Assessing the Burden of Neglected Tropical Diseases in Low-Income Communities: Challenges and Solutions
Abstract
1. Introduction
2. Prevalence and Impact of NTDs in Low-Income Communities
2.1. Global Burden and Distribution of NTDs
2.2. Co-Infections and Polyparasitism
- (a)
- Drug interactions: treatment for one parasite may negatively interact with treatment for another, reducing efficacy or increasing the risk of adverse effects.
- (b)
- Exacerbation of symptoms: treating one infection could exacerbate the symptoms of another, particularly if the immune response plays a significant role in the pathogenesis of either disease.
- (c)
- Masking of diagnosis: the presence of one parasite could mask the symptoms of another, making diagnosis and appropriate treatment more challenging.
- (d)
- Altered immune response: one infection may alter the host’s immune response, making them more susceptible to a second infection or less responsive to treatment for either.
- (a)
- Test-and-Not-Treat: This strategy involves screening individuals for Loa loa microfilaremia before administering ivermectin. Those with high micro-filarial loads are excluded from treatment to avoid the risk of SAEs. Kamgno et al. describes such a strategy implemented in the Okola district [26].
- (b)
- (c)
- Improved diagnostics: Accurate and rapid diagnostic tools for both Loa loa and Onchocerca volvulus are crucial for effective implementation of test-and-treat or alternative treatment strategies.
2.3. The Impact of Climate Change on NTDs
2.3.1. Soil-Transmitted Helminth Infections
Epidemiology
The Current Landscape, Achievements, and Challenges in Disease Control
2.3.2. Lymphatic Filariasis
Epidemiology
The Current Landscape, Achievements, and Challenges in Disease Control
2.3.3. Schistosomiasis
Epidemiology
