Interferon-Stimulated Genes and Immune Metabolites as Broad-Spectrum Biomarkers for Viral Infections
Abstract
:1. Introduction
2. Type I Interferon Response
3. ddhCTP Derivatives
4. MxA
5. ISG15
6. IFI27
7. IFI44L
8. CXCL10
9. Practical Considerations for Clinical Application
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mueller, A.A.; Tamura, T.; Crowley, C.P.; DeGrado, J.R.; Haider, H.; Jezmir, J.L.; Keras, G.; Penn, E.H.; Massaro, A.F.; Kim, E.Y. Inflammatory Biomarker Trends Predict Respiratory Decline in COVID-19 Patients. Cell Rep. Med. 2020, 1, 100144. [Google Scholar] [CrossRef]
- Villoteau, A.; Asfar, M.; Otekpo, M.; Loison, J.; Gautier, J.; Annweiler, C.; GERIA-COVID Study Group. Elevated C-reactive protein in early COVID-19 predicts worse survival among hospitalized geriatric patients. PLoS ONE 2021, 16, e0256931. [Google Scholar] [CrossRef]
- Tong-Minh, K.; van der Does, Y.; Engelen, S.; de Jong, E.; Ramakers, C.; Gommers, D.; van Gorp, E.; Endeman, H. High procalcitonin levels associated with increased intensive care unit admission and mortality in patients with a COVID-19 infection in the emergency department. BMC Infect. Dis. 2022, 22, 165. [Google Scholar] [CrossRef]
- Fauter, M.; Viel, S.; Zaepfel, S.; Pradat, P.; Fiscus, J.; Villard, M.; Garnier, L.; Walzer, T.; Seve, P.; Henry, T.; et al. Low glycosylated ferritin is a sensitive biomarker of severe COVID-19. Cell Mol. Immunol. 2020, 17, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Mohn, N.; Skripuletz, T.; Senel, M.; Tumani, H.; Pessler, F.; Suhs, K.W.; Stangel, M. Differentiation of viral and autoimmune central nervous system inflammation by kynurenine pathway. Ann. Clin. Transl. Neurol. 2021, 8, 2228–2234. [Google Scholar] [CrossRef] [PubMed]
- Haroun, R.A.; Osman, W.H.; Amin, R.E.; Hassan, A.K.; Abo-Shanab, W.S.; Eessa, A.M. Circulating plasma miR-155 is a potential biomarker for the detection of SARS-CoV-2 infection. Pathology 2022, 54, 104–110. [Google Scholar] [CrossRef]
- Li, H.K.; Kaforou, M.; Rodriguez-Manzano, J.; Channon-Wells, S.; Moniri, A.; Habgood-Coote, D.; Gupta, R.K.; Mills, E.A.; Arancon, D.; Lin, J.; et al. Discovery and validation of a three-gene signature to distinguish COVID-19 and other viral infections in emergency infectious disease presentations: A case-control and observational cohort study. Lancet Microbe 2021, 2, e594–e603. [Google Scholar] [CrossRef] [PubMed]
- Lo Sasso, B.; Gambino, C.M.; Scichilone, N.; Giglio, R.V.; Bivona, G.; Scazzone, C.; Muratore, R.; Milano, S.; Barbagallo, M.; Agnello, L.; et al. Clinical Utility of Midregional Proadrenomedullin in Patients with COVID-19. Lab. Med. 2021, 52, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Michels, M.; Djamiatun, K.; Faradz, S.M.; Koenders, M.M.; de Mast, Q.; van der Ven, A.J. High plasma mid-regional pro-adrenomedullin levels in children with severe dengue virus infections. J. Clin. Virol. 2011, 50, 8–12. [Google Scholar] [CrossRef]
- Wu, Z.; Geng, N.; Liu, Z.; Pan, W.; Zhu, Y.; Shan, J.; Shi, H.; Han, Y.; Ma, Y.; Liu, B. Presepsin as a prognostic biomarker in COVID-19 patients: Combining clinical scoring systems and laboratory inflammatory markers for outcome prediction. Virol. J. 2024, 21, 96. [Google Scholar] [CrossRef]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368, m1091. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhou, W.; Yan, X.; Guo, T.; Wang, B.; Xia, H.; Ye, L.; Xiong, J.; Jiang, Z.; Liu, Y.; et al. Prognostic Value of C-Reactive Protein in Patients With Coronavirus 2019. Clin. Infect. Dis. 2020, 71, 2174–2179. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Karn, E.; Trivedi, K.; Kumar, P.; Chauhan, G.; Kumari, A.; Pant, P.; Munisamy, M.; Prakash, J.; Sarkar, P.G.; et al. Procalcitonin as a predictive marker in COVID-19: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0272840. [Google Scholar] [CrossRef]
