Advancements and Challenges in Addressing Zoonotic Viral Infections with Epidemic and Pandemic Threats
Abstract
:1. Introduction
| Zoonotic Viral Infection | Causative Agent | Reservoir Host(s) | Transmission Host(s) | Mode of Transmission to Human | Human-to-Human Transmission |
|---|---|---|---|---|---|
| Chikungunya Fever | Chikungunya virus | Non-human primates [14,15] | Mosquitoes; Aedes aegypti and Aedes albopictus [16] | Mosquito bite | No |
| Ebola/Marburg Hemorrhagic Fever | Ebola virus, Marburg virus | Fruit bats [17,18,19,20] | Gorillas and Chimpanzees [21], African Green Monkey [22] | Contact with body fluids of infected animals | Yes [22] |
| Yellow Fever | Yellow Fever virus | Non-human primates [23,24,25] | Aedes Mosquitoes [25] | Mosquito bite | No |
| Monkeypox | Mpox virus | Non-human primates [26,27], rodents [28,29] | Rodents [30], Monkeys [27] | Contact with body fluids or mucosal lesions of infected animals | Rare [30] |
| Nipah Virus Infection | Nipah virus | Bats (fruit bats) [31], flying-foxes [32] | Bats [33,34], Pigs [35] | Contact with body fluids or respiratory secretions of infected animals, consumption of contaminated date palm sap | Yes [36] |
| Lassa Fever | Lassa virus | Rodents (multimammate mouse) [37,38] | Rodents [39] | Direct exposure to rodent excreta, bodily fluids or indirect exposure via contaminated surfaces and food | Yes [40] |
| Rift Valley Fever | Rift Valley Fever virus | Livestock (sheep, cattle, goats, camels, donkeys) [41,42] | Mosquitoes [43,44,45,46] | Mosquito bite, direct contact with body fluids of infected animals | No |
| Tick-borne Encephalitis | Tick-borne Encephalitis virus | Rodents [47] | Ticks [48,49] | Tick bite | No |
| Japanese Encephalitis | Japnese Encephalitis virus | Birds [50], pigs [51,52] | Culex Mosquitoes [53,54,55] | Mosquito bite | No |
| West Nile Fever | West Nile virus | Wild Birds [56] | Culex Mosquitoes [57,58,59] | Mosquito bite | No |
| Hantavirus Infection | Hantavirus | Rodents; mice [60], rats [61], vole [62] Non-rodents; shrew [63], mole [64] | Rodents [65,66] | Rodent bite or inhalation of aerosolized rodent excreta | Rare (e.g., Andes virus) [67,68,69] |
| Crimean-Congo Hemorrhagic Fever | Crimean-Congo Hemorrhagic Fever virus | Cattle, goat, sheep, hare, wild boars [70] | Ticks [71,72,73] | Tick bite or direct contact with blood or secretions of infected animal | Yes [74,75] |
| Severe Fever with Thrombocytopenia Syndrome (SFTS) | SFTS virus | Cats [76], dog [77], cattle [78]; sheep, chicken, minks, pig [79]; goat, rodent [80] | Ticks [81,82,83] | Direct contact with the bodily fluids of an infected animal [84] | Yes [85,86] |
| Influenza | Influenza A, B, C, D viruses | Wild birds [87] | Horses, Poultry, Duck, Chicken, Turkey, Pigs, Horses, Whales, Seals, Mink [88] | Direct contact with infected animals, aerosols, contaminated surfaces | Yes |
| Virus | Genome Type | Genome Structure | Viral Family | Genus | Therapeutics | Licensed/Emerging Vaccines |
|---|---|---|---|---|---|---|
| Chikungunya virus | RNA | ss (+) | Togaviridae | Alphavirus | No standard treatment, NSAIDs, DMARDs [89], supportive care | VLA1553 [90] |
| Ebolavirus, Marburgvirus | RNA | ss (−) | Filoviridae | Ebolavirus Marburgvirus | mAb114 [91], REGN-EB3 [92], supportive care | rVSV-ZEBOV (Ervebo) [93], no licensed vaccine for Marburg |
| Yellow fever virus | RNA | ss (+) | Flaviviridae | Flavivirus | Supportive care, analgesics and antipyretics, avoidance of Aspirin and NSAIDs | YF-17D [94] |