The Current Landscape, Achievements, and Challenges in Disease Control
2.3.4. Trachoma
Epidemiology
The Current Landscape, Achievements, and Challenges in Disease Control
2.3.5. Onchocerciasis
Epidemiology
The Current Landscape, Achievements, and Challenges in Disease Control
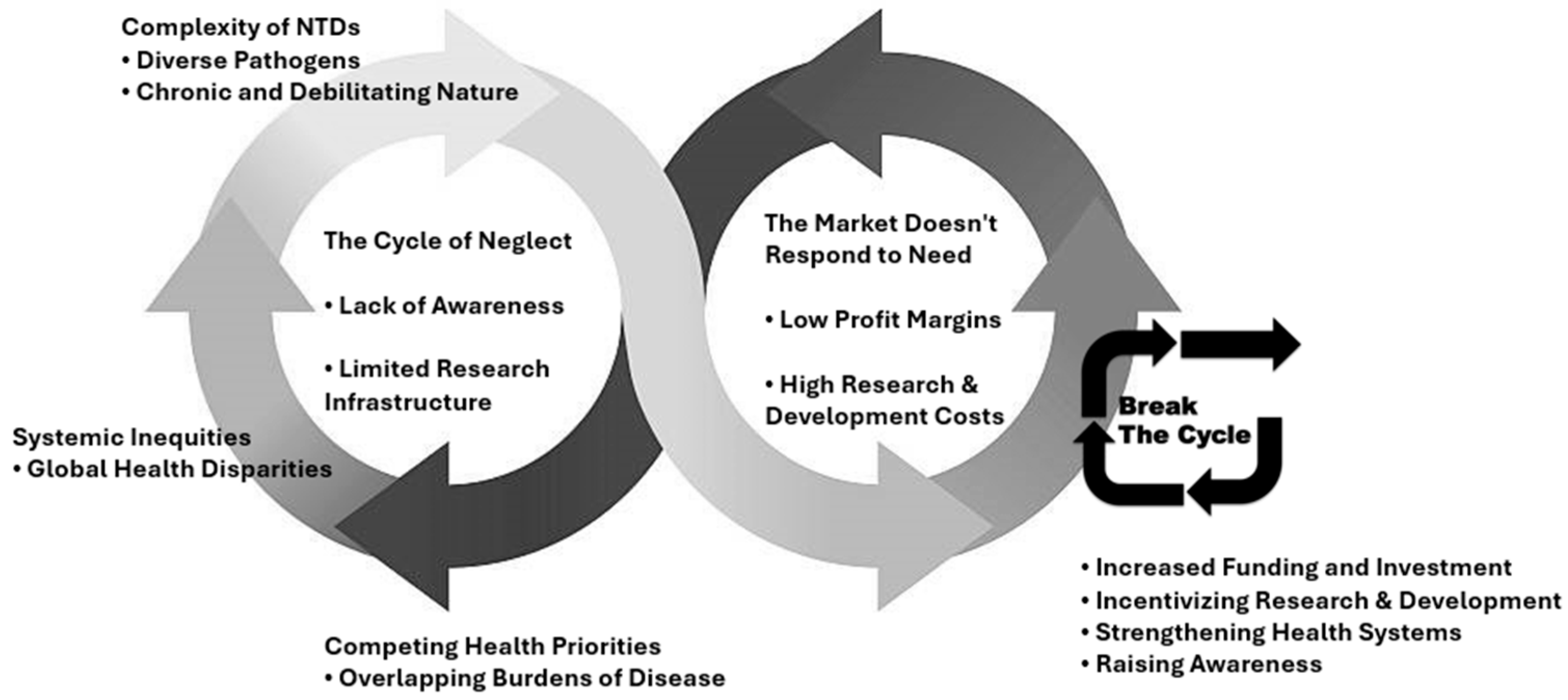
3. Challenges
3.1. Addressing the Lack of Therapeutic Resources for NTDs
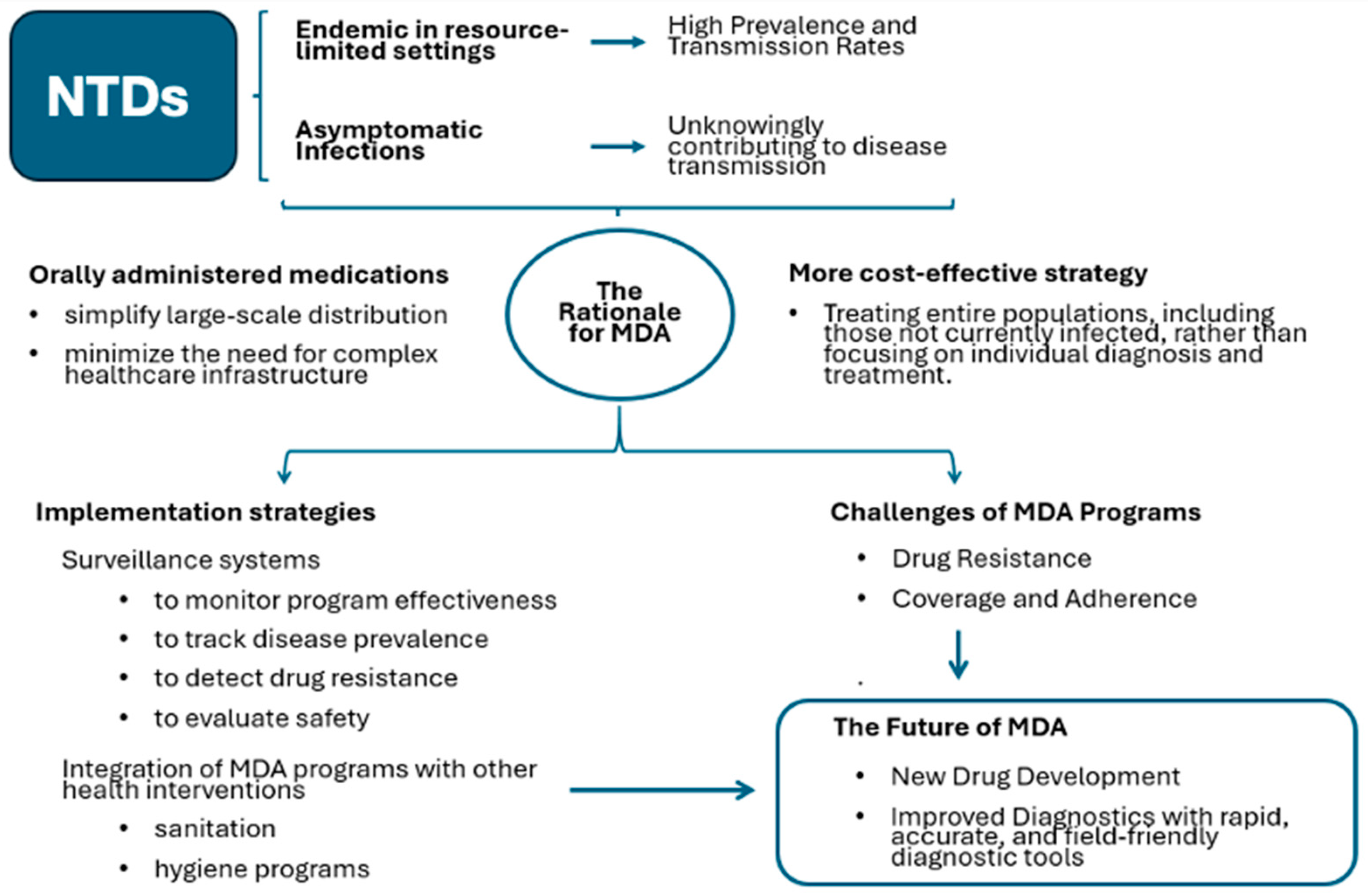
3.2. MDA Programs
4. Solutions
4.1. Importance of Health Education in NTD Control
4.2. Challenges and Strategies for Effective Health Education and Community Engagement
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
Appendix A.1
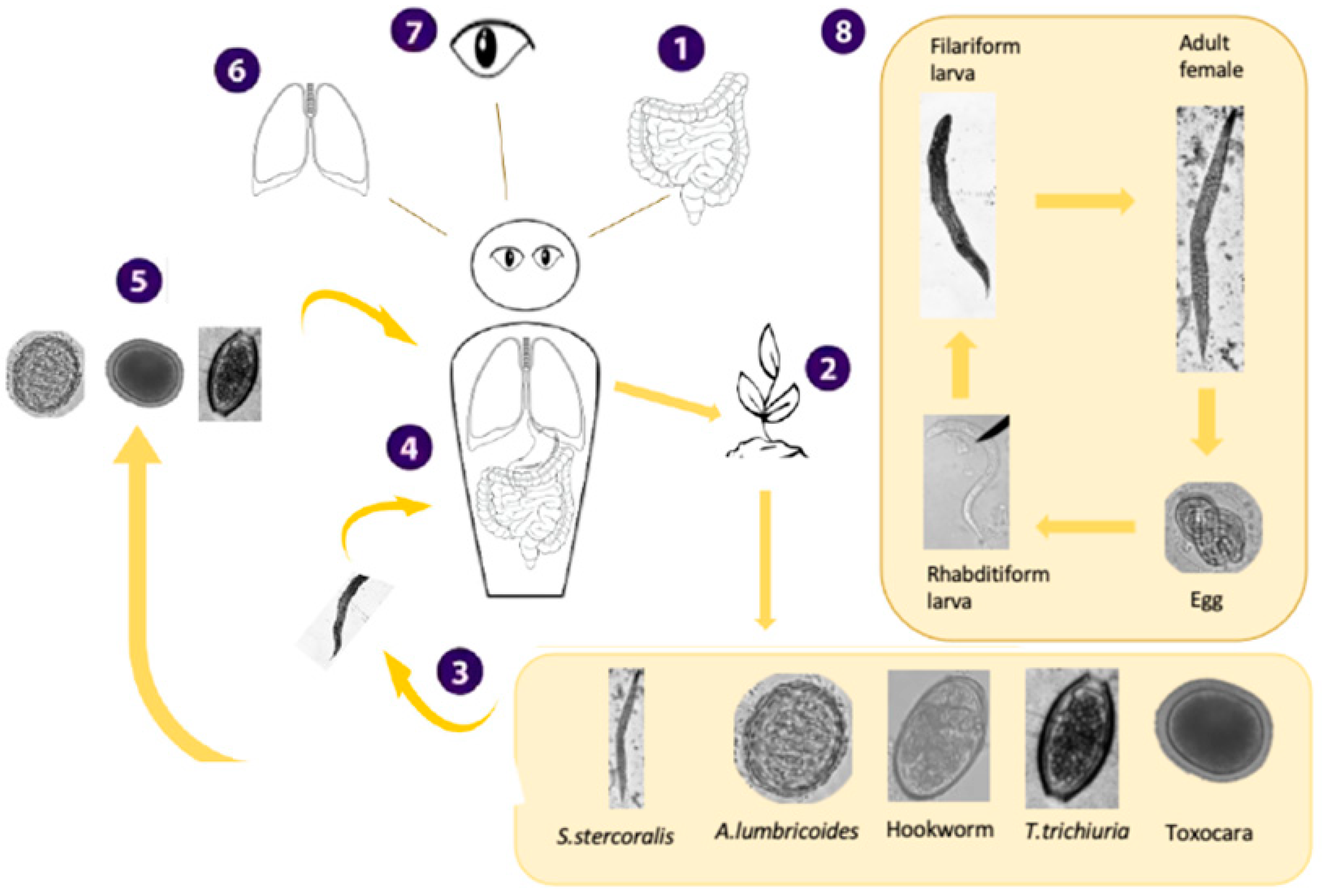
Appendix A2
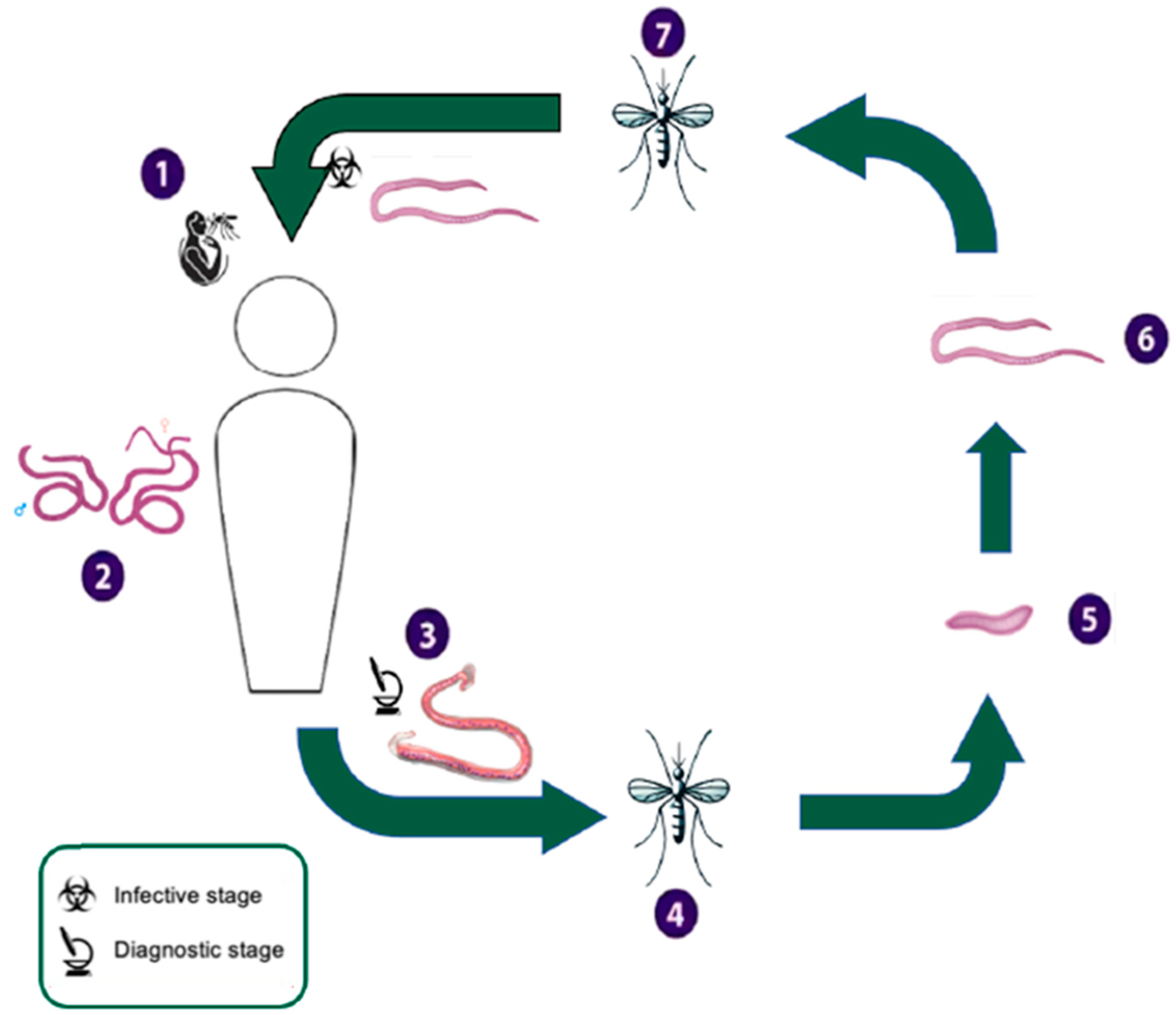
Appendix A.3

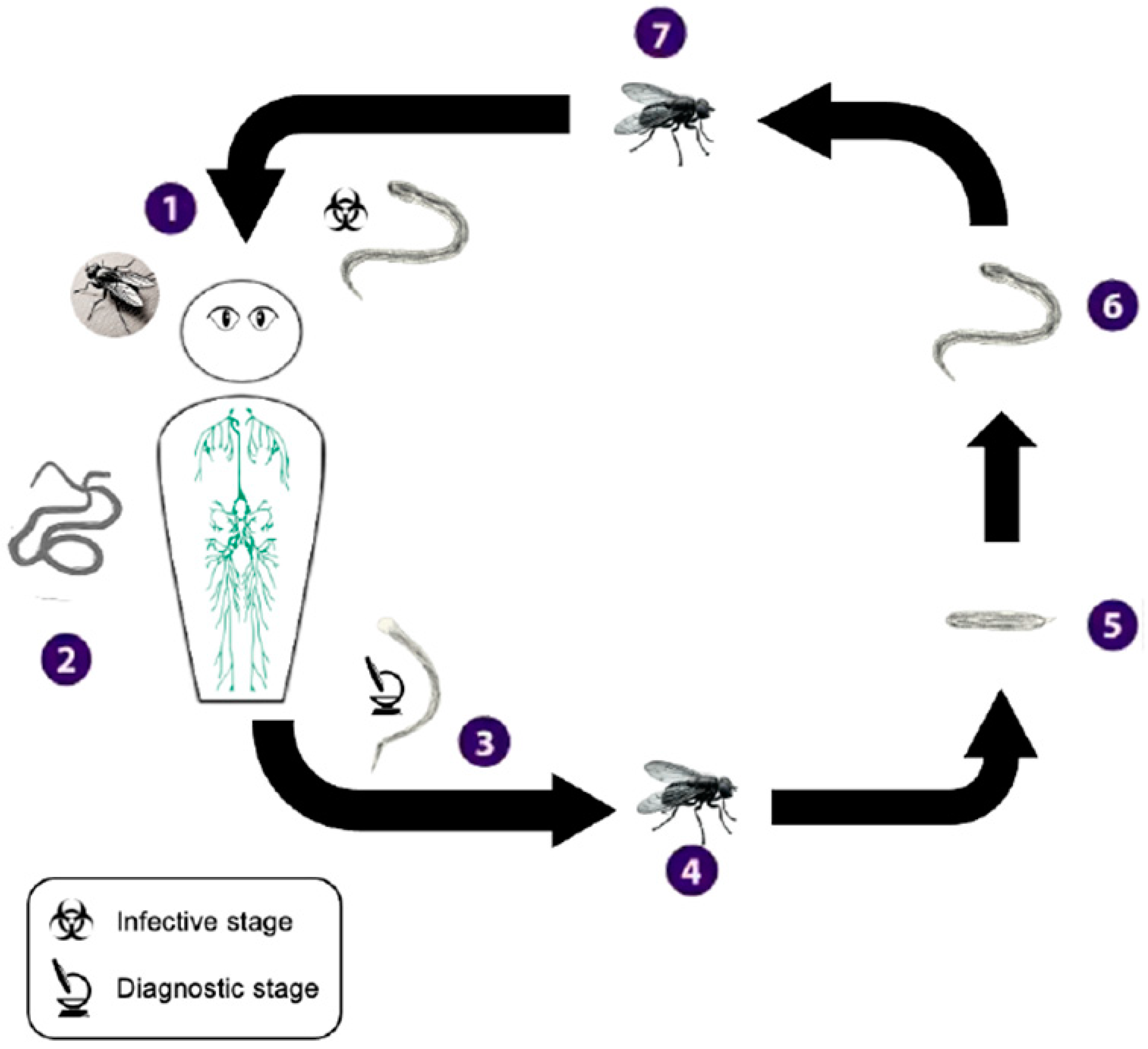
Appendix A.4
References
- Hotez, P.J.; Fenwick, A.; Savioli, L.; Molyneux, D.H. Rescuing the bottom billion through control of neglected tropical diseases. Lancet 2009, 373, 1570–1575. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, D.H.; Malecela, M.N. Neglected Tropical Diseases and the Millennium Development Goals-why the other diseases matter: Reality versus rhetoric. Parasites Vectors 2011, 4, 234. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Molyneux, D.H.; Fenwick, A.; Kumaresan, J.; Sachs, S.E.; Sachs, J.D.; Savioli, L. Control of neglected tropical diseases. N. Engl. J. Med. 2007, 357, 1018–1027. [Google Scholar] [CrossRef]
- Dreyer, G.; Addiss, D.; Dreyer, P.; Noroes, J. Basic lymphoedema management: Treatment and prevention of problems associated with lymphatic filariasis. Int. J. Infect. Dis. 2002. [Google Scholar]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- Peters, D.H.; Garg, A.; Bloom, G.; Walker, D.G.; Brieger, W.R.; Hafizur Rahman, M. Poverty and access to health care in developing countries. Ann. N. Y. Acad. Sci. 2008, 1136, 161–171. [Google Scholar] [CrossRef]
