Phylogenetic Analysis of Varicella–Zoster Virus in Cerebrospinal Fluid from Individuals with Acute Central Nervous System Infection: An Exploratory Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. VZV Identification
2.3. DNA Extraction and Purification
2.4. Discrimination Test for v-Oka Vaccine Strain
2.5. VZV Genotyping
2.6. Intercontinental Dissemination of VZV Clades
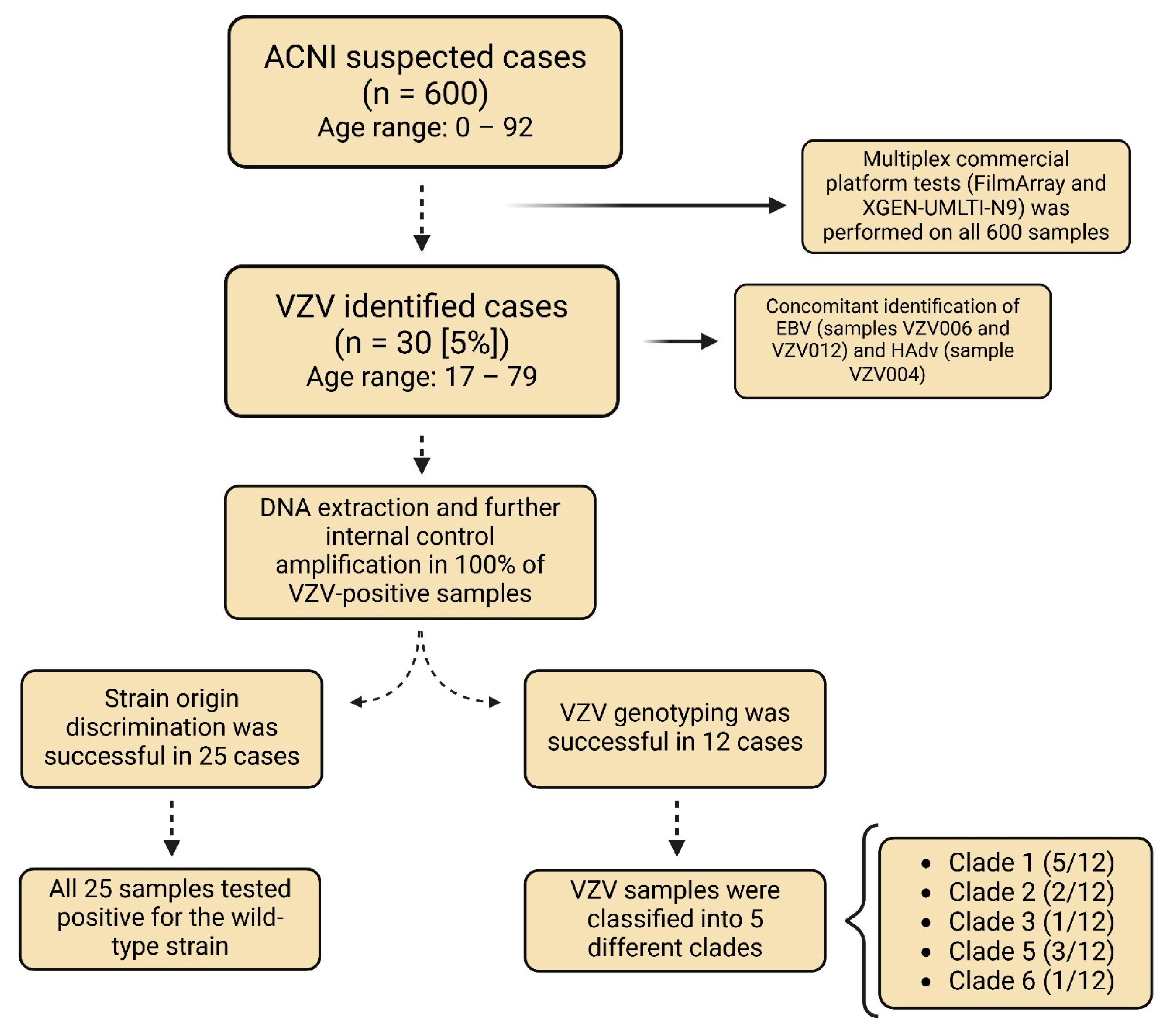
3. Results
3.1. Participants and Genetic Classification of VZV Isolates
3.1.1. VZV Identification
3.1.2. Identification of Other Viruses
3.1.3. DNA Extraction Quality Assessment
3.1.4. Discrimination Test for v-Oka Vaccine Strain
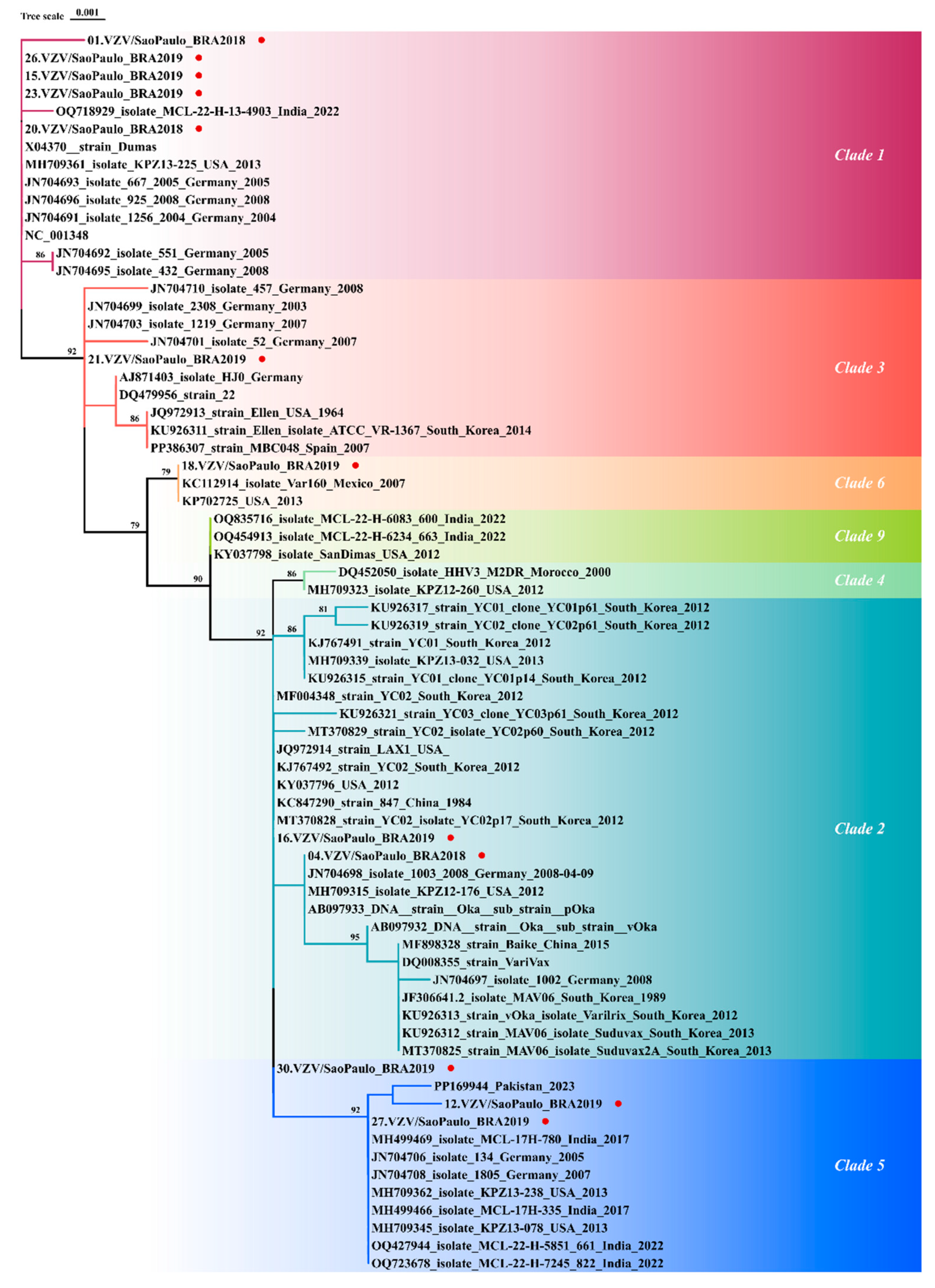
3.1.5. VZV Genotyping
3.2. Phylogenetic Classification and Nucleotide Identity
3.3. Intercontinental Dissemination of VZV Isolates from Brazil and from Other Origins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arvin, A.M. Varicella-zoster virus. Clin. Microbiol. Rev. 1996, 1, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Steiner, I.; Kennedy, P.G.; Pachner, A.R. The neurotropic herpes viruses: Herpes simplex and varicella-zoster. Lancet Neurol. 2007, 6, 1015–1028. [Google Scholar] [CrossRef] [PubMed]
- Rivest, P.; Bédard, L.; Valiquette, L.; Mills, E.; Lebel, H.M.; Lavoie, G.; Carsley, J. Severe complications associated with varicella: Province of Quebec, April 1994 to March 1996. Can. J. Infect. Dis. 2001, 12, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, R.; Miron, D.; Lumelsky, D.; Horovitz, Y. Severe meningoencephalitis due to late reactivation of varicella-zoster virus in an immunocompetent child. J. Child Neurol. 2010, 25, 87–90. [Google Scholar] [CrossRef]
- Hakami, M.A.; Khan, F.R.; Abdulaziz, O.; Alshaghdali, K.; Hazazi, A.; Aleissi, A.F.; Abalkhail, A.; Alotaibi, B.S.; Alhazmi, A.Y.M.; Kukreti, N.; et al. Varicella-zoster virus-related neurological complications: From infection to immunomodulatory therapies. Rev. Med. Virol. 2024, 34, e2554. [Google Scholar] [CrossRef] [PubMed]
- Bastos, M.S.; Lessa, N.; Naveca, F.G.; Monte, R.L.; Braga, W.S.; Figueiredo, L.T.M.; Ramasawmy, R.; Mourao, M.P.G. Detection of Herpesvirus, Enterovirus, and Arbovirus Infection in Patients with Suspected Central Nervous System Viral Infection in the Western Brazilian Amazon. J. Med. Virol. 2014, 86, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.B.; Candiani, T.M.; Franco-Luiz, A.P.M.; Almeida, G.M.F.; Abrahão, J.S.; Rios, M.; Coimbra, R.S.; Kroon, E.G. Etiological agents of viral meningitis in children from a dengue-endemic area, Southeast region of Brazil. J. Neurol. Sci. 2017, 375, 390–394. [Google Scholar] [CrossRef]
