Evaluating the Use of Sacran, a Polysaccharide Isolated from Aphanothece sacrum, as a Possible Microbicide for Preventing HIV-1 Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Sacran Gel
2.2. Cell Lines and Culture
2.3. Virus Preparation
2.4. Flow Cytometric Detection of HIV-1-Infected Cells
2.5. Cytotoxicity Assay
2.6. Cell–Cell Fusion Assay
2.7. Transwell Experiments to Test the Barrier Function of Sacran
2.8. Transwell Experiments for TZM-bl Assay
2.9. TZM-bl Assay
2.10. Quantification of Synergistic Effects of Sacran with Anti-HIV-1 Agents
2.11. Statistical Analysis
3. Results
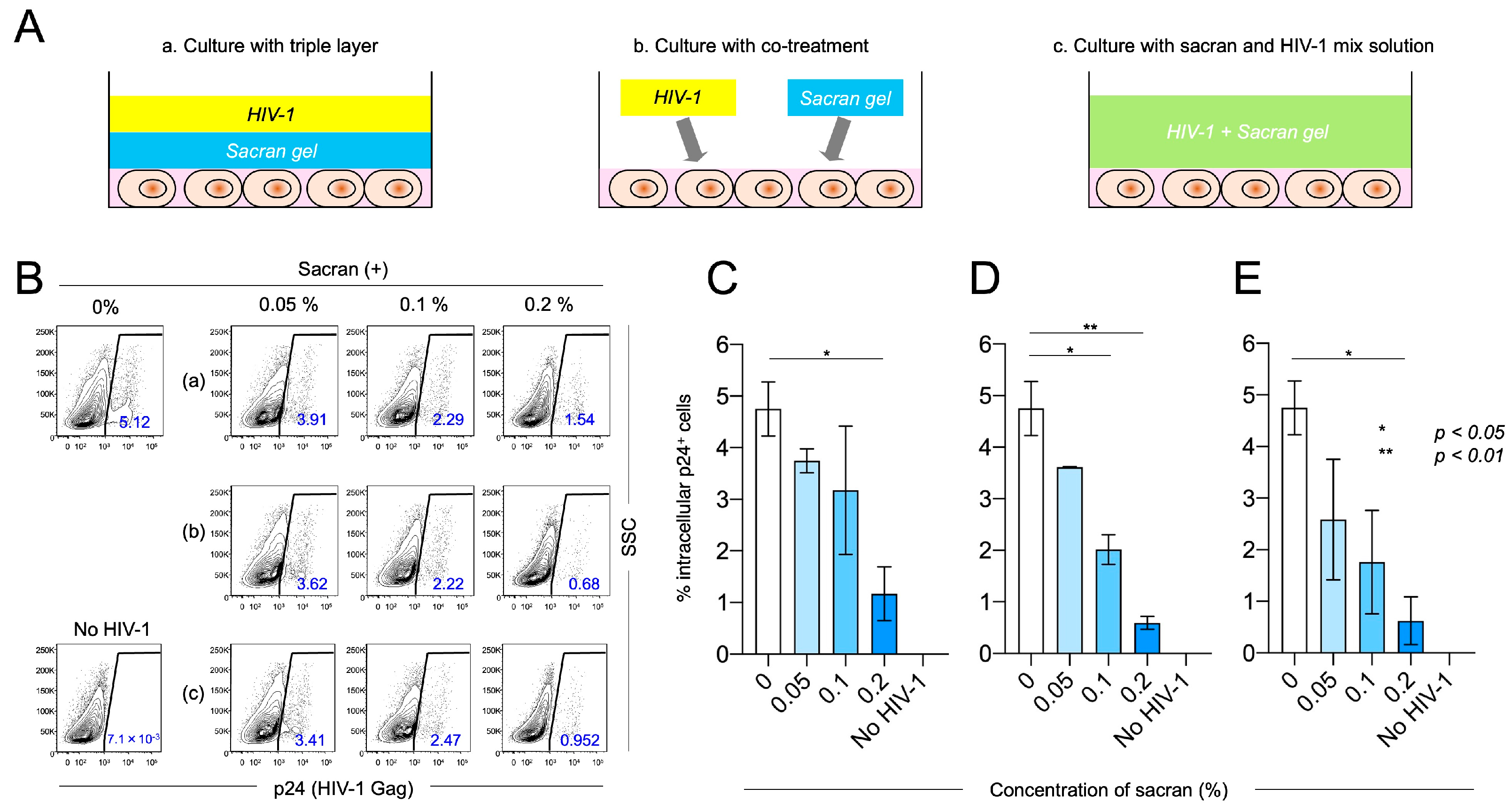
3.1. Sacran Inhibited HIV-1 Infection
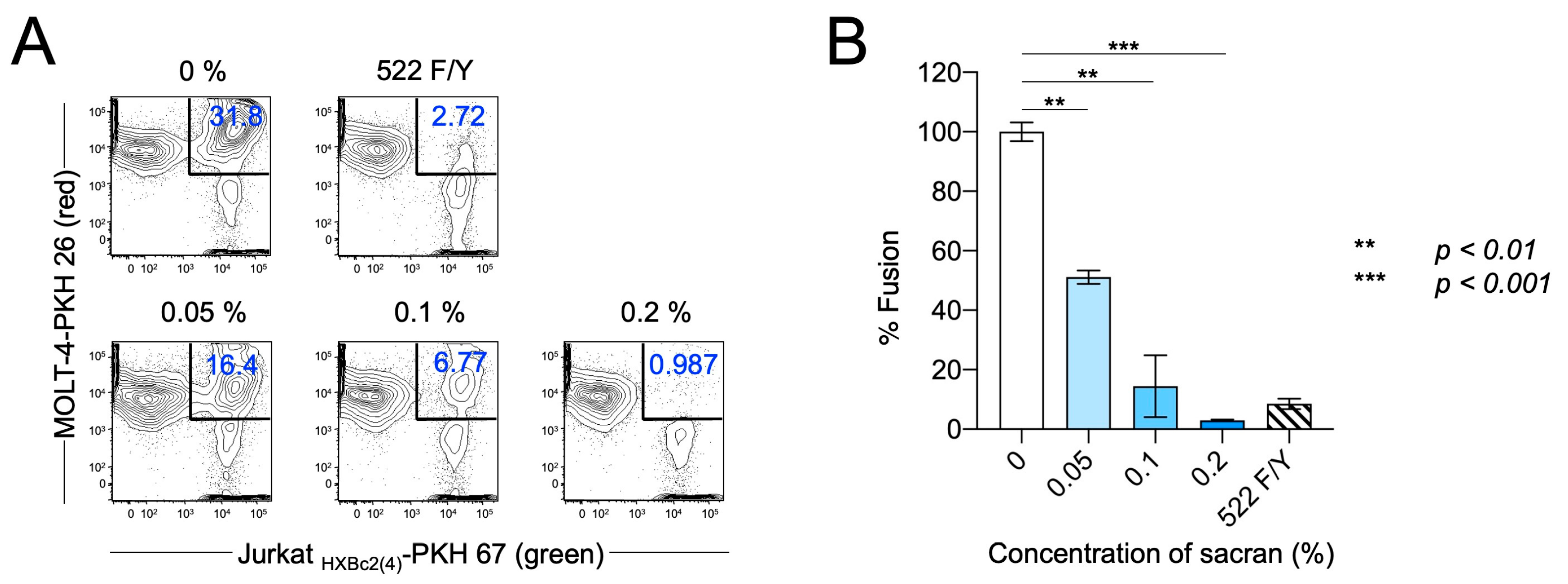