- Gautam, S.; Cohen, A.J.; Stahl, Y.; Valda Toro, P.; Young, G.M.; Datta, R.; Yan, X.; Ristic, N.T.; Bermejo, S.D.; Sharma, L.; et al. Severe respiratory viral infection induces procalcitonin in the absence of bacterial pneumonia. Thorax 2020, 75, 974–981. [Google Scholar] [CrossRef]
- Yu, Z.; Jing, H.; Hongtao, P.; Furong, J.; Yuting, J.; Xu, S.; Venge, P. Distinction between bacterial and viral infections by serum measurement of human neutrophil lipocalin (HNL) and the impact of antibody selection. J. Immunol. Methods 2016, 432, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, S.H. Use of common blood parameters for the differential diagnosis of childhood infections. PLoS ONE 2022, 17, e0273236. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Cai, Y.; Shao, Y. Comparison of presepsin and Mid-regional pro-adrenomedullin in the diagnosis of sepsis or septic shock: A systematic review and meta-analysis. BMC Infect. Dis. 2023, 23, 288. [Google Scholar] [CrossRef] [PubMed]
- Florkowski, C.M. Sensitivity, specificity, receiver-operating characteristic (ROC) curves and likelihood ratios: Communicating the performance of diagnostic tests. Clin. Biochem. Rev. 2008, 29 (Suppl. S1), S83–S87. [Google Scholar] [PubMed]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]
- Habibzadeh, F.; Habibzadeh, P.; Yadollahie, M. On determining the most appropriate test cut-off value: The case of tests with continuous results. Biochem. Med. 2016, 26, 297–307. [Google Scholar] [CrossRef]
- Mehta, R.; Chekmeneva, E.; Jackson, H.; Sands, C.; Mills, E.; Arancon, D.; Li, H.K.; Arkell, P.; Rawson, T.M.; Hammond, R.; et al. Antiviral metabolite 3′-deoxy-3′,4′-didehydro-cytidine is detectable in serum and identifies acute viral infections including COVID-19. Med 2022, 3, 204–215.e206. [Google Scholar] [CrossRef] [PubMed]
- Sala, S.; Nitschke, P.; Masuda, R.; Gray, N.; Lawler, N.G.; Wood, J.M.; Buckler, J.N.; Berezhnoy, G.; Bolanos, J.; Boughton, B.A.; et al. Integrative Molecular Structure Elucidation and Construction of an Extended Metabolic Pathway Associated with an Ancient Innate Immune Response in COVID-19 Patients. J. Proteome Res. 2024, 23, 956–970. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, O.; Broman, N.; Waris, M.; Vuorinen, T.; Peltola, V.; Loyttyniemi, E.; Oksi, J.; Feuth, T. Association of human myxovirus resistance protein A with severity of COVID-19. BMC Infect. Dis. 2022, 22, 755. [Google Scholar] [CrossRef] [PubMed]
- Toivonen, L.; Schuez-Havupalo, L.; Rulli, M.; Ilonen, J.; Pelkonen, J.; Melen, K.; Julkunen, I.; Peltola, V.; Waris, M. Blood MxA protein as a marker for respiratory virus infections in young children. J. Clin. Virol. 2015, 62, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Piri, R.; Yahya, M.; Ivaska, L.; Toivonen, L.; Lempainen, J.; Nuolivirta, K.; Tripathi, L.; Waris, M.; Peltola, V. Myxovirus Resistance Protein A as a Marker of Viral Cause of Illness in Children Hospitalized with an Acute Infection. Microbiol. Spectr. 2022, 10, e0203121. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, I.; Dubos, F.; Lobert, P.E.; Houssin, C.; Degas, V.; Sardet, A.; Decoster, A.; Dewilde, A.; Martinot, A.; Hober, D. Diagnosis of viral infections using myxovirus resistance protein A (MxA). Pediatrics 2015, 135, e985–e993. [Google Scholar] [CrossRef] [PubMed]
- Maria, N.I.; Brkic, Z.; Waris, M.; van Helden-Meeuwsen, C.G.; Heezen, K.; van de Merwe, J.P.; van Daele, P.L.; Dalm, V.A.; Drexhage, H.A.; Versnel, M.A. MxA as a clinically applicable biomarker for identifying systemic interferon type I in primary Sjogren’s syndrome. Ann. Rheum. Dis. 2014, 73, 1052–1059. [Google Scholar] [CrossRef]
- Huijser, E.; van Helden-Meeuwsen, C.G.; Groot, N.; Bodewes, I.L.A.; Wahadat, M.J.; Schreurs, M.W.J.; van Daele, P.L.A.; Dalm, V.; van Laar, J.A.M.; van Hagen, P.M.; et al. MxA is a clinically applicable biomarker for type I interferon activation in systemic lupus erythematosus and systemic sclerosis. Rheumatology 2019, 58, 1302–1303. [Google Scholar] [CrossRef] [PubMed]