- Dupuis, M.; Hull, R.; Wang, H.; Nattanmai, S.; Glasheen, B.; Fusco, H.; Dzigua, L.; Markey, K.; Tavakoli, N.P. Molecular Detection of Viral Causes of Encephalitis and Meningitis in New York State. J. Med. Virol. 2011, 83, 2172–2181. [Google Scholar] [CrossRef] [PubMed]
- Bryant, P.; Yildirim, T.; Griesemer, S.B.; Shaw, K.; Ehrbar, D.; St. George, K. Vaccine Strain and Wild-Type Clades of Varicella-Zoster Virus in Central Nervous System and Non-CNS Disease, New York State, 2004–2019. J. Clin. Microbiol. 2022, 60, e02381-21. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.; McCollum, A.M.; Radford, K.; Hughes, C.; Lopez, A.S.; Guagliardo, S.A.J.; Nguete, B.; Likafi, T.; Kabamba, J.; Malekani, J.; et al. Varicella in Tshuapa Province, Democratic Republic of Congo, 2009–2014. Trop. Med. Int. Health 2019, 24, 839–848. [Google Scholar] [CrossRef]
- Loparev, V.N.; Rubtcova, E.N.; Bostik, V.; Govil, D.; Birch, C.J.; Druce, J.D.; Schmid, D.S.; Croxson, M.C. Identification of Five Major and Two Minor Genotypes of Varicella-Zoster Virus Strains: A Practical Two-Amplicon Approach Used To Genotype Clinical Isolates in Australia and New Zealand. J. Virol. 2007, 81, 12758–12765. [Google Scholar] [CrossRef] [PubMed]
- Bastos, M.d.S.; Folster, J.; Alvarenga, O.P.d.; Sampaio, D.d.A.; Rabelo, R.M.P.; João, G.A.P.; Lacerda, M.V.G.d.; Schmid, D.S. Genotypes of clinical varicella-zoster virus isolates from Manaus, Brazil. Rev. Soc. Bras. Med. Trop. 2019, 52, e20180166. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.R.M.d.; Monteiros, T.A.F.; Linhares, A.d.C.; Costa, I.B.; Kaiano, J.H.L.; Oliveira, D.d.S.; Ramos, F.L.d.P.; Sousa, R.C.M. de Varicella-zoster virus: Identification of genotypes in cases of varicella and herpes zoster in the Municipalities of Ananindeua, Belém and Marituba, Pará State, Brazil. Rev. Pan-Amaz. Saúde 2016, 7, 31–41. [Google Scholar] [CrossRef]
- Barrett-Muir, W.; Scott, F.T.; Aaby, P.; John, J.; Matondo, P.; Chaudhry, Q.L.; Siqueira, M.; Poulsen, A.; Yaminishi, K.; Breuer, J. Genetic variation of varicella-zoster virus: Evidence for geographical separation of strains. J. Med. Virol. 2003, 70, S42–S47. [Google Scholar] [CrossRef] [PubMed]
- Muir, W.B.; Nichols, R.; Breuer, J. Phylogenetic Analysis of Varicella-Zoster Virus: Evidence of Intercontinental Spread of Genotypes and Recombination. J. Virol. 2002, 76, 1971–1979. [Google Scholar] [CrossRef]
- Norberg, P.; Depledge, D.P.; Kundu, S.; Atkinson, C.; Brown, J.; Haque, T.; Hussaini, Y.; MacMahon, E.; Molyneaux, P.; Papaevangelou, V.; et al. Recombination of Globally Circulating Varicella-Zoster Virus. J. Virol. 2015, 89, 7133–7146. [Google Scholar] [CrossRef] [PubMed]
