion potential and genomic organization. Viruses 2015, 7, 1927–1959. [Google Scholar] [CrossRef]
- Patterson, E.I.; Villinger, J.; Muthoni, J.N.; Dobel-Ober, L.; Hughes, G.L. Exploiting insect-specific viruses as a novel strategy to control vector-borne disease. Curr. Opin. Insect Sci. 2020, 39, 50–56. [Google Scholar] [CrossRef]
- Hall, R.A.; Bielefeldt-Ohmann, H.; McLean, B.J.; O’Brien, C.A.; Colmant, A.M.; Piyasena, T.B.; Harrison, J.J.; Newton, N.D.; Barnard, R.T.; Prow, N.A.; et al. Commensal viruses of mosquitoes: Host restriction, transmission, and interaction with arboviral pathogens. Evol. Bioinform. Online 2016, 12, 35–44. [Google Scholar] [CrossRef]
- Hobson-Peters, J.; Yam, A.W.; Lu, J.W.; Setoh, Y.X.; May, F.J.; Kurucz, N.; Walsh, S.; Prow, N.A.; Davis, S.S.; Weir, R.; et al. A new insect-specific flavivirus from northern Australia suppresses replication of West Nile virus and Murray Valley encephalitis virus in co-infected mosquito cells. PLoS ONE 2013, 8, e56534. [Google Scholar] [CrossRef]
- Carvalho, V.L.; Long, M.T. Insect-Specific Viruses: An overview and their relationship to arboviruses of concern to humans and animals. Virology 2021, 557, 34–43. [Google Scholar] [CrossRef]
- Nouri, S.; Matsumura, E.E.; Kuo, Y.W.; Falk, B.W. Insect-specific viruses: From discovery to potential translational applications. Curr. Opin. Virol. 2018, 33, 33–41. [Google Scholar] [CrossRef]
- Vasilakis, N.; Guzman, H.; Firth, C.; Forrester, N.L.; Widen, S.G.; Wood, T.G.; Rossi, S.L.; Ghedin, E.; Popov, V.; Blasdell, K.R.; et al. Mesoniviruses are mosquito-specific viruses with extensive geographic distribution and host range. Virol. J. 2014, 11, 97. [Google Scholar] [CrossRef]
- Vasilakis, N.; Forrester, N.L.; Palacios, G.; Nasar, F.; Savji, N.; Rossi, S.L.; Guzman, H.; Wood, T.G.; Popov, V.; Gorchakov, R.; et al. Negevirus: A proposed new taxon of insect-specific viruses with wide geographic distribution. J. Virol. 2013, 87, 2475–2488. [Google Scholar] [CrossRef]
- Boshra, H. An Overview of the Infectious Cycle of Bunyaviruses. Viruses 2022, 14, 2139. [Google Scholar] [CrossRef] [PubMed]
- Newton, N.D.; Colmant, A.M.G.; O’Brien, C.A.; Ledger, E.; Paramitha, D.; Bielefeldt-Ohmann, H.; Watterson, D.; McLean, B.J.; Hall-Mendelin, S.; Warrilow, D.; et al. Genetic, Morphological and Antigenic Relationships between Mesonivirus Isolates from Australian Mosquitoes and Evidence for Their Horizontal Transmission. Viruses 2020, 12, 1159. [Google Scholar] [CrossRef] [PubMed]
- Nasar, F.; Haddow, A.D.; Tesh, R.B.; Weaver, S.C. Eilat virus displays a narrow mosquito vector range. Parasit. Vectors 2014, 7, 595. [Google Scholar] [CrossRef]
- Elrefaey, A.M.; Abdelnabi, R.; Rosales Rosas, A.L.; Wang, L.; Basu, S.; Delang, L. Understanding the mechanisms underlying host restriction of insect-specific viruses. Viruses 2020, 12, 964. [Google Scholar] [CrossRef]
- Cook, S.; Moureau, G.; Kitchen, A.; Gould, E.A.; de Lamballerie, X.; Holmes, E.C.; Harbach, R.E. Molecular evolution of the insect-specific flaviviruses. J. Gen. Virol. 2012, 93, 223–234. [Google Scholar] [CrossRef]
