The Influence of the Temperature on Effectiveness of Selected Disinfectants Against African Swine Fever Virus (ASFV)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Virus Stock Preparation
2.3. Disinfectants
2.4. Diluent and Interfering Substances
2.5. Temperature Conditions
2.6. Test Conditions
2.7. Cytotoxicity Reduction
2.8. Test Controls
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jurado, C.; Fernández-Carrión, E.; Mur, L.; Rolesu, S.; Laddomada, A.; Sánchez-Vizcaíno, J.M. Why is African swine fever still present in Sardinia? Transbound. Emerg. Dis. 2018, 65, 557–566. [Google Scholar] [CrossRef]
- European Commission. Commission Recognises Sardinia and Sweden as Free of African Swine Fever. Available online: https://ec.europa.eu/commission/presscorner/detail/en/mex_24_4841 (accessed on 22 October 2024).
- Blome, S.; Franzke, K.; Beer, M. African swine fever—A review of current knowledge. Virus Res. 2020, 287, 198099. [Google Scholar] [CrossRef]
- Li, X.; Tian, K. African swine fever in China. Vet. Rec. 2018, 183, 300–301. [Google Scholar] [CrossRef]
- Ma, J.; Chen, H.; Gao, X.; Xiao, J.; Wang, H. African swine fever emerging in China: Distribution characteristics and high-risk areas. Prev. Vet. Med. 2020, 175, 104861. [Google Scholar] [CrossRef]
- USDA. Livestock and Poultry: World Markets and Trade; USDA: Washington, DC, USA, 2023.
- Krajowy Ośrodek Wsparcia Rolnictwa. Sytuacja Podażowo-Popytowa i Cenowa na Rynku Wieprzowiny; Krajowy Ośrodek Wsparcia Rolnictwa: Warsaw, Poland, 2023.
- GIW General Veterinary Inspectorate/Główny Inspektorat Weterynarii. Afrykanski Pomór Świń (ASF). Available online: https://bip.wetgiw.gov.pl/asf/mapa/ (accessed on 22 October 2024).
- World Organisation for Animal Health (WOAH). African Swine Fever (ASF)—Situation Report 47; World Organisation for Animal Health (WAHIS): Paris, France, 2024. [Google Scholar]
- Long, T.H. Summary of Information African Swine Fever Vaccine in Viet Nam. Available online: https://rr-asia.woah.org/app/uploads/2023/09/8-5-vietnam-asf_vaccines.pdf (accessed on 22 October 2024).
- Manso-Ribeiro, J.; Nunes-Petisca, J.L.; Lopez-Frazao, F.; Sobral, M. Vaccination against ASF. Bull. Off. Int. Epizoot. 1963, 60, 921–937. [Google Scholar]
- Bessems, E. The effect of practical conditions on the efficacy of disinfectants. Int. Biodeterior. Biodegrad. 1998, 41, 177–183. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nation; World Organization for Animal Health; World Bank Animal Production and Health. Good Practices for Biosecurity in the Pig Sector; FAO: Rome, Italy, 2010; ISBN 9789251065075. [Google Scholar]
- United States Deparment of Agriculture. The Foreign Animal Disease Preparedness & Response Plan. In Standard Operating Procedures: 15. Cleaning and Disinfection; United States Deparment of Agriculture: Annapolis, MD, USA, 2018. [Google Scholar]
- Stone, S.S.; Hess, W.R. Effects of some disinfectants on African swine fever virus. Appl. Microbiol. 1973, 25, 115–122. [Google Scholar] [CrossRef]