- Metz, M.; Gualdoni, G.A.; Winkler, H.M.; Warenits, A.M.; Stockl, J.; Burgmann, H.; Winkler, S.; Oesterreicher, Z.A. MxA for differentiating viral and bacterial infections in adults: A prospective, exploratory study. Infection 2023, 51, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Halminen, M.; Ilonen, J.; Julkunen, I.; Ruuskanen, O.; Simell, O.; Makela, M.J. Expression of MxA protein in blood lymphocytes discriminates between viral and bacterial infections in febrile children. Pediatr. Res. 1997, 41, 647–650. [Google Scholar] [CrossRef]
- Chieux, V.; Hober, D.; Chehadeh, W.; Harvey, J.; Alm, G.; Cousin, J.; Ducoulombier, H.; Wattre, P. MxA protein in capillary blood of children with viral infections. J. Med. Virol. 1999, 59, 547–551. [Google Scholar] [CrossRef]
- Sampson, D.L.; Fox, B.A.; Yager, T.D.; Bhide, S.; Cermelli, S.; McHugh, L.C.; Seldon, T.A.; Brandon, R.A.; Sullivan, E.; Zimmerman, J.J.; et al. A Four-Biomarker Blood Signature Discriminates Systemic Inflammation Due to Viral Infection Versus Other Etiologies. Sci. Rep. 2017, 7, 2914. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, S.; Banerjee, U.; Dr, G.D.; Kandukuru, R.; Thakur, C.; Chakravortty, D.; Balaji, K.N.; Singh, A.; Chandra, N. VB(10), a new blood biomarker for differential diagnosis and recovery monitoring of acute viral and bacterial infections. eBioMedicine 2021, 67, 103352. [Google Scholar] [CrossRef] [PubMed]
- Mirzalieva, O.; Juncker, M.; Schwartzenburg, J.; Desai, S. ISG15 and ISGylation in Human Diseases. Cells 2022, 11, 538. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.M.; Shojaei, M.; Parnell, G.P.; Huang, S.; Nalos, M.; Teoh, S.; O’Connor, K.; Schibeci, S.; Phu, A.L.; Kumar, A.; et al. A novel immune biomarker IFI27 discriminates between influenza and bacteria in patients with suspected respiratory infection. Eur. Respir. J. 2017, 49, 1602098. [Google Scholar] [CrossRef] [PubMed]
- Min, Z.; Ye, Z.; Gang, L.; Boyu, D.; Xueyan, X. IFI27 as a potential indicator for severe Enterovirus 71-infected hand foot and mouth disease. Virus Res. 2020, 289, 198149. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhu, X.; Wu, M.; Jiang, L.; Wang, F.; He, S. IFI27 may predict and evaluate the severity of respiratory syncytial virus infection in preterm infants. Hereditas 2021, 158, 3. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, M.; Shamshirian, A.; Monkman, J.; Grice, L.; Tran, M.; Tan, C.W.; Teo, S.M.; Rodrigues Rossi, G.; McCulloch, T.R.; Nalos, M.; et al. IFI27 transcription is an early predictor for COVID-19 outcomes, a multi-cohort observational study. Front. Immunol. 2022, 13, 1060438. [Google Scholar] [CrossRef]
- Xu, N.; Hao, F.; Dong, X.; Yao, Y.; Guan, Y.; Yang, L.; Chen, F.; Zheng, F.; Li, Q.; Liu, W.; et al. A two-transcript biomarker of host classifier genes for discrimination of bacterial from viral infection in acute febrile illness: A multicentre discovery and validation study. Lancet Digit. Health 2021, 3, e507–e516. [Google Scholar] [CrossRef]
- Herberg, J.A.; Kaforou, M.; Wright, V.J.; Shailes, H.; Eleftherohorinou, H.; Hoggart, C.J.; Cebey-Lopez, M.; Carter, M.J.; Janes, V.A.; Gormley, S.; et al. Diagnostic Test Accuracy of a 2-Transcript Host RNA Signature for Discriminating Bacterial vs Viral Infection in Febrile Children. JAMA 2016, 316, 835–845. [Google Scholar] [CrossRef]
- Naveca, F.G.; Pontes, G.S.; Chang, A.Y.; Silva, G.; Nascimento, V.A.D.; Monteiro, D.; Silva, M.S.D.; Abdalla, L.F.; Santos, J.H.A.; Almeida, T.A.P.; et al. Analysis of the immunological biomarker profile during acute Zika virus infection reveals the overexpression of CXCL10, a chemokine linked to neuronal damage. Mem. Inst. Oswaldo Cruz 2018, 113, e170542. [Google Scholar] [CrossRef] [PubMed]
