Analysis of Hepatic Lentiviral Vector Transduction: Implications for Preclinical Studies and Clinical Gene Therapy Protocols
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Lentiviral Particles Production, Concentration, and Titration
2.3. Lentiviral Vector Administration and in Vivo Imagine
2.4. Tissue Vector Copy Number
2.5. Sleep Phenotyping and Behavior Analysis
2.6. Statistical and QTL Analyses
3. Results
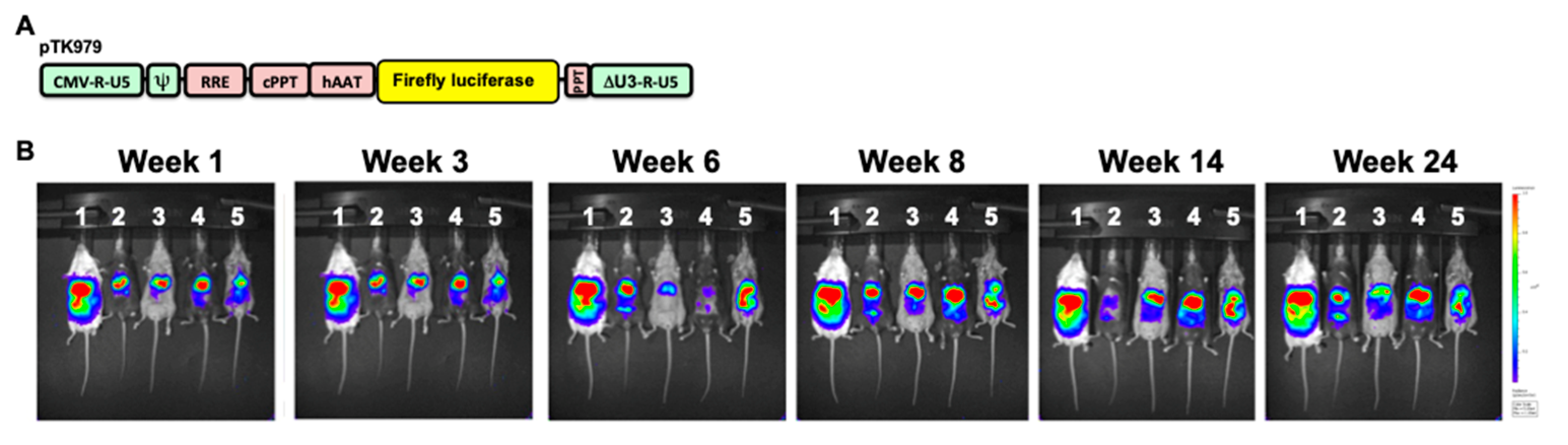
3.1. Overall Approach
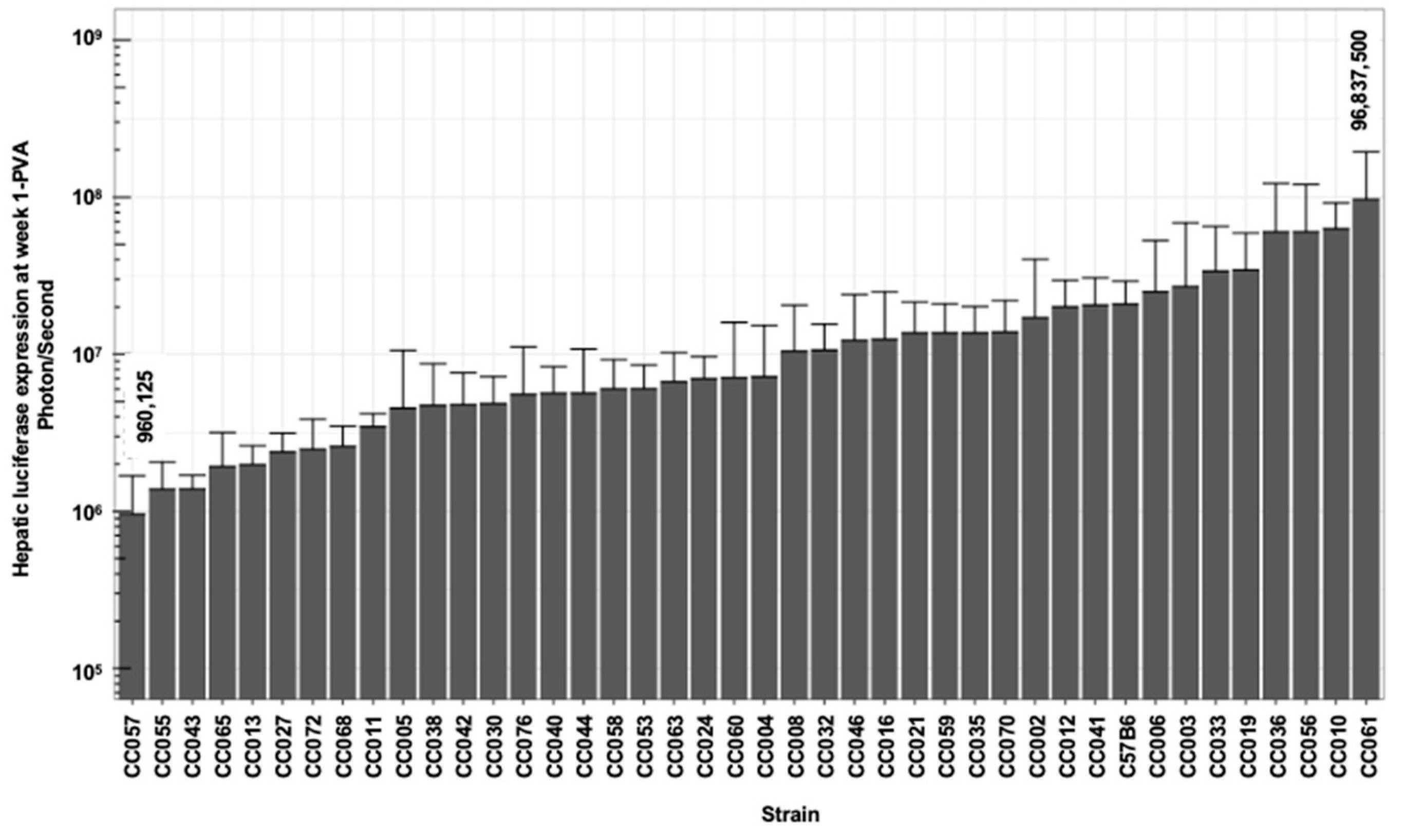
3.2. Broad Range of Lentiviral Vector-Mediated Hepatic Luciferase Expression Among 41 Collaborative Cross Mouse Strains
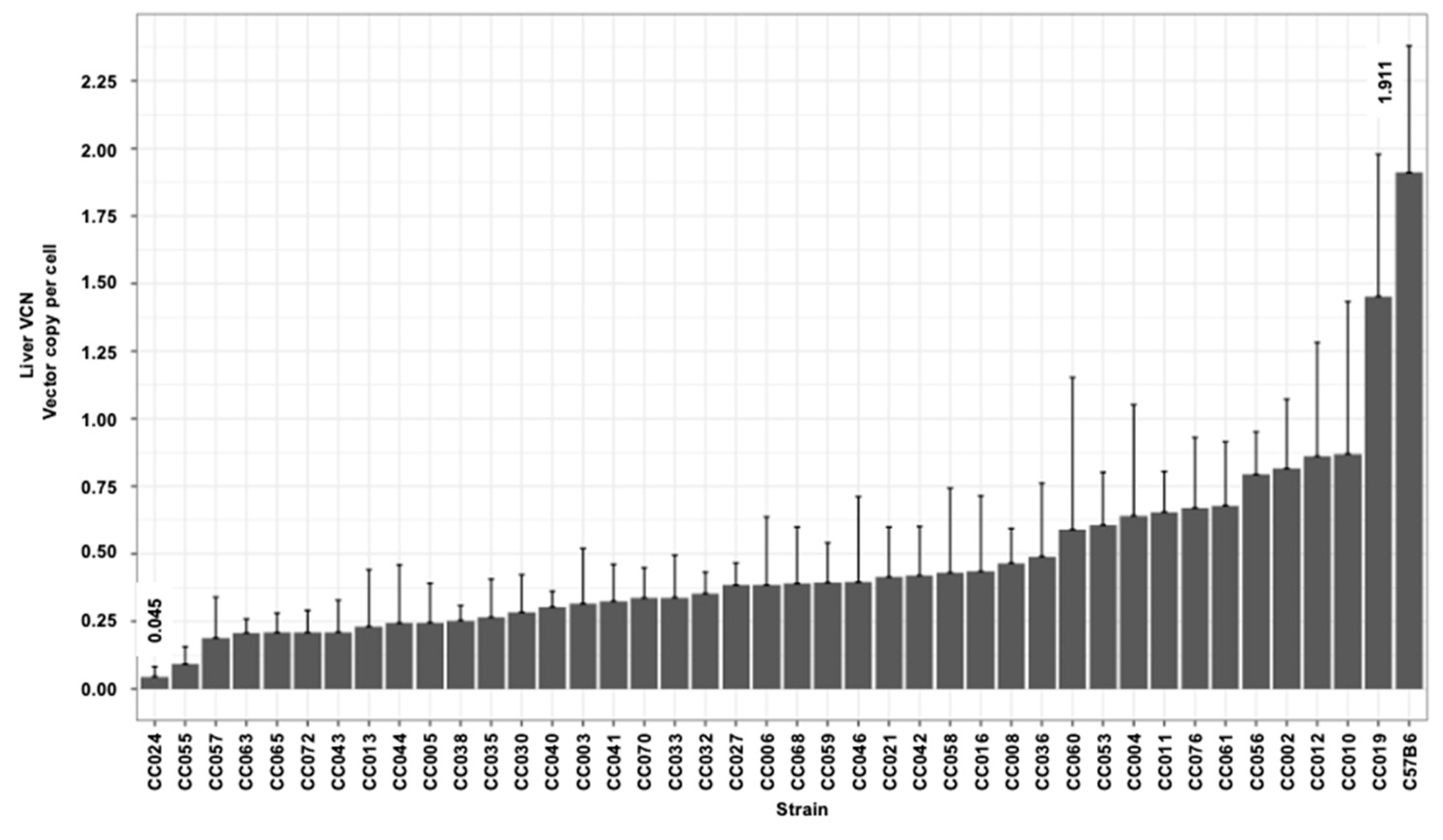
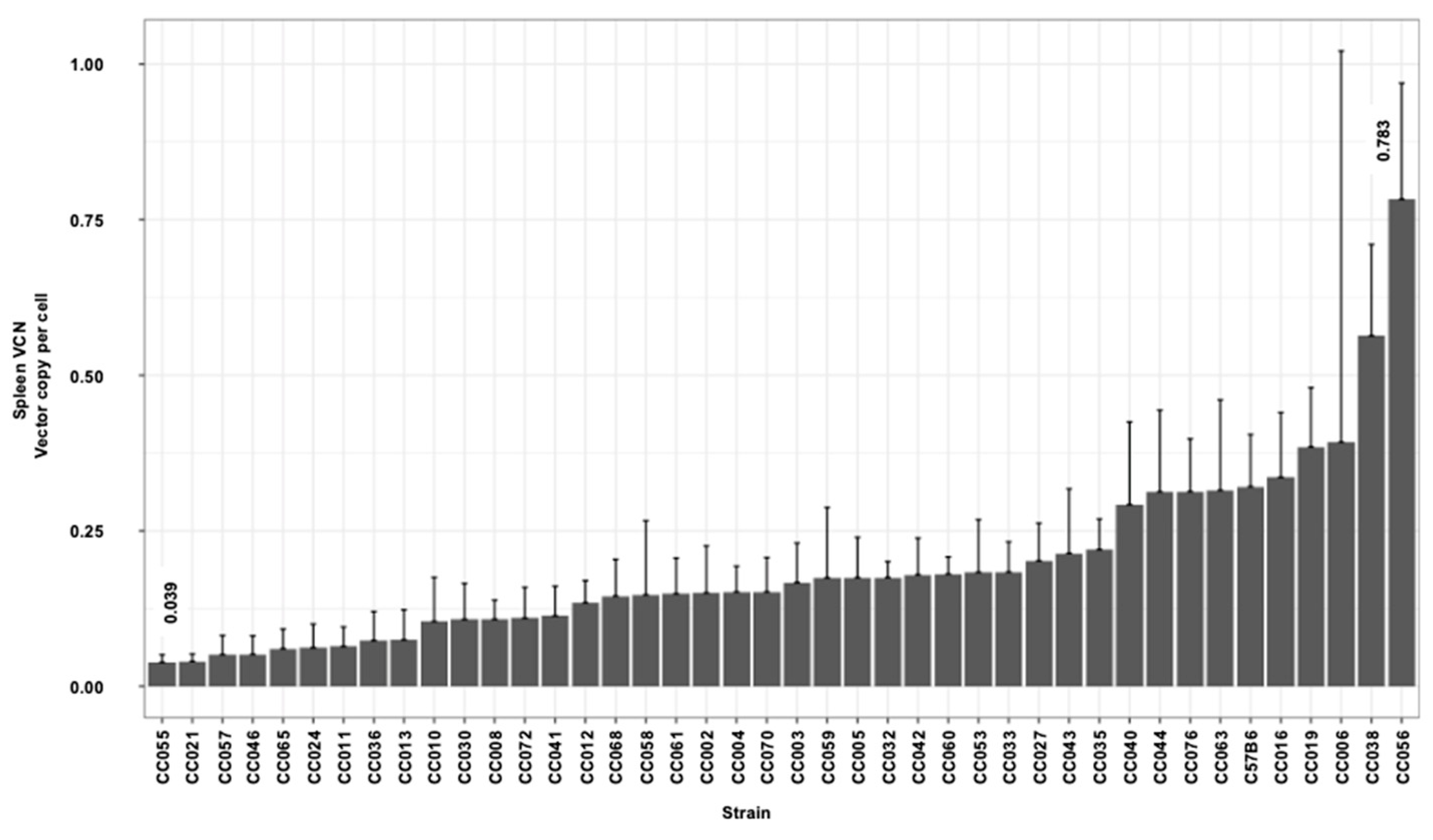
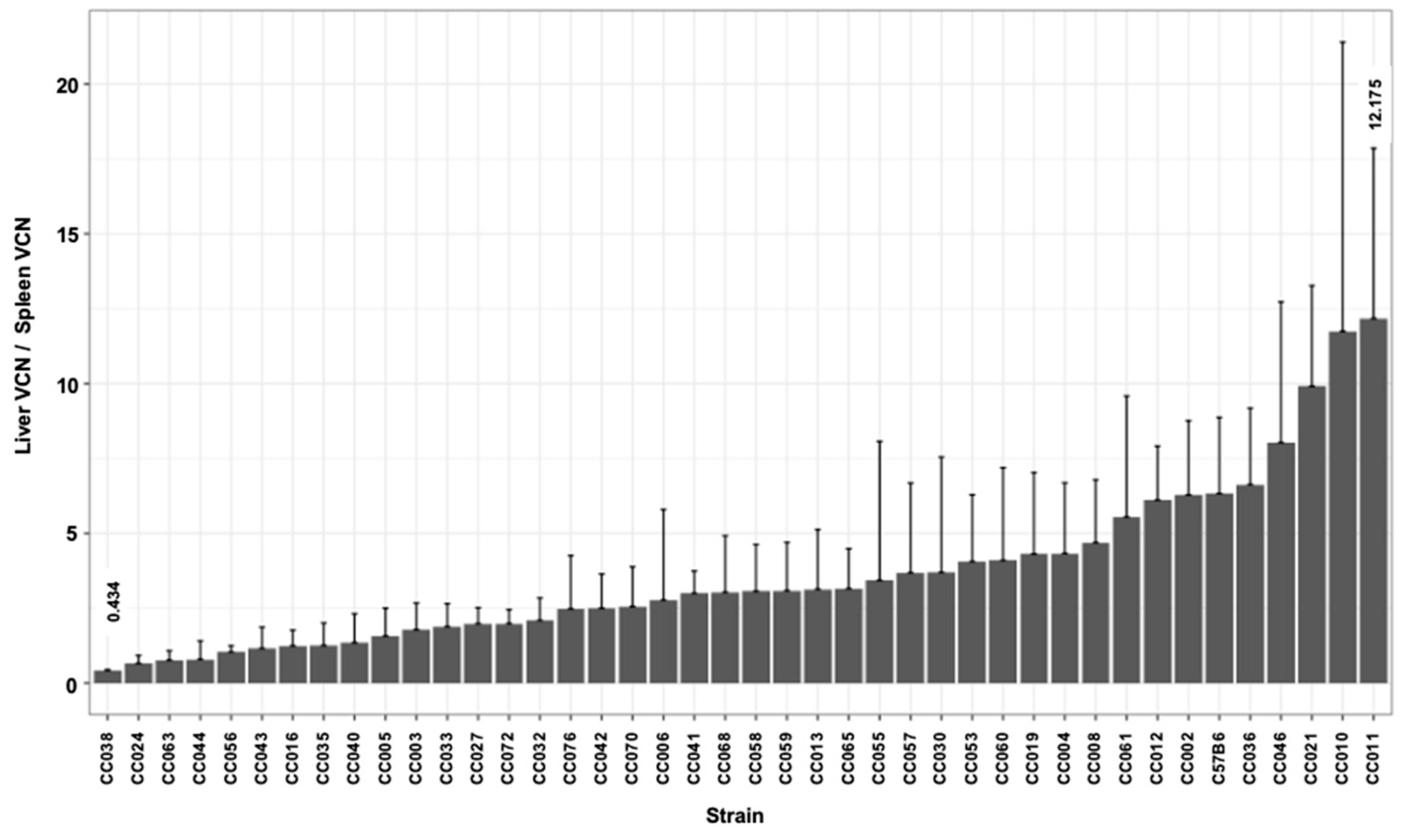
3.3. Hepatic and Splenic Lentiviral Vector Genome Copy Number per Cell (VCN) in CC Mouse Strains
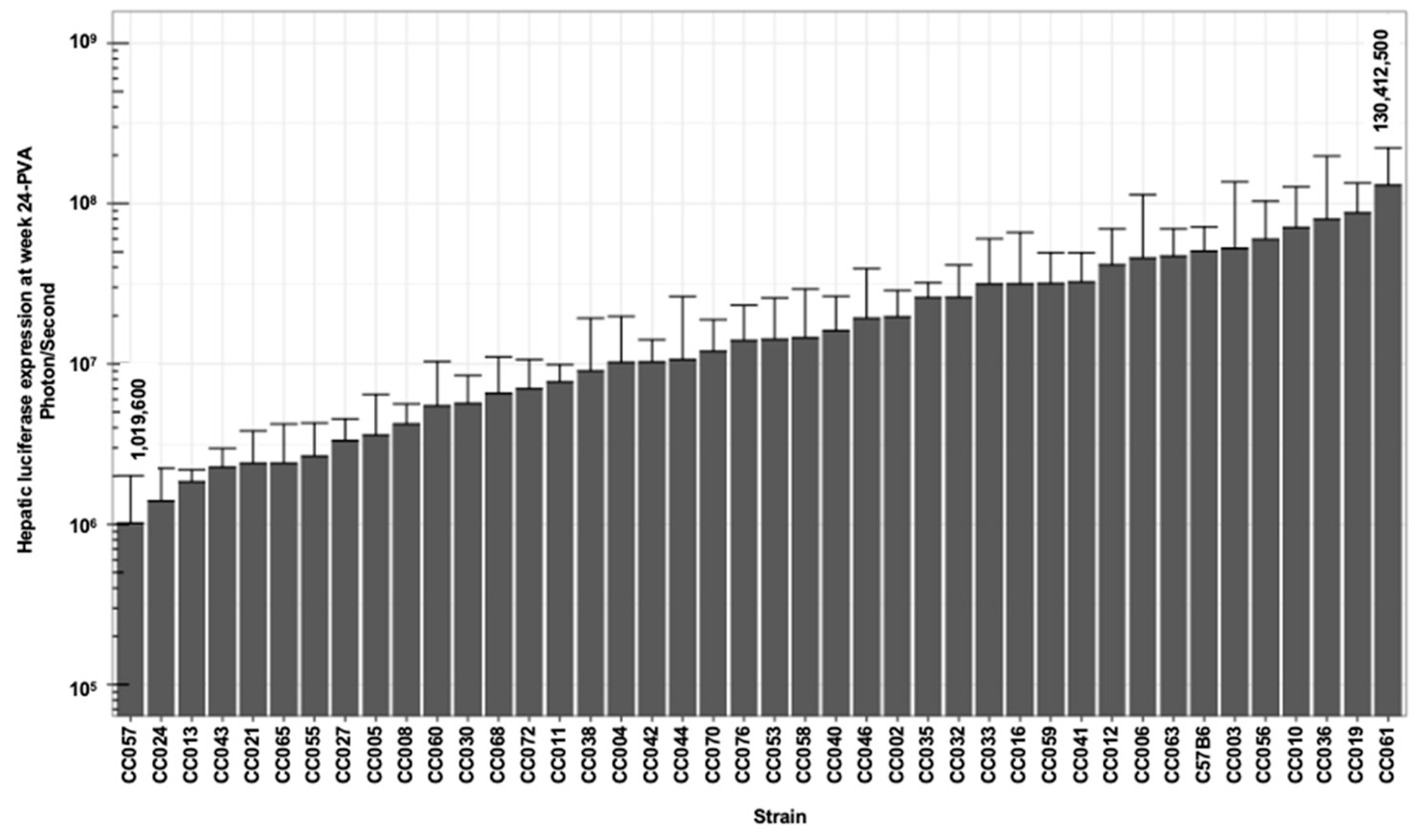
3.4. The Kinetics of Hepatic Luciferase Expression Level in CC Mouse Strains
3.5. Lentiviral Vector-Specific Activity in CC Mouse Strains
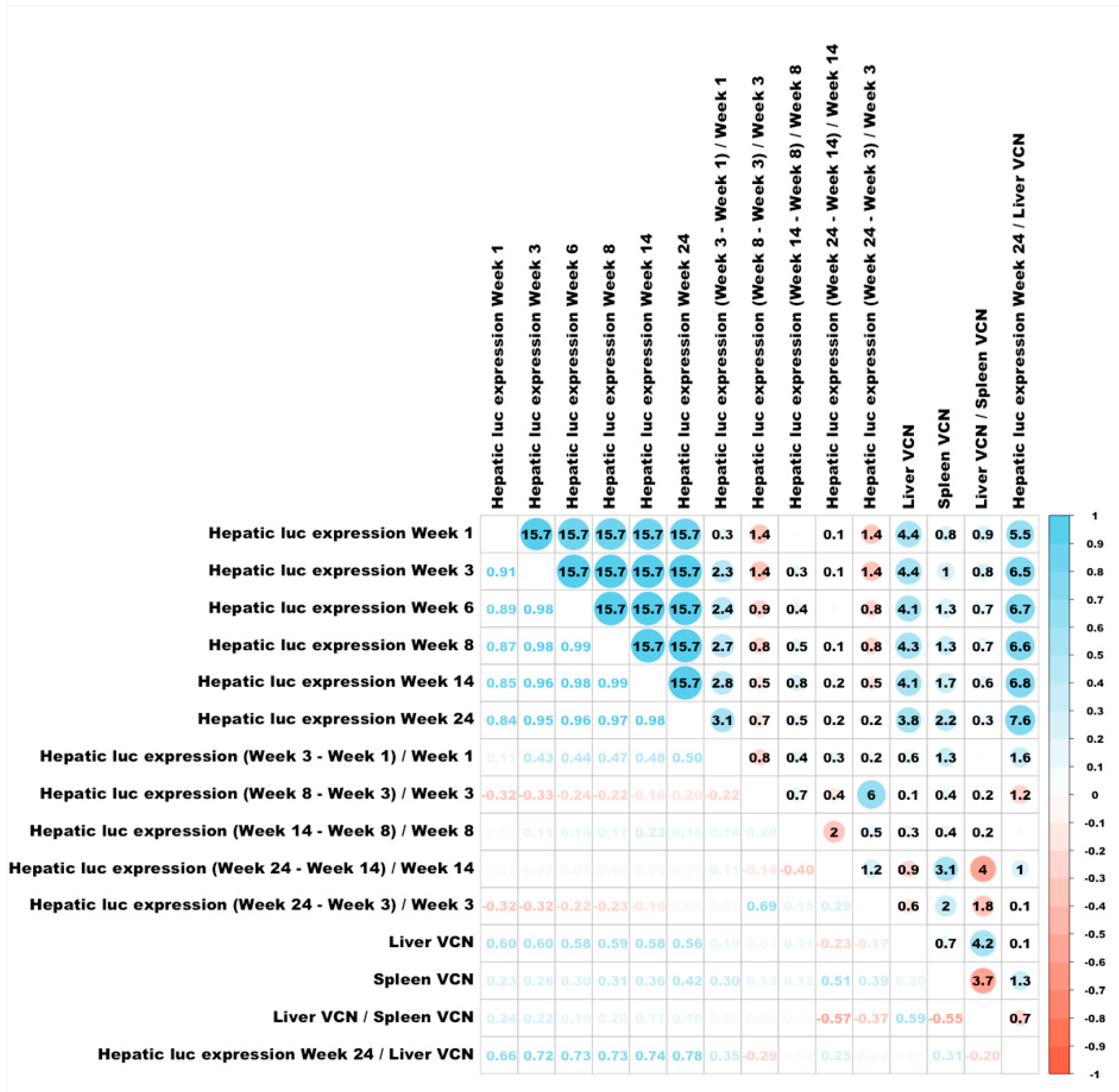
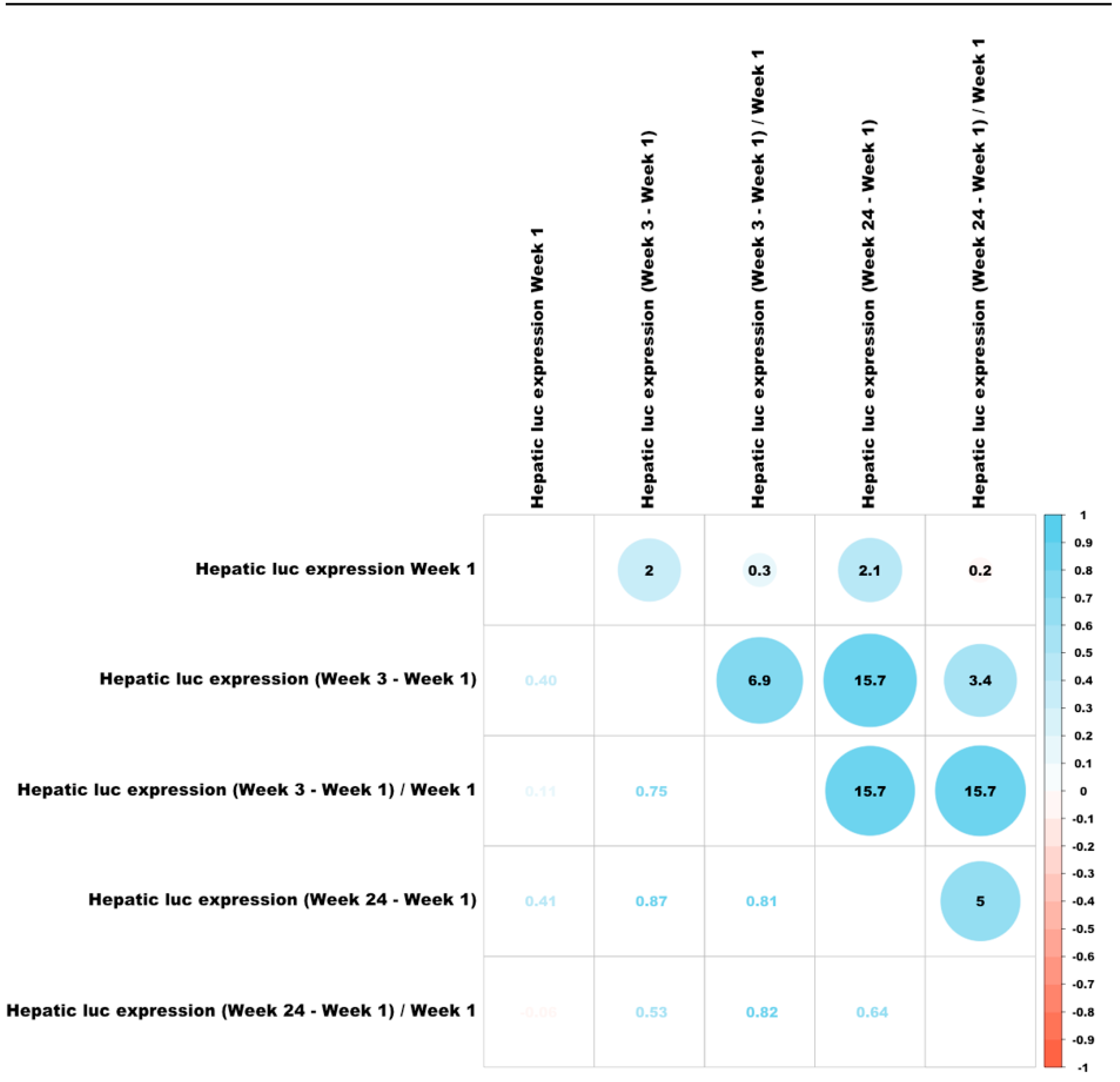
3.6. Correlations Between Different Characteristics of Hepatic Transduction by Lentiviral Vectors
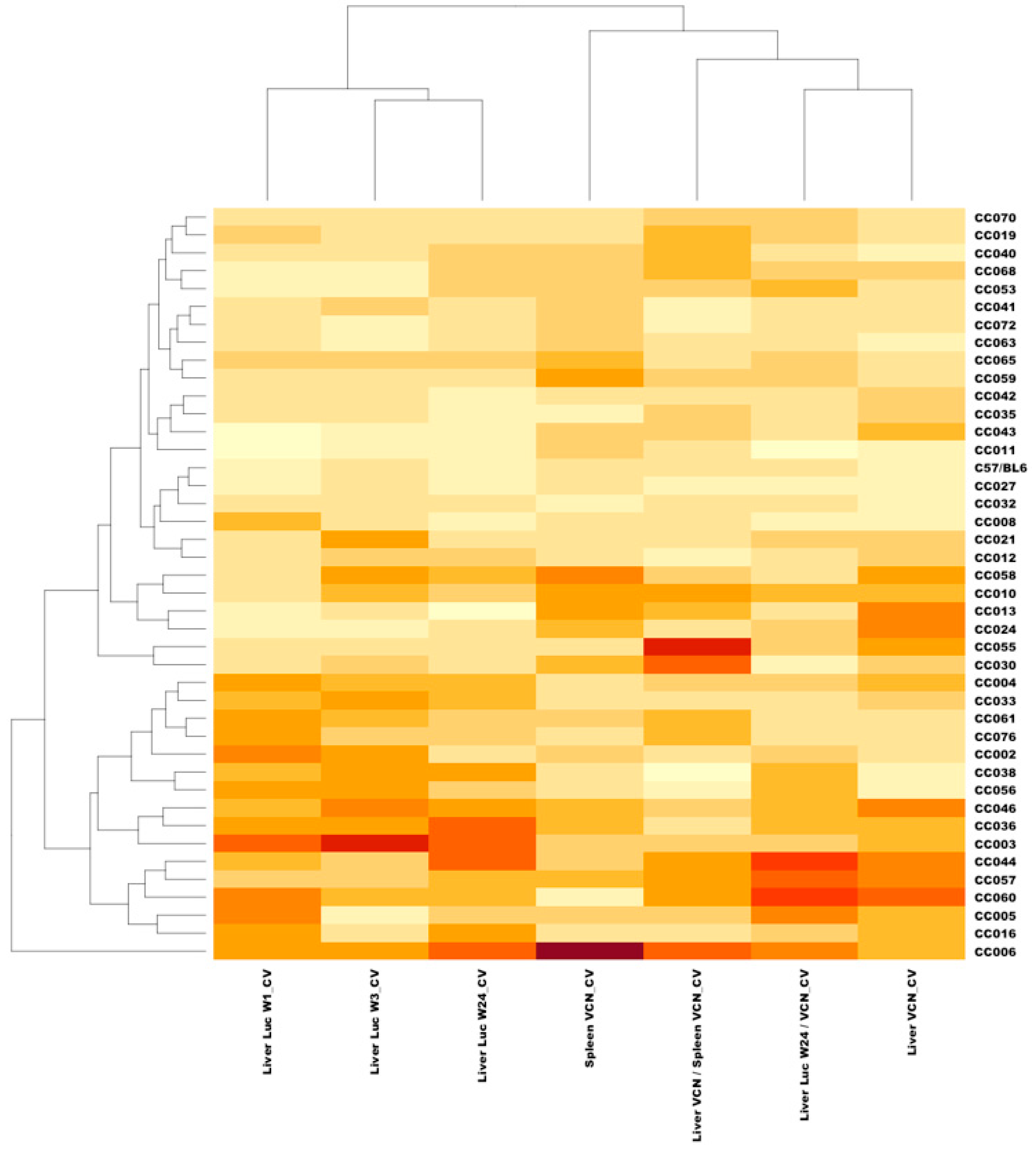
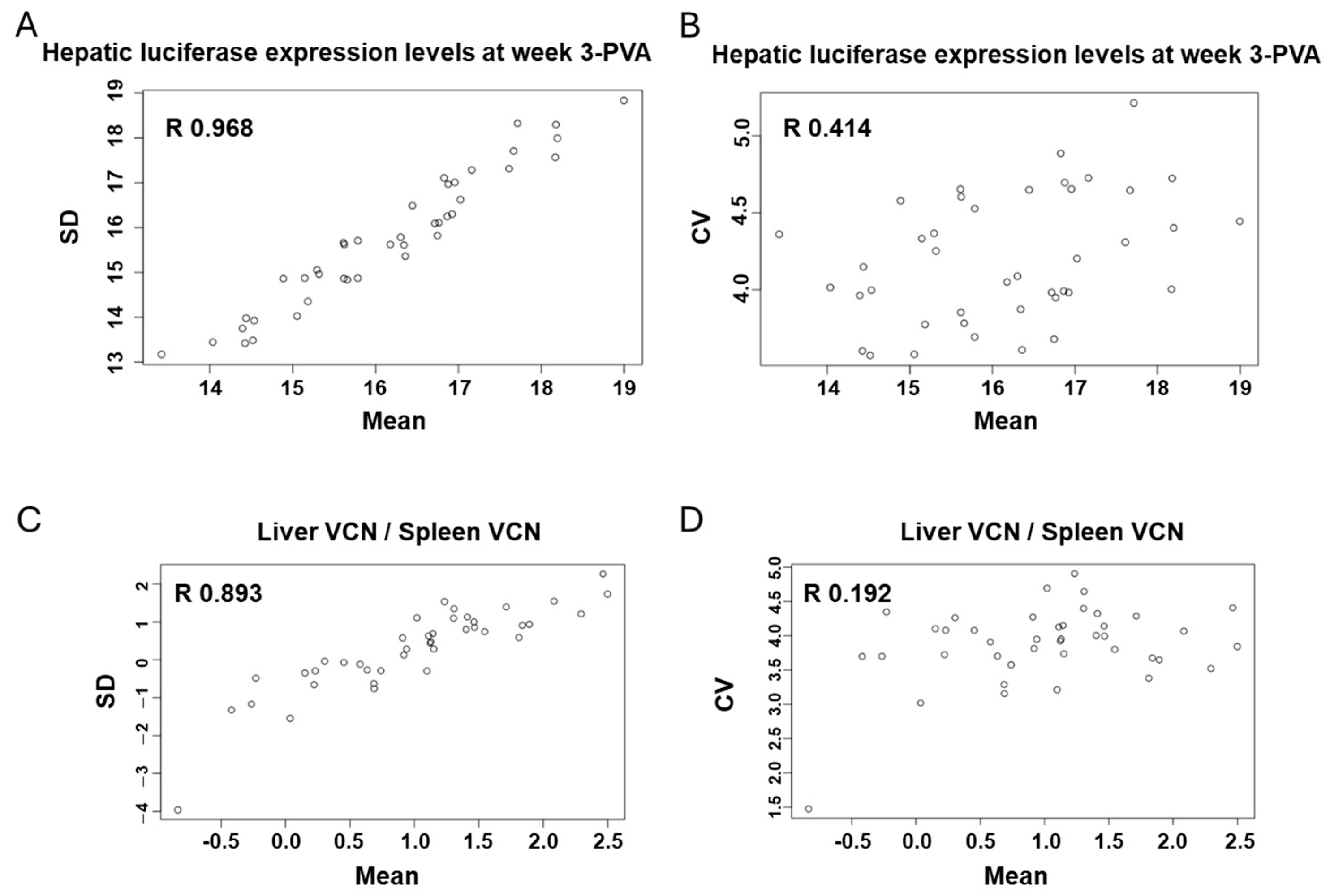
3.7. In-Strain Variabilities in Characteristics of Hepatic Transgene Expression
3.8. The Overall Effect of the Host Genetics on Hepatic Transduction by Lentiviral Vectors
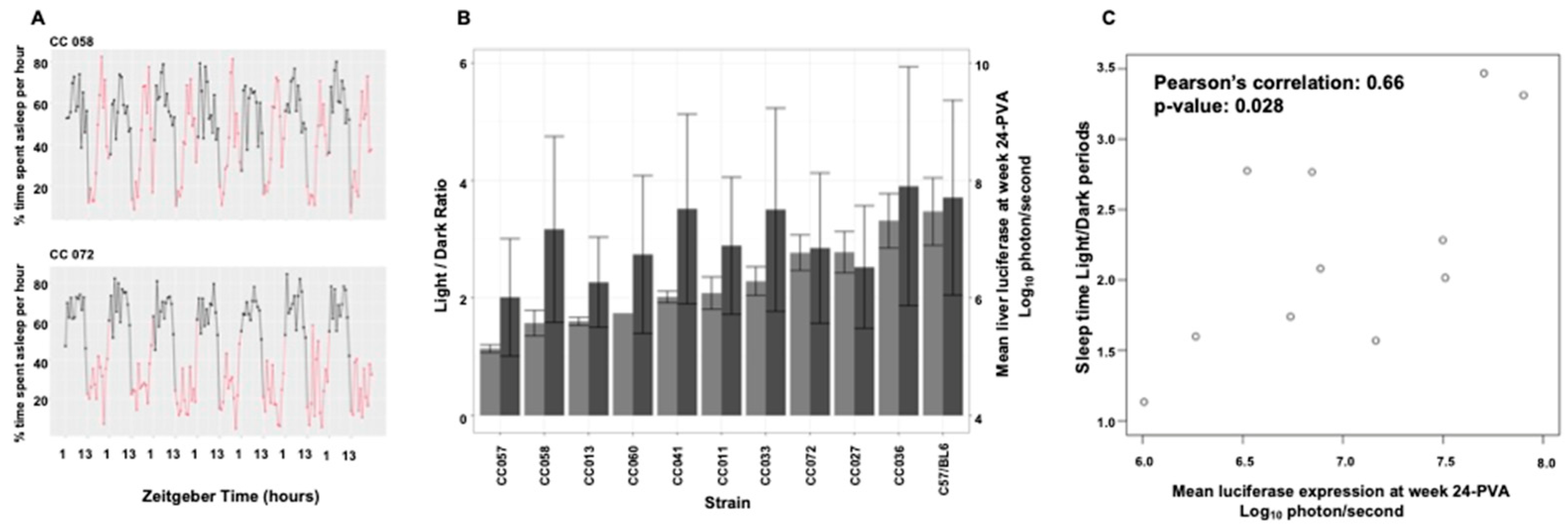
3.9. Mouse Strain-Specific Sleep Patterns as an Intrinsic Environmental Factor Affecting Hepatic Transduction by Lentiviral Vectors
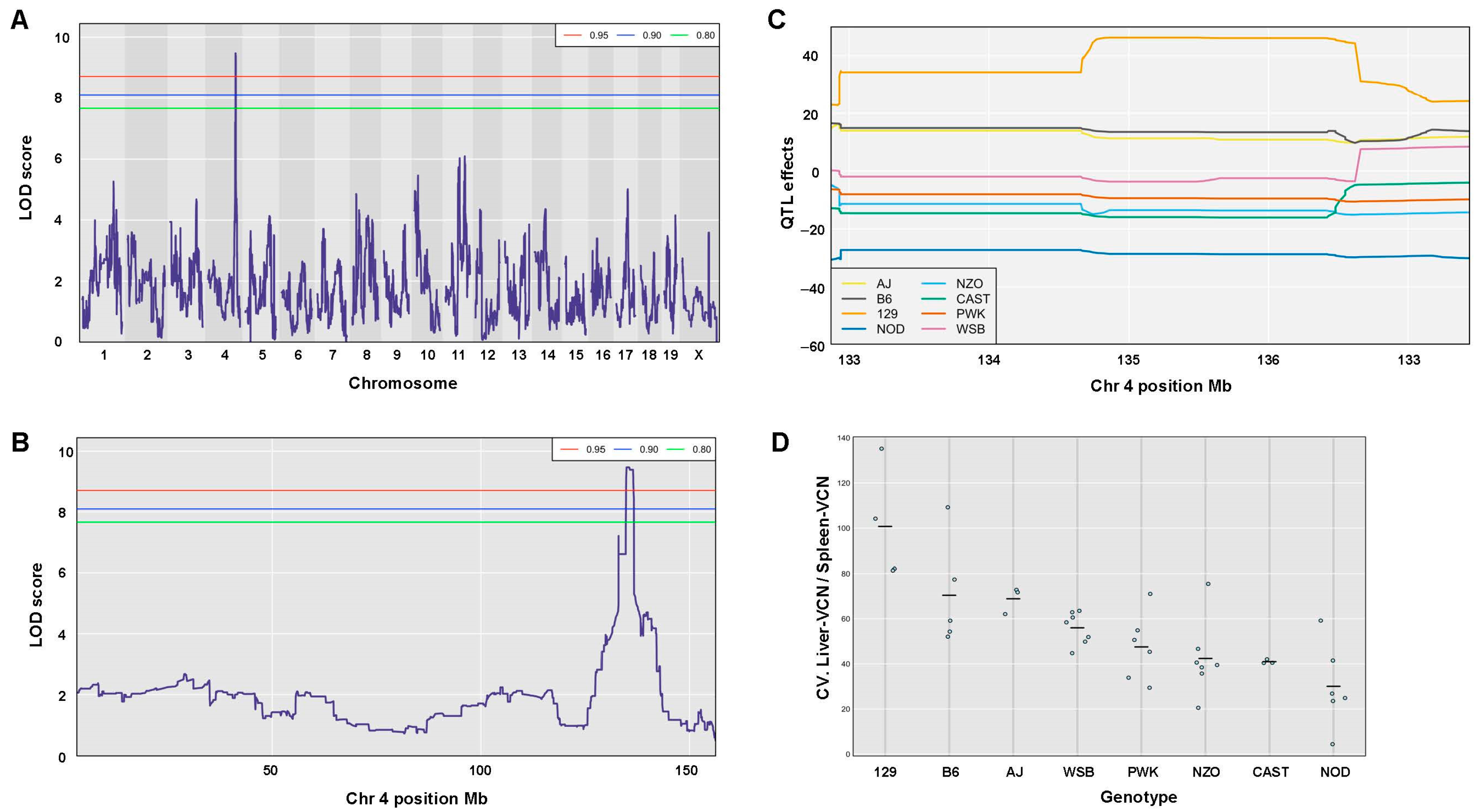
3.10. Identification and Characterization of QTLs Associated with Traits of Hepatic Lentiviral Vector Transduction
3.11. QTL Association with CV of the Ratio Between Liver VCN to Spleen VCN
3.12. QTL Association with CV of Hepatic Luciferase Expression at Week 3 PVA
3.13. Identification of a QTL Associated with Changes in Hepatic Luciferase Expression Between Weeks 1 and 3 PVA
4. Discussion
5. Clinical Relevance
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheridan, C. First approval in sight for Novartis’ CAR-T therapy after panel vote. Nat. Biotechnol. 2017, 35, 691–693. [Google Scholar] [CrossRef]
- Marcucci, K.T.; Jadlowsky, J.K.; Hwang, W.T.; Suhoski-Davis, M.; Gonzalez, V.E.; Kulikovskaya, I.; Gupta, M.; Lacey, S.F.; Plesa, G.; Chew, A.; et al. Retroviral and Lentiviral Safety Analysis of Gene-Modified T Cell Products and Infused HIV and Oncology Patients. Mol. Ther. 2018, 26, 269–279. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- Ledford, H. FDA advisers back gene therapy for rare form of blindness. Nature 2017, 550, 314. [Google Scholar] [CrossRef]
- Ogbonmide, T.; Rathore, R.; Rangrej, S.B.; Hutchinson, S.; Lewis, M.; Ojilere, S.; Carvalho, V.; Kelly, I. Gene Therapy for Spinal Muscular Atrophy (SMA): A Review of Current Challenges and Safety Considerations for Onasemnogene Abeparvovec (Zolgensma). Cureus 2023, 15, e36197. [Google Scholar] [CrossRef]
- Follenzi, A.; Battaglia, M.; Lombardo, A.; Annoni, A.; Roncarolo, M.G.; Naldini, L. Targeting lentiviral vector expression to hepatocytes limits transgene-specific immune response and establishes long-term expression of human antihemophilic factor IX in mice. Blood 2004, 103, 3700–3709. [Google Scholar] [CrossRef]
- Zhao, Y.; Keating, K.; Thorpe, R. Comparison of toxicogenomic profiles of two murine strains treated with HIV-1-based vectors for gene therapy. Toxicol. Appl. Pharmacol. 2007, 225, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Suwanmanee, T.; Ferris, M.T.; Hu, P.; Gui, T.; Montgomery, S.A.; Pardo-Manuel de Villena, F.; Kafri, T. Toward Personalized Gene Therapy: Characterizing the Host Genetic Control of Lentiviral-Vector-Mediated Hepatic Gene Delivery. Mol. Ther. Methods Clin. Dev. 2017, 5, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Cavazzana-Calvo, M.; Payen, E.; Negre, O.; Wang, G.; Hehir, K.; Fusil, F.; Down, J.; Denaro, M.; Brady, T.; Westerman, K.; et al. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature 2010, 467, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, H.B.; Cooray, S.; Gilmour, K.C.; Parsley, K.L.; Adams, S.; Howe, S.J.; Al Ghonaium, A.; Bayford, J.; Brown, L.; Davies, E.G.; et al. Long-term persistence of a polyclonal T cell repertoire after gene therapy for X-linked severe combined immunodeficiency. Sci. Transl. Med. 2011, 3, 97ra79. [Google Scholar] [CrossRef]
- Hacein-Bey-Abina, S.; Von Kalle, C.; Schmidt, M.; McCormack, M.P.; Wulffraat, N.; Leboulch, P.; Lim, A.; Osborne, C.S.; Pawliuk, R.; Morillon, E.; et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 2003, 302, 415–419. [Google Scholar] [CrossRef]
- Lek, A.; Wong, B.; Keeler, A.; Blackwood, M.; Ma, K.; Huang, S.; Sylvia, K.; Batista, A.R.; Artinian, R.; Kokoski, D.; et al. Death after High-Dose rAAV9 Gene Therapy in a Patient with Duchenne’s Muscular Dystrophy. N. Engl. J. Med. 2023, 389, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Manno, C.S.; Pierce, G.F.; Arruda, V.R.; Glader, B.; Ragni, M.; Rasko, J.J.; Ozelo, M.C.; Hoots, K.; Blatt, P.; Konkle, B.; et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006, 12, 342–347. [Google Scholar] [CrossRef]