- Molyneux, D.H.; Savioli, L.; Engels, D. Neglected tropical diseases: Progress towards addressing the chronic pandemic. Lancet 2017, 389, 312–325. [Google Scholar] [CrossRef]
- Strunz, E.C.; Addiss, D.G.; Stocks, M.E.; Ogden, S.; Utzinger, J.; Freeman, M.C. Water, sanitation, hygiene, and soil-transmitted helminth infection: A systematic review and meta-analysis. PLoS Med. 2014, 11, e1001620. [Google Scholar] [CrossRef]
- Hotez, P.J.; Pecoul, B.; Rijal, S.; Boehme, C.; Aksoy, S.; Malecela, M.; Tapia-Conyer, R.; Reeder, J.C. Eliminating the neglected tropical diseases: Translational science and new technologies. PLoS Negl. Trop. Dis. 2016, 10, e0003895. [Google Scholar] [CrossRef]
- Miguel, E.; Kremer, M. Worms: Identifying impacts on education and health in the presence of treatment externalities. Econometrica 2004, 72, 159–217. [Google Scholar] [CrossRef]
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030. Available online: https://www.who.int/publications/i/item/9789240010352 (accessed on 17 September 2024).
- WHO Methods and Data Sources for Global Burden of Disease Estimates 2000–2021. Available online: https://cdn.who.int/media/docs/default-source/gho-documents/global-health-estimates/ghe2021_daly_methods.pdf?sfvrsn=690b16c3_1 (accessed on 17 September 2024).
- Sakho, F.; Badila, C.F.; Dembele, B.; Diaby, A.; Camara, A.K.; Lamah, L.; Reid, S.D.; Weng, A.; Fuller, B.B.; Sanchez, K.A.; et al. Implementation of mass drug administration for neglected tropical diseases in Guinea during the COVID-19 pandemic. PLoS Negl. Trop. Dis. 2021, 15, e0009807. [Google Scholar] [CrossRef] [PubMed]
- Jesudason, T. Global progress report on neglected tropical diseases. Lancet Infect. Dis. 2024, 24, e420. [Google Scholar] [CrossRef]