- Landry, M.L.; Foxman, E.F. Antiviral Response in the Nasopharynx Identifies Patients with Respiratory Virus Infection. J. Infect. Dis. 2018, 217, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Pastor, L.; Casellas, A.; Ruperez, M.; Carrillo, J.; Maculuve, S.; Jairoce, C.; Paredes, R.; Blanco, J.; Naniche, D. Interferon-gamma-Inducible Protein 10 (IP-10) as a Screening Tool to Optimize Human Immunodeficiency Virus RNA Monitoring in Resource-Limited Settings. Clin. Infect. Dis. 2017, 65, 1670–1675. [Google Scholar] [CrossRef]
- Semmler, G.; Griebler, H.; Aberle, S.W.; Stiasny, K.; Richter, L.; Holzmann, H.; Weseslindtner, L. Elevated CXCL10 Serum Levels in Measles Virus Primary Infection and Reinfection Correlate with the Serological Stage and Hospitalization Status. J. Infect. Dis. 2020, 222, 2030–2034. [Google Scholar] [CrossRef] [PubMed]
- Jusof, F.F.; Lim, C.K.; Aziz, F.N.; Soe, H.J.; Raju, C.S.; Sekaran, S.D.; Guillemin, G.J. The Cytokines CXCL10 and CCL2 and the Kynurenine Metabolite Anthranilic Acid Accurately Predict Patients at Risk of Developing Dengue with Warning Signs. J. Infect. Dis. 2022, 226, 1964–1973. [Google Scholar] [CrossRef] [PubMed]
- Lore, N.I.; De Lorenzo, R.; Rancoita, P.M.V.; Cugnata, F.; Agresti, A.; Benedetti, F.; Bianchi, M.E.; Bonini, C.; Capobianco, A.; Conte, C.; et al. CXCL10 levels at hospital admission predict COVID-19 outcome: Hierarchical assessment of 53 putative inflammatory biomarkers in an observational study. Mol. Med. 2021, 27, 129. [Google Scholar] [CrossRef]
- Azzurri, A.; Sow, O.Y.; Amedei, A.; Bah, B.; Diallo, S.; Peri, G.; Benagiano, M.; D’Elios, M.M.; Mantovani, A.; Del Prete, G. IFN-gamma-inducible protein 10 and pentraxin 3 plasma levels are tools for monitoring inflammation and disease activity in Mycobacterium tuberculosis infection. Microbes Infect. 2005, 7, 1–8. [Google Scholar] [CrossRef]
- Vargas-Inchaustegui, D.A.; Hogg, A.E.; Tulliano, G.; Llanos-Cuentas, A.; Arevalo, J.; Endsley, J.J.; Soong, L. CXCL10 production by human monocytes in response to Leishmania braziliensis infection. Infect. Immun. 2010, 78, 301–308. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, T.E.; Dos Santos, L.I.; Gomes, A.O.; Carneiro, A.; Machado, A.S.; Coelho-Dos-Reis, J.G.; Peruhype-Magalhaes, V.; Bela, S.R.; Andrade, G.M.Q.; Vasconcelos-Santos, D.V.; et al. Putative biomarkers for early diagnosis and prognosis of congenital ocular toxoplasmosis. Sci. Rep. 2020, 10, 16757. [Google Scholar] [CrossRef]
- Cheemarla, N.R.; Hanron, A.; Fauver, J.R.; Bishai, J.; Watkins, T.A.; Brito, A.F.; Zhao, D.; Alpert, T.; Vogels, C.B.F.; Ko, A.I.; et al. Nasal host response-based screening for undiagnosed respiratory viruses: A pathogen surveillance and detection study. Lancet Microbe 2023, 4, e38–e46. [Google Scholar] [CrossRef] [PubMed]
- Armah, H.B.; Wilson, N.O.; Sarfo, B.Y.; Powell, M.D.; Bond, V.C.; Anderson, W.; Adjei, A.A.; Gyasi, R.K.; Tettey, Y.; Wiredu, E.K.; et al. Cerebrospinal fluid and serum biomarkers of cerebral malaria mortality in Ghanaian children. Malar. J. 2007, 6, 147. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Armah, H.B.; Tongren, J.E.; Ned, R.M.; Wilson, N.O.; Crawford, S.; Joel, P.K.; Singh, M.P.; Nagpal, A.C.; Dash, A.P.; et al. Plasma IP-10, apoptotic and angiogenic factors associated with fatal cerebral malaria in India. Malar. J. 2008, 7, 83. [Google Scholar] [CrossRef]
- Wu, X.; Lai, X.; Tu, H.; Zou, H.; Cao, J. Elevated serum CXCL10 in patients with Clostridium difficile infection are associated with disease severity. Int. Immunopharmacol. 2019, 72, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, Y.; Li, H.; Yang, D.; Zhou, Y.; Chen, Z.; Zhang, Y. Serum CXCL10/IP-10 may be a potential biomarker for severe Mycoplasma pneumoniae pneumonia in children. BMC Infect. Dis. 2021, 21, 909. [Google Scholar] [CrossRef]