- Juszkiewicz, M.; Walczak, M.; Mazur-Panasiuk, N.; Woźniakowski, G. Virucidal effect of chosen disinfectants against African swine fever virus (ASFV)—Preliminary studies. Pol. J. Vet. Sci. 2019, 22, 777–780. [Google Scholar] [CrossRef]
- Juszkiewicz, M.; Walczak, M.; Mazur-Panasiuk, N.; Woźniakowski, G. Effectiveness of chemical compounds used against african swine fever virus in commercial available disinfectants. Pathogens 2020, 9, 878. [Google Scholar] [CrossRef]
- Frost, L.; Tully, M.; Dixon, L.; Hicks, H.M.; Bennett, J.; Stokes, I.; Marsella, L.; Gubbins, S.; Batten, C. Evaluation of the Efficacy of Commercial Disinfectants against African Swine Fever Virus. Pathogens 2023, 12, 855. [Google Scholar] [CrossRef]
- Krug, P.W.; Davis, T.; O’Brien, C.; LaRocco, M.; Rodriguez, L.L. Disinfection of transboundary animal disease viruses on surfaces used in pork packing plants. Vet. Microbiol. 2018, 219, 219–225. [Google Scholar] [CrossRef]
- Hierholzer, J.C.; Killington, R.A. Virus isolation and quantitation. In Virology Methods Manual; Academy Press: Cambridge, MA, USA, 1996; pp. 25–46. [Google Scholar] [CrossRef]
- Mayr, A.; Bachmann, P.A.; Bibrack, B.; Wittmann, G. Virologische Arbeitsmethoden: Band II (Serologie); Gustav Fischer Verlag: Jena, Germany, 1977. [Google Scholar]
- Cycle, L. African Swine Fever Aetiology; World Organisation for Animal Health (WOAH): Paris, France, 2013; pp. 1–500. [Google Scholar]
- PN-EN:14675-2015; Chemical Disinfectants and Antiseptics-Quantitative Suspension Test for The Evaluation of Virucidal Activity of Chemical Disinfectants and Antiseptics Used in the Veterinary Area—Test Method and Requirements (Phase 2, Step 1). Polish Committee for Standardization: Warsaw, Poland, 2013.
- Food and Agriculture Organization of the United Nations. African Swine Fever (ASF) Situation Update in Asia & Pacific; FAO: Rome, Italy, 2024. [Google Scholar]
- Stefanello, A.; Fracari, J.C.; Silva, M.; Lemos, J.G.; Garcia, M.V.; dos Santos, B.A.; Copetti, M.V. Influence of type, concentration, exposure time, temperature, and presence of organic load on the antifungal efficacy of industrial sanitizers against Aspergillus brasiliensis (ATCC 16404). Food Microbiol. 2021, 97, 103740. [Google Scholar] [CrossRef]
- Aksoy, A.; El Kahlout, K.; Yardimci, H. Comparative Evaluation of the Effects of Binzalkonium Chloride, Iodine, Gluteraldehyde and Hydrogen Peroxide Disinfectants against Avian Salmonellae Focusing on Genotypic Resistance Pattern of the Salmonellae Serotypes toward Benzalkonium Chloride. Braz. J. Poult. Sci. 2020, 22, 1055. [Google Scholar] [CrossRef]
- Jang, Y.; Lee, J.; So, B.; Lee, K.; Yun, S.; Lee, M.; Choe, N. Evaluation of changes induced by temperature, contact time, and surface in the efficacies of disinfectants against avian influenza virus. Poult. Sci. 2014, 93, 70–76. [Google Scholar] [CrossRef]
- Krug, P.W.; Lee, L.J.; Eslami, A.C.; Larson, C.R.; Rodriguez, L. Chemical disinfection of high-consequence transboundary animal disease viruses on nonporous surfaces. Biologicals 2011, 39, 231–235. [Google Scholar] [CrossRef]