- Dawaki, S.; Al-Mekhlafi, H.M.; Ithoi, I. The burden and epidemiology of polyparasitism among rural communities in Kano State, Nigeria. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 169–182. [Google Scholar] [CrossRef]
- Pullan, R.; Brooker, S. The health impact of polyparasitism in humans: Are we under-estimating the burden of parasitic diseases? Parasitology 2008, 135, 783–794. [Google Scholar] [CrossRef]
- Hürlimann, E.; Yapi, R.B.; Houngbedji, C.A. The epidemiology of polyparasitism and implications for morbidity in two rural communities of Côte d’Ivoire. Parasites Vectors 2014, 7, 81. [Google Scholar] [CrossRef]
- Keiser, J.; N’Goran, E.K.; Traoré, M.; Lohourignon, K.L.; Singer, B.H.; Lengeler, C.; Tanner, M.; Utzinger, J. Polyparasitism with Schistosoma mansoni, geohelminths, and intestinal protozoa in rural Côte d’Ivoire. J. Parasitol. 2002, 88, 461–466. [Google Scholar]
- Hotez, P.J.; Molyneux, D.H.; Fenwick, A.; Ottesen, E.; Ehrlich Sachs, S.; Sachs, J.D. Incorporating a rapid-impact package for neglected tropical diseases with programs for HIV/AIDS, tuberculosis, and malaria: A comprehensive pro-poor health policy and strategy for the developing world. PLoS Med. 2006, 3, e102. [Google Scholar] [CrossRef]
- Donohue, R.E.; Cross, Z.K.; Michael, E. The extent, nature, and pathogenic consequences of helminth polyparasitism in humans: A meta-analysis. PLoS Negl. Trop. Dis. 2019, 13, e0007455. [Google Scholar] [CrossRef]
- World Health Organization. WHO Policy Recommendation on Seasonal Malaria Chemoprevention (SMC) for Plasmodium falciparum Malaria Control in Highly Seasonal Transmission Areas of the Sahel Sub-Region in Africa (WHO/HTM/GMP/2012.02). 2012. Available online: https://apps.who.int/iris/handle/10665/337978 (accessed on 5 November 2024).