- Lee, A.J.; Ashkar, A.A. The Dual Nature of Type I and Type II Interferons. Front. Immunol. 2018, 9, 2061. [Google Scholar] [CrossRef]
- Tau, G.; Rothman, P. Biologic functions of the IFN-gamma receptors. Allergy 1999, 54, 1233–1251. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Dai, T.; He, X.; Zhang, Z.; Xie, F.; Wang, S.; Zhang, L.; Zhou, F. The interactions between cGAS-STING pathway and pathogens. Signal Transduct. Target. Ther. 2020, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed]
- Platanias, L.C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 2005, 5, 375–386. [Google Scholar] [CrossRef]
- Schoggins, J.W. Interferon-Stimulated Genes: What Do They All Do? Annu. Rev. Virol. 2019, 6, 567–584. [Google Scholar] [CrossRef]
- Schoggins, J.W. Interferon-stimulated genes: Roles in viral pathogenesis. Curr. Opin. Virol. 2014, 6, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Fink, J.; Gu, F.; Ling, L.; Tolfvenstam, T.; Olfat, F.; Chin, K.C.; Aw, P.; George, J.; Kuznetsov, V.A.; Schreiber, M.; et al. Host gene expression profiling of dengue virus infection in cell lines and patients. PLoS Negl. Trop. Dis. 2007, 1, e86. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Serrano, E.E.; Gizzi, A.S.; Arnold, J.J.; Grove, T.L.; Almo, S.C.; Cameron, C.E. Viperin Reveals Its True Function. Annu. Rev. Virol. 2020, 7, 421–446. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.C.; Laurent-Rolle, M.; Pawlak, J.B.; Xia, H.; Kunte, A.; Hee, J.S.; Lim, J.; Harris, L.D.; Wood, J.M.; Evans, G.B.; et al. Viperin triggers ribosome collision-dependent translation inhibition to restrict viral replication. Mol. Cell 2022, 82, 1631–1642.e6. [Google Scholar] [CrossRef] [PubMed]
- Gizzi, A.S.; Grove, T.L.; Arnold, J.J.; Jose, J.; Jangra, R.K.; Garforth, S.J.; Du, Q.; Cahill, S.M.; Dulyaninova, N.G.; Love, J.D.; et al. A naturally occurring antiviral ribonucleotide encoded by the human genome. Nature 2018, 558, 610–614. [Google Scholar] [CrossRef]
- Pawlak, J.B.; Hsu, J.C.; Xia, H.; Han, P.; Suh, H.W.; Grove, T.L.; Morrison, J.; Shi, P.Y.; Cresswell, P.; Laurent-Rolle, M. CMPK2 restricts Zika virus replication by inhibiting viral translation. PLoS Pathog. 2023, 19, e1011286. [Google Scholar] [CrossRef]
- Zhu, M.; Lv, J.; Wang, W.; Guo, R.; Zhong, C.; Antia, A.; Zeng, Q.; Li, J.; Liu, Q.; Zhou, J.; et al. CMPK2 is a host restriction factor that inhibits infection of multiple coronaviruses in a cell-intrinsic manner. PLoS Biol. 2023, 21, e3002039. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, P.; Chauhan, M.; Rajeev, T.; Chakraborty, R.; Bisht, K.; Madan, M.; Shankaran, D.; Ramalingam, S.; Gandotra, S.; Rao, V. The mitochondrial gene-CMPK2 functions as a rheostat for macrophage homeostasis. Front. Immunol. 2022, 13, 935710. [Google Scholar] [CrossRef]
- Helbig, K.J.; Teh, M.Y.; Crosse, K.M.; Monson, E.A.; Smith, M.; Tran, E.N.; Standish, A.J.; Morona, R.; Beard, M.R. The interferon stimulated gene viperin, restricts Shigella. flexneri in vitro. Sci. Rep. 2019, 9, 15598. [Google Scholar] [CrossRef]
- Haller, O.; Staeheli, P.; Schwemmle, M.; Kochs, G. Mx GTPases: Dynamin-like antiviral machines of innate immunity. Trends Microbiol. 2015, 23, 154–163. [Google Scholar] [CrossRef]
- Zav’yalov, V.P.; Hamalainen-Laanaya, H.; Korpela, T.K.; Wahlroos, T. Interferon-Inducible Myxovirus Resistance Proteins: Potential Biomarkers for Differentiating Viral from Bacterial Infections. Clin. Chem. 2019, 65, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Matzinger, S.R.; Carroll, T.D.; Dutra, J.C.; Ma, Z.M.; Miller, C.J. Myxovirus resistance gene A (MxA) expression suppresses influenza A virus replication in alpha interferon-treated primate cells. J. Virol. 2013, 87, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Arnheiter, H.; Skuntz, S.; Noteborn, M.; Chang, S.; Meier, E. Transgenic mice with intracellular immunity to influenza virus. Cell 1990, 62, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Gordien, E.; Rosmorduc, O.; Peltekian, C.; Garreau, F.; Brechot, C.; Kremsdorf, D. Inhibition of hepatitis B virus replication by the interferon-inducible MxA protein. J. Virol. 2001, 75, 2684–2691. [Google Scholar] [CrossRef]