- Kurnia, N.; Kaitana, Y.; Salaki, C.L.; Mandey, L.C.; Tuda, J.S.B.; Tallei, T.E. Study of dengue virus transovarial transmission in Aedes spp. in Ternate City using streptavidin-biotin-peroxidase complex immunohistochemistry. Infect. Dis. Rep. 2022, 14, 765–771. [Google Scholar] [CrossRef]
- Goenaga, S.; Goenaga, J.; Boaglio, E.R.; Enria, D.A.; Levis, S.D.C. Superinfection exclusion studies using West Nile virus and Culex flavivirus strains from Argentina. Mem. Inst. Oswaldo Cruz. 2020, 115, e200012. [Google Scholar] [CrossRef]
- Kenney, J.L.; Solberg, O.D.; Langevin, S.A.; Brault, A.C. Characterization of a novel insect-specific flavivirus from Brazil: Potential for inhibition of infection of arthropod cells with medically important flaviviruses. J. Gen. Virol. 2014, 95, 2796–2808. [Google Scholar] [CrossRef]
- McLean, B.J.; Hall-Mendelin, S.; Webb, C.E.; Bielefeldt-Ohmann, H.; Ritchie, S.A.; Hobson-Peters, J.; Hall, R.A.; van den Hurk, A.F. The insect-specific Parramatta River virus is vertically transmitted by Aedes vigilax mosquitoes and suppresses replication of pathogenic flaviviruses in vitro. Vector Borne Zoonotic Dis. 2021, 21, 208–215. [Google Scholar] [CrossRef]
- Hall-Mendelin, S.; McLean, B.J.; Bielefeldt-Ohmann, H.; Hobson-Peters, J.; Hall, R.A.; van den Hurk, A.F. The insect-specific Palm Creek virus modulates West Nile virus infection in and transmission by Australian mosquitoes. Parasit. Vectors 2016, 9, 414. [Google Scholar] [CrossRef]
- de Almeida, J.P.; Aguiar, E.R.; Armache, J.N.; Olmo, R.P.; Marques, J.T. The virome of vector mosquitoes. Curr. Opin. Virol. 2021, 49, 7–12. [Google Scholar] [CrossRef]
- Laureti, M.; Narayanan, D.; Rodriguez-Andres, J.; Fazakerley, J.K.; Kedzierski, L. Flavivirus receptors: Diversity, identity, and cell entry. Front. Immunol. 2018, 9, 2180. [Google Scholar] [CrossRef]
- Lindenbach, B.D.; Randal, G.; Bartenschlager, R.; Rice, C.M. Flaviviridae: The viruses and their replication. In Fields Virology: Emerging Viruses; Howley, P.M., Knipe, D.M., Whelan, S., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2020. [Google Scholar]
- Howley, P.M. Fields Virology, 7th ed.; Whelan, S., Ed.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2020; Volume 1, p. 840. [Google Scholar]
- Harrison, J.J.; Hobson-Peters, J.; Colmant, A.M.G.; Koh, J.; Newton, N.D.; Warrilow, D.; Bielefeldt-Ohmann, H.; Piyasena, T.B.H.; O’Brien, C.A.; Vet, L.J.; et al. Antigenic characterization of new lineage II insect-specific flaviviruses in Australian mosquitoes and identification of host restriction factors. mSphere 2020, 5. [Google Scholar] [CrossRef]
- Stollar, V.; Thomas, V.L. An agent in the Aedes aegypti cell line (Peleg) which causes fusion of Aedes albopictus cells. Virology 1975, 64, 367–377. [Google Scholar] [CrossRef]
- Sang, R.C.; Gichogo, A.; Gachoya, J.; Dunster, M.D.; Ofula, V.; Hunt, A.R.; Crabtree, M.B.; Miller, B.R.; Dunster, L.M. Isolation of a new flavivirus related to cell fusing agent virus (CFAV) from field-collected flood-water Aedes mosquitoes sampled from a dambo in central Kenya. Arch. Virol. 2003, 148, 1085–1093. [Google Scholar] [CrossRef]
- Crabtree, M.B.; Sang, R.C.; Stollar, V.; Dunster, L.M.; Miller, B.R. Genetic and phenotypic characterization of the newly described insect flavivirus, Kamiti River virus. Arch. Virol. 2003, 148, 1095–1118. [Google Scholar] [CrossRef]