- Raper, S.E.; Yudkoff, M.; Chirmule, N.; Gao, G.P.; Nunes, F.; Haskal, Z.J.; Furth, E.E.; Propert, K.J.; Robinson, M.B.; Magosin, S.; et al. A pilot study of in vivo liver-directed gene transfer with an adenoviral vector in partial ornithine transcarbamylase deficiency. Hum. Gene Ther. 2002, 13, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Kachanov, A.; Kostyusheva, A.; Brezgin, S.; Karandashov, I.; Ponomareva, N.; Tikhonov, A.; Lukashev, A.; Pokrovsky, V.; Zamyatnin, A.A., Jr.; Parodi, A.; et al. The menace of severe adverse events and deaths associated with viral gene therapy and its potential solution. Med. Res. Rev. 2024, 44, 2112–2193. [Google Scholar] [CrossRef] [PubMed]
- Wills, C.A.; Drago, D.; Pietrusko, R.G. Clinical holds for cell and gene therapy trials: Risks, impact, and lessons learned. Mol. Ther. Methods Clin. Dev. 2023, 31, 101125. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, Z.; Aiken, C. HIV-1 uncoating: Connection to nuclear entry and regulation by host proteins. Virology 2014, 454–455, 371–379. [Google Scholar] [CrossRef]
- Dochi, T.; Nakano, T.; Inoue, M.; Takamune, N.; Shoji, S.; Sano, K.; Misumi, S. Phosphorylation of human immunodeficiency virus type 1 capsid protein at serine 16, required for peptidyl-prolyl isomerase-dependent uncoating, is mediated by virion-incorporated extracellular signal-regulated kinase 2. J. Gen. Virol. 2014, 95, 1156–1166. [Google Scholar] [CrossRef]
- Goff, S.P. Host factors exploited by retroviruses. Nat. Rev. Microbiol. 2007, 5, 253–263. [Google Scholar] [CrossRef]
- Hilditch, L.; Towers, G.J. A model for cofactor use during HIV-1 reverse transcription and nuclear entry. Curr. Opin. Virol. 2014, 4, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Taltynov, O.; Desimmie, B.A.; Demeulemeester, J.; Christ, F.; Debyser, Z. Cellular cofactors of lentiviral integrase: From target validation to drug discovery. Mol. Biol. Int. 2012, 2012, 863405. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Cross, C. The genome architecture of the Collaborative Cross mouse genetic reference population. Genetics 2012, 190, 389–401. [Google Scholar] [CrossRef]
- Ferris, M.T.; Aylor, D.L.; Bottomly, D.; Whitmore, A.C.; Aicher, L.D.; Bell, T.A.; Bradel-Tretheway, B.; Bryan, J.T.; Buus, R.J.; Gralinski, L.E.; et al. Modeling host genetic regulation of influenza pathogenesis in the collaborative cross. PLoS Pathog. 2013, 9, e1003196. [Google Scholar] [CrossRef]
- Gralinski, L.E.; Ferris, M.T.; Aylor, D.L.; Whitmore, A.C.; Green, R.; Frieman, M.B.; Deming, D.; Menachery, V.D.; Miller, D.R.; Buus, R.J.; et al. Genome Wide Identification of SARS-CoV Susceptibility Loci Using the Collaborative Cross. PLoS Genet. 2015, 11, e1005504. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A.L.; Okumura, A.; Ferris, M.T.; Green, R.; Feldmann, F.; Kelly, S.M.; Scott, D.P.; Safronetz, D.; Haddock, E.; LaCasse, R.; et al. Host genetic diversity enables Ebola hemorrhagic fever pathogenesis and resistance. Science 2014, 346, 987–991. [Google Scholar] [CrossRef]
- Rogala, A.R.; Morgan, A.P.; Christensen, A.M.; Gooch, T.J.; Bell, T.A.; Miller, D.R.; Godfrey, V.L.; de Villena, F.P. The Collaborative Cross as a resource for modeling human disease: CC011/Unc, a new mouse model for spontaneous colitis. Mamm. Genome 2014, 25, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Baker, R.E.; Proulx, M.K.; Mishra, B.B.; Long, J.E.; Park, S.W.; Lee, H.N.; Kiritsy, M.C.; Bellerose, M.M.; Olive, A.J.; et al. Host-pathogen genetic interactions underlie tuberculosis susceptibility in genetically diverse mice. eLife 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Bayer, M.; Kantor, B.; Cockrell, A.; Ma, H.; Zeithaml, B.; Li, X.; McCown, T.; Kafri, T. A large U3 deletion causes increased in vivo expression from a nonintegrating lentiviral vector. Mol. Ther. 2008, 16, 1968–1976. [Google Scholar] [CrossRef] [PubMed]
- Cockrell, A.S.; Kafri, T. Gene delivery by lentivirus vectors. Mol. Biotechnol. 2007, 36, 184–204. [Google Scholar] [CrossRef]
- Cockrell, A.S.; Ma, H.; Fu, K.; McCown, T.J.; Kafri, T. A trans-lentiviral packaging cell line for high-titer conditional self-inactivating HIV-1 vectors. Mol. Ther. 2006, 14, 276–284. [Google Scholar] [CrossRef]
- Kafri, T.; van Praag, H.; Ouyang, L.; Gage, F.H.; Verma, I.M. A packaging cell line for lentivirus vectors. J. Virol. 1999, 73, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Suwanmanee, T.; Hu, G.; Gui, T.; Bartholomae, C.C.; Kutschera, I.; von Kalle, C.; Schmidt, M.; Monahan, P.E.; Kafri, T. Integration-deficient lentiviral vectors expressing codon-optimized R338L human FIX restore normal hemostasis in Hemophilia B mice. Mol. Ther. 2014, 22, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.C.; Gay, S.M.; Armstrong, M.L.; Pazhayam, N.M.; Reisdorph, N.; Diering, G.H. Tonic endocannabinoid signaling supports sleep through development in both sexes. Sleep 2022, 45, zsac083. [Google Scholar] [CrossRef] [PubMed]
- Mang, G.M.; Nicod, J.; Emmenegger, Y.; Donohue, K.D.; O’Hara, B.F.; Franken, P. Evaluation of a piezoelectric system as an alternative to electroencephalogram/electromyogram recordings in mouse sleep studies. Sleep 2014, 37, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.E.; Flores, J.E.; Deshpande, H.; Picazo, J.A.; Xie, X.S.; Franken, P.; Heller, H.C.; Grahn, D.A.; O’Hara, B.F. Pattern recognition of sleep in rodents using piezoelectric signals generated by gross body movements. IEEE Trans. Biomed. Eng. 2007, 54, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Donohue, K.D.; Medonza, D.C.; Crane, E.R.; O’Hara, B.F. Assessment of a non-invasive high-throughput classifier for behaviours associated with sleep and wake in mice. Biomed. Eng. Online 2008, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Yaghouby, F.; Donohue, K.D.; O’Hara, B.F.; Sunderam, S. Noninvasive dissection of mouse sleep using a piezoelectric motion sensor. J. Neurosci. Methods 2016, 259, 90–100. [Google Scholar] [CrossRef]