3.2. Sacran Inhibited HIV-1 Envelope-Dependent Cell Fusion
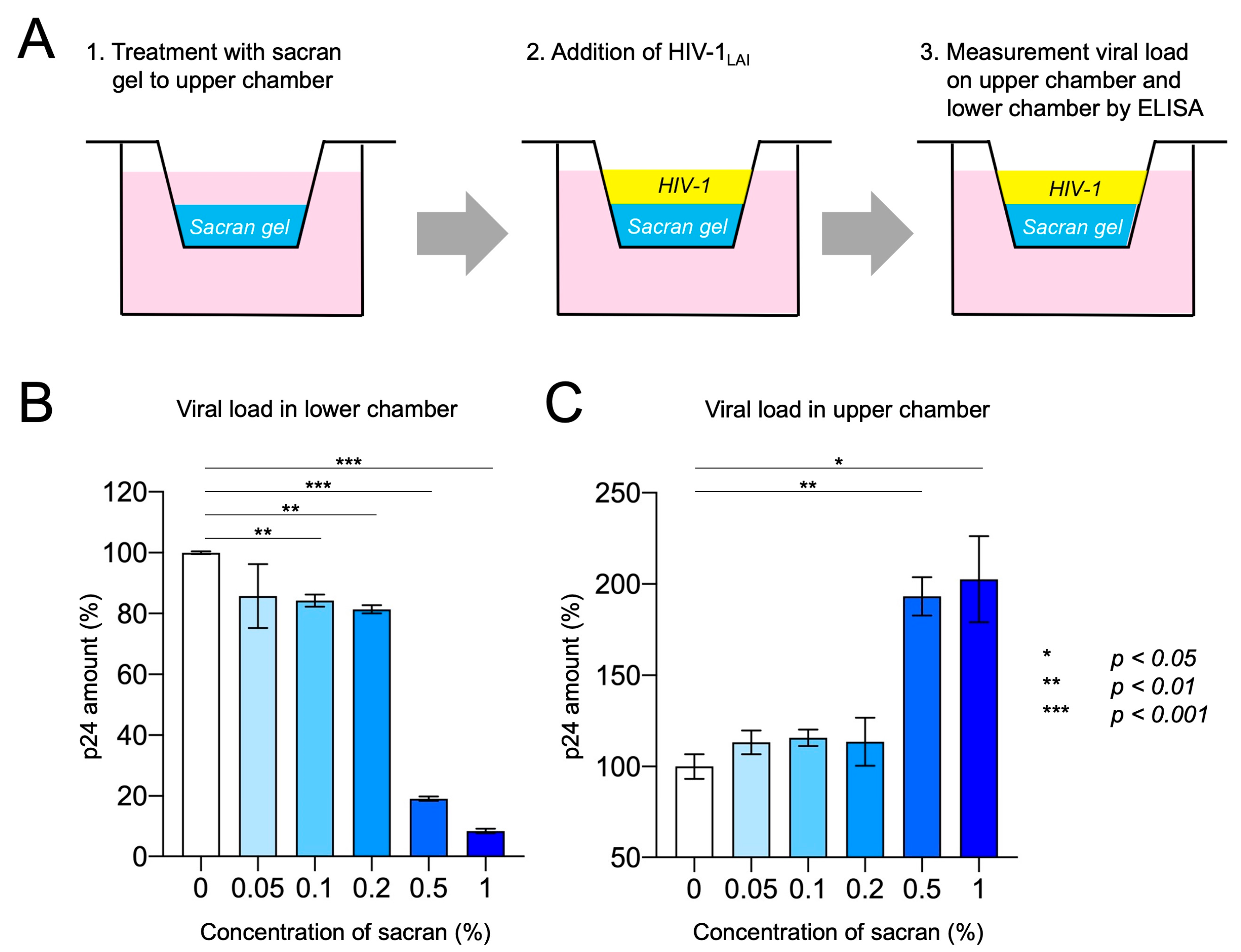
3.3. Sacran Inhibited Viral Diffusion and Captured HIV-1
3.4. Synergistic HIV-1 Inhibition of Sacran and Anti-HIV-1 Drugs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNAIDS. 2023 Global AIDS Update. 2024. Available online: http://www.unaids.org/en (accessed on 21 September 2024).