- Depledge, D.P.; Cudini, J.; Kundu, S.; Atkinson, C.; Brown, J.R.; Haque, T.; Houldcroft, C.J.; Koay, E.S.; McGill, F.; Milne, R.; et al. High Viral Diversity and Mixed Infections in Cerebral Spinal Fluid from Cases of Varicella Zoster Virus Encephalitis. J. Infect. Dis. 2018, 218, 1592–1601. [Google Scholar] [CrossRef]
- Breuer, J. Molecular Genetic Insights Into Varicella Zoster Virus (VZV), the vOka Vaccine Strain, and the Pathogenesis of Latency and Reactivation. J. Infect. Dis. 2018, 218 (Suppl. S2), S75–S80. [Google Scholar] [CrossRef]
- LaRussa, P.; Lungu, O.; Hardy, I.; Gershon, A.; Steinberg, S.P.; Silverstein, S. Restriction fragment length polymorphism of polymerase chain reaction products from vaccine and wild-type varicella-zoster virus isolates. J. Virol. 1992, 66, 1016–1020. [Google Scholar] [CrossRef]
- Luan, L.; Shen, X.; Qiu, J.; Jing, Y.; Zhang, J.; Wang, J.; Zhang, J.; Dong, C. Seroprevalence and molecular characteristics of varicella-zoster virus infection in Chinese children. BMC Infect. Dis. 2019, 19, 643. [Google Scholar] [CrossRef] [PubMed]
- Mocarski, E.S. Arvin, A., Campadelli-Fiume, G., Mocarski, E., Moore, P.S., Roizman, B., Whitley, R., Yamanish, K., Eds.; Comparative analysis of herpesvirus-common proteins. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Grose, C.; Tyler, S.; Peters, G.; Hiebert, J.; Stephens, G.M.; Ruyechan, W.T.; Jackson, W.; Storlie, J.; Tipples, G.A. Complete DNA Sequence Analyses of the First Two Varicella-Zoster Virus Glycoprotein E (D150N) Mutant Viruses Found in North America: Evolution of Genotypes with an Accelerated Cell Spread Phenotype. J. Virol. 2004, 78, 6799–6807. [Google Scholar] [CrossRef] [PubMed]
- Zell, R.; Taudien, S.; Pfaff, F.; Wutzler, P.; Platzer, M.; Sauerbrei, A. Sequencing of 21 Varicella-Zoster Virus Genomes Reveals Two Novel Genotypes and Evidence of Recombination. J. Virol. 2012, 86, 1608–1622. [Google Scholar] [CrossRef] [PubMed]
- Honorato, L.; Ferreira, N.E.; Domingues, R.B.; Senne, C.; Leite, F.B.V.d.M.; Santos, M.V.d.; Fernandes, G.B.P.; Paião, H.G.O.; Vilas Boas, L.S.; da Costa, A.C.; et al. Evaluation of enterovirus concentration, species identification, and cerebrospinal fluid parameters in patients of different ages with aseptic meningitis in São Paulo, Brazil. J. Med. Virol. 2024, 96, e29471. [Google Scholar] [CrossRef] [PubMed]
- Emery, S.L.; Erdman, D.D.; Bowen, M.D.; Newton, B.R.; Winchell, J.M.; Meyer, R.F.; Tong, S.; Cook, B.T.; Holloway, B.P.; McCaustland, K.A.; et al. Real-Time Reverse Transcription–Polymerase Chain Reaction Assay for SARS-associated Coronavirus. Emerg. Infect. Dis. 2004, 10, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Campsall, P.A.; Au, N.H.C.; Prendiville, J.S.; Speert, D.P.; Tan, R.; Thomas, E.E. Detection and Genotyping of Varicella-Zoster Virus by TaqMan Allelic Discrimination Real-Time PCR. J. Clin. Microbiol. 