- Lord, J.S.; Gay, S.M.; Harper, K.M.; Nikolova, V.D.; Smith, K.M.; Moy, S.S.; Diering, G.H. Early life sleep disruption potentiates lasting sex-specific changes in behavior in genetically vulnerable Shank3 heterozygous autism model mice. Mol. Autism 2022, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.C.; Joyce, K.K.; Lord, J.S.; Harper, K.M.; Nikolova, V.D.; Cohen, T.J.; Moy, S.S.; Diering, G.H. Sleep Disruption Precedes Forebrain Synaptic Tau Burden and Contributes to Cognitive Decline in a Sex-Dependent Manner in the P301S Tau Transgenic Mouse Model. eNeuro 2024, 11. [Google Scholar] [CrossRef] [PubMed]
- Dixon, W.J. Simplified Estimation from Censored Normal Samples. Ann. Math. Stat. 1960, 31, 385–391. [Google Scholar] [CrossRef]
- Burdick, R.C.; Morse, M.; Rouzina, I.; Williams, M.C.; Hu, W.S.; Pathak, V.K. HIV-1 uncoating requires long double-stranded reverse transcription products. Sci. Adv. 2024, 10, eadn7033. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.D.; Sitia, G.; Annoni, A.; Hauben, E.; Sergi, L.S.; Zingale, A.; Roncarolo, M.G.; Guidotti, L.G.; Naldini, L. In vivo administration of lentiviral vectors triggers a type I interferon response that restricts hepatocyte gene transfer and promotes vector clearance. Blood 2007, 109, 2797–2805. [Google Scholar] [CrossRef]
- Elmer, J.L.; Hay, A.D.; Kessler, N.J.; Bertozzi, T.M.; Ainscough, E.A.C.; Ferguson-Smith, A.C. Genomic properties of variably methylated retrotransposons in mouse. Mob. DNA 2021, 12, 6. [Google Scholar] [CrossRef]
- Kazachenka, A.; Bertozzi, T.M.; Sjoberg-Herrera, M.K.; Walker, N.; Gardner, J.; Gunning, R.; Pahita, E.; Adams, S.; Adams, D.; Ferguson-Smith, A.C. Identification, Characterization, and Heritability of Murine Metastable Epialleles: Implications for Non-genetic Inheritance. Cell 2018, 175, 1717. [Google Scholar] [CrossRef] [PubMed]
- Nellaker, C.; Keane, T.M.; Yalcin, B.; Wong, K.; Agam, A.; Belgard, T.G.; Flint, J.; Adams, D.J.; Frankel, W.N.; Ponting, C.P. The genomic landscape shaped by selection on transposable elements across 18 mouse strains. Genome Biol. 2012, 13, R45. [Google Scholar] [CrossRef]
- Rand, E.; Ben-Porath, I.; Keshet, I.; Cedar, H. CTCF elements direct allele-specific undermethylation at the imprinted H19 locus. Curr. Biol. 2004, 14, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Strogantsev, R.; Takahashi, N.; Kazachenka, A.; Lorincz, M.C.; Hemberger, M.; Ferguson-Smith, A.C. ZFP57 regulation of transposable elements and gene expression within and beyond imprinted domains. Epigenetics Chromatin 2019, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Bennetts, J.S.; Fowles, L.F.; Berkman, J.L.; van Bueren, K.L.; Richman, J.M.; Simpson, F.; Wicking, C. Evolutionary conservation and murine embryonic expression of the gene encoding the SERTA domain-containing protein CDCA4 (HEPP). Gene 2006, 374, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Stratton, M.S.; Bagchi, R.A.; Felisbino, M.B.; Hirsch, R.A.; Smith, H.E.; Riching, A.S.; Enyart, B.Y.; Koch, K.A.; Cavasin, M.A.; Alexanian, M.; et al. Dynamic Chromatin Targeting of BRD4 Stimulates Cardiac Fibroblast Activation. Circ. Res. 2019, 125, 662–677. [Google Scholar] [CrossRef]
- Ainouze, M.; Rochefort, P.; Parroche, P.; Roblot, G.; Tout, I.; Briat, F.; Zannetti, C.; Marotel, M.; Goutagny, N.; Auron, P.; et al. Human papillomavirus type 16 antagonizes IRF6 regulation of IL-1beta. PLoS Pathog. 2018, 14, e1007158. [Google Scholar] [CrossRef]
- Blanton, S.H.; Cortez, A.; Stal, S.; Mulliken, J.B.; Finnell, R.H.; Hecht, J.T. Variation in IRF6 contributes to nonsyndromic cleft lip and palate. Am. J. Med. Genet. A 2005, 137A, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Gou, Y.; Zhang, T.; Xu, J. Transcription Factors in Craniofacial Development: From Receptor Signaling to Transcriptional and Epigenetic Regulation. Curr. Top. Dev. Biol. 2015, 115, 377–410. [Google Scholar] [CrossRef]
- Taniguchi, T.; Ogasawara, K.; Takaoka, A.; Tanaka, N. IRF family of transcription factors as regulators of host defense. Annu. Rev. Immunol. 2001, 19, 623–655. [Google Scholar] [CrossRef]
- Deng, M.; Tam, J.W.; Wang, L.; Liang, K.; Li, S.; Zhang, L.; Guo, H.; Luo, X.; Zhang, Y.; Petrucelli, A.; et al. TRAF3IP3 negatively regulates cytosolic RNA induced anti-viral signaling by promoting TBK1 K48 ubiquitination. Nat. Commun. 2020, 11, 2193. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Li, J.; Zhang, R.; Cai, Y.; Wang, C.; Qi, S.; Chen, S.; Liang, X.; Qi, N.; Hou, F. TRAF3IP3 mediates the recruitment of TRAF3 to MAVS for antiviral innate immunity. EMBO J. 2019, 38, e102075. [Google Scholar] [CrossRef] [PubMed]
- Aryal, N.K.; Wasylishen, A.R.; Pant, V.; Riley-Croce, M.; Lozano, G. Loss of digestive organ expansion factor (Diexf) reveals an essential role during murine embryonic development that is independent of p53. Oncotarget 2017, 8, 103996–104006. [Google Scholar] [CrossRef]