- Afolabi, M.O.; Diaw, A.; Sall, F.B.; Diédhiou, A.; Seck, A.; Camara, B.; Niang, D.; Manga, I.A.; Mbaye, I.; Sougou, N.M.; et al. Provider and User Acceptability of Integrated Treatment for the Control of Malaria and Helminths in Saraya, South-Eastern Senegal. Am. J. Trop. Med. Hyg. 2023, 109, 1047–1056. [Google Scholar] [CrossRef]
- Gyapong, J.O.; Gyapong, M.; Yellu, N.; Anakwah, K.; Amofah, G.; Bockarie, M.; Adjei, S. 2010 Integration of control of neglected tropical diseases into health-care systems: Challenges and opportunities. Lancet 2010, 375, 160–165. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Integrating Neglected Tropical Diseases into Global Health and Development. 2017. Available online: https://www.who.int/publications/i/item/9789241565448 (accessed on 5 November 2024).
- Kamgno, J.; Pion, S.D.; Chesnais, C.B.; Bakalar, M.H.; D’Ambrosio, M.V.; Mackenzie, C.D.; Nana-Djeunga, H.C.; Gounoue-Kamkumo, R.; Njitchouang, G.R.; Nwane, P.; et al. A Test-and-Not-Treat Strategy for Onchocerciasis in Loa loa-Endemic Areas. N. Engl. J. Med. 2017, 377, 2044–2052. [Google Scholar] [CrossRef]
- Blok, D.J.; Kamgno, J.; Pion, S.D.; Nana-Djeunga, H.C.; Niamsi-Emalio, Y.; Chesnais, C.B.; Mackenzie, C.D.; Klion, A.D.; Fletcher, D.A.; Nutman, T.B.; et al. Feasibility of Onchocerciasis Elimination Using a Test-and-not-treat Strategy in Loa loa Co-endemic Areas. Clin. Infect. Dis. 2021, 72, e1047–e1055. [Google Scholar] [CrossRef] [PubMed]
- Ojurongbe, O.; Akindele, A.A.; Adeleke, M.A.; Oyedeji, M.O.; Adedokun, S.A.; Ojo, J.F.; Akinleye, C.A.; Bolaji, O.S.; Adefioye, O.A.; Adeyeba, O.A. Co-endemicity of loiasis and onchocerciasis in rain forest communities in southwestern Nigeria. PLoS Negl. Trop. Dis. 2015, 9, e0003633. [Google Scholar] [CrossRef] [PubMed]
- Trotignon, G.; Dixon, R.; Atekem, K.; Senyonjo, L.; Kamgno, J.; Biholong, D.; Jones, I.; Nditanchou, R. Cost of implementing a doxycycline test-and-treat strategy for onchocerciasis elimination among settled and semi-nomadic groups in Cameroon. PLoS Negl. Trop. Dis. 2023, 17, e0011670. [Google Scholar] [CrossRef]
- Nditanchou, R.; Dixon, R.; Atekem, K.; Akongo, S.; Biholong, B.; Ayisi, F.; Nwane, P.; Wilhelm, A.; Basnet, S.; Selby, R.; et al. Acceptability of test and treat with doxycycline against Onchocerciasis in an area of persistent transmission in Massangam Health District, Cameroon. PLoS Negl. Trop. Dis. 2023, 17, e0011185. [Google Scholar] [CrossRef]
- Pavia, G.; Branda, F.; Ciccozzi, A.; Romano, C.; Locci, C.; Azzena, I.; Pascale, N.; Marascio, N.; Quirino, A.; Gigliotti, S.; et al. The issue of climate change and the spread of tropical diseases in Europe and Italy: Vector biology, disease transmission, genome-based monitoring and public health implications. Infect. Dis. 2024, 11, 1–16. [Google Scholar] [CrossRef]
- Ceccarelli, G.; Branda, F.; Giovanetti, M.; Ciccozzi, M.; Scarpa, F. The urgent need for arbovirus surveillance and control following a catastrophic event: The case of the DANA flood event in Valencia. New Microbes New Infect. 2024, 62, 101547. [Google Scholar] [CrossRef]
- Booth, M. Climate Change and the Neglected Tropical Diseases. Adv. Parasitol. 2018, 100, 39–126. [Google Scholar] [CrossRef]
- Tidman, R.; Abela-Ridder, B.; de Castañeda, R.R. The impact of climate change on neglected tropical diseases: A systematic review. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 147–168. [Google Scholar] [CrossRef]
- Acosta-España, J.D.; Romero-Alvarez, D.; Luna, C.; Rodriguez-Morales, A.J. Infectious disease outbreaks in the wake of natural flood disasters: Global patterns and local implications. Infez. Med. 2024, 32, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Caminade, C.; McIntyre, K.M.; Jones, A.E. Impact of recent and future climate change on vector-borne diseases. Ann. N. Y. Acad. Sci. 2019, 1436, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Kelly-Hope, L.A.; Harding-Esch, E.M.; Willems, J.; Ahmed, F.; Sanders, A.M. Conflict-climate-displacement: A cross-sectional ecological study determining the burden, risk and need for strategies for neglected tropical disease programmes in Africa. BMJ Open 2023, 13, e071557. [Google Scholar] [CrossRef] [PubMed]
- Villabona-Arenas, C.J.; de Oliveira, J.L.; Capra, C.d.S.; Balarini, K.; Loureiro, M.; Fonseca, C.R.T.P.; 587 Passos, S.D.; Zanotto, P.M.d.A. Detection of four dengue serotypes suggests rise in hyperendemicity 588 in urban centers of Brazil. PLoS Negl. Trop. Dis. 2014, 8, e2620. [Google Scholar] [CrossRef]
- Jourdan, P.M.; Lamberton, P.H.L.; Fenwick, A.; Addiss, D.G. Soil-transmitted helminth infections. Lancet 2018, 391, 252–265. [Google Scholar] [CrossRef]
- World Health Organization. Soil-Transmitted Helminth Infections. Available online: https://www.who.int/news-room/fact-sheets/detail/neglected-tropical-diseases (accessed on 17 September 2024).
- Chen, J.; Gong, Y.; Chen, Q.; Li, S.; Zhou, Y. Global burden of soil-transmitted helminth infections, 1990–2021. Infect. Dis. Poverty 2024, 13, 77. [Google Scholar] [CrossRef]
- Pullan, R.L.; Smith, J.L.; Jasrasaria, R.; Brooker, S.J. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasites Vectors 2014, 7, 37. [Google Scholar] [CrossRef]
- Vaz Nery, S.; Pickering, A.J.; Abate, E.; Asmare, A.; Barrett, L.; Benjamin-Chung, J.; Bundy, D.A.; Clasen, T.; Clements, A.C.; Colford, J.M.; et al. The role of water, sanitation and hygiene interventions in reducing soil-transmitted helminths: Interpreting the evidence and identifying next steps. Parasites Vectors 2019, 15, e0009625. [Google Scholar] [CrossRef]
- Ercumen, A.; Benjamin-Chung, J.; Arnold, B.F.; Lin, A.; Hubbard, A.E.; Stewart, C. Effects of water, sanitation, handwashing and nutritional interventions on soil-transmitted helminth infections in young children: A cluster-ransomized controlled trial in rural Bangladesh. PLoS Negl. Trop. Dis. 2019, 13, e0007323. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Preventive Chemotherapy to Control Soil-Transmitted Helminth Infections in at-Risk Population Groups; WHO: Geneva, Switzerland, 2017.