2004, 42, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrei, A.; Wutzler, P. Different genotype pattern of varicella-zoster virus obtained from patients with varicella and zoster in Germany. J. Med. Virol. 2007, 79, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- LaRussa, P.; Steinbcrg, S.; Arvin, A.; Dwyer, D.; Burgess, M.; Menegus, M.; Rekrut, K.; Yamanishi, K.; Gershon, A. Polymerase chain reaction and restriction fragment length polymorphism analysis of varicella-zoster virus isolates from the united states and other parts of the world. J. Infect. Dis. 1998, 178, S64–S66. [Google Scholar] [CrossRef]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; Varlamov, A.; Vaskin, Y.; Efremov, I.; German Grehov, O.G.; Kandrov, D.; Rasputin, K.; Syabro, M.; et al. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- de Oliveira Lopes, A.; Spitz, N.; Martinelli, K.G.; de Paula, A.V.; de Castro Conde Toscano, A.L.; Braz-Silva, P.H.; dos Santos Barbosa Netto, J.; Tozetto-Mendoza, T.R.; de Paula, V.S. Introduction of human gammaherpesvirus 8 genotypes A, B, and C into Brazil from multiple geographic regions. Virus Res. 2020, 276, 197828. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Poplin, V.; Boulware, D.R.; Bahr, N.C. Methods for rapid diagnosis of meningitis etiology in adults. Biomark. Med. 2020, 14, 459–479. [Google Scholar] [CrossRef]
- Aksamit, A.J.; Berkowitz, A.L. Meningitis. Contin. Lifelong Learn. Neurol. 2021, 27, 836–854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zuo, Y.; Jiang, L.; Peng, Y.; Huang, X.; Zuo, L. Epstein-Barr Virus and Neurological Diseases. Front. Mol. Biosci. 2022, 8, 816098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, W.; Ornelles, D.A.; Gooding, L.R. Modeling Adenovirus Latency in Human Lymphocyte Cell Lines. J. Virol. 2010, 84, 8799–8810. [Google Scholar] [CrossRef] [PubMed]
- El-Duah, P.; Sylverken, A.A.; Owusu, M.; Amoako, Y.A.; Yeboah, R.; Gorman, R.; Nyarko-Afriyie, E.; Schneider, J.; Jones, T.C.; Bonney, J.; et al. Genetic characterization of varicella-zoster and HIV-1 viruses from the cerebrospinal fluid of a co-infected encephalitic patient, Ghana. Virol. J. 2022, 19, 122. [Google Scholar] [CrossRef]
- Chesky, M.; Scalco, R.; Failace, L.; Read, S.; Jobim, L.F. Polymerase chain reaction for the laboratory diagnosis of aseptic meningitis and encephalitis. Arq. Neuropsiquiatr. 2000, 58, 836–842. [Google Scholar] [CrossRef]
- DATASUS: Meningite—Casos Confirmados Notificados no Sistema de Informação de Agravos de Notificação—Brasil [Internet]. Ministério da Saúde. 2022. Available online: http://tabnet.datasus.gov.br/cgi/deftohtm.exe?sinannet/cnv/meninbr.def (accessed on 15 November 2023).