- Gaviraghi, M.; Vivori, C.; Tonon, G. How Cancer Exploits Ribosomal RNA Biogenesis: A Journey beyond the Boundaries of rRNA Transcription. Cells 2019, 8, 1098. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Pappa, S.; Diez-Villanueva, A.; Mallona, I.; Custodio, J.; Barrero, M.J.; Peinado, M.A.; Jorda, M. Tissue and cancer-specific expression of DIEXF is epigenetically mediated by an Alu repeat. Epigenetics 2020, 15, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Charette, J.M.; Baserga, S.J. The DEAD-box RNA helicase-like Utp25 is an SSU processome component. RNA 2010, 16, 2156–2169. [Google Scholar] [CrossRef] [PubMed]
- González-Serna, D.; Shi, C.; Kerick, M.; Hankinson, J.; Ding, J.; McGovern, A.; Tutino, M.; Villanueva Martin, G.; Ortego-Centeno, N.; Callejas, J.L.; et al. P152 Allele-Associated Chromatin Interactions In Primary Immune Cells Uncover Mechanisms Of Gene Regulation. Rheumatology 2023, 62, ii96. [Google Scholar] [CrossRef]
- Colomer-Lluch, M.; Ruiz, A.; Moris, A.; Prado, J.G. Restriction Factors: From Intrinsic Viral Restriction to Shaping Cellular Immunity Against HIV-1. Front. Immunol. 2018, 9, 2876. [Google Scholar] [CrossRef] [PubMed]
- Pertel, T.; Hausmann, S.; Morger, D.; Zuger, S.; Guerra, J.; Lascano, J.; Reinhard, C.; Santoni, F.A.; Uchil, P.D.; Chatel, L.; et al. TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature 2011, 472, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Brass, A.L.; Dykxhoorn, D.M.; Benita, Y.; Yan, N.; Engelman, A.; Xavier, R.J.; Lieberman, J.; Elledge, S.J. Identification of host proteins required for HIV infection through a functional genomic screen. Science 2008, 319, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Konig, R.; Zhou, Y.; Elleder, D.; Diamond, T.L.; Bonamy, G.M.; Irelan, J.T.; Chiang, C.Y.; Tu, B.P.; De Jesus, P.D.; Lilley, C.E.; et al. Global analysis of host-pathogen interactions that regulate early-stage HIV-1 replication. Cell 2008, 135, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xu, M.; Huang, Q.; Gates, A.T.; Zhang, X.D.; Castle, J.C.; Stec, E.; Ferrer, M.; Strulovici, B.; Hazuda, D.J.; et al. Genome-scale RNAi screen for host factors required for HIV replication. Cell Host Microbe 2008, 4, 495–504. [Google Scholar] [CrossRef]
- Borjabad, A.; Dong, B.; Chao, W.; Volsky, D.J.; Potash, M.J. Innate immune responses reverse HIV cognitive disease in mice: Profile by RNAseq in the brain. Virology 2024, 589, 109917. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.J.; Borjabad, A.; Hadas, E.; Kelschenbach, J.; Kim, B.H.; Chao, W.; Arancio, O.; Suh, J.; Polsky, B.; McMillan, J.; et al. EcoHIV infection of mice establishes latent viral reservoirs in T cells and active viral reservoirs in macrophages that are sufficient for induction of neurocognitive impairment. PLoS Pathog. 2018, 14, e1007061. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Murphy, S.L.; Giles-Davis, W.; Edmonson, S.; Xiang, Z.; Li, Y.; Lasaro, M.O.; High, K.A.; Ertl, H.C. Pre-existing AAV capsid-specific CD8+ T cells are unable to eliminate AAV-transduced hepatocytes. Mol. Ther. 2007, 15, 792–800. [Google Scholar] [CrossRef]
- Schutgens, R.E.G. Gene Therapy for Hemophilia A: How Long Will It Last? Hemasphere 2022, 6, e720. [Google Scholar] [CrossRef]
- Uchil, P.D.; Quinlan, B.D.; Chan, W.T.; Luna, J.M.; Mothes, W. TRIM E3 ligases interfere with early and late stages of the retroviral life cycle. PLoS Pathog. 2008, 4, e16. [Google Scholar] [CrossRef] [PubMed]
- Churchill, G.A.; Airey, D.C.; Allayee, H.; Angel, J.M.; Attie, A.D.; Beatty, J.; Beavis, W.D.; Belknap, J.K.; Bennett, B.; Berrettini, W.; et al. The Collaborative Cross, a community resource for the genetic analysis of complex traits. Nat. Genet. 2004, 36, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Durrant, C.; Tayem, H.; Yalcin, B.; Cleak, J.; Goodstadt, L.; de Villena, F.P.; Mott, R.; Iraqi, F.A. Collaborative Cross mice and their power to map host susceptibility to Aspergillus fumigatus infection. Genome Res. 2011, 21, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Elbahesh, H.; Schughart, K. Genetically diverse CC-founder mouse strains replicate the human influenza gene expression signature. Sci. Rep. 2016, 6, 26437. [Google Scholar] [CrossRef]
- Green, R.; Wilkins, C.; Thomas, S.; Sekine, A.; Ireton, R.C.; Ferris, M.T.; Hendrick, D.M.; Voss, K.; Pardo-Manuel de Villena, F.; Baric, R.S.; et al. Transcriptional profiles of WNV neurovirulence in a genetically diverse Collaborative Cross population. Genom. Data 2016, 10, 137–140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leist, S.R.; Pilzner, C.; van den Brand, J.M.; Dengler, L.; Geffers, R.; Kuiken, T.; Balling, R.; Kollmus, H.; Schughart, K. Influenza H3N2 infection of the collaborative cross founder strains reveals highly divergent host responses and identifies a unique phenotype in CAST/EiJ mice. BMC Genom. 2016, 17, 143. [Google Scholar] [CrossRef] [PubMed]
- Lore, N.I.; Iraqi, F.A.; Bragonzi, A. Host genetic diversity influences the severity of Pseudomonas aeruginosa pneumonia in the Collaborative Cross mice. BMC Genet. 2015, 16, 106. [Google Scholar] [CrossRef]
- Vered, K.; Durrant, C.; Mott, R.; Iraqi, F.A. Susceptibility to Klebsiella pneumonaie infection in collaborative cross mice is a complex trait controlled by at least three loci acting at different time points. BMC Genom. 2014, 15, 865. [Google Scholar] [CrossRef]