- de Souza, D.K.; Dorlo, T.P.C. Safe mass drug administration for neglected tropical diseases. Lancet 2018, 6, 1054–1055. [Google Scholar] [CrossRef]
- Farrell, S.H.; Coffeng, L.E.; Truscott, J.E.; Werkman, M.; Toor, J.; de Vlas, S.J. Investigating the effectiveness of current and modified World Health Organization guidelines for the control of soil-transmitted helminth infections. Clin. Infect. Dis. 2018, 66, S253–S259. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Soil-Transmitted Helminth Infections. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections (accessed on 5 November 2024).
- Speich, B.; Moser, W.; Ali, S.M.; Ame, S.M.; Albonico, M.; Hattendori, J. Efficacy and reinfection with soil-transmitted helminths 18-weeks post-treatment with albendazole-ivermectin, albendazole-mebendazole, albendazole-oxantel pamoate and mebendazole. Parasites Vectors 2016, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Crossing the billion. Lymphatic Filariasis, Onchocerciasis, Schistosomiasis, Soil-Transmitted Helminthiases and Trachoma: Preventive Chemotherapy for Neglected Tropical Diseases; World Health Organization: Geneva, Switzerland, 2017.
- Chong, N.S.; Smith, S.R.; Werkman, M.; Anderson, R.M. Modelling the ability of mass drug administration to interrupt soil-transmitted helminth transmission: Community-based deworming in Kenya as a case study. PLoS Negl. Trop. Dis. 2021, 215, e0009625. [Google Scholar] [CrossRef] [PubMed]
- Newman, T.E.; Juergens, A.L. Filariasis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK556012/ (accessed on 8 August 2023).
- Cromwell, E.A.; Schmidt, C.A.; Kwong, K.T.; Pigott, D.M.; Mupfasoni, D.; Biswas, G.; Shirude, S.; Hill, E.; Donkers, K.M.; Abdoli, A.; et al. Local Burden of Disease 2019 Neglected Tropical Diseases Collaborators. The global distribution of lymphatic filariasis, 2000–2018: A geospatial analysis. Lancet Glob. Health 2020, 8, e1186–e1194, Erratum in Lancet Glob. Health 2021, 9, e1371. [Google Scholar] [CrossRef] [PubMed]
- Stolk, W.A.; Swaminathan, S.; van Oortmarssen, G.J.; Das, P.K.; Habbema, J.D.F. Prospects for elimination of Bancroftian filariasis by mass drug treatment in Pondicherry, India: A simulation study. J. Infect. Dis. 2003, 188, 1371–1381. [Google Scholar] [CrossRef]
- Koudou, B.G.; de Souza, D.K.; Biritwum, N.K.; Bougma, R.; Aboulaye, M.; Elhassan, E.; Bush, S.; Molyneux, D.H. Elimination of lymphatic filariasis in west African urban areas: Is implementation of mass drug administration necessary? Lancet Infect. Dis. 2018, 18, e214–e220. [Google Scholar] [CrossRef]
- Stillwaggon, E.; Sawers, L.; Rout, J.; Addiss, D.; Fox, L. Economic costs and benefits of a community-based lymphedema management program for lymphatic filariasis in Odisha State, India. Am. J. Trop. Med. Hyg. 2016, 95, 877. [Google Scholar] [CrossRef]
- CDC. About Schistosomiasis. 2024. Available online: https://www.cdc.gov/schistosomiasis/about/index.html (accessed on 5 November 2024).
- Ceccarelli, G.; d’Ettorre, G.; Riccardo, F.; Ceccarelli, C.; Chiaretti, M.; Picciarella, A.; Pacifici, L.E.; Vullo, V. Urinary schistosomiasis in asylum seekers in Italy: An emergency currently undervalued. J. Immigr. Minor. Health 2013, 15, 846–850. [Google Scholar] [CrossRef]
- Ezeamama, A.E.; Bustinduy, A.L.; Nkwata, A.K.; Martinez, L.; Pabalan, N.; Boivin, M.J.; King, C.H. Cognitive deficits and educational loss in children with schistosome infection—A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2018, 12, e0005524. [Google Scholar] [CrossRef]
- Lai, Y.S.; Biedermann, P.; Ekpo, U.F.; Garba, A.; Mathieu, E.; Midzi, N.; Mwinzi, P.; N’Goran, E.K.; Raso, G.; Assaré, R.K.; et al. Spatial distribution of schistosomiasis and treatment needs in sub-Saharan Africa: A systematic review and geostatistical analysis. Lancet Infect. Dis. 2015, 15, 927–940. [Google Scholar] [CrossRef]
- Montgomery, S.; Evan Secor, W. Schistosomiasis-CDC Yellow Book. 2024. Available online: https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/schistosomiasis (accessed on 3 November 2024).
- Rothe, C.; Zimmer, T.; Schunk, M.; Wallrauch, C.; Helfrich, K.; Gültekin, F.; Bretzel, G.; Allienne, J.F.; Boissier, J. Developing Endemicity of Schistosomiasis, Corsica, France. Emerg. Infect. Dis. 2021, 27, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Wellinghausen, N.; Moné, H.; Mouahid, G. A family cluster of schistosomiasis acquired in Solenzara River, Corsica (France)—Solenzara River is clearly a transmission site for schistosomiasis in Corsica. Parasitol. Res. 2022, 121, 2449–2452. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.R.; Burton, M.J.; Haddad, D.; West, S.; Wright, H. Trachoma. Lancet 2014, 384, 2142–2152. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.W.; Burton, M.J.; Gower, E.W.; Harding-Esch, E.M.; Oldenburg, C.E.; Taylor, H.R.; Traoré, L. Trachoma. Nat. Rev. Dis. Primers 2022, 8, 32. [Google Scholar] [CrossRef]
- Tian, L.; Wang, N.L. Trachoma control: The SAFE strategy. Int. J. Ophthalmol. 2018, 11, 1887–1888. [Google Scholar] [CrossRef]
- Gordon, C.A.; Kurscheid, J.; Jones, M.K.; Gray, D.J.; McManus, D.P. Soil-transmitted helminths in tropical Australia and Asia. Trop. Med. Infect. Dis. 2017, 2, 56. [Google Scholar] [CrossRef]
- Sanders, A.M.; Dixon, R.; Stuck, L.; Kelly, M.; Woods, G.; Muheki, E.M.; Baayenda, G.; Masika, M.; Kafanikhale, H.; Mwingira, U.; et al. Evaluation of facial cleanliness and environmental improvement activities: Lessons learned from Malawi, Tanzania, and Uganda. PLoS Negl. Trop. Dis. 2021, 15, e0009962. [Google Scholar] [CrossRef]
- World Health Organization. Onchocerciasis. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/onchocerciasis (accessed on 30 October 2024).