- Olmo, R.P.; Todjro, Y.M.H.; Aguiar, E.; de Almeida, J.P.P.; Ferreira, F.V.; Armache, J.N.; de Faria, I.J.S.; Ferreira, A.G.A.; Amadou, S.C.G.; Silva, A.T.S.; et al. Mosquito vector competence for dengue is modulated by insect-specific viruses. Nat. Microbiol. 2023, 8, 135–149. [Google Scholar] [CrossRef]
- Bolling, B.G.; Olea-Popelka, F.J.; Eisen, L.; Moore, C.G.; Blair, C.D. Transmission dynamics of an insect-specific flavivirus in a naturally infected Culex pipiens laboratory colony and effects of co-infection on vector competence for West Nile virus. Virology 2012, 427, 90–97. [Google Scholar] [CrossRef]
- Barbanti-Brodano, G.; Swetly, P.; Koprowski, H. Superinfection of simian virus 40-transformed permissive cells with simian virus 40. J. Virol. 1970, 6, 644–651. [Google Scholar] [CrossRef]
- Burivong, P.; Pattanakitsakul, S.N.; Thongrungkiat, S.; Malasit, P.; Flegel, T.W. Markedly reduced severity of Dengue virus infection in mosquito cell cultures persistently infected with Aedes albopictus densovirus (AalDNV). Virology 2004, 329, 261–269. [Google Scholar] [CrossRef]
- Pepin, K.M.; Lambeth, K.; Hanley, K.A. Asymmetric competitive suppression between strains of dengue virus. BMC Microbiol. 2008, 8, 28. [Google Scholar] [CrossRef]
- Baidaliuk, A.; Miot, E.F.; Lequime, S.; Moltini-Conclois, I.; Delaigue, F.; Dabo, S.; Dickson, L.B.; Aubry, F.; Merkling, S.H.; Cao-Lormeau, V.M.; et al. Cell-fusing agent virus reduces arbovirus dissemination in Aedes aegypti mosquitoes In Vivo. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Whelan, S.; Goldman, N. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol. Biol. Evol. 2001, 18, 691–699. [Google Scholar] [CrossRef]
- Goenaga, S.; Kenney, J.L.; Duggal, N.K.; Delorey, M.; Ebel, G.D.; Zhang, B.; Levis, S.C.; Enria, D.A.; Brault, A.C. Potential for co-infection of a mosquito-specific flavivirus, Nhumirim virus, to block West Nile virus transmission in mosquitoes. Viruses 2015, 7, 5801–5812. [Google Scholar] [CrossRef]
- da Costa, C.F.; da Silva, A.V.; do Nascimento, V.A.; de Souza, V.C.; Monteiro, D.; Terrazas, W.C.M.; Dos Passos, R.A.; Nascimento, S.; Lima, J.B.P.; Naveca, F.G. Evidence of vertical transmission of Zika virus in field-collected eggs of Aedes aegypti in the Brazilian Amazon. PLoS Negl. Trop. Dis. 2018, 12, e0006594. [Google Scholar] [CrossRef]
- Rosen, L.; Shroyer, D.A.; Tesh, R.B.; Freier, J.E.; Lien, J.C. Transovarial transmission of dengue viruses by mosquitoes: Aedes albopictus and Aedes aegypti. Am. J. Trop. Med. Hyg. 1983, 32, 1108–1119. [Google Scholar] [CrossRef]
- Rosen, L. Further observations on the mechanism of vertical transmission of flaviviruses by Aedes mosquitoes. Am. J. Trop. Med. Hyg. 1988, 39, 123–126. [Google Scholar] [CrossRef]
- Agboli, E.; Leggewie, M.; Altinli, M.; Schnettler, E. Mosquito-specific viruses-transmission and interaction. Viruses 2019, 11, 873. [Google Scholar] [CrossRef]
- Lequime, S.; Paul, R.E.; Lambrechts, L. Determinants of arbovirus vertical transmission in mosquitoes. PLoS Pathog. 2016, 12, e1005548. [Google Scholar] [CrossRef]