- Abu-Toamih Atamni, H.J.; Ziner, Y.; Mott, R.; Wolf, L.; Iraqi, F.A. Glucose tolerance female-specific QTL mapped in collaborative cross mice. Mamm. Genome 2017, 28, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Dorman, A.; Baer, D.; Tomlinson, I.; Mott, R.; Iraqi, F.A. Genetic analysis of intestinal polyp development in Collaborative Cross mice carrying the Apc (Min/+) mutation. BMC Genet. 2016, 17, 46. [Google Scholar] [CrossRef][Green Version]
- Reilly, K.M. Using the Collaborative Cross to Study the Role of Genetic Diversity in Cancer-Related Phenotypes. Cold Spring Harb. Protoc. 2016, 2016, pdb.prot079178. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Langley, S.A.; Zhou, Y.X.; Jen, K.Y.; Sun, Q.; Brislawn, C.; Rojas, C.M.; Wahl, K.L.; Wang, T.; et al. Diverse tumour susceptibility in Collaborative Cross mice: Identification of a new mouse model for human gastric tumourigenesis. Gut 2019, 68, 1942–1952. [Google Scholar] [CrossRef]
- Mao, J.H.; Langley, S.A.; Huang, Y.; Hang, M.; Bouchard, K.E.; Celniker, S.E.; Brown, J.B.; Jansson, J.K.; Karpen, G.H.; Snijders, A.M. Identification of genetic factors that modify motor performance and body weight using Collaborative Cross mice. Sci. Rep. 2015, 5, 16247. [Google Scholar] [CrossRef] [PubMed]
- Phillippi, J.; Xie, Y.; Miller, D.R.; Bell, T.A.; Zhang, Z.; Lenarcic, A.B.; Aylor, D.L.; Krovi, S.H.; Threadgill, D.W.; de Villena, F.P.; et al. Using the emerging Collaborative Cross to probe the immune system. Genes. Immun. 2014, 15, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Hollis, E.R., 2nd; Kadoya, K.; Hirsch, M.; Samulski, R.J.; Tuszynski, M.H. Efficient retrograde neuronal transduction utilizing self-complementary AAV1. Mol. Ther. 2008, 16, 296–301. [Google Scholar] [CrossRef]
- Dalgaard, K.; Landgraf, K.; Heyne, S.; Lempradl, A.; Longinotto, J.; Gossens, K.; Ruf, M.; Orthofer, M.; Strogantsev, R.; Selvaraj, M.; et al. Trim28 Haploinsufficiency Triggers Bi-stable Epigenetic Obesity. Cell 2016, 164, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Miska, E.A.; Ferguson-Smith, A.C. Transgenerational inheritance: Models and mechanisms of non-DNA sequence-based inheritance. Science 2016, 354, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Duhl, D.M.; Vrieling, H.; Miller, K.A.; Wolff, G.L.; Barsh, G.S. Neomorphic agouti mutations in obese yellow mice. Nat. Genet. 1994, 8, 59–65. [Google Scholar] [CrossRef]
- Morgan, H.D.; Sutherland, H.G.; Martin, D.I.; Whitelaw, E. Epigenetic inheritance at the agouti locus in the mouse. Nat. Genet. 1999, 23, 314–318. [Google Scholar] [CrossRef]
- Elmer, J.L.; Ferguson-Smith, A.C. Strain-Specific Epigenetic Regulation of Endogenous Retroviruses: The Role of Trans-Acting Modifiers. Viruses 2020, 12, 810. [Google Scholar] [CrossRef] [PubMed]
- Rakyan, V.K.; Blewitt, M.E.; Druker, R.; Preis, J.I.; Whitelaw, E. Metastable epialleles in mammals. Trends Genet. 2002, 18, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, M.; Kessler, N.J.; Hellenthal, G.; Silver, M.J. Metastable epialleles in humans. Trends Genet. 2024, 40, 52–68. [Google Scholar] [CrossRef]
- Bergman, Y.; Cedar, H. DNA methylation dynamics in health and disease. Nat. Struct. Mol. Biol. 2013, 20, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Kafri, T.; Ariel, M.; Brandeis, M.; Shemer, R.; Urven, L.; McCarrey, J.; Cedar, H.; Razin, A. Developmental pattern of gene-specific DNA methylation in the mouse embryo and germ line. Genes Dev. 1992, 6, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.; Dean, W.; Erhardt, S.; Hajkova, P.; Surani, A.; Walter, J.; Reik, W. Resistance of IAPs to methylation reprogramming may provide a mechanism for epigenetic inheritance in the mouse. Genesis 2003, 35, 88–93. [Google Scholar] [CrossRef]
- Reik, W.; Dean, W.; Walter, J. Epigenetic reprogramming in mammalian development. Science 2001, 293, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.P.; Chaillet, J.R.; Bestor, T.H. Transcription of IAP endogenous retroviruses is constrained by cytosine methylation. Nat. Genet. 1998, 20, 116–117. [Google Scholar] [CrossRef]
- Ondicova, M.; Oakey, R.J.; Walsh, C.P. Is imprinting the result of “friendly fire” by the host defense system? PLoS Genet. 2020, 16, e1008599. [Google Scholar] [CrossRef] [PubMed]
- Slotkin, R.K.; Martienssen, R. Transposable elements and the epigenetic regulation of the genome. Nat. Rev. Genet. 2007, 8, 272–285. [Google Scholar] [CrossRef]
- Karimi, M.M.; Goyal, P.; Maksakova, I.A.; Bilenky, M.; Leung, D.; Tang, J.X.; Shinkai, Y.; Mager, D.L.; Jones, S.; Hirst, M.; et al. DNA methylation and SETDB1/H3K9me3 regulate predominantly distinct sets of genes, retroelements, and chimeric transcripts in mESCs. Cell Stem Cell 2011, 8, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Kano, H.; Kurahashi, H.; Toda, T. Genetically regulated epigenetic transcriptional activation of retrotransposon insertion confers mouse dactylaplasia phenotype. Proc. Natl. Acad. Sci. USA 2007, 104, 19034–19039. [Google Scholar] [CrossRef] [PubMed]
- Maeda-Smithies, N.; Hiller, S.; Dong, S.; Kim, H.S.; Bennett, B.J.; Kayashima, Y. Ectopic expression of the Stabilin2 gene triggered by an intracisternal A particle (IAP) element in DBA/2J strain of mice. Mamm. Genome 2020, 31, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Plamondon, J.A.; Harris, M.J.; Mager, D.L.; Gagnier, L.; Juriloff, D.M. The clf2 gene has an epigenetic role in the multifactorial etiology of cleft lip and palate in the A/WySn mouse strain. Birth Defects Res. A Clin. Mol. Teratol. 2011, 91, 716–727. [Google Scholar] [CrossRef]