- Staeheli, P.; Pavlovic, J. Inhibition of vesicular stomatitis virus mRNA synthesis by human MxA protein. J. Virol. 1991, 65, 4498–4501. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Schaulies, S.; Schneider-Schaulies, J.; Schuster, A.; Bayer, M.; Pavlovic, J.; ter Meulen, V. Cell type-specific MxA-mediated inhibition of measles virus transcription in human brain cells. J. Virol. 1994, 68, 6910–6917. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Horisberger, M.A. Combined action of mouse alpha and beta interferons in influenza virus-infected macrophages carrying the resistance gene Mx. J. Virol. 1984, 49, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Killip, M.J.; Staeheli, P.; Randall, R.E.; Jackson, D. The human interferon-induced MxA protein inhibits early stages of influenza A virus infection by retaining the incoming viral genome in the cytoplasm. J. Virol. 2013, 87, 13053–13058. [Google Scholar] [CrossRef]
- Verhelst, J.; Parthoens, E.; Schepens, B.; Fiers, W.; Saelens, X. Interferon-inducible protein Mx1 inhibits influenza virus by interfering with functional viral ribonucleoprotein complex assembly. J. Virol. 2012, 86, 13445–13455. [Google Scholar] [CrossRef]
- Tong-Minh, K.; van Hooijdonk, S.; Versnel, M.A.; van Helden-Meeuwsen, C.G.; van Hagen, P.M.; van Gorp, E.C.M.; Endeman, H.; van der Does, Y.; Dalm, V.; Dik, W.A. Blood myxovirus resistance protein-1 measurement in the diagnostic work-up of suspected COVID-19 infection in the emergency department. Immun. Inflamm. Dis. 2022, 10, e609. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, D.; Jorns, C.; Stertz, S.; Boisson-Dupuis, S.; Thimme, R.; Weidmann, M.; Casanova, J.L.; Haller, O.; Kochs, G. Induction of MxA gene expression by influenza A virus requires type I or type III interferon signaling. J. Virol. 2007, 81, 7776–7785. [Google Scholar] [CrossRef] [PubMed]
- Perng, Y.-C.; Lenschow, D.J. ISG15 in antiviral immunity and beyond. Nat. Rev. Microbiol. 2018, 16, 423–439. [Google Scholar] [CrossRef] [PubMed]
- Lenschow, D.J.; Lai, C.; Frias-Staheli, N.; Giannakopoulos, N.V.; Lutz, A.; Wolff, T.; Osiak, A.; Levine, B.; Schmidt, R.E.; Garcia-Sastre, A.; et al. IFN-stimulated gene 15 functions as a critical antiviral molecule against influenza, herpes, and Sindbis viruses. Proc. Natl. Acad. Sci. USA 2007, 104, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Werneke, S.W.; Schilte, C.; Rohatgi, A.; Monte, K.J.; Michault, A.; Arenzana-Seisdedos, F.; Vanlandingham, D.L.; Higgs, S.; Fontanet, A.; Albert, M.L.; et al. ISG15 is critical in the control of Chikungunya virus infection independent of UbE1L mediated conjugation. PLoS Pathog. 2011, 7, e1002322. [Google Scholar] [CrossRef]
- Schwartzenburg, J.; Reed, R.; Koul, H.; Zea, A.H.; Shellito, J.; Miele, L.; Crabtree, J.S.; Desai, S. ISGylation is increased in the peripheral blood mononuclear cells derived from symptomatic COVID-19 patients. Exp. Biol. Med. 2022, 247, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Yang, D.; Wang, J.; Xu, Y.; Wang, X.; Qin, Y.; Tian, R.; Chen, S.; Xie, Q.; Liu, N.; et al. ISG12a Restricts Hepatitis C Virus Infection through the Ubiquitination-Dependent Degradation Pathway. J. Virol. 2016, 90, 6832–6845. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Tan, C.W.; Ribeiro Dos Santos Miggiolaro, A.F.; Monkman, J.; SadeghiRad, H.; Bhuva, D.D.; Motta Junior, J.D.S.; Busatta Vaz de Paula, C.; Nagashima, S.; Baena, C.P.; et al. Profiling of lung SARS-CoV-2 and influenza virus infection dissects virus-specific host responses and gene signatures. Eur. Respir. J. 2022, 59, 2101881. [Google Scholar] [CrossRef]