- Grunnill, M.; Boots, M. How important is vertical tansmission of dengue viruses by mosquitoes (Diptera: Culicidae)? J. Med. Entomol. 2016, 53, 1–19. [Google Scholar] [CrossRef]
- Ferreira-de-Lima, V.H.; Lima-Camara, T.N. Natural vertical transmission of dengue virus in Aedes aegypti and Aedes albopictus: A systematic review. Parasit. Vectors 2018, 11, 77. [Google Scholar] [CrossRef]
- Bergren, N.A.; Kading, R.C. The ecological significance and implications of transovarial transmission among the vector-borne bunyaviruses: A review. Insects 2018, 9, 173. [Google Scholar] [CrossRef]
- Dahiya, N.; Yadav, M.; Yadav, A.; Sehrawat, N. Zika virus vertical transmission in mosquitoes: A less understood mechanism. J. Vector Borne Dis. 2022, 59, 37–44. [Google Scholar] [CrossRef]
- Moraes, A.; Cortelli, F.C.; Miranda, T.B.; Aquino, D.R.; Cortelli, J.R.; Guimaraes, M.I.A.; Costa, F.O.; Cortelli, S.C. Transovarial transmission of dengue 1 virus in Aedes aegypti larvae: Real-time PCR analysis in a Brazilian city with high mosquito population density. Can. J. Microbiol. 2018, 64, 393–400. [Google Scholar] [CrossRef]
- Maia, L.M.S.; Bezerra, M.C.F.; Costa, M.C.S.; Souza, E.M.; Oliveira, M.E.B.; Ribeiro, A.L.M.; Miyazaki, R.D.; Slhessarenko, R.D. Natural vertical infection by dengue virus serotype 4, Zika virus and Mayaro virus in Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus. Med. Vet. Entomol. 2019, 33, 437–442. [Google Scholar] [CrossRef]
- Adams, B.; Boots, M. How important is vertical transmission in mosquitoes for the persistence of dengue? Insights from a mathematical model. Epidemics 2010, 2, 1–10. [Google Scholar] [CrossRef]
- Gutierrez-Bugallo, G.; Rodriguez-Roche, R.; Diaz, G.; Perez, M.; Mendizabal, M.E.; Peraza, I.; Vazquez, A.A.; Alvarez, M.; Rodriguez, M.; Bisset, J.A.; et al. Spatio-temporal distribution of vertically transmitted dengue viruses by Aedes aegypti (Diptera: Culicidae) from Arroyo Naranjo, Havana, Cuba. Trop. Med. Int. Health 2018, 23, 1342–1349. [Google Scholar] [CrossRef]
- Danis-Lozano, R.; Diaz-Gonzalez, E.E.; Malo-Garcia, I.R.; Rodriguez, M.H.; Ramos-Castaneda, J.; Juarez-Palma, L.; Ramos, C.; Lopez-Ordonez, T.; Mosso-Gonzalez, C.; Fernandez-Salas, I. Vertical transmission of dengue virus in Aedes aegypti and its role in the epidemiological persistence of dengue in Central and Southern Mexico. Trop. Med. Int. Health 2019, 24, 1311–1319. [Google Scholar] [CrossRef]
- Apodaca-Medina, A.I.; Torres-Avendano, J.I.; Rendon-Maldonado, J.G.; Torres-Montoya, E.H.; Flores-Lopez, B.A.; Del Angel, R.M.; Velarde-Felix, J.S.; Salomon-Soto, V.M.; Castillo-Ureta, H. First evidence of vertical infection of dengue Virus 2 in Aedes aegypti mosquitoes from Sinaloa, Mexico. Vector Borne Zoonotic Dis. 2018, 18, 231–233. [Google Scholar] [CrossRef]