- McConville, C.; Boyd, P.; Major, I. Efficacy of Tenofovir 1% Vaginal Gel in Reducing the Risk of HIV-1 and HSV-2 Infection. Clin. Med. Insights Womens Health 2014, 7, CMWH-S10353. [Google Scholar] [CrossRef]
- Musekiwa, A.; Fernando, N.B.; Abariga, S.A. Effectiveness of Vaginal Microbicides in Preventing HIV Transmission. Trop. Med. Int. Health 2020, 25, 790–802. [Google Scholar] [CrossRef]
- Parikh, U.M.; Mellors, J.W. How Could HIV-1 Drug Resistance Impact Preexposure Prophylaxis for HIV Prevention? Curr. Opin. HIV AIDS 2022, 17, 213–221. [Google Scholar] [CrossRef]
- Cutler, B.; Justman, J. Vaginal Microbicides and the Prevention of HIV Transmission. Lancet Infect. Dis. 2008, 8, 685–697. [Google Scholar] [CrossRef]
- Veazey, R.S.; Ketas, T.A.; Klasse, P.J.; Davison, D.K.; Singletary, M.; Green, L.C.; Greenberg, M.L.; Moore, J.P. Tropism-Independent Protection of Macaques against Vaginal Transmission of Three SHIVs by the HIV-1 Fusion Inhibitor T-1249. Proc. Natl. Acad. Sci. USA 2008, 105, 10531–10536. [Google Scholar] [CrossRef]
- Abdool Karim, Q.; Abdool Karim, S.S.; Frohlich, J.A.; Grobler, A.C.; Baxter, C.; Mansoor, L.E.; Kharsany, A.B.; Sibeko, S.; Mlisana, K.P.; Omar, Z.; et al. Effectiveness and Safety of Tenofovir Gel, an Antiretroviral Microbicide, for the Prevention of HIV Infection in Women. Science 2010, 329, 1168–1174. [Google Scholar] [CrossRef]
- Marrazzo, J.M.; Ramjee, G.; Richardson, B.A.; Gomez, K.; Mgodi, N.; Nair, G.; Palanee, T.; Nakabiito, C.; van der Straten, A.; Noguchi, L.; et al. Tenofovir-Based Preexposure Prophylaxis for HIV Infection among African Women. N. Engl. J. Med. 2015, 372, 509–518. [Google Scholar] [CrossRef]
- Maiko, K.; Okajima, T.B.; Kaneso, Y.; Hirata, K.; Fukusaki, E.; Kajiyama, S.; Kaneko, T. Supergiant Ampholytic Sugar Chains with Imbalanced Charge Ratio Form Saline Ultra-Absorbent Hydrogels. Macromolecules 2008, 41, 4061–4536. [Google Scholar] [CrossRef]
- Okajima, M.K.; Miyazato, S.; Kaneko, T. Cyanobacterial Megamolecule Sacran Efficiently Forms LC Gels with Very Heavy Metal Ions. Langmuir 2009, 25, 8526–8531. [Google Scholar] [CrossRef]
- Okajima, M.K.; Nakamura, M.; Mitsumata, T.; Kaneko, T. Cyanobacterial Polysaccharide Gels with Efficient Rare-Earth-Metal Sorption. Biomacromolecules 2010, 11, 1773–1778. [Google Scholar] [CrossRef] [PubMed]
- Okajima, M.K.; Higashi, T.; Asakawa, R.; Mitsumata, T.; Kaneko, D.; Kaneko, T.; Ogawa, T.; Kurata, H.; Isoda, S. Gelation Behavior by the Lanthanoid Adsorption of the Cyanobacterial Extracellular Polysaccharide. Biomacromolecules 2010, 11, 3172–3177. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, K.; Tanida, Y.; Hata, K.; Hayashi, T.; Higashi, T.; Ishitsuka, Y.; Kondo, Y.; Irie, T.; Kaneko, S.; Arima, H. Potential Use of a Megamolecular Polysaccharide Sacran as a Hydrogel-Based Sustained Release System. Chem. Pharm. Bull. 2014, 62, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Puluhulawa, L.E.; Joni, I.M.; Mohammed, A.F.A.; Arima, H.; Wathoni, N. The Use of Megamolecular Polysaccharide Sacran in Food and Biomedical Applications. Molecules 2021, 26, 3362. [Google Scholar] [CrossRef] [PubMed]
- Yusa, K.; Maeda, Y.; Fujioka, A.; Monde, K.; Harada, S. Isolation of TAK-779-Resistant HIV-1 from an R5 HIV-1 GP120 V3 Loop Library. J. Biol. Chem. 2005, 280, 30083–30090. [Google Scholar] [CrossRef]