- Frallonardo, L.; Di Gennaro, F.; Panico, G.G.; Novara, R.; Pallara, E.; Cotugno, S.; Guido, G.; De Vita, E.; Ricciardi, A.; Totaro, V.; et al. Onchocerciasis: Current knowledge and future goals. Front. Trop. Dis. 2022, 3, 986884. [Google Scholar] [CrossRef]
- Tekle, A.H.; Zouré, H.G.; Noma, M.; Boussinesq, M.; Coffeng, L.E.; Stolk, W.A.; Remme, J.H. Progress towards onchocerciasis elimination in the participating countries of the African Programme for Onchocerciasis Control: Epidemiological evaluation results. Infect. Dis. Poverty 2016, 5, 1–25. [Google Scholar] [CrossRef]
- Senyonjo, L.; Oye, J.; Bakajika, D.; Biholong, B.; Tekle, A.; Boakye, D.; Schmidt, E.; Elhassan, E. Factors associated with ivermectin non-compliance and its potential role in sustaining Onchocerca volvulus transmission in the west region of Cameroon. PLoS Negl. Trop. Dis. 2016, 10, e0004905. [Google Scholar] [CrossRef]
- Boussinesq, M.; Fobi, G.; Kuesel, A.C. Alternative treatment strategies to accelerate the elimination of onchocerciasis. Int. Health 2018, 10 (Suppl 1), i40–i48. [Google Scholar] [CrossRef] [PubMed]
- Jacob, B.; Michael, E.; Unnasch, T.R. Community-Directed Vector Control to Accelerate Onchocerciasis Elimination. Pathogens 2024, 13, 268. [Google Scholar] [CrossRef] [PubMed]
- Wanji, S.; Nji, T.M.; Hamill, L. Implementation of test-and-treat with doxycycline and temephos ground larviciding as alternative strategies for accelerating onchocerciasis elimination in an area of loiasis co-endemicity: The COUNTDOWN consortium multi-disciplinary study protocol. Parasites Vectors 2019, 12, 574. [Google Scholar] [CrossRef] [PubMed]
- Ensor, T.; Cooper, S. Overcoming barriers to health service access: Influencing the demand side. Health Policy Plan. 2004, 19, 69–79. [Google Scholar] [CrossRef]
- Russell, S. The economic burden of illness for households in developing countries: A review of studies focusing on malaria, tuberculosis, and human immunodeficiency virus/acquired immunodeficiency syndrome. The Intolerable Burden of Malaria II: What’s New. What’s Needed. Am. J. Trop. Med. Hyg. 2004, 71 (Suppl. 2). [Google Scholar]
- Kickbusch, I.; Wait, S.; Maag, D. Navigating Health: The Role of Health Literacy; International Longevity Centre UK: London, UK, 2006; Available online: https://ilcuk.org.uk/wp-content/uploads/2018/10/NavigatingHealth.pdf (accessed on 5 November 2024).
- Engels, D.; Zhou, X.N. Neglected tropical diseases: An effective global response to local poverty-related disease priorities. Infect. Dis. Poverty 2020, 9, 9–17. [Google Scholar] [CrossRef]
- Turner, H.C.; Stolk, W.A.; Solomon, A.W.; King, J.D.; Montresor, A.; Molyneux, D.H.; Toor, J. Are current preventive chemotherapy strategies for controlling and eliminating neglected tropical diseases cost-effective? BMJ Glob. Health 2021, 6, e005456. [Google Scholar] [CrossRef]
- Malecela, M.N. Reflections on the decade of the neglected tropical diseases. Int. Health 2019, 11, 338–340. [Google Scholar] [CrossRef]
- Fitzpatrick, C.; Nwankwo, U.; Lenk, E.; de Vlas, S.J.; Bundy, D.A. An investment case for ending neglected tropical diseases. In Major Infectious Diseases; The World Bank: Washington, DC, USA, 2018. [Google Scholar]
- Rust, J.; Clark, A.; Woodgate, M.; Koch, C.; Mohammed, T.; Steinmann, P.; Krentel, A.; Torres-Vitolas, C.A.; Carlin, A.; Pavluck, A. Innovate to eliminate: A prerequisite in NTD programmes. Int. Health 2022, 14 (Suppl. 2), ii20–ii24. [Google Scholar] [CrossRef]
- Richards, F.O.; Eigege, A.; Umaru, J.; Kahansim, B.; Adelamo, S.; Kadimbo, J.; Danboyi, J.; Mafuyai, H.; Saka, Y.; Noland, G.S.; et al. The Interruption of Transmission of Human Onchocerciasis by an Annual Mass Drug Administration Program in Plateau and Nasarawa States, Nigeria. Am. J. Trop. Med. Hyg. 2020, 102, 582–592. [Google Scholar] [CrossRef]
- Njenga, S.M.; Kanyi, H.; Okoyo, C.; Githinji, E.; Mwatele, C.; Matendechero, S.H.; Omondi, W.P.; Gitahi, P.N.; Owaga, C.; Onsongo, J.K.; et al. Triple-drug therapy with ivermectin, diethylcarbamazine and albendazole for the acceleration of lymphatic filariasis elimination in Kenya: Programmatic implementation and results of the first impact assessment. PLoS Negl. Trop. Dis. 2024, 18, e0011942. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Geleta, D.; Tafese, G.; Tafese, T.; Behaksira, S.; Solomon, H.; Oljira, B.; Miecha, H.; Gemechu, L.; Debebe, K.; et al. Perceptions and acceptability of co-administered albendazole, ivermectin and azithromycin mass drug administration, among the health workforce and recipient communities in Ethiopia. PLoS Negl. Trop. Dis. 2023, 17, e0011332. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Tafese, G.; Tafese, T.; Behaksra, S.W.; Solomon, H.; Oljira, B.; Miecha, H.; Debebe, K.A.; Kebede, B.; Gebre, T.; et al. Safety of integrated mass drug administration of azithromycin, albendazole and ivermectin versus standard treatment regimens: A cluster-randomised trial in Ethiopia. EClinicalMedicine 2023, 59, 101984. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, C.L.; Dean, L.; Thomson, R.; Garner, P. Community drug distributors for mass drug administration in neglected tropical disease programmes: Systematic review and analysis of policy documents. J. Glob. Health 2019, 9, 020414. [Google Scholar] [CrossRef]
- Taylor, M.; Thomas, R.; Oliver, S.; Garner, P. Community views on mass drug administration for filariasis: A qualitative evidence synthesis. Cochrane Database Syst. Rev. 2022, 2. [Google Scholar]
- Hoefle-Bénard, J.; Salloch, S. Mass drug administration for neglected tropical disease control and elimination: A systematic review of ethical reasons. BMJ Glob. Health 2024, 9, e013439. [Google Scholar] [CrossRef]
- Maddren, R.; Phillips, A.; Rayment Gomez, S.; Forbes, K.; Collyer, B.S.; Kura, K.; Anderson, R. Individual longitudinal compliance to neglected tropical disease mass drug administration programmes, a systematic review. PLoS Negl. Trop. Dis. 2023, 17, e0010853. [Google Scholar] [CrossRef]