- Torres-Avendano, J.I.; Apodaca-Medina, A.I.; Castillo-Ureta, H.; Rendon-Maldonado, J.G.; Torres-Montoya, E.H.; Cota-Medina, A.; Rios-Tostado, J.J.; Zazueta-Moreno, J.M. Natural vertical transmission of dengue virus serotype 4 in Aedes aegypti larvae from urban areas in Sinaloa, Mexico. Vector Borne Zoonotic Dis. 2021, 21, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Kirstein, O.D.; Talavera, G.A.; Wei, Z.; Ciau-Carrilo, K.J.; Koyoc-Cardena, E.; Puerta-Guardo, H.; Rodriguez-Martin, E.; Medina-Barreiro, A.; Mendoza, A.C.; Piantadosi, A.L.; et al. Natural Aedes-borne virus infection detected in male adult Aedes aegypti (Diptera: Culicidae) collected from urban settings in Merida, Yucatan, Mexico. J. Med. Entomol. 2022, 59, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Suzan, M.; Zarate, S.; Torres-Flores, J.; Correa-Morales, F.; Gonzalez-Acosta, C.; Sevilla-Reyes, E.E.; Lira, R.; Alcaraz-Estrada, S.L.; Yocupicio-Monroy, M. Natural vertical transmission of Zika virus in larval Aedes aegypti populations, Morelos, Mexico. Emerg. Infect. Dis. 2019, 25, 1477–1484. [Google Scholar] [CrossRef]
- Ajamma, Y.U.; Onchuru, T.O.; Ouso, D.O.; Omondi, D.; Masiga, D.K.; Villinger, J. Vertical transmission of naturally occurring Bunyamwera and insect-specific flavivirus infections in mosquitoes from islands and mainland shores of Lakes Victoria and Baringo in Kenya. PLoS Negl. Trop. Dis. 2018, 12, e0006949. [Google Scholar] [CrossRef]
- Peinado, S.A.; Aliota, M.T.; Blitvich, B.J.; Bartholomay, L.C. Biology and transmission dynamics of Aedes flavivirus. J. Med. Entomol. 2022, 59, 659–666. [Google Scholar] [CrossRef]
- Logan, R.A.E.; Quek, S.; Muthoni, J.N.; von Eicken, A.; Brettell, L.E.; Anderson, E.R.; Villena, M.E.N.; Hegde, S.; Patterson, G.T.; Heinz, E.; et al. Vertical and horizontal transmission of cell fusing agent virus in Aedes aegypti. Appl. Environ. Microbiol. 2022, 88, e0106222. [Google Scholar] [CrossRef]
- Bolling, B.G.; Eisen, L.; Moore, C.G.; Blair, C.D. Insect-specific flaviviruses from Culex mosquitoes in Colorado, with evidence of vertical transmission. Am. J. Trop. Med. Hyg. 2011, 85, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Saiyasombat, R.; Bolling, B.G.; Brault, A.C.; Bartholomay, L.C.; Blitvich, B.J. Evidence of efficient transovarial transmission of Culex flavivirus by Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 2011, 48, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Tang, W.; Briggs, C.; Hopson, H.; Juarez, J.G.; Garcia-Luna, S.M.; de Valdez, M.W.; Badillo-Vargas, I.E.; Borucki, M.K.; Frank, M.; et al. Cell fusing agent virus (Flavivirus) infection in Aedes aegypti in Texas: Seasonality, comparison by trap type, and individual viral loads. Arch. Virol. 2020, 165, 1769–1776. [Google Scholar] [CrossRef]
- Colmant, A.M.G.; Hobson-Peters, J.; Bielefeldt-Ohmann, H.; van den Hurk, A.F.; Hall-Mendelin, S.; Chow, W.K.; Johansen, C.A.; Fros, J.; Simmonds, P.; Watterson, D.; et al. A new clade of insect-specific faviviruses from Australian Anopheles mosquitoes displays species-specific host restriction. mSphere 2017, 2. [Google Scholar] [CrossRef]
- Lutomiah, J.J.; Mwandawiro, C.; Magambo, J.; Sang, R.C. Infection and vertical transmission of Kamiti river virus in laboratory bred Aedes aegypti mosquitoes. J. Insect Sci. 2007, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Huang, E.; Guo, X.; Xiong, Y.; Xie, J.; Cai, T.; Du, Y.; Wu, Q.; Guo, S.; Han, W.; et al. Cell fusing agent virus isolated from Aag2 cells does not vertically transmit in Aedes aegypti via artificial infection. Parasit. Vectors 2023, 16, 402. [Google Scholar] [CrossRef] [PubMed]