- Scotta, M.C.; Paternina-de la Ossa, R.; Lumertz, M.S.; Jones, M.H.; Mattiello, R.; Pinto, L.A. Early impact of universal varicella vaccination on childhood varicella and herpes zoster hospitalizations in Brazil. Vaccine 2018, 36, 280–284. [Google Scholar] [CrossRef]
- Wutzler, P.; Bonanni, P.; Burgess, M.; Gershon, A.; Sáfadi, M.A.; Casabona, G. Varicella vaccination—The global experience. Expert Rev. Vaccines 2017, 16, 833–843. [Google Scholar] [CrossRef] [PubMed]
- González, I.; Molina-Ortega, A.; Pérez-Romero, P.; Echevarría, J.E.; He, L.; Tarragó, D. Varicella-zoster virus clades circulating in Spain over two decades. J. Clin. Virol. 2019, 110, 17–21. [Google Scholar] [CrossRef]
- Depledge, D.P.; Breuer, J. Arvin, A.M., Moffat, J.F., Abendroth, A., Oliver, S.L., Eds.; Varicella-Zoster Virus-Genetics, Molecular Evolution and Recombination. In Varicella-Zoster Virus; Current Topics in Microbiology and Immunology; Springer: Cham, Switzerland, 2010; pp. 1–23. [Google Scholar]
- Quinlivan, M.; Hawrami, K.; Barrett-Muir, W.; Aaby, P.; Arvin, A.; Chow, V.T.; John, T.J.; Matondo, P.; Peiris, M.; Poulsen, A.; et al. The molecular epidemiology of varicella-zoster virus: Evidence for geographic segregation. J. Infect. Dis. 2002, 186, 888–894. [Google Scholar] [CrossRef]
- Dayan, G.H.; Panero, M.S.; Debbag, R.; Urquiza, A.; Molina, M.; Prieto, S.; del Carmen Perego, M.; Scagliotti, G.; Galimberti, D.; Carroli, G.; et al. Varicella Seroprevalence and Molecular Epidemiology of Varicella-Zoster Virus in Argentina, 2002. J. Clin. Microbiol. 2004, 42, 5698–5704. [Google Scholar] [CrossRef] [PubMed]
- Condori-Yujra, F.J.; Jiménez-Vásquez, V.A.; Enríquez-Alva, G.W.; Cabezudo-Pillpe, N.E. Genetic characterization of varicella zoster virus isolated from complicated clinical cases in Peru in 2016 and 2017. Rev. Peru. Med. Exp. Salud Publica 2018, 35, 533. [Google Scholar] [CrossRef]
- Rodríguez-Castillo, A.; Vaughan, G.; Ramírez-González, J.E.; González-Durán, E.; Gudiño-Rosales, J.C.; Escobar-Gutiérrez, A. Genetic variation of varicella-zoster virus strains circulating in Mexico City. J. Clin. Virol. 2009, 46, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Sahay, R.R.; Yadav, P.D.; Majumdar, T.; Patil, S.; Sarkale, P.; Shete, A.M.; Chaubal, G.; Dange, V.R.; Patil, S.; Nyayanit, D.A.; et al. Clinico-epidemiological investigation on Varicella Zoster Virus indicates multiple clade circulation in Maharashtra state, India. Heliyon 2018, 4, e00757. [Google Scholar] [CrossRef] [PubMed]
- Pontremoli, C.; Forni, D.; Clerici, M.; Cagliani, R.; Sironi, M. Possible European Origin of Circulating Varicella Zoster Virus Strains. J. Infect. Dis. 2020, 221, 1286–1294. [Google Scholar] [CrossRef]
- Boštíková, V.; Sleha, R.; Boštík, P. Genotyping of Varicella Zoster Virus Clinical Isolates from the Czech Republic. Cent. Eur. J. Public Health 2016, 24, 331–332. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Xu, S.; Maple, P.A.C.; Xu, W.; Brown, K.E. Differentiation between wild-type and vaccines strains of varicella zoster virus (VZV) based on four single nucleotide polymorphisms. Epidemiol. Infect. 2017, 145, 2618–2625. [Google Scholar] [CrossRef]
- Breuer, J.; Grose, C.; Norberg, P.; Tipples, G.; Schmid, D.S. A proposal for a common nomenclature for viral clades that form the species varicella-zoster virus: Summary of VZV Nomenclature Meeting 2008, Barts and the London School of Medicine and Dentistry, 24-25 July 2008. J. Gen. Virol. 2010, 91, 821–828. [Google Scholar] [CrossRef]
- Schmidt-Chanasit, J.; Sauerbrei, A. Evolution and world-wide distribution of varicella-zoster virus clades. Infect. Genet. Evol. 2011, 11, 1–10. [Google Scholar] [CrossRef]
- Grose, C. Pangaea and the Out-of-Africa Model of Varicella-Zoster Virus Evolution and Phylogeography. J. Virol. 2012, 86, 9558–9565. [Google Scholar] [CrossRef] [PubMed]
- Breuer, J. The origin and migration of varicella zoster virus strains. J. Infect. Dis. 2020, 221, 1213–1215. [Google Scholar] [CrossRef] [PubMed]

 The red lines represent sequences from Clade 3,
The red lines represent sequences from Clade 3,  the turquoise lines represent sequences from Clade 1,
the turquoise lines represent sequences from Clade 1,  the blue lines represent sequences from Clade 6, and
the blue lines represent sequences from Clade 6, and  the green lines represent sequences from Clades 1, 2, and 5. GenBank accession numbers for the reference sequences are as follows: AJ871403, AY379115, AY379116, DQ457052, DQ674250, EU154348, FJ425229–FJ425272, FJ425272, JF306641, JN704690–JN704710, JQ972913, JQ972914, KC112914, KC847290, KF130961, KF130961- KF130965, KF811485, KJ767491, KJ767492, KJ808816, KP702725, KP902647–KP902660, KT360938–KT360942, KU926311–KU926322, KX352173–KX352185, KY037796, KY037797, LT984514–LT984535, MF004348, MF503712–MF503738, MF898328, MH499466–MH499469, MH709309–MH709377, MT358898–MT358918, MT370825–MT370830, MW545806–MW545808, MZ465781–MZ465905, OQ427944, OQ718929, OQ723678, OQ871571, OQ916049, OQ916050, OR689713, OR770642, OR885927, OR898439–OR898444, OR915870, OR958827–OR958829, OR988144–OR988146, OR992036–OR992041, PP003316–PP003321, PP169944, PP261331, PP261332, and PP386307. Adobe Illustrator version CC2022. The origin-colored points do not represent a specific location and are only representative of the country of origin.