- Blewitt, M.E.; Chong, S.; Whitelaw, E. How the mouse got its spots. Trends Genet. 2004, 20, 550–554. [Google Scholar] [CrossRef]
- Daxinger, L.; Harten, S.K.; Oey, H.; Epp, T.; Isbel, L.; Huang, E.; Whitelaw, N.; Apedaile, A.; Sorolla, A.; Yong, J.; et al. An ENU mutagenesis screen identifies novel and known genes involved in epigenetic processes in the mouse. Genome Biol. 2013, 14, R96. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Hore, T.A.; Reik, W. Reprogramming the methylome: Erasing memory and creating diversity. Cell Stem Cell 2014, 14, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi, T.M.; Elmer, J.L.; Macfarlan, T.S.; Ferguson-Smith, A.C. KRAB zinc finger protein diversification drives mammalian interindividual methylation variability. Proc. Natl. Acad. Sci. USA 2020, 117, 31290–31300. [Google Scholar] [CrossRef] [PubMed]
- Bernal, A.J.; Dolinoy, D.C.; Huang, D.; Skaar, D.A.; Weinhouse, C.; Jirtle, R.L. Adaptive radiation-induced epigenetic alterations mitigated by antioxidants. FASEB J. 2013, 27, 665–671. [Google Scholar] [CrossRef]
- Dolinoy, D.C. The agouti mouse model: An epigenetic biosensor for nutritional and environmental alterations on the fetal epigenome. Nutr. Rev. 2008, 66 (Suppl. S1), S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Faulk, C.; Barks, A.; Liu, K.; Goodrich, J.M.; Dolinoy, D.C. Early-life lead exposure results in dose- and sex-specific effects on weight and epigenetic gene regulation in weanling mice. Epigenomics 2013, 5, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Jirtle, R.L. The Agouti mouse: A biosensor for environmental epigenomics studies investigating the developmental origins of health and disease. Epigenomics 2014, 6, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Kaminen-Ahola, N.; Ahola, A.; Maga, M.; Mallitt, K.A.; Fahey, P.; Cox, T.C.; Whitelaw, E.; Chong, S. Maternal ethanol consumption alters the epigenotype and the phenotype of offspring in a mouse model. PLoS Genet. 2010, 6, e1000811. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi, T.M.; Becker, J.L.; Blake, G.E.T.; Bansal, A.; Nguyen, D.K.; Fernandez-Twinn, D.S.; Ozanne, S.E.; Bartolomei, M.S.; Simmons, R.A.; Watson, E.D.; et al. Variably methylated retrotransposons are refractory to a range of environmental perturbations. Nat. Genet. 2021, 53, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S.; Sieli, P.T.; Warzak, D.A.; Ellersieck, M.R.; Pennington, K.A.; Roberts, R.M. Maternal exposure to bisphenol A and genistein has minimal effect on A(vy)/a offspring coat color but favors birth of agouti over nonagouti mice. Proc. Natl. Acad. Sci. USA 2013, 110, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Blewitt, M.E.; Vickaryous, N.K.; Hemley, S.J.; Ashe, A.; Bruxner, T.J.; Preis, J.I.; Arkell, R.; Whitelaw, E. An N-ethyl-N-nitrosourea screen for genes involved in variegation in the mouse. Proc. Natl. Acad. Sci. USA 2005, 102, 7629–7634. [Google Scholar] [CrossRef] [PubMed]
- Preis, J.I.; Downes, M.; Oates, N.A.; Rasko, J.E.; Whitelaw, E. Sensitive flow cytometric analysis reveals a novel type of parent-of-origin effect in the mouse genome. Curr. Biol. 2003, 13, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Razin, A.; Szyf, M.; Kafri, T.; Roll, M.; Giloh, H.; Scarpa, S.; Carotti, D.; Cantoni, G.L. Replacement of 5-methylcytosine by cytosine: A possible mechanism for transient DNA demethylation during differentiation. Proc. Natl. Acad. Sci. USA 1986, 83, 2827–2831. [Google Scholar] [CrossRef] [PubMed]
- Razin, A.; Webb, C.; Szyf, M.; Yisraeli, J.; Rosenthal, A.; Naveh-Many, T.; Sciaky-Gallili, N.; Cedar, H. Variations in DNA methylation during mouse cell differentiation in vivo and in vitro. Proc. Natl. Acad. Sci. USA 1984, 81, 2275–2279. [Google Scholar] [CrossRef]
- Szyf, M.; Eliasson, L.; Mann, V.; Klein, G.; Razin, A. Cellular and viral DNA hypomethylation associated with induction of Epstein-Barr virus lytic cycle. Proc. Natl. Acad. Sci. USA 1985, 82, 8090–8094. [Google Scholar] [CrossRef] [PubMed]
- Young, P.R.; Tilghman, S.M. Induction of alpha-fetoprotein synthesis in differentiating F9 teratocarcinoma cells is accompanied by a genome-wide loss of DNA methylation. Mol. Cell. Biol. 1984, 4, 898–907. [Google Scholar] [CrossRef]
- Takahashi, N.; Coluccio, A.; Thorball, C.W.; Planet, E.; Shi, H.; Offner, S.; Turelli, P.; Imbeault, M.; Ferguson-Smith, A.C.; Trono, D. ZNF445 is a primary regulator of genomic imprinting. Genes Dev. 2019, 33, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.; de Iaco, A.; Sun, M.A.; Bruno, M.; Tinkham, M.; Hoang, D.; Mitra, A.; Ralls, S.; Trono, D.; Macfarlan, T.S. KRAB-zinc finger protein gene expansion in response to active retrotransposons in the murine lineage. eLife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.S.; Beitzel, B.F.; Schroder, A.R.; Shinn, P.; Chen, H.; Berry, C.C.; Ecker, J.R.; Bushman, F.D. Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol. 2004, 2, E234. [Google Scholar] [CrossRef]