- Nag, D.K.; Efner, K. Cell fusing agent virus rarely transmits vertically in artificially infected laboratory-colonized Aedes aegypti mosquitoes. Parasit. Vectors 2024, 17, 177. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Gutierrez, M.A.; Guzman, H.; Thangamani, S.; Vasilakis, N.; Tesh, R.B. Experimental Infection with and maintenance of cell fusing agent virus (Flavivirus) in Aedes aegypti. Am. J. Trop. Med. Hyg. 2017, 97, 299–304. [Google Scholar] [CrossRef]
- Wen, D.; Ding, L.S.; Zhang, Y.; Li, X.; Zhang, X.; Yuan, F.; Zhao, T.; Zheng, A. Suppression of flavivirus transmission from animal hosts to mosquitoes with a mosquito-delivered vaccine. Nat. Commun. 2022, 13, 7780. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, J.; Yang, L.; Li, C.; Chen, R.; Xie, Z.; Ren, R. A predominant dengue virus-1 endemic strain and the vector competence of Aedes albopictus from Guangzhou City, China. Acta Trop. 2019, 199, 104975. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vargas, I.; Harrington, L.C.; Doty, J.B.; Black, W.C.t.; Olson, K.E. Demonstration of efficient vertical and venereal transmission of dengue virus type-2 in a genetically diverse laboratory strain of Aedes aegypti. PLoS Negl. Trop. Dis. 2018, 12, e0006754. [Google Scholar] [CrossRef]
- Comeau, G.; Zinna, R.A.; Scott, T.; Ernst, K.; Walker, K.; Carriere, Y.; Riehle, M.A. Vertical transmission of Zika virus in Aedes aegypti produces potentially infectious progeny. Am. J. Trop. Med. Hyg. 2020, 103, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Guo, J.; Li, Y. Wolbachia wPip blocks Zika virus transovarial transmission in Aedes albopictus. Microbiol. Spectr. 2022, 10, e0263321. [Google Scholar] [CrossRef]
- Goncalves, D.D.S.; Hue, K.D.T.; Thuy, V.T.; Tuyet, N.V.; Thi, G.N.; Thi Thuy, V.H.; Xuan, T.H.T.; Thi, D.L.; Vo, L.T.; Le Anh Huy, H.; et al. Assessing the vertical transmission potential of dengue virus in field-reared Aedes aegypti using patient-derived blood meals in Ho Chi Minh City, Vietnam. Parasit. Vectors 2020, 13, 468. [Google Scholar] [CrossRef]
- Anderson, J.F.; Main, A.J.; Ferrandino, F.J. Horizontal and vertical transmission of West Nile virus by Aedes vexans (Diptera: Culicidae). J. Med. Entomol. 2020, 57, 1614–1618. [Google Scholar] [CrossRef]
- Chaves, B.A.; Junior, A.B.V.; Silveira, K.R.D.; Paz, A.D.C.; Vaz, E.; Araujo, R.G.P.; Rodrigues, N.B.; Campolina, T.B.; Orfano, A.D.S.; Nacif-Pimenta, R.; et al. Vertical transmission of Zika virus (Flaviviridae, Flavivirus) in Amazonian Aedes aegypti (Diptera: Culicidae) delays egg hatching and larval development of progeny. J. Med. Entomol. 2019, 56, 1739–1744. [Google Scholar] [CrossRef]
- Zimler, R.A.; Alto, B.W. Vertical transmission of Zika virus by Florida Aedes aegypti and Ae. albopictus. Insects 2023, 14, 289. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, C.; Deng, Y.; Jiang, Y.; Sun, A.; Liu, Q.; Dong, Y.; Xing, D.; Cao, W.; Qin, C.; et al. Vector competence and vertical transmission of Zika virus in Aedes albopictus (Diptera: Culicidae). Vector Borne Zoonotic Dis. 2020, 20, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jiang, Y.; Li, C.; Gao, J.; Zhao, T.; Zhang, H.; Li, C.; Xing, D.; Dong, Y.; Zhao, T.; et al. Survival and replication of Zika virus in diapause eggs of Aedes albopictus from Beijing, China. Front. Microbiol. 2022, 13, 924334. [Google Scholar] [CrossRef] [PubMed]