- Hu, W.; Shimoda, H.; Tsuchiya, Y.; Kishi, M.; Hayasaka, D. pH—Dependent virucidal effects of weak acids against pathogenic viruses. Trop. Med. Health 2024, 52, 9. [Google Scholar] [CrossRef]
- Ren, Z.; Han, J.; Zhang, X.; Yan, Z.; Wei, Q. Effective of different industrial disinfection in subzero cold—Chain environment. Sci. Rep. 2024, 14, 12651. [Google Scholar] [CrossRef]
- Ujile, A.A.; Dirinna, A.; Konne, J. Evaluating the Thermodynamics and Kinetics of Production of Caustic Soda from Brine. J. Mod. Phys. 2018, 9, 99–111. [Google Scholar] [CrossRef]
- Donnell, G.M.C. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- Rasmussen, K.E.; Albrechtsen, J. Glutaraldehyde. The Influence of pH, Temperature, and Buffering on the Polymerization Rate. Histochemistry 1974, 26, 19–26. [Google Scholar] [CrossRef]
- McGinley, H.R.; Enzien, M.V.; Jenneman, G.; Harris, J. Studies on the Chemical Stability of Glutaraldehyde in Produced Water. In Proceedings of the Paper Presented at the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 11–13 April 2011. [Google Scholar]
- Sirtes, G.; Waltimo, T.; Schaetzle, M.; Zehnder, M. The Effects of Temperature on Sodium Hypochlorite Short-Term Stability, Pulp Dissolution Capacity, and Antimicrobial Efficacy. J. Endod. 2005, 31, 669–671. [Google Scholar] [CrossRef]
- Jones, L.A., Jr.; Hoffman, R.K.; Phillips, C.R. Sporicidal Activity of Sodium Hypochlorite at Subzero Temperatures. Appl. Microbiol. 1968, 16, 787–791. [Google Scholar] [CrossRef]
- Maillard, J.Y.; Hann, A.C.; Baubet, V.; Perrin, R. Efficacy and mechanisms of action of sodium hypochlorite on Pseudomonas aeruginosa PAO1 phage F116. J. Appl. Microbiol. 1998, 85, 925–932. [Google Scholar] [CrossRef]
- Estrela, C.; Barbin, E.L.; Pécora, J.D. Mechanism of Action of Sodium Hypochlorite. Braz. Dent. J. 2002, 13, 113–117. [Google Scholar] [CrossRef]
- Clarkson, R.M.; Moule, A.J.; Podlich, H.M. The shelf-life of sodium hypochlorite irrigating solutions. Aust. Dent. J. 2001, 46, 269–276. [Google Scholar] [CrossRef]
- Diomedi, A.; Chacón, E.; Delpiano, L.; Hervé, B.; Jemenao, M.I.; Medel, M.; Quintanilla, M.; Riedel, G.; Tinoco, J. Antisépticos y desinfectantes: Apuntando al uso racional. Recomendaciones del Comité Consultivo de Infecciones Asociadas a la Atención de Salud, Sociedad Chilena de Infectología. Rev. Chil. Infectol. 2017, 34, 156–174. [Google Scholar] [CrossRef]
- Gorman, S.P.; Scott, E.M.; Russell, A.D. Antimicrobial Activity, Uses and Mechanism of Action of Glutaraldehyde. J. Appl. Bacteriol. 1980, 48, 161–190. [Google Scholar] [CrossRef]
- Eickmann, U.; Bloch, M.; Falcy, M.; Halsen, G.; Merz, B. Use of disinfectants in the health care sector: Chemical hazards and preventive measures Factsheet 3: Hazards of chemical disinfectants. In International Social Security Association (ISSA); ISSA International Section on Prevention of Occupational Risks in Health Services: Hamburg, Germany, 2014; pp. 1–26. ISBN 978-92-843-6191-5. [Google Scholar]
- Chemical Safety in the Workplace. In Guidance Notes on Safe Use of Chemical Disinfectants; Labour Department: Hong Kong, China, 2007; pp. 1–40.