- Gifford, R.J.; Katzourakis, A.; Tristem, M.; Pybus, O.G.; Winters, M.; Shafer, R.W. A transitional endogenous lentivirus from the genome of a basal primate and implications for lentivirus evolution. Proc. Natl. Acad. Sci. USA 2008, 105, 20362–20367. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, C.; Maxfield, D.G.; Goodman, S.M.; Feschotte, C. Parallel germline infiltration of a lentivirus in two Malagasy lemurs. PLoS Genet. 2009, 5, e1000425. [Google Scholar] [CrossRef]
- Kambol, R.; Gatseva, A.; Gifford, R.J. An endogenous lentivirus in the germline of a rodent. Retrovirology 2022, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Katzourakis, A.; Tristem, M.; Pybus, O.G.; Gifford, R.J. Discovery and analysis of the first endogenous lentivirus. Proc. Natl. Acad. Sci. USA 2007, 104, 6261–6265. [Google Scholar] [CrossRef] [PubMed]
- Campillos, M.; Doerks, T.; Shah, P.K.; Bork, P. Computational characterization of multiple Gag-like human proteins. Trends Genet. 2006, 22, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Pastuzyn, E.D.; Day, C.E.; Kearns, R.B.; Kyrke-Smith, M.; Taibi, A.V.; McCormick, J.; Yoder, N.; Belnap, D.M.; Erlendsson, S.; Morado, D.R.; et al. The Neuronal Gene Arc Encodes a Repurposed Retrotransposon Gag Protein that Mediates Intercellular RNA Transfer. Cell 2018, 173, 275. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.A.; Nagy-Szakal, D.; Kellermayer, R. Human metastable epiallele candidates link to common disorders. Epigenetics 2013, 8, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Sulli, G.; Manoogian, E.N.C.; Taub, P.R.; Panda, S. Training the Circadian Clock, Clocking the Drugs, and Drugging the Clock to Prevent, Manage, and Treat Chronic Diseases. Trends Pharmacol. Sci. 2018, 39, 812–827. [Google Scholar] [CrossRef] [PubMed]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84. [Google Scholar] [CrossRef]
- Edgar, R.S.; Stangherlin, A.; Nagy, A.D.; Nicoll, M.P.; Efstathiou, S.; O’Neill, J.S.; Reddy, A.B. Cell autonomous regulation of herpes and influenza virus infection by the circadian clock. Proc. Natl. Acad. Sci. USA 2016, 113, 10085–10090. [Google Scholar] [CrossRef]
- Nilsson, E.E.; Skinner, M.K. Environmentally induced epigenetic transgenerational inheritance of disease susceptibility. Transl. Res. 2015, 165, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R.; Daskalakis, N.P.; Bierer, L.M.; Bader, H.N.; Klengel, T.; Holsboer, F.; Binder, E.B. Holocaust Exposure Induced Intergenerational Effects on FKBP5 Methylation. Biol. Psychiatry 2016, 80, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Heard, E.; Martienssen, R.A. Transgenerational epigenetic inheritance: Myths and mechanisms. Cell 2014, 157, 95–109. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, Z.; Hou, W.; Li, Z.; Cheng, S.; Green, L.A.; Wang, Y.; Wen, X.; Cai, L.; Clauss, M.; et al. HIV Tat protein affects circadian rhythmicity by interfering with the circadian system. HIV Med. 2014, 15, 565–570. [Google Scholar] [CrossRef]
- Silvestri, M.; Rossi, G.A. Melatonin: Its possible role in the management of viral infections—A brief review. Ital. J. Pediatr. 2013, 39, 61. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Oshima, I.; Tomita, T.; Ebihara, S. Melatonin content of the pineal gland in different mouse strains. J. Pineal Res. 1989, 7, 195–204. [Google Scholar] [CrossRef]
- Kasahara, T.; Abe, K.; Mekada, K.; Yoshiki, A.; Kato, T. Genetic variation of melatonin productivity in laboratory mice under domestication. Proc. Natl. Acad. Sci. USA 2010, 107, 6412–6417. [Google Scholar] [CrossRef] [PubMed]
- Biffi, A. Hematopoietic Stem Cell Gene Therapy for Storage Disease: Current and New Indications. Mol. Ther. 2017, 25, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Ferrua, F.; Cicalese, M.P.; Galimberti, S.; Giannelli, S.; Dionisio, F.; Barzaghi, F.; Migliavacca, M.; Bernardo, M.E.; Calbi, V.; Assanelli, A.A.; et al. Lentiviral haemopoietic stem/progenitor cell gene therapy for treatment of Wiskott-Aldrich syndrome: Interim results of a non-randomised, open-label, phase 1/2 clinical study. Lancet Haematol. 2019, 6, e239–e253. [Google Scholar] [CrossRef] [PubMed]
- Kohn, D.B. Gene therapy for adenosine deaminase severe combined immune deficiency-An unexpected journey of four decades. Immunol. Rev. 2024, 322, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. Inadvertent Transfer of Murine VL30 Retrotransposons to CAR-T Cells. bioRxiv 2022. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.L.; Pasquini, M.C.; Connolly, J.E.; Porter, D.L.; Gustafson, M.P.; Boelens, J.J.; Horwitz, E.M.; Grupp, S.A.; Maus, M.V.; Locke, F.L.; et al. Unanswered questions following reports of secondary malignancies after CAR-T cell therapy. Nat. Med. 2024, 30, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Segura, E.E.R.; Ayoub, P.G.; Hart, K.L.; Kohn, D.B. Gene Therapy for beta-Hemoglobinopathies: From Discovery to Clinical Trials. Viruses 2023, 15, 713. [Google Scholar] [CrossRef]
- Kafkafi, N.; Agassi, J.; Chesler, E.J.; Crabbe, J.C.; Crusio, W.E.; Eilam, D.; Gerlai, R.; Golani, I.; Gomez-Marin, A.; Heller, R.; et al. Reproducibility and replicability of rodent phenotyping in preclinical studies. Neurosci. Biobehav. Rev. 2018, 87, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Mosedale, M.; Cai, Y.; Eaddy, J.S.; Corty, R.W.; Nautiyal, M.; Watkins, P.B.; Valdar, W. Identification of Candidate Risk Factor Genes for Human Idelalisib Toxicity Using a Collaborative Cross Approach. Toxicol. Sci. 2019, 172, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Aiuti, A.; Biasco, L.; Scaramuzza, S.; Ferrua, F.; Cicalese, M.P.; Baricordi, C.; Dionisio, F.; Calabria, A.; Giannelli, S.; Castiello, M.C.; et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science 2013, 341, 1233151. [Google Scholar] [CrossRef]