- Sweeney, T.E.; Wong, H.R.; Khatri, P. Robust classification of bacterial and viral infections via integrated host gene expression diagnostics. Sci. Transl. Med. 2016, 8, 346ra391. [Google Scholar] [CrossRef] [PubMed]
- Almansa, R.; Herrero-Rodriguez, C.; Martinez-Huelamo, M.; Vicente-Andres, M.D.P.; Nieto-Barbero, J.A.; Martin-Ballesteros, M.; Rodilla-Carvajal, M.D.M.; de la Fuente, A.; Ortega, A.; Alonso-Ramos, M.J.; et al. A host transcriptomic signature for identification of respiratory viral infections in the community. Eur. J. Clin. Investig. 2021, 51, e13626. [Google Scholar] [CrossRef]
- Rao, A.M.; Popper, S.J.; Gupta, S.; Davong, V.; Vaidya, K.; Chanthongthip, A.; Dittrich, S.; Robinson, M.T.; Vongsouvath, M.; Mayxay, M.; et al. A robust host-response-based signature distinguishes bacterial and viral infections across diverse global populations. Cell Rep. Med. 2022, 3, 100842. [Google Scholar] [CrossRef]
- Busse, D.C.; Habgood-Coote, D.; Clare, S.; Brandt, C.; Bassano, I.; Kaforou, M.; Herberg, J.; Levin, M.; Eleouet, J.F.; Kellam, P.; et al. Interferon-Induced Protein 44 and Interferon-Induced Protein 44-Like Restrict Replication of Respiratory Syncytial Virus. J. Virol. 2020, 94, 10–1128. [Google Scholar] [CrossRef]
- Schoggins, J.W.; Wilson, S.J.; Panis, M.; Murphy, M.Y.; Jones, C.T.; Bieniasz, P.; Rice, C.M. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 2011, 472, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, T.; Naito, T.; Murata, Y.; Matsuda, H.; Ohtani, M.; Hiramatsu, K.; Nishizawa, T.; Okamoto, H.; Nakamoto, Y. Regulatory function of interferon-inducible 44-like for hepatitis B virus covalently closed circular DNA in primary human hepatocytes. Hepatol. Res. 2022, 52, 141–152. [Google Scholar] [CrossRef]
- DeDiego, M.L.; Martinez-Sobrido, L.; Topham, D.J. Novel Functions of IFI44L as a Feedback Regulator of Host Antiviral Responses. J. Virol. 2019, 93, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, I.; Rodriguez-Manzano, J.; Moniri, A.; Kaforou, M.; Herberg, J.A.; Levin, M.; Georgiou, P. Translation of a Host Blood RNA Signature Distinguishing Bacterial from Viral Infection Into a Platform Suitable for Development as a Point-of-Care Test. JAMA Pediatr. 2021, 175, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Elemam, N.M.; Talaat, I.M.; Maghazachi, A.A. CXCL10 Chemokine: A Critical Player in RNA and DNA Viral Infections. Viruses 2022, 14, 2445. [Google Scholar] [CrossRef] [PubMed]
- Dufour, J.H.; Dziejman, M.; Liu, M.T.; Leung, J.H.; Lane, T.E.; Luster, A.D. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J. Immunol. 2002, 168, 3195–3204. [Google Scholar] [CrossRef]
- Klein, R.S.; Lin, E.; Zhang, B.; Luster, A.D.; Tollett, J.; Samuel, M.A.; Engle, M.; Diamond, M.S. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J. Virol. 2005, 79, 11457–11466. [Google Scholar] [CrossRef] [PubMed]
- Rathakrishnan, A.; Wang, S.M.; Hu, Y.; Khan, A.M.; Ponnampalavanar, S.; Lum, L.C.; Manikam, R.; Sekaran, S.D. Cytokine expression profile of dengue patients at different phases of illness. PLoS ONE 2012, 7, e52215. [Google Scholar] [CrossRef]
- Becerra, A.; Warke, R.V.; Martin, K.; Xhaja, K.; de Bosch, N.; Rothman, A.L.; Bosch, I. Gene expression profiling of dengue infected human primary cells identifies secreted mediators in vivo. J. Med. Virol. 2009, 81, 1403–1411. [Google Scholar] [CrossRef]
- Madhurantakam, S.; Lee, Z.J.; Naqvi, A.; Prasad, S. Importance of IP-10 as a biomarker of host immune response: Critical perspective as a target for biosensing. Curr. Res. Biotechnol. 2023, 5, 100130. [Google Scholar] [CrossRef]