- Konopka, J.K.; Chatterjee, P.; LaMontagne, C.; Brown, J. Environmental impacts of mass drug administration programs: Exposures, risks, and mitigation of antimicrobial resistance. Infect. Dis. Poverty 2022, 11, 78. [Google Scholar] [CrossRef]
- Pokharel, S.; Adhikari, B.; Johnson, T.; Cheah, P.Y. Interventions to address antimicrobial resistance: An ethical analysis of key tensions and how they apply in low-income and middle-income countries. BMJ Glob. Health 2024, 9, e012874. [Google Scholar] [CrossRef]
- Parker, M.; Allen, T.I. Will mass drug administration eliminate lymphatic filariasis? Evidence from northern coastal Tanzania. J. Biosoc. Sci. 2013, 45, 517–545. [Google Scholar] [CrossRef]
- Campbell, C.; Cornish, F. Towards a “Fourth generation” of approaches to HIV/AIDS management: Creating contexts for effective community mobilisation. AIDS Care 2010, 22 (Suppl. 2), 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Rifkin, S.B. Examining the links between community participation and health outcomes: A review of the literature. Health Policy Plan. 2014, 29 (Suppl. 2), ii98–ii106. [Google Scholar] [CrossRef] [PubMed]
- Amazigo, U.V.; Brieger, W.R.; Katabarwa, M.; Akogun, O.; Ntep, M.; Boatin, B.; N’doyo, J.; Noma, M.; Seketeli, A. The challenges of community-directed treatment with ivermectin (CDTI) within the African Programme for Onchocerciasis Control (APOC). Ann. Trop. Med. Parasitol. 2002, 96 (Suppl. 1), S41–S58. [Google Scholar] [CrossRef] [PubMed]
- Easterly, W. The trouble with the sustainable development goals. Curr. Hist. 2015, 114, 322. [Google Scholar] [CrossRef]
- Laverack, G.; Labonte, R. A planning framework for community empowerment goals within health promotion. Health Policy Plan. 2000, 15, 255–262. [Google Scholar] [CrossRef]
- Morgan, L.M. Community participation in health: Perpetual allure, persistent challenge. Health Policy Plan. 2001, 16, 221–230. [Google Scholar] [CrossRef]

| Climate Variation | Impact on Environment/Vector/Host | NTD Affected | Impact on Transmission/Distribution | Public Health Implications |
|---|---|---|---|---|
| Increased temperature | Increased vector breeding rates (e.g., mosquitoes), shorter incubation periods for pathogens | Plasmodium spp. (malaria), Dengue, Zika, Chikungunya, Leishmaniasis spp. (leishmaniasis) | Increased transmission potential, wider vector distribution, faster disease development | Enhanced vector control, surveillance, rapid diagnostic testing, and public awareness campaigns |
| Altered Rainfall Patterns | Increased breeding sites for vectors (e.g., stagnant water), changes in intermediate host populations, contamination of water sources | Schistosomiasis, Lymphatic Filariasis, Soil-Transmitted Helminths, Guinea worm disease, waterborne NTDs | Altered transmission dynamics, shifts in disease distribution, increased risk of waterborne outbreaks | Improved water and sanitation infrastructure, targeted interventions based on rainfall patterns, water quality monitoring |
| Extreme Weather Events (e.g., floods, droughts, heatwaves) | Disruption of sanitation systems, displacement of populations, contamination of water sources, increased stress on individuals and healthcare systems | Vibrio Cholera, Typhoid, other waterborne diseases, vector-borne diseases | Increased risk of outbreaks, wider spread of disease, reduced access to healthcare | Disaster preparedness plans, rapid response mechanisms, access to safe water and sanitation, strengthening of healthcare infrastructure |
| Sea Level Rise | Salinization of freshwater sources, coastal erosion, displacement of populations, loss of arable land | Various NTDs, particularly in coastal regions, foodborne NTDs | Altered disease ecology, potential shifts in disease distribution, food insecurity | Climate change adaptation strategies, relocation of vulnerable communities, food security programs |
| Ocean Acidification | Changes in marine ecosystems, impacting intermediate hosts or vectors | Foodborne trematodiases | Potential changes in transmission dynamics, impacts on food security | Monitoring of marine ecosystems, sustainable aquaculture practices, food safety regulations |
| Increased Humidity | Increased vector survival and biting rates | Many vector-borne NTDs, including Dengue, Zika, and Chikungunya | Increased transmission potential | Enhanced vector control measures, public health messaging about protective measures |
| Changes in wind patterns | Dispersal of vectors and pathogens over wider areas | Various vector-borne NTDs | Potential introduction of diseases to new regions | Surveillance for emerging diseases, vector control measures adapted to wind patterns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branda, F.; Ali, A.Y.; Ceccarelli, G.; Albanese, M.; Binetti, E.; Giovanetti, M.; Ciccozzi, M.; Scarpa, F. Assessing the Burden of Neglected Tropical Diseases in Low-Income Communities: Challenges and Solutions. Viruses 2025, 17, 29. https://doi.org/10.3390/v17010029
Branda F, Ali AY, Ceccarelli G, Albanese M, Binetti E, Giovanetti M, Ciccozzi M, Scarpa F. Assessing the Burden of Neglected Tropical Diseases in Low-Income Communities: Challenges and Solutions. Viruses. 2025; 17(1):29. https://doi.org/10.3390/v17010029
Chicago/Turabian StyleBranda, Francesco, Abdisalam Yusuf Ali, Giancarlo Ceccarelli, Mattia Albanese, Erica Binetti, Marta Giovanetti, Massimo Ciccozzi, and Fabio Scarpa. 2025. "Assessing the Burden of Neglected Tropical Diseases in Low-Income Communities: Challenges and Solutions" Viruses 17, no. 1: 29. https://doi.org/10.3390/v17010029
APA StyleBranda, F., Ali, A. Y., Ceccarelli, G., Albanese, M., Binetti, E., Giovanetti, M., Ciccozzi, M., & Scarpa, F. (2025). Assessing the Burden of Neglected Tropical Diseases in Low-Income Communities: Challenges and Solutions. Viruses, 17(1), 29. https://doi.org/10.3390/v17010029