the green lines represent sequences from Clades 1, 2, and 5. GenBank accession numbers for the reference sequences are as follows: AJ871403, AY379115, AY379116, DQ457052, DQ674250, EU154348, FJ425229–FJ425272, FJ425272, JF306641, JN704690–JN704710, JQ972913, JQ972914, KC112914, KC847290, KF130961, KF130961- KF130965, KF811485, KJ767491, KJ767492, KJ808816, KP702725, KP902647–KP902660, KT360938–KT360942, KU926311–KU926322, KX352173–KX352185, KY037796, KY037797, LT984514–LT984535, MF004348, MF503712–MF503738, MF898328, MH499466–MH499469, MH709309–MH709377, MT358898–MT358918, MT370825–MT370830, MW545806–MW545808, MZ465781–MZ465905, OQ427944, OQ718929, OQ723678, OQ871571, OQ916049, OQ916050, OR689713, OR770642, OR885927, OR898439–OR898444, OR915870, OR958827–OR958829, OR988144–OR988146, OR992036–OR992041, PP003316–PP003321, PP169944, PP261331, PP261332, and PP386307. Adobe Illustrator version CC2022. The origin-colored points do not represent a specific location and are only representative of the country of origin.
 The red lines represent sequences from Clade 3,
The red lines represent sequences from Clade 3,  the turquoise lines represent sequences from Clade 1,
the turquoise lines represent sequences from Clade 1,  the blue lines represent sequences from Clade 6, and
the blue lines represent sequences from Clade 6, and  the green lines represent sequences from Clades 1, 2, and 5. GenBank accession numbers for the reference sequences are as follows: AJ871403, AY379115, AY379116, DQ457052, DQ674250, EU154348, FJ425229–FJ425272, FJ425272, JF306641, JN704690–JN704710, JQ972913, JQ972914, KC112914, KC847290, KF130961, KF130961- KF130965, KF811485, KJ767491, KJ767492, KJ808816, KP702725, KP902647–KP902660, KT360938–KT360942, KU926311–KU926322, KX352173–KX352185, KY037796, KY037797, LT984514–LT984535, MF004348, MF503712–MF503738, MF898328, MH499466–MH499469, MH709309–MH709377, MT358898–MT358918, MT370825–MT370830, MW545806–MW545808, MZ465781–MZ465905, OQ427944, OQ718929, OQ723678, OQ871571, OQ916049, OQ916050, OR689713, OR770642, OR885927, OR898439–OR898444, OR915870, OR958827–OR958829, OR988144–OR988146, OR992036–OR992041, PP003316–PP003321, PP169944, PP261331, PP261332, and PP386307. Adobe Illustrator version CC2022. The origin-colored points do not represent a specific location and are only representative of the country of origin.