- Cao, J.; Park, I.W.; Cooper, A.; Sodroski, J. Molecular Determinants of Acute Single-Cell Lysis by Human Immunodeficiency Virus Type 1. J. Virol. 1996, 70, 1340–1354. [Google Scholar] [CrossRef]
- Matsuda, K.; Hattori, S.; Kariya, R.; Komizu, Y.; Kudo, E.; Goto, H.; Taura, M.; Ueoka, R.; Kimura, S.; Okada, S. Inhibition of HIV-1 Entry by the Tricyclic Coumarin GUT-70 through the Modification of Membrane Fluidity. Biochem. Biophys. Res. Commun. 2015, 457, 288–294. [Google Scholar] [CrossRef]
- Matsuda, K.; Hattori, S.; Komizu, Y.; Kariya, R.; Ueoka, R.; Okada, S. Cepharanthine Inhibited HIV-1 Cell-Cell Transmission and Cell-Free Infection via Modification of Cell Membrane Fluidity. Bioorg. Med. Chem. Lett. 2014, 24, 2115–2117. [Google Scholar] [CrossRef] [PubMed]
- Hattori, S.; Ide, K.; Nakata, H.; Harada, H.; Suzu, S.; Ashida, N.; Kohgo, S.; Hayakawa, H.; Mitsuya, H.; Okada, S. Potent Activity of a Nucleoside Reverse Transcriptase Inhibitor, 4′-Ethynyl-2-Fluoro-2′-Deoxyadenosine, against Human Immunodeficiency Virus Type 1 Infection in a Model Using Human Peripheral Blood Mononuclear Cell-Transplanted NOD/SCID Janus Kinase 3 Knockout Mice. Antimicrob. Agents Chemother. 2009, 53, 3887–3893. [Google Scholar] [CrossRef]
- Schols, D.; Struyf, S.; Van Damme, J.; Este, J.A.; Henson, G.; De Clercq, E. Inhibition of T-Tropic HIV Strains by Selective Antagonization of the Chemokine Receptor CXCR4. J. Exp. Med. 1997, 186, 1383–1388. [Google Scholar] [CrossRef]
- Wild, C.; Greenwell, T.; Matthews, T. A Synthetic Peptide from HIV-1 gp41 Is a Potent Inhibitor of Virus-Mediated Cell-Cell Fusion. AIDS Res. Hum. Retroviruses 1993, 9, 1051–1053. [Google Scholar] [CrossRef]
- Young, S.D.; Britcher, S.F.; Tran, L.O.; Payne, L.S.; Lumma, W.C.; Lyle, T.A.; Huff, J.R.; Anderson, P.S.; Olsen, D.B.; Carroll, S.S.; et al. L-743, 726 (DMP-266): A Novel, Highly Potent Nonnucleoside Inhibitor of the Human Immunodeficiency Virus Type 1 Reverse Transcriptase. Antimicrob. Agents Chemother. 1995, 39, 2602–2605. [Google Scholar] [CrossRef] [PubMed]
- Lai, B.E.; Geonnotti, A.R.; Desoto, M.G.; Montefiori, D.C.; Katz, D.F. Semi-Solid Gels Function as Physical Barriers to Human Immunodeficiency Virus Transport In Vitro. Antiviral Res. 2010, 88, 143–151. [Google Scholar] [CrossRef][Green Version]
- Chutiwitoonchai, N.; Hiyoshi, M.; Mwimanzi, P.; Ueno, T.; Adachi, A.; Ode, H.; Sato, H.; Fackler, O.T.; Okada, S.; Suzu, S. The Identification of a Small Molecule Compound That Reduces HIV-1 Nef-Mediated Viral Infectivity Enhancement. PLoS ONE 2011, 6, e27696. [Google Scholar] [CrossRef][Green Version]
- Chou, T.C.; Talalay, P. Analysis of Combined Drug Effects—A New Look at a Very Old Problem. Trends Pharmacol. Sci. 1983, 4, 450–454. [Google Scholar] [CrossRef]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The Combined Effects of Multiple Drugs or Enzyme Inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Chou, T.C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Dorr, P.; Westby, M.; Dobbs, S.; Griffin, P.; Irvine, B.; Macartney, M.; Mori, J.; Rickett, G.; Smith-Burchnell, C.; Napier, C.; et al. Maraviroc (UK-427,857), a Potent, Orally Bioavailable, and Selective Small-Molecule Inhibitor of Chemokine Receptor CCR5 with Broad-Spectrum Anti-Human Immunodeficiency Virus Type 1 Activity. Antimicrob. Agents Chemother. 2005, 49, 4721–4732. [Google Scholar] [CrossRef] [PubMed]