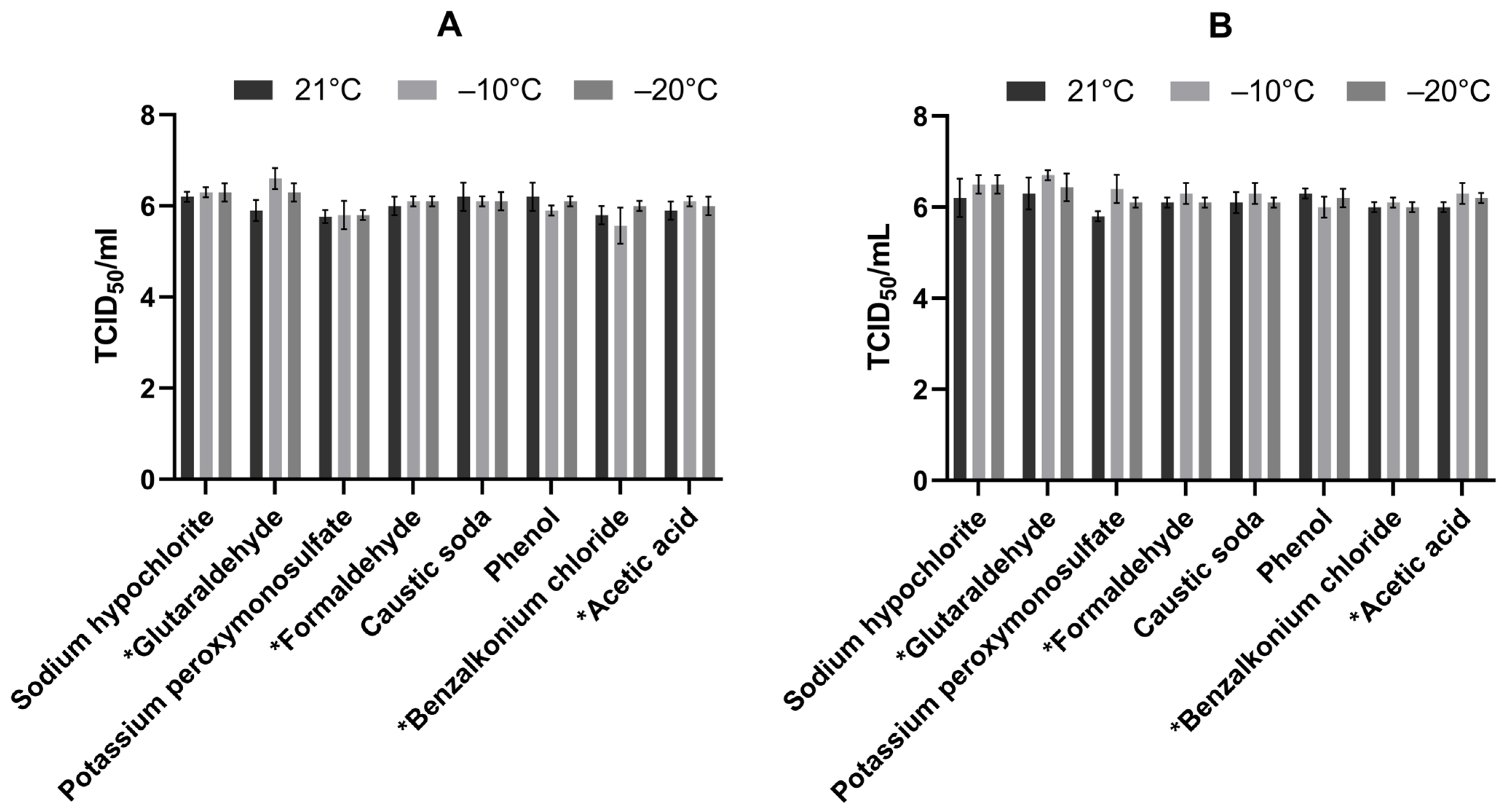
| Active Substance | Temperature (C) | Log10 Difference ** (±SD) (TCID50/mL) | Virucidal Effect (Difference ≥ 4 Log10) | ||
|---|---|---|---|---|---|
| BSA | BSA + YE | BSA | BSA + YE | ||
| Sodium hypochlorite (1%) | 21 | 4.7 (±0.11) | 4.7 (±0.42) | Yes | Yes |
| −10 | 4.8 (±0.11) | 5.0 (±0.2) | Yes | Yes | |
| −20 | 4.8 (±0.12) | 5.0 (±0.2) | Yes | Yes | |
| Glutaraldehyde (0.1%) * | 21 | 4.2 (±0.23) | 4.8 (±0.35) | Yes | Yes |
| −10 | 5.1 (±0.23) | 5.2 (±0.11) | Yes | Yes | |
| −20 | 4.8 (±0.2) | 5.0 (±0.2) | Yes | Yes | |
| Potassium peroxymonosulfate (0.5%) | 21 | 4.2 (±0.23) | 4.3 (±0.11) | Yes | Yes |
| −10 | 4.3 (±0.31) | 4.9 (±0.31) | Yes | Yes | |
| −20 | 4.3 (±0.11) | 4.6 (±0.11) | Yes | Yes | |
| Formaldehyde (0.4%) * | 21 | 2.0 (±0.2) | 2.1 (±0.11) | N/A | N/A |
| −10 | 1.9 (±0.31) | 2.2 (±0.31) | N/A | N/A | |
| −20 | 2.1 (±0.11) | 2.1 (±0.11) | N/A | N/A | |
| Caustic soda (2%) | 21 | 4.2 (±0.31) | 4.1 (±0.11) | Yes | Yes |
| −10 | 4.1 (±0.11) | 4.3 (±0.23) | Yes | Yes | |
| −20 | 4.1 (±0.11) | 4.1 (±0.11) | Yes | Yes | |
| Phenol (1%) | 21 | 4.2 (±0.31) | 4.1 (±0.11) | Yes | Yes |
| −10 | 4.1 (±0.11) | 4.3 (±0.23) | Yes | Yes | |
| −20 | 4.1 (±0.11) | 4.1 (±011) | Yes | Yes | |
| Benzalkonium chloride (1%) * | 21 | 4.0 (±0.2) | 3.5 (±0.11) | Yes | No |
| −10 | 3.1 (±0.11) | 3.3 (±0.23) | N/A | N/A | |