the green lines represent sequences from Clades 1, 2, and 5. GenBank accession numbers for the reference sequences are as follows: AJ871403, AY379115, AY379116, DQ457052, DQ674250, EU154348, FJ425229–FJ425272, FJ425272, JF306641, JN704690–JN704710, JQ972913, JQ972914, KC112914, KC847290, KF130961, KF130961- KF130965, KF811485, KJ767491, KJ767492, KJ808816, KP702725, KP902647–KP902660, KT360938–KT360942, KU926311–KU926322, KX352173–KX352185, KY037796, KY037797, LT984514–LT984535, MF004348, MF503712–MF503738, MF898328, MH499466–MH499469, MH709309–MH709377, MT358898–MT358918, MT370825–MT370830, MW545806–MW545808, MZ465781–MZ465905, OQ427944, OQ718929, OQ723678, OQ871571, OQ916049, OQ916050, OR689713, OR770642, OR885927, OR898439–OR898444, OR915870, OR958827–OR958829, OR988144–OR988146, OR992036–OR992041, PP003316–PP003321, PP169944, PP261331, PP261332, and PP386307. Adobe Illustrator version CC2022. The origin-colored points do not represent a specific location and are only representative of the country of origin.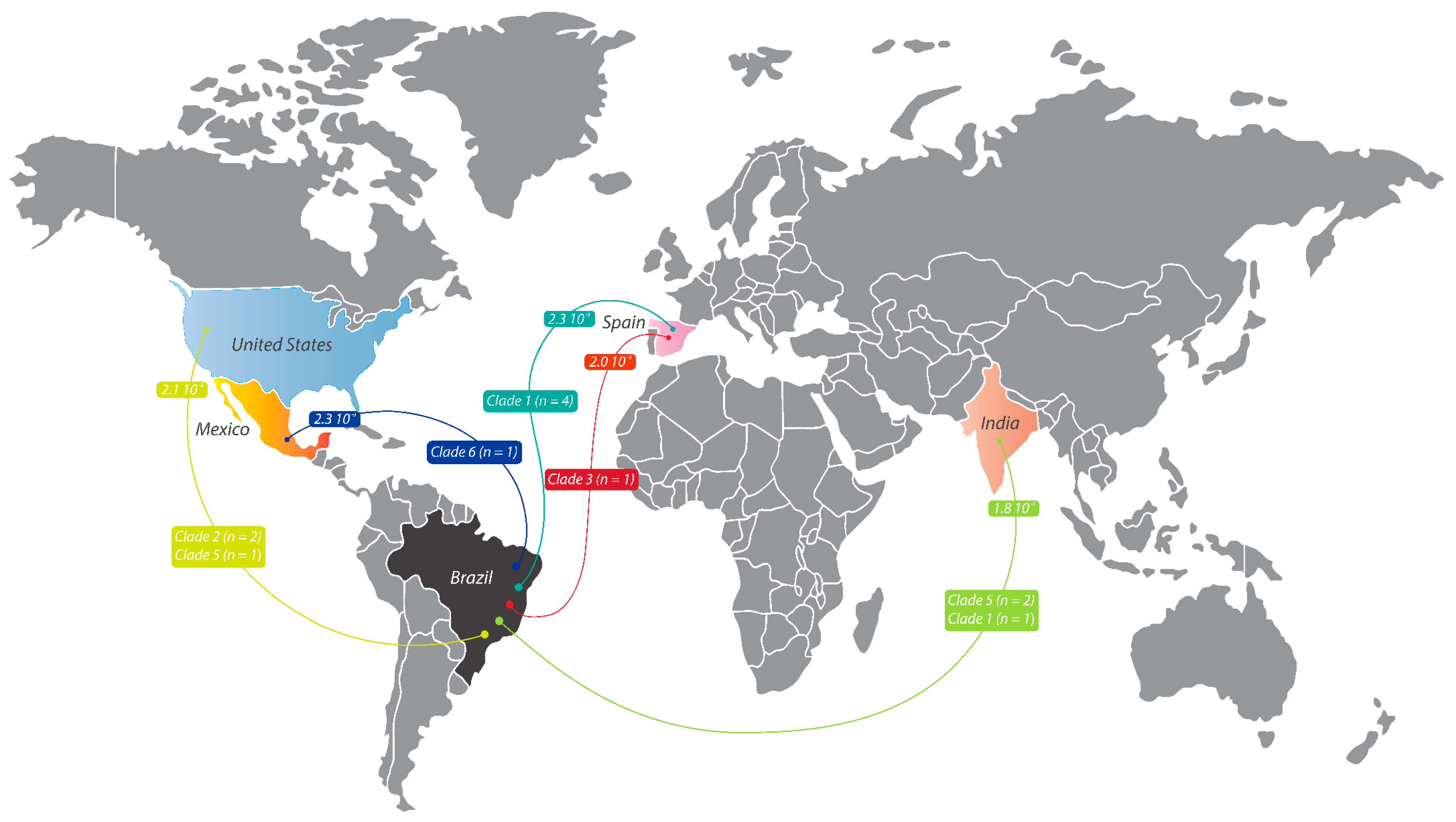
| Open Reading Frame (ORF) | Sequence 5′-3′ | Authors | |
|---|---|---|---|
| p22R1f | 22 | GGGTTTTGTATGAGCGTTGG | Loparev [11] Sauerbrei [27] |
| p22R1r | CCCCCGAGGTTCGTAATATC | ||
| PstA38 | 38 | TTGAACAATCACGAACCGTT | LaRussa [28] |
| PstB38 | CGGGTGAACCGTATTCTGAG | ||
| Fok54 | 54 | TCCCTTCATGCCCGTTACAT | LaRussa [19] |
| Nla54 | GGAACCCCTGCACCATTAAA | ||
| 62F | 62 | GGCCTTGGAAACCACATGATCG | Luan [20] |
| 62r | CGTCTCCCGTTCCGCATGTAG |
| Sample | Sex | Age | Co-Infection | Origin Type | Clade | Total bp Size (Concatenated Sequences) | Nucleotide Average Identity * with Prototypes from the Same Clade |
|---|---|---|---|---|---|---|---|
| VZV001 | Female | 48 | Not detected | WT | 1 | 1203 bp | 99.2% |
| VZV002 | Female | 72 | Not detected | ND | NS | - | - |
| VZV003 | Male | 67 | Not detected | ND | NS | - | - |
| VZV004 | Female | 58 | HAdv | WT | 2 | 909 bp | 99.6% |
| VZV005 | Male | 67 | Not detected | ND | NS | - | - |
| VZV006 | Male | 48 | EBV | WT | NS | - | - |
| VZV007 | Male | 79 | Not detected | WT | NS | - | - |
| VZV008 | Male | 17 | Not detected | WT | NS | - | - |
| VZV009 | Male | 53 | Not detected | WT | NS | - | - |
| VZV010 | Male | 32 | Not detected | ND | NS | - | - |
| VZV011 | Female | 31 | Not detected | ND | NS | - | - |
| VZV012 | Female | 34 | EBV | WT | 5 | 804 bp | 99.7% |
| VZV013 | Female | 57 | Not detected | WT | NS | - | - |
| VZV014 | Female | 62 | Not detected | WT | NS | - | - |
| VZV015 | Male | 38 | Not detected | WT | 1 | 507 bp | 100% |
| VZV016 | Female | 51 | Not detected | WT | 2 | 1203 bp | 99.45% |
| VZV017 | Female | 30 | Not detected | WT | NS | - | - |
| VZV018 | Male | 28 | Not detected | WT | 6 | 507 bp | 100% |
| VZV019 | Male | 30 | Not detected | WT | NS | - | - |
| VZV020 | Male | 41 | Not detected | WT | 1 | 213 bp | 100% |
| VZV021 | Female | 17 | Not detected | WT | 3 | 906 bp | 99.9% |
| VZV022 | Male | 35 | Not detected | WT | NS | - | - |