- Nunez, A.I.; Talavera, S.; Birnberg, L.; Rivas, R.; Pujol, N.; Verdun, M.; Aranda, C.; Berdugo, M.; Busquets, N. Evidence of Zika virus horizontal and vertical transmission in Aedes albopictus from Spain but not infectious virus in saliva of the progeny. Emerg. Microbes. Infect. 2020, 9, 2236–2244. [Google Scholar] [CrossRef]
- Torres, F.J.; Parry, R.; Hugo, L.E.; Slonchak, A.; Newton, N.D.; Vet, L.J.; Modhiran, N.; Pullinger, B.; Wang, X.; Potter, J.; et al. Reporter flaviviruses as tools to demonstrate homologous and heterologous superinfection Exclusion. Viruses 2022, 14, 1501. [Google Scholar] [CrossRef]
- Lee, W.S.; Webster, J.A.; Madzokere, E.T.; Stephenson, E.B.; Herrero, L.J. Mosquito antiviral defense mechanisms: A delicate balance between innate immunity and persistent viral infection. Parasit. Vectors 2019, 12, 165. [Google Scholar] [CrossRef]
- Samuel, G.H.; Adelman, Z.N.; Myles, K.M. Antiviral immunity and virus-mediated antagonism in disease vector mosquitoes. Trends Microbiol. 2018, 26, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Blair, C.D. Deducing the role of virus genome-derived PIWI-associated RNAs in the mosquito-arbovirus arms race. Front. Genet. 2019, 10, 1114. [Google Scholar] [CrossRef]
- Rodriguez-Andres, J.; Axford, J.; Hoffmann, A.; Fazakerley, J. Mosquito transgenerational antiviral immunity is mediated by vertical transfer of virus DNA sequences and RNAi. iScience 2024, 27, 108598. [Google Scholar] [CrossRef]
- Suzuki, Y.; Baidaliuk, A.; Miesen, P.; Frangeul, L.; Crist, A.B.; Merkling, S.H.; Fontaine, A.; Lequime, S.; Moltini-Conclois, I.; Blanc, H.; et al. Non-retroviral endogenous viral element limits cognate virus replication in Aedes aegypti ovaries. Curr. Biol. 2020, 30, 3495–3506.e6. [Google Scholar] [CrossRef]
- Goic, B.; Stapleford, K.A.; Frangeul, L.; Doucet, A.J.; Gausson, V.; Blanc, H.; Schemmel-Jofre, N.; Cristofari, G.; Lambrechts, L.; Vignuzzi, M.; et al. Virus-derived DNA drives mosquito vector tolerance to arboviral infection. Nat. Commun. 2016, 7, 12410. [Google Scholar] [CrossRef] [PubMed]
- Palatini, U.; Contreras, C.A.; Gasmi, L.; Bonizzoni, M. Endogenous viral elements in mosquito genomes: Current knowledge and outstanding questions. Curr. Opin. Insect Sci. 2022, 49, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Crochu, S.; Cook, S.; Attoui, H.; Charrel, R.N.; De Chesse, R.; Belhouchet, M.; Lemasson, J.J.; de Micco, P.; de Lamballerie, X. Sequences of flavivirus-related RNA viruses persist in DNA form integrated in the genome of Aedes spp. mosquitoes. J. Gen. Virol. 2004, 85, 1971–1980. [Google Scholar] [CrossRef]