| −20 | 3.1 (±0.11) | 3.1 (±011) | N/A | N/A | |
| Acetic acid (3%) * | 21 | 4.0 (±0.2) | 4.1 (±0.11) | Yes | Yes |
| −10 | 4.1 (±0.11) | 3.8 (±0.2) | Yes | Yes | |
| −20 | 4.1 (±0.11) | 4.1 (±0.11) | Yes | Yes | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juszkiewicz, M.; Walczak, M.; Woźniakowski, G.; Pejsak, Z.; Podgórska, K. The Influence of the Temperature on Effectiveness of Selected Disinfectants Against African Swine Fever Virus (ASFV). Viruses 2025, 17, 156. https://doi.org/10.3390/v17020156
Juszkiewicz M, Walczak M, Woźniakowski G, Pejsak Z, Podgórska K. The Influence of the Temperature on Effectiveness of Selected Disinfectants Against African Swine Fever Virus (ASFV). Viruses. 2025; 17(2):156. https://doi.org/10.3390/v17020156
Chicago/Turabian StyleJuszkiewicz, Małgorzata, Marek Walczak, Grzegorz Woźniakowski, Zygmunt Pejsak, and Katarzyna Podgórska. 2025. "The Influence of the Temperature on Effectiveness of Selected Disinfectants Against African Swine Fever Virus (ASFV)" Viruses 17, no. 2: 156. https://doi.org/10.3390/v17020156
APA StyleJuszkiewicz, M., Walczak, M., Woźniakowski, G., Pejsak, Z., & Podgórska, K. (2025). The Influence of the Temperature on Effectiveness of Selected Disinfectants Against African Swine Fever Virus (ASFV). Viruses, 17(2), 156. https://doi.org/10.3390/v17020156