- Cervia, J.S.; Smith, M.A. Enfuvirtide (T-20): A Novel Human Immunodeficiency Virus Type 1 Fusion Inhibitor. Clin. Infect. Dis. 2003, 37, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Matthews, T.; Salgo, M.; Greenberg, M.; Chung, J.; DeMasi, R.; Bolognesi, D. Enfuvirtide: The First Therapy to Inhibit the Entry of HIV-1 into Host CD4 Lymphocytes. Nat. Rev. Drug Discov. 2004, 3, 215–225. [Google Scholar] [CrossRef]
- Veazey, R.S.; Klasse, P.J.; Schader, S.M.; Hu, Q.; Ketas, T.J.; Lu, M.; Marx, P.A.; Dufour, J.; Colonno, R.J.; Shattock, R.J.; et al. Protection of Macaques from Vaginal SHIV Challenge by Vaginally Delivered Inhibitors of Virus-Cell Fusion. Nature 2005, 438, 99–102. [Google Scholar] [CrossRef]
- Ketas, T.J.; Schader, S.M.; Zurita, J.; Teo, E.; Polonis, V.; Lu, M.; Klasse, P.J.; Moore, J.P. Entry Inhibitor-Based Microbicides Are Active In Vitro against HIV-1 Isolates from Multiple Genetic Subtypes. Virology 2007, 364, 431–440. [Google Scholar] [CrossRef][Green Version]
- Sattentau, Q.J. Cell-to-Cell Spread of Retroviruses. Viruses 2010, 2, 1306–1321. [Google Scholar] [CrossRef]
- Schaeffer, D.J.; Krylov, V.S. Anti-HIV Activity of Extracts and Compounds from Algae and Cyanobacteria. Ecotoxicol. Environ. Saf. 2000, 45, 208–227. [Google Scholar] [CrossRef]
- Damonte, E.; Neyts, J.; Pujol, C.A.; Snoeck, R.; Andrei, G.; Ikeda, S.; Witvrouw, M.; Reymen, D.; Haines, H.; Matulewicz, M.C.; et al. Antiviral Activity of a Sulphated Polysaccharide from the Red Seaweed Nothogenia Fastigiata. Biochem. Pharmacol. 1994, 47, 2187–2192. [Google Scholar] [CrossRef]
- Ngatu, N.R.; Okajima, M.K.; Yokogawa, M.; Hirota, R.; Eitoku, M.; Muzembo, B.A.; Dumavibhat, N.; Takaishi, M.; Sano, S.; Kaneko, T.; et al. Anti-Inflammatory Effects of Sacran, a Novel Polysaccharide from Aphanothece Sacrum, on 2,4,6-Trinitrochlorobenzene-Induced Allergic Dermatitis In Vivo. Ann. Allergy Asthma Immunol. 2012, 108, 117–122. [Google Scholar] [CrossRef]


| Sacran (%) | Plerixafor (nM) | Effect | Combination Index |
| 10−5 | 10 | 0.178 | 0.72172 |
| 10−4 | 30 | 0.355 | 0.58724 |
| 10−3 | 50 | 0.623 | 0.32316 |
| Sacran (%) | Enfuvirtide (nM) | Effect | Combination Index |
| 10−5 | 30 | 0.443 | 0.55077 |
| 10−4 | 50 | 0.606 | 0.51666 |
| 10−3 | 100 | 0.781 | 0.51390 |
| Sacran (%) | EFV (nM) | Effect | Combination Index |
| 10−4 | 10 | 0.478 | 0.58724 |
| 10−3 | 30 | 0.714 | 0.32316 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuda, K.; Kariya, R.; Maeda, K.; Okada, S. Evaluating the Use of Sacran, a Polysaccharide Isolated from Aphanothece sacrum, as a Possible Microbicide for Preventing HIV-1 Infection. Viruses 2024, 16, 1501. https://doi.org/10.3390/v16091501
Matsuda K, Kariya R, Maeda K, Okada S. Evaluating the Use of Sacran, a Polysaccharide Isolated from Aphanothece sacrum, as a Possible Microbicide for Preventing HIV-1 Infection. Viruses. 2024; 16(9):1501. https://doi.org/10.3390/v16091501
Chicago/Turabian StyleMatsuda, Kouki, Ryusho Kariya, Kenji Maeda, and Seiji Okada. 2024. "Evaluating the Use of Sacran, a Polysaccharide Isolated from Aphanothece sacrum, as a Possible Microbicide for Preventing HIV-1 Infection" Viruses 16, no. 9: 1501. https://doi.org/10.3390/v16091501
APA StyleMatsuda, K., Kariya, R., Maeda, K., & Okada, S. (2024). Evaluating the Use of Sacran, a Polysaccharide Isolated from Aphanothece sacrum, as a Possible Microbicide for Preventing HIV-1 Infection. Viruses, 16(9), 1501. https://doi.org/10.3390/v16091501