- Piyasena, T.B.H.; Newton, N.D.; Hobson-Peters, J.; Vet, L.J.; Setoh, Y.X.; Bielefeldt-Ohmann, H.; Khromykh, A.A.; Hall, R.A. Chimeric viruses of the insect-specific flavivirus Palm Creek with structural proteins of vertebrate-infecting flaviviruses identify barriers to replication of insect-specific flaviviruses in vertebrate cells. J. Gen. Virol. 2019, 100, 1580–1586. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, D.; Yuan, F.; Yan, Y.; Wang, Z.; Liu, P.; Yu, Q.; Zhang, X.; Wang, X.; Zheng, A. Replication is the key barrier during the dual-host adaptation of mosquito-borne flaviviruses. Proc. Natl. Acad. Sci. USA 2022, 119, e2110491119. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Y.; Li, S.; Yuan, F.; Wen, D.; Jia, N.; Xiong, T.; Zhang, X.; Zheng, A. Broad host tropism of flaviviruses during the entry stage. Microbiol. Spectr. 2023, 11, e0528122. [Google Scholar] [CrossRef] [PubMed]
- Junglen, S.; Korries, M.; Grasse, W.; Wieseler, J.; Kopp, A.; Hermanns, K.; Leon-Juarez, M.; Drosten, C.; Kummerer, B.M. Host range restriction of insect-specific flaviviruses occurs at several levels of the viral life cycle. mSphere 2017, 2. [Google Scholar] [CrossRef]
- Schnettler, E.; Sreenu, V.B.; Mottram, T.; McFarlane, M. Wolbachia restricts insect-specific flavivirus infection in Aedes aegypti cells. J. Gen. Virol. 2016, 97, 3024–3029. [Google Scholar] [CrossRef]
- Diaz, S.; Camargo, C.; Avila, F.W. Characterization of the reproductive tract bacterial microbiota of virgin, mated, and blood-fed Aedes aegypti and Aedes albopictus females. Parasit. Vectors 2021, 14, 592. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Shi, P.; Li, J.; Niu, J.; Chen, J.; Wang, G.; Wu, L.; Chen, L.; Yang, Z.; et al. A naturally isolated symbiotic bacterium suppresses flavivirus transmission by Aedes mosquitoes. Science 2024, 384, eadn9524. [Google Scholar] [CrossRef]
- do Nascimento, R.M.; Campolina, T.B.; Chaves, B.A.; Delgado, J.L.F.; Godoy, R.S.M.; Pimenta, P.F.P.; Secundino, N.F.C. The influence of culture-dependent native microbiota in Zika virus infection in Aedes aegypti. Parasit. Vectors 2022, 15, 57. [Google Scholar] [CrossRef]
- Garrigos, M.; Garrido, M.; Panisse, G.; Veiga, J.; Martinez-de la Puente, J. Interactions between West Nile virus and the microbiota of Culex pipiens vectors: A literature review. Pathogens 2023, 12, 1287. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peterson, A.J.; Hall, R.A.; Harrison, J.J.; Hobson-Peters, J.; Hugo, L.E. Unleashing Nature’s Allies: Comparing the Vertical Transmission Dynamics of Insect-Specific and Vertebrate-Infecting Flaviviruses in Mosquitoes. Viruses 2024, 16, 1499. https://doi.org/10.3390/v16091499
Peterson AJ, Hall RA, Harrison JJ, Hobson-Peters J, Hugo LE. Unleashing Nature’s Allies: Comparing the Vertical Transmission Dynamics of Insect-Specific and Vertebrate-Infecting Flaviviruses in Mosquitoes. Viruses. 2024; 16(9):1499. https://doi.org/10.3390/v16091499
Chicago/Turabian StylePeterson, Alyssa J., Roy A. Hall, Jessica J. Harrison, Jody Hobson-Peters, and Leon E. Hugo. 2024. "Unleashing Nature’s Allies: Comparing the Vertical Transmission Dynamics of Insect-Specific and Vertebrate-Infecting Flaviviruses in Mosquitoes" Viruses 16, no. 9: 1499. https://doi.org/10.3390/v16091499
APA StylePeterson, A. J., Hall, R. A., Harrison, J. J., Hobson-Peters, J., & Hugo, L. E. (2024). Unleashing Nature’s Allies: Comparing the Vertical Transmission Dynamics of Insect-Specific and Vertebrate-Infecting Flaviviruses in Mosquitoes. Viruses, 16(9), 1499. https://doi.org/10.3390/v16091499