| VZV023 | Female | 31 | Not detected | WT | 1 | 804 bp | 99.8% |
| VZV024 | Female | 72 | Not detected | WT | NS | - | - |
| VZV025 | Female | 66 | Not detected | WT | NS | - | - |
| VZV026 | Male | 42 | Not detected | WT | 1 | 696 bp | - |
| VZV027 | Male | 39 | Not detected | WT | 5 | 1203 bp | 99.5% |
| VZV028 | Female | 20 | Not detected | WT | NS | - | - |
| VZV029 | Female | 30 | Not detected | WT | NS | - | - |
| VZV030 | Male | 28 | Not detected | WT | 5 | 906 bp | 99.2% |
| Sample | VZV Clade | CSF Collection Year | ORF 22 Accession Numbers | ORF 38 Accession Numbers | ORF 54 Accession Numbers | ORF 62 Accession Numbers |
|---|---|---|---|---|---|---|
| VZV001 | 1 | 2018 | OR770642 | OR885927 | OR992036 | OR988147 |
| VZV004 | 2 | 2018 | OR958829 | NS | OR992037 | OR988148 |
| VZV012 | 5 | 2019 | OR988144 | OR898439 | OR992038 | NS |
| VZV015 | 1 | 2019 | NS | OR898440 | OR992039 | NS |
| VZV016 | 2 | 2019 | OR988145 | OR898441 | OR992040 | OR988149 |
| VZV018 | 6 | 2019 | NS | OR898442 | OR992041 | NS |
| VZV020 | 1 | 2018 | NS | NS | PP003316 | NS |
| VZV021 | 3 | 2019 | NS | OR898443 | PP003317 | OR988150 |
| VZV023 | 1 | 2019 | OR988146 | OR898444 | PP003318 | NS |
| VZV026 | 1 | 2019 | NS | OR915870 | PP003319 | NS |
| VZV027 | 5 | 2019 | OR689713 | OR958827 | PP003320 | OR988151 |
| VZV030 | 5 | 2019 | NS | OR958828 | PP003321 | OR988152 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paião, H.G.O.; da Costa, A.C.; Ferreira, N.E.; Honorato, L.; Santos, B.M.d.; de Matos, M.L.M.; Domingues, R.B.; Senne, C.A.; Lopes, A.d.O.; de Paula, V.S.; et al. Phylogenetic Analysis of Varicella–Zoster Virus in Cerebrospinal Fluid from Individuals with Acute Central Nervous System Infection: An Exploratory Study. Viruses 2025, 17, 286. https://doi.org/10.3390/v17020286
Paião HGO, da Costa AC, Ferreira NE, Honorato L, Santos BMd, de Matos MLM, Domingues RB, Senne CA, Lopes AdO, de Paula VS, et al. Phylogenetic Analysis of Varicella–Zoster Virus in Cerebrospinal Fluid from Individuals with Acute Central Nervous System Infection: An Exploratory Study. Viruses. 2025; 17(2):286. https://doi.org/10.3390/v17020286
Chicago/Turabian StylePaião, Heuder G. O., Antônio C. da Costa, Noely E. Ferreira, Layla Honorato, Bianca M. dos Santos, Maria L. M. de Matos, Renan B. Domingues, Carlos A. Senne, Amanda de O. Lopes, Vanessa S. de Paula, and et al. 2025. "Phylogenetic Analysis of Varicella–Zoster Virus in Cerebrospinal Fluid from Individuals with Acute Central Nervous System Infection: An Exploratory Study" Viruses 17, no. 2: 286. https://doi.org/10.3390/v17020286
APA StylePaião, H. G. O., da Costa, A. C., Ferreira, N. E., Honorato, L., Santos, B. M. d., de Matos, M. L. M., Domingues, R. B., Senne, C. A., Lopes, A. d. O., de Paula, V. S., Witkin, S. S., Tozetto-Mendoza, T. R., & Mendes-Correa, M. C. (2025). Phylogenetic Analysis of Varicella–Zoster Virus in Cerebrospinal Fluid from Individuals with Acute Central Nervous System Infection: An Exploratory Study. Viruses, 17(2), 286. https://doi.org/10.3390/v17020286