- Katneni, U.K.; Alexaki, A.; Hunt, R.C.; Hamasaki-Katagiri, N.; Hettiarachchi, G.K.; Kames, J.M.; McGill, J.R.; Holcomb, D.D.; Athey, J.C.; Lin, B.; et al. Structural, functional, and immunogenicity implications of F9 gene recoding. Blood Adv. 2022, 6, 3932–3944. [Google Scholar] [CrossRef]


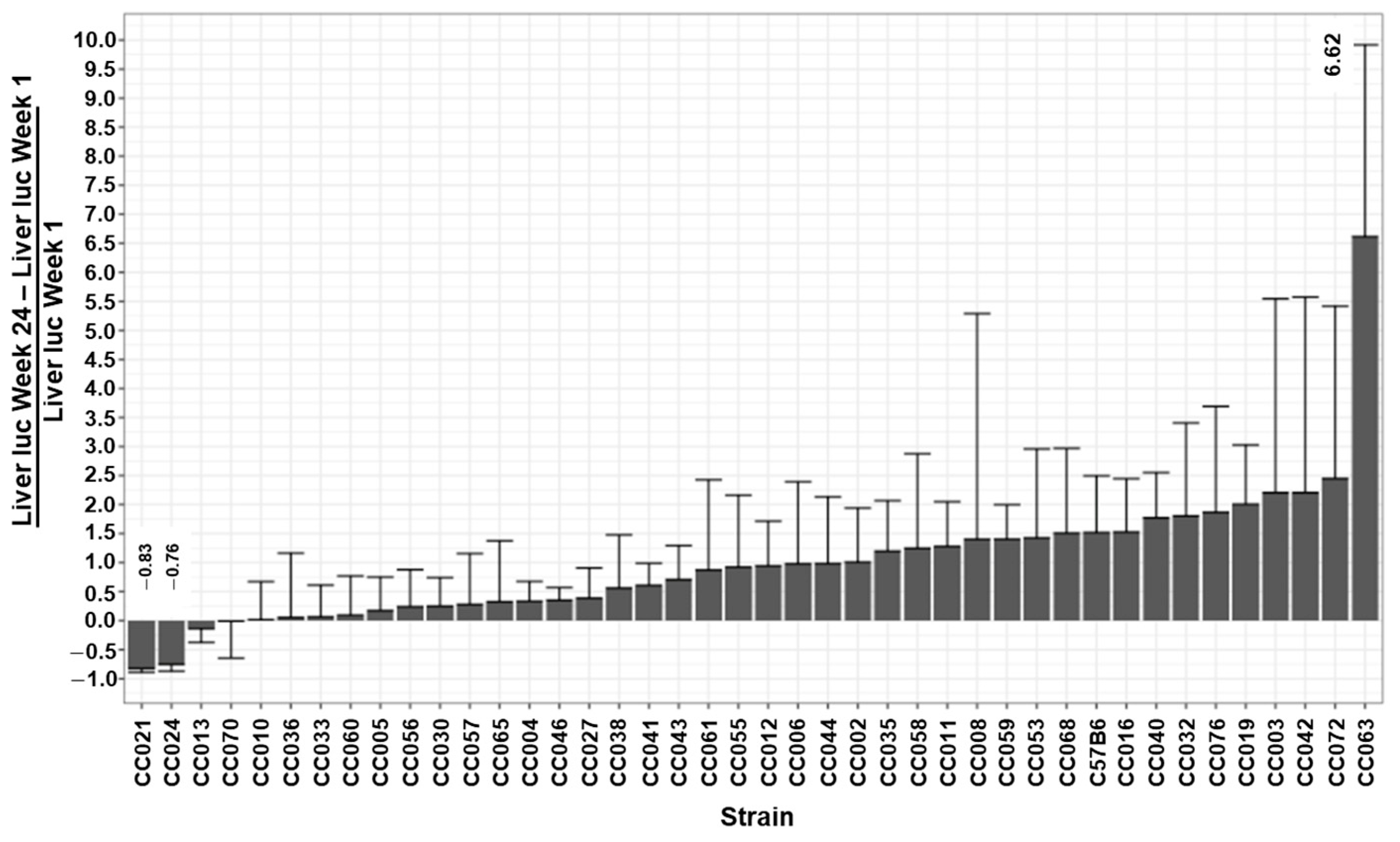
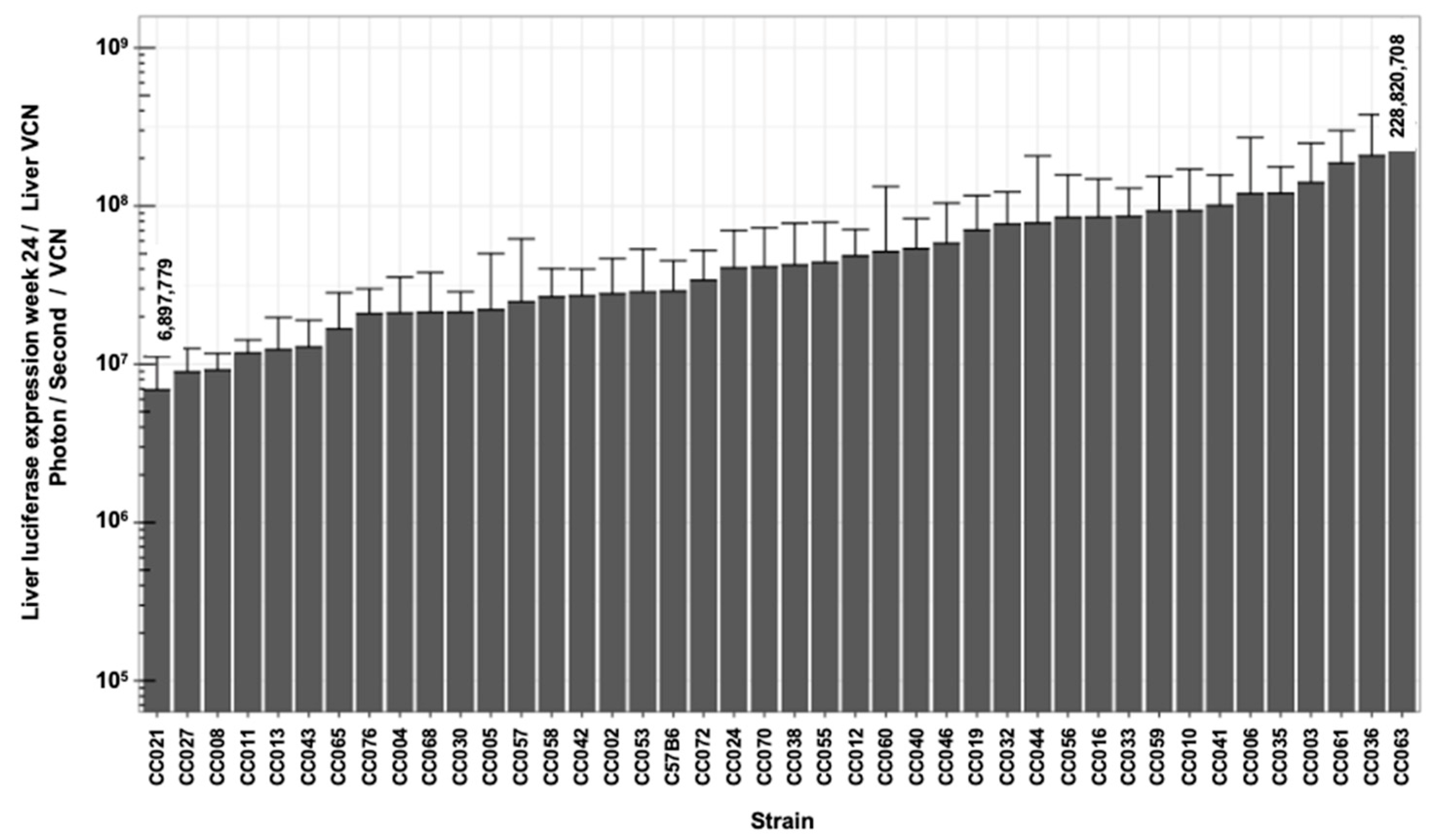
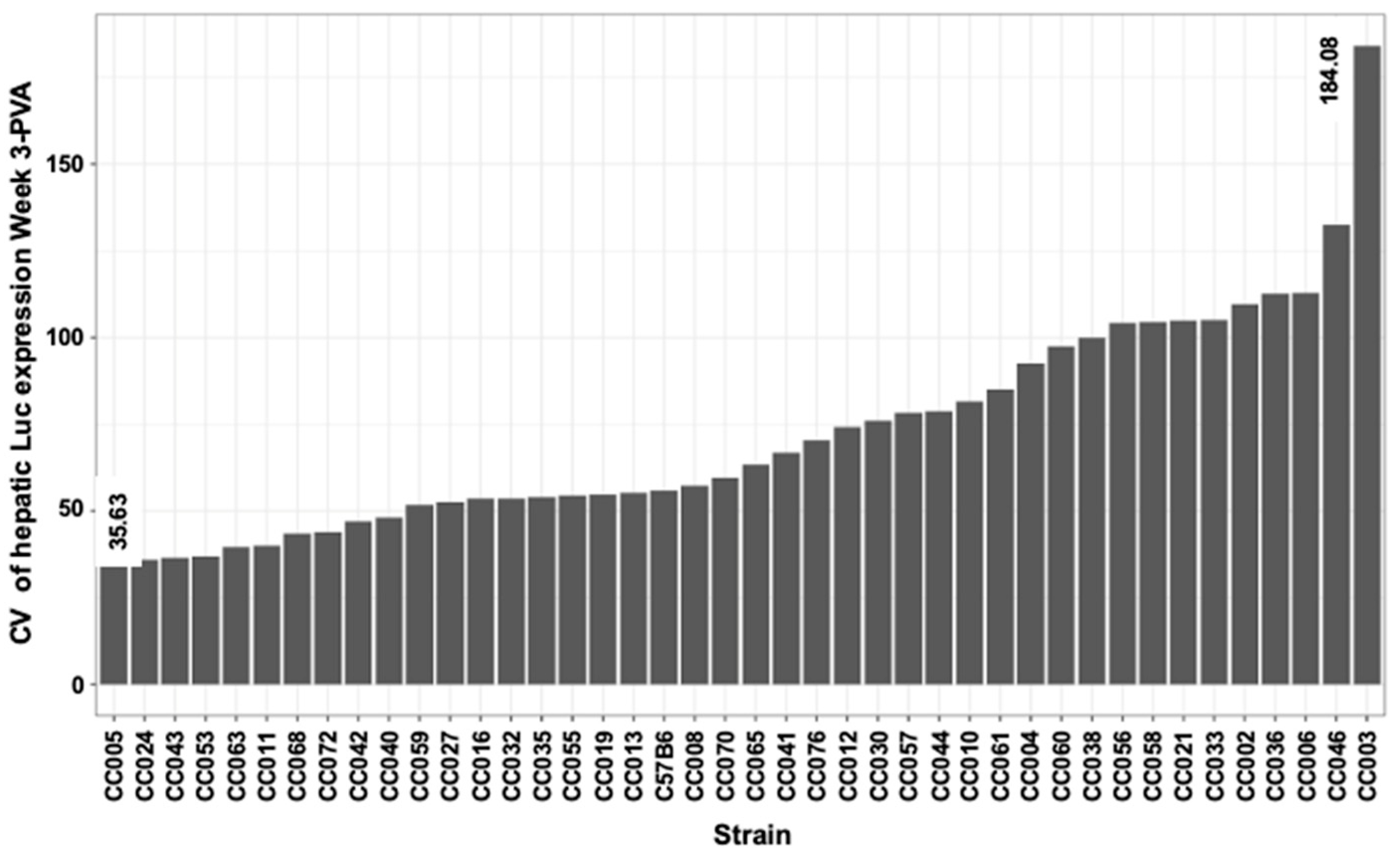
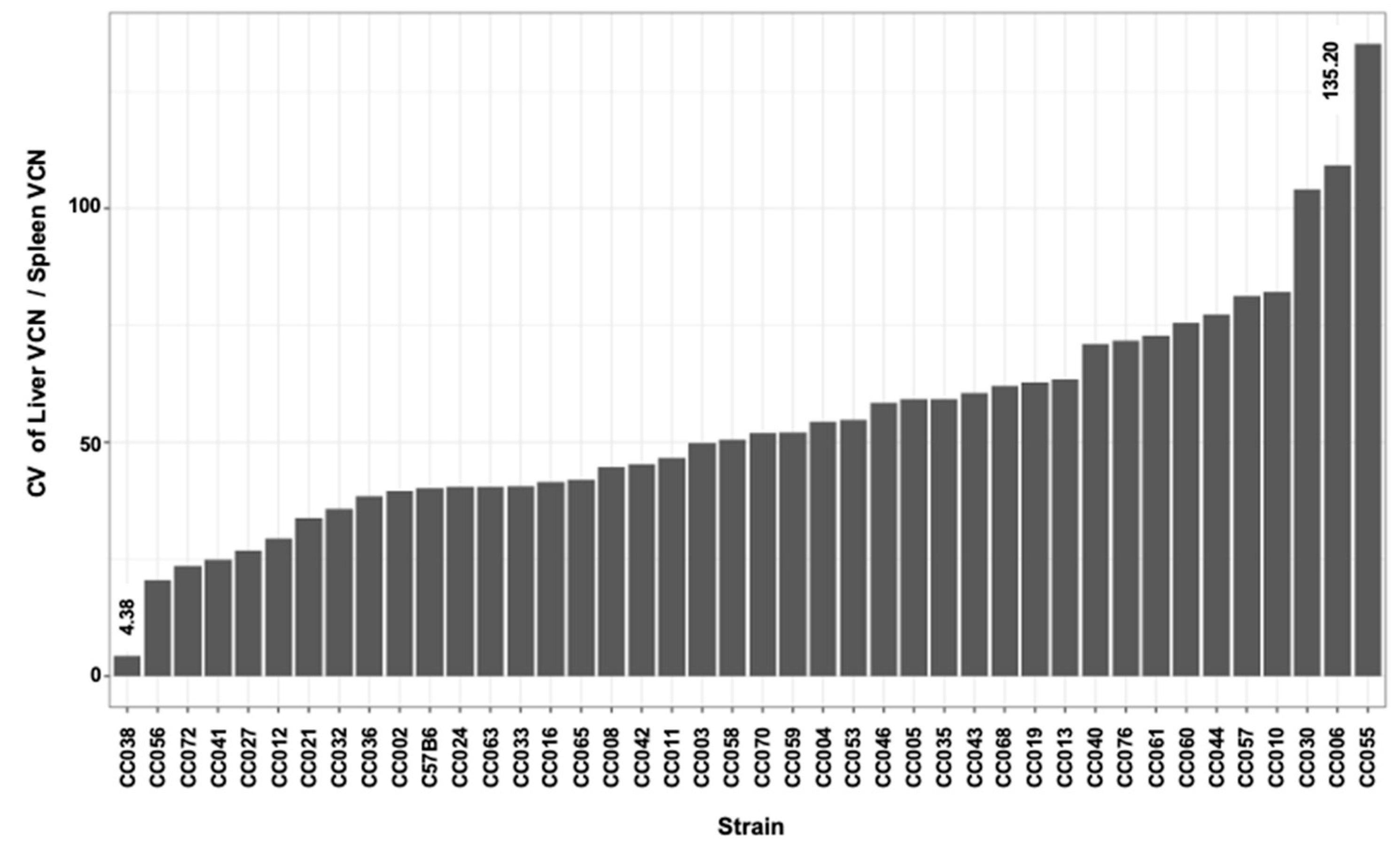
| Luc W1 photon/s | Luc W3 photon/s | Luc W6 photon/s | Luc W8 photon/s | Luc W14 photon/s | Luc W24 photon/s | |||
|---|---|---|---|---|---|---|---|---|
| Min | 960,125 | 670,875 | 614,943 | 640,300 | 978,000 | 1,016,900 | −0.625 | −0.158 |
| First Quantile | 4,735,500 | 4,040,833 | 5,517,083 | 4,660,000 | 5,528,750 | 5,524,792 | 0.036 | 0.140 |
| Median | 7,154,583 | 12,271,667 | 14,277,083 | 12,921,700 | 14,581,267 | 14,131,083 | 0.421 | 0.308 |
| Mean | 16,051,377 | 22,468,382 | 25,291,655 | 26,118,529 | 26,426,337 | 25,063,259 | 0.519 | 0.386 |
| Third Quantile | 19,337,500 | 22,962,083 | 30,437,188 | 30,902,917 | 35,147,708 | 32,247,500 | 0.984 | 0.617 |
| Max | 96,837,500 | 177,962,500 | 163,275,000 | 178,150,000 | 193,912,500 | 130,412,500 | 2.150 | 1.619 |
| Liver VCN Vector-genome/Cell | Spleen VCN Vector-genome/Cell | Vector SA (Specific Activity) | ||||||
| Min | −0.419 | −0.522 | −0.481 | −0.158 | 0.045 | 0.039 | 0.434 | 6,897,779 |
| First Quantile | −0.058 | −0.099 | 0.148 | 0.140 | 0.256 | 0.108 | 1.809 | 21,511,854 |
| Median | 0.020 | 0.093 | 0.467 | 0.308 | 0.387 | 0.159 | 3.053 | 41,832,255 |
| Mean | 0.088 | 0.156 | 0.461 | 0.386 | 0.471 | 0.194 | 3.665 | 60,354,896 |
| Third Quantile | 0.234 | 0.267 | 0.726 | 0.617 | 0.602 | 0.218 | 4.329 | 84,925,752 |
| Max | 0.627 | 1.220 | 1.561 | 1.619 | 1.911 | 0.783 | 12.175 | 228,820,708 |
| Luc W1 | Luc W3 | Luc W24 | Liver VCN | Spleen VCN | Vector SA (Luc W24/VCN) | ||
|---|---|---|---|---|---|---|---|
| Min | 20.77 | 35.63 | 18.51 | 19.33 | 14.81 | 4.38 | 20.89 |
| First Quantile | 46.87 | 52.04 | 50.02 | 32.54 | 27.44 | 40.26 | 50.43 |
| Median | 57.68 | 61.48 | 64.75 | 45.54 | 39.63 | 51.26 | 65.33 |
| Mean | 70.81 | 72.54 | 72.13 | 49.01 | 43.66 | 54.26 | 71.02 |
| Third Quantile | 98.57 | 96.20 | 90.83 | 64.62 | 51.40 | 63.37 | 79.82 |
| Max | 155.21 | 184.08 | 159.87 | 95.44 | 160.22 | 135.20 | 165.26 |
| Phenotype | Heritability (%) | p-Value |
|---|---|---|
| Liver Luc Week 1 | 44.94 | <0.0001 |
| Liver Luc Week 3 | 49.20 | <0.0001 |
| Liver Luc Week 6 | 43.19 | <0.0001 |
| Liver Luc Week 8 | 49.51 | <0.0001 |
| Liver Luc Week 14 | 50.28 | <0.0001 |
| Liver Luc Week 24 | 45.45 | <0.0001 |
| 35.60 | <0.0001 | |
| 23.01 | 0.0370 | |
| 14.79 | 0.6819 | |
| 15.79 | 0.6130 | |
| 18.92 | 0.3018 | |
| 43.44 | <0.0001 | |
| Liver VCN | 59.02 | <0.0001 |
| Spleen VCN | 53.77 | <0.0001 |
| Liver VCN/Spleen VCN | 54.28 | <0.0001 |
| Liver Luc Week 24/Liver VCN | 49.03 | <0.0001 |
| Phenotype * | Sig | p-Value | LOD | Chr | Position (Mb) | Lower Bound (Mb) | Upper Bound (Mb) | Interval Width (Mb) | Heritability (%) | # of Genes | # of Pseudogenes |
|---|---|---|---|---|---|---|---|---|---|---|---|
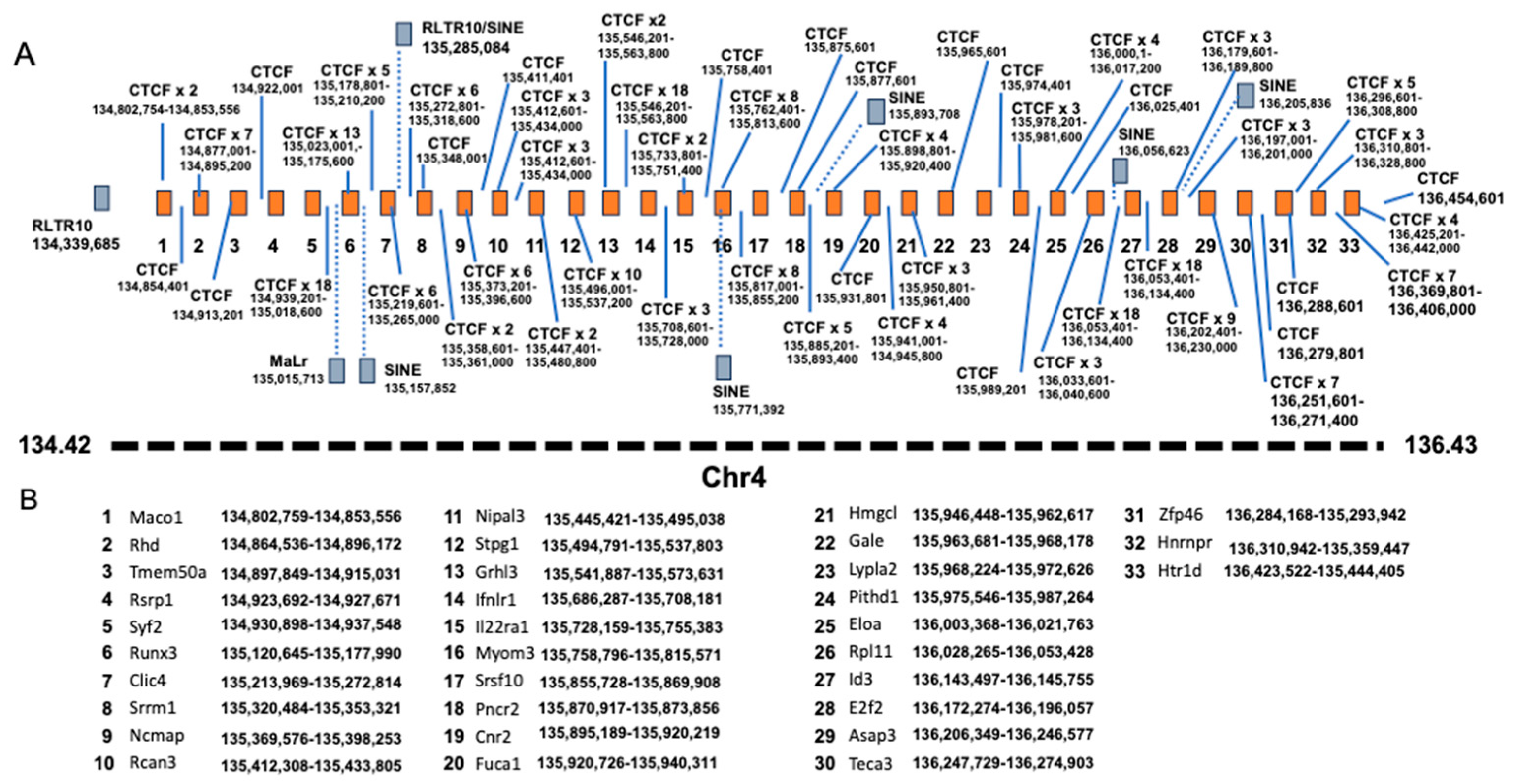
| CV. | 0.05 | 0.008 | 9.47 | 4 | 135.41 | 134.82 | 136.43 | 1.61 | 65.48 | 57 | 11 |
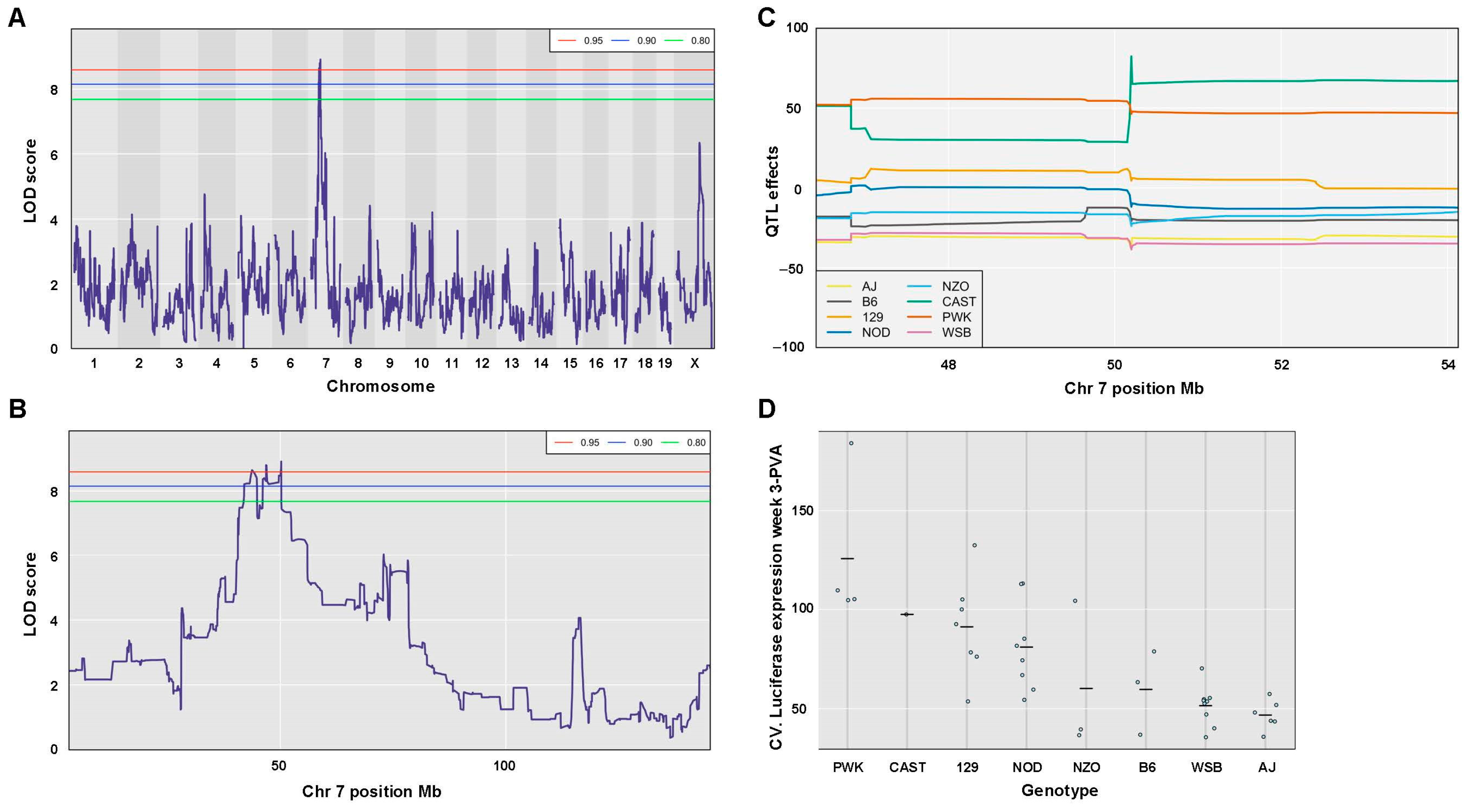
| CV. Liver Luc Week 3 | 0.05 | 0.030 | 8.92 | 7 | 50.19 | 40.97 | 50.25 | 9.28 | 63.26 | 372 | 134 |
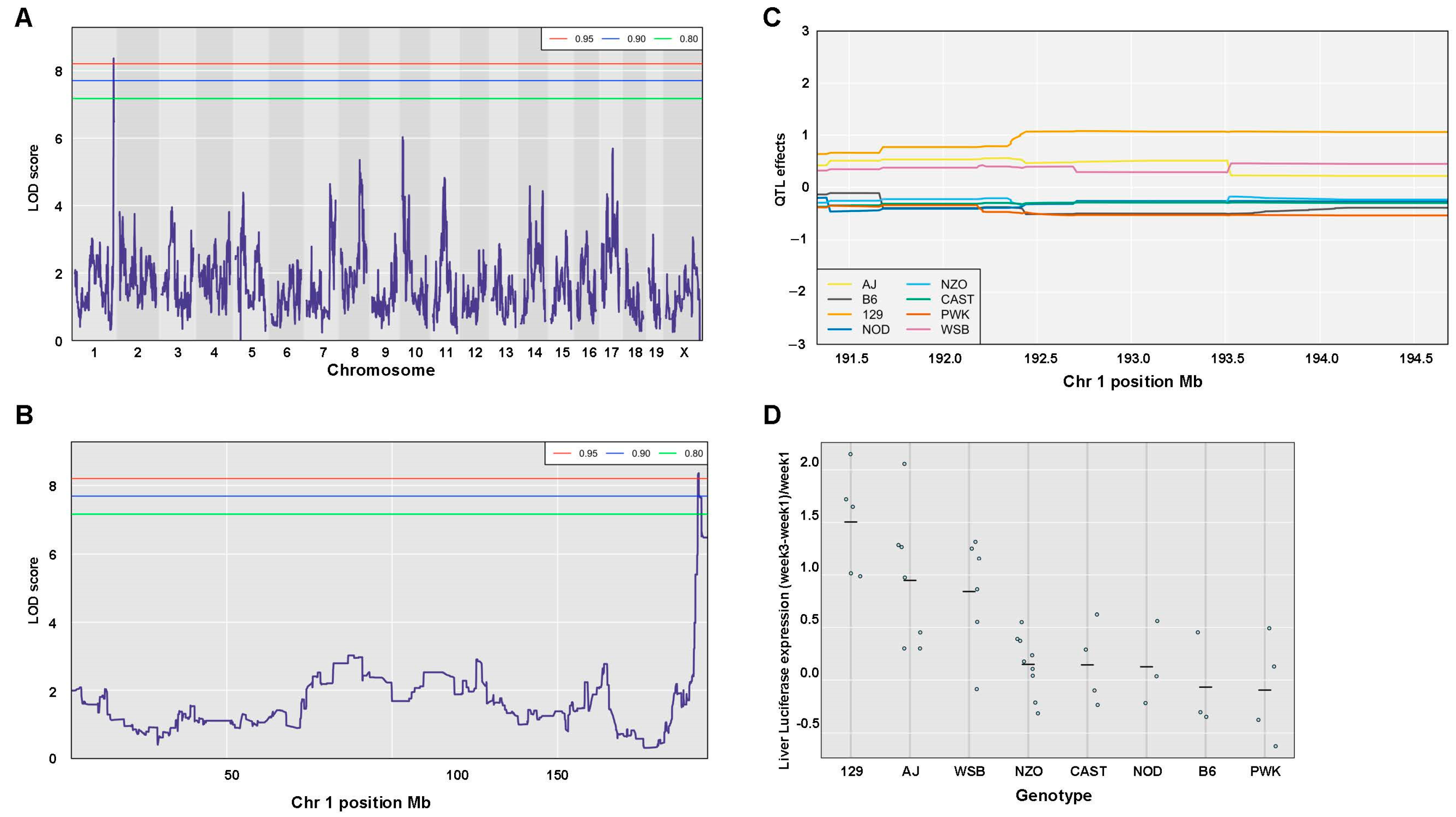
| 0.05 | 0.040 | 8.37 | 1 | 192.69 | 192.36 | 193.71 | 1.35 | 60.92 | 34 | 4 | |
| CV. Vector SA (Liver Luc Week 24/Liver VCN) | 0.10 | 0.064 | 9.72 | 8 | 99.03 | 99.03 | 100.59 | 1.56 | 66.45 | ||
| Liver Luc Week 1 | 0.10 | 0.081 | 11.27 | 2 | 154.29 | 152.95 | 156.38 | 3.43 | 71.79 | ||
| Liver VCN/Spleen VCN | 0.10 | 0.091 | 9.86 | 4 | 19.80 | 14.75 | 19.80 | 5.05 | 66.95 | ||
| Liver Luc Week 6 | 0.20 | 0.143 | 10.12 | 1 | 37.56 | 37.54 | 41.37 | 3.83 | 67.91 | ||
| 0.20 | 0.159 | 7.34 | 8 | 36.09 | 31.11 | 61.36 | 30.25 | 56.16 | |||
| CV. Spleen VCN | 0.20 | 0.166 | 11.90 | 19 | 27.49 | 24.77 | 28.06 | 3.29 | 73.72 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, P.; Hao, Y.; Tang, W.; Diering, G.H.; Zou, F.; Kafri, T. Analysis of Hepatic Lentiviral Vector Transduction: Implications for Preclinical Studies and Clinical Gene Therapy Protocols. Viruses 2025, 17, 276. https://doi.org/10.3390/v17020276
Hu P, Hao Y, Tang W, Diering GH, Zou F, Kafri T. Analysis of Hepatic Lentiviral Vector Transduction: Implications for Preclinical Studies and Clinical Gene Therapy Protocols. Viruses. 2025; 17(2):276. https://doi.org/10.3390/v17020276
Chicago/Turabian StyleHu, Peirong, Yajing Hao, Wei Tang, Graham H. Diering, Fei Zou, and Tal Kafri. 2025. "Analysis of Hepatic Lentiviral Vector Transduction: Implications for Preclinical Studies and Clinical Gene Therapy Protocols" Viruses 17, no. 2: 276. https://doi.org/10.3390/v17020276
APA StyleHu, P., Hao, Y., Tang, W., Diering, G. H., Zou, F., & Kafri, T. (2025). Analysis of Hepatic Lentiviral Vector Transduction: Implications for Preclinical Studies and Clinical Gene Therapy Protocols. Viruses, 17(2), 276. https://doi.org/10.3390/v17020276