- Oved, K.; Cohen, A.; Boico, O.; Navon, R.; Friedman, T.; Etshtein, L.; Kriger, O.; Bamberger, E.; Fonar, Y.; Yacobov, R.; et al. A novel host-proteome signature for distinguishing between acute bacterial and viral infections. PLoS ONE 2015, 10, e0120012. [Google Scholar] [CrossRef] [PubMed]
- Mastboim, N.S.; Angel, A.; Shaham, O.; Ber, T.I.; Navon, R.; Simon, E.; Rosenberg, M.; Israeli, Y.; Hainrichson, M.; Avni, N.; et al. An immune-protein score combining TRAIL, IP-10 and CRP for predicting severe COVID-19 disease. Cytokine 2023, 169, 156246. [Google Scholar] [CrossRef] [PubMed]
- van Houten, C.B.; de Groot, J.A.H.; Klein, A.; Srugo, I.; Chistyakov, I.; de Waal, W.; Meijssen, C.B.; Avis, W.; Wolfs, T.F.W.; Shachor-Meyouhas, Y.; et al. A host-protein based assay to differentiate between bacterial and viral infections in preschool children (OPPORTUNITY): A double-blind, multicentre, validation study. Lancet Infect. Dis. 2017, 17, 431–440. [Google Scholar] [CrossRef] [PubMed]
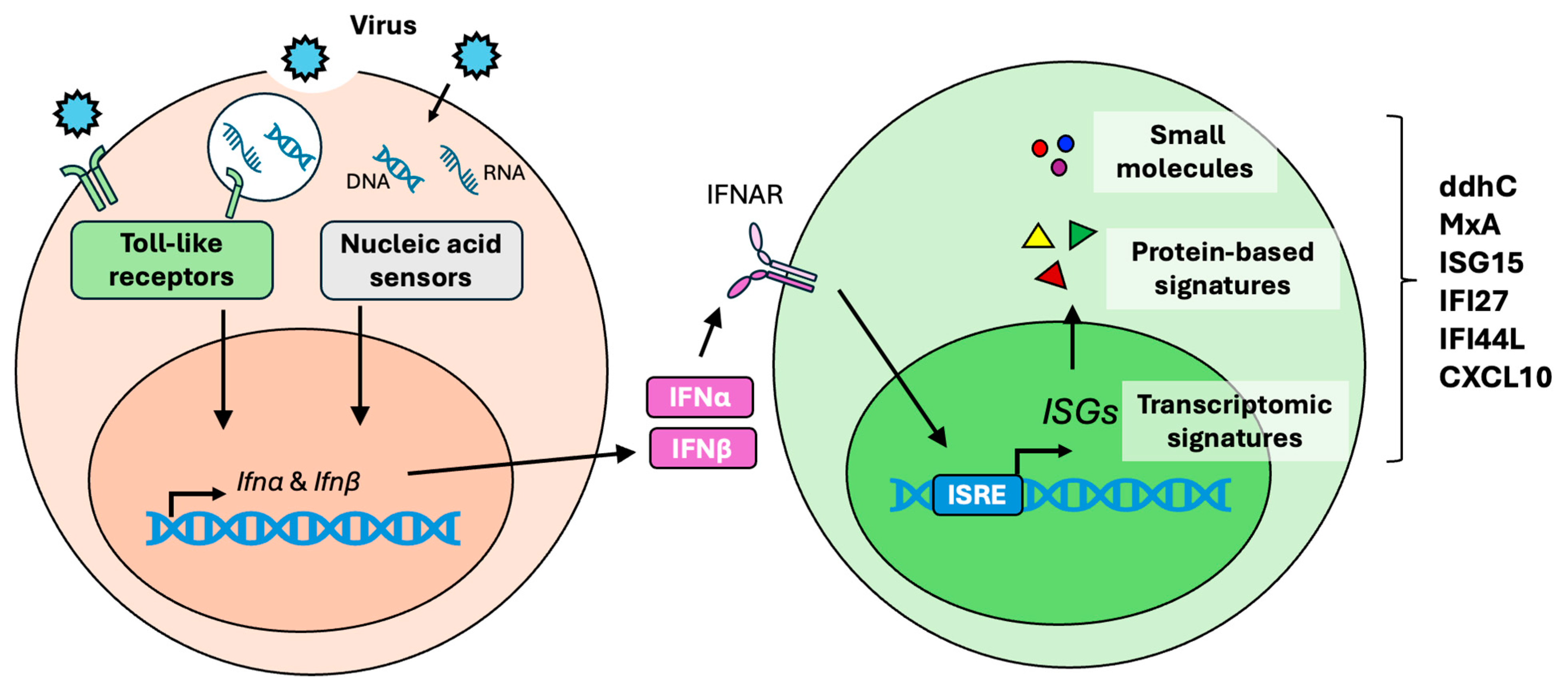
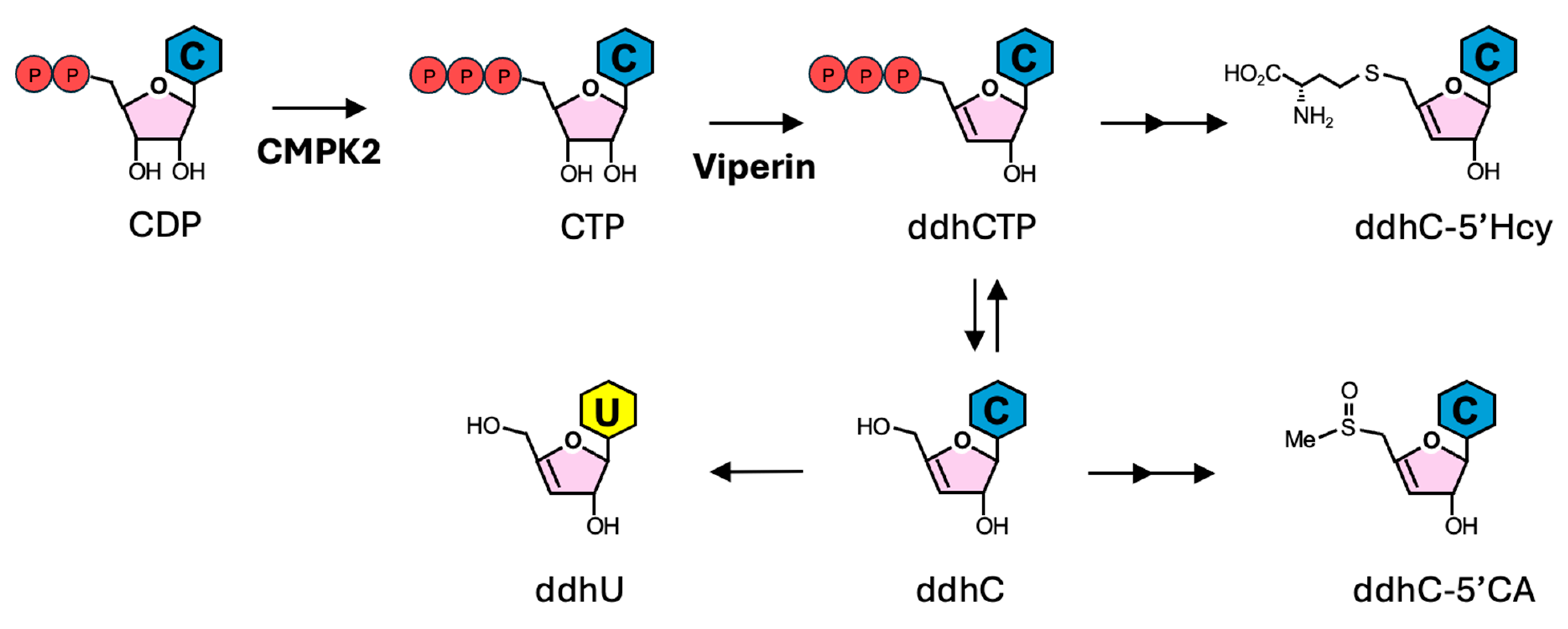
| Biomarker | Sample Source | Substance Type | Detection Approach | Pathogen and Etiology | Refs. |
|---|---|---|---|---|---|
| ddhC | Serum Urine | Nucleoside derivative | NMR LC-MS/MS | SARS-CoV-2 Influenza viruses Adenovirus Dengue virus Rotavirus Respiratory syncytial virus Varicella-zoster virus Measles virus | [21,22] |
| MxA | Blood Monocytes Lymphocytes | Protein | ELISA Immunochemiluminescent assay Flow cytometry | Rotavirus Respiratory syncytial virus Influenza virus SARS-CoV-2 Rhinovirus Human bocavirus Human metapneumovirus Parainfluenza virus Rhinovirus Adenovirus Enterovirus Herpes simplex virus Epstein–Barr virus Bacteria * Autoimmune diseases * | [23,24,25,26,27,28,29,30,31] |
| ISG15 | Whole blood PBMCs | RNA Protein(ISGylation) | RNA-Seq | Rhinovirus Adenovirus Respiratory syncytial virus Influenza virus SARS-CoV-2 Enterovirus Human metapneumovirus HIV-1 Herpes virus Varicella-zoster virus Epstein–Barr virus Cytomegalovirus Dengue virus Cancer * | [32,33,34] |
| IFI27 | Blood PBMCs | RNA | RT-qPCR | Influenza virus Enterovirus 71 Respiratory syncytial virus Human metapneumovirus Herpes virus Cytomegalovirus Adenovirus Rhinovirus SARS-CoV-2 Dengue virus | [33,35,36,37,38] |
| IFI44L | Blood | RNA | Reverse Transcriptase Loop-Mediated Isothermal Amplification (RT-LAMP) RT-PCR | Adenovirus Influenza virus Respiratory syncytial virus Rotavirus Enterovirus Epstein–Barr virus Epidemic hemorrhagic fever virus Severe fever with thrombocytopenia syndrome virus | [39,40] |
| CXCL10 | Nasopharyngeal swab Plasma Serum Cerebrospinal fluid | RNA Protein | RT-qPCR Microbeads multiplex immunoassay ELISA Magnetic Luminex Assay Cytometric bead array | Adenovirus Human metapneumovirus Influenza virus Parainfluenza virus Respiratory syncytial virus Rhinovirus SARS-CoV-2 Zika virus Dengue virus Measles virus HIV-1 Bacteria * Parasites * Autoimmune diseases * Cancer * | [41,42,43,44,45,46,47,48,49,50,51,52,53,54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-H.; Laurent-Rolle, M.; Grove, T.L.; Hsu, J.C.-C. Interferon-Stimulated Genes and Immune Metabolites as Broad-Spectrum Biomarkers for Viral Infections. Viruses 2025, 17, 132. https://doi.org/10.3390/v17010132
Huang C-H, Laurent-Rolle M, Grove TL, Hsu JC-C. Interferon-Stimulated Genes and Immune Metabolites as Broad-Spectrum Biomarkers for Viral Infections. Viruses. 2025; 17(1):132. https://doi.org/10.3390/v17010132
Chicago/Turabian StyleHuang, Chien-Hsin, Maudry Laurent-Rolle, Tyler L. Grove, and Jack Chun-Chieh Hsu. 2025. "Interferon-Stimulated Genes and Immune Metabolites as Broad-Spectrum Biomarkers for Viral Infections" Viruses 17, no. 1: 132. https://doi.org/10.3390/v17010132
APA StyleHuang, C.-H., Laurent-Rolle, M., Grove, T. L., & Hsu, J. C.-C. (2025). Interferon-Stimulated Genes and Immune Metabolites as Broad-Spectrum Biomarkers for Viral Infections. Viruses, 17(1), 132. https://doi.org/10.3390/v17010132