| Monkeypox virus | DNA | ds | Poxviridae | Orthopoxvirus | Tecovirimat (TPOXX or ST-246) [95] | JYNNEOS (MVA-BN), LC16m18 and ACAM2000 [96] |
| Nipah virus | RNA | ss (−) | Paramyxoviridae | Henipavirus | Ribavirin [97], m102.4 [98], hu1F5 [99] | No licensed vaccine, mRNA 1215 (NCT05398796), ChAdOx1 NipahB ISRCTN87634044 |
| Lassa virus | RNA | ss (−) segmented | Arenaviridae | Mammarenavirus | Supportive care | No licensed vaccine, MV-LASV, EBS-LASV, INO-4500 and rVSVΔG-LASV-GPC [100] |
| Rift Valley fever virus | RNA | ss (−) segmented | Phenuiviridae | Phlebovirus | Supportive care | No licensed vaccine, TSI-GSD-200, hRVFV-4s and ChAdOx1 RVF [101] |
| Tick-borne encephalitis virus | RNA | ss (+) | Flaviviridae | Flavivirus | Supportive care | FSME-IMMUN (TicoVac) [102] |
| Japanese encephalitis virus | RNA | ss (+) | Flaviviridae | Flavivirus | Supportive care | SA14-14-2, IXIARO, ChimeriVax (IMOJEV) [103] |
| West Nile virus | RNA | ss (+) | Flaviviridae | Flavivirus | Supportive care | No licensed vaccine, ChimeriVax-WN02 [104], HydroVax-001 [105], WN/DEN4delta30 [106] |
| Hantaviruses | RNA | ss (−) segmented | Hantaviridae | Orthohantavirus | Supportive care | Hantavax licensed in China and South Korea [107] |
| Crimean-Congo hemorrhagic fever virus | RNA | ss (−) segmented | Nairoviridae | Orthonairovirus | Supportive care | No Licensed vaccine, ChAdOx2 CCHF (ISRCTN12351734) |
| SFTS virus | RNA | ss (−) segmented | Phenuiviridae | Banyangvirus | Supportive care, favipiravir [108] | No licensed vaccine |
| Influenza | RNA | ss (−) segmented | Orthomyxoviridae | Alphainfluenzavirus (A), Betainfluenzavirus (B), Gammainfluenzavirus (C), Deltainfluenzavirus (D) | Oseltamivir, Zanamivir, Peramivir, Baloxavir marboxil [109] | Inactivated (Fluzone, Fluarix, FluLaval, Afluria, Vaxigrip), live attenuated (FluMist), recombinant (Flublock) [110,111] |
2. Chikungunya Virus (CHIKV)
3. Ebola and Marburg Virus
4. Yellow Fever Virus (YFV)
5. Mpox Virus
6. Nipah Virus (NiV)
7. Lassa Virus (LASV)
8. Rift Valley Fever Virus
9. Tick-Borne Encephalitis Virus (TBEV)
10. Japanese Encephalitis Virus (JEV)
11. West Nile Virus (WNV)
12. Hantavirus
13. Crimean-Congo Hemorrhagic Fever Virus (CCHFV)
14. Severe Fever with Thrombocytopenia Syndrome Virus (SFTSV)
15. Influenza Virus
16. Challenges and Future Perspectives
17. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grzybek, M.; Tolkacz, K.; Sironen, T.; Maki, S.; Alsarraf, M.; Behnke-Borowczyk, J.; Biernat, B.; Nowicka, J.; Vaheri, A.; Henttonen, H.; et al. Zoonotic Viruses in Three Species of Voles from Poland. Animals 2020, 10, 1820. [Google Scholar] [CrossRef]
- Kruger, D.H.; Ulrich, R.G.; Hofmann, J. Hantaviruses as zoonotic pathogens in Germany. Dtsch. Arztebl. Int. 2013, 110, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Skowron, K.; Bauza-Kaszewska, J.; Grudlewska-Buda, K.; Wiktorczyk-Kapischke, N.; Zacharski, M.; Bernaciak, Z.; Gospodarek-Komkowska, E. Nipah Virus-Another Threat From the World of Zoonotic Viruses. Front. Microbiol. 2021, 12, 811157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zheng, M.; Zhang, Y.; Feng, G.; Peng, C.; Li, C.; Li, Y.; Zhang, H.; Li, N.; Xiao, P. Multiple Novel Mosquito-Borne Zoonotic Viruses Revealed in Pangolin Virome. Front. Cell. Infect. Microbiol. 2022, 12, 874003. [Google Scholar] [CrossRef]
- Weidinger, P.; Kolodziejek, J.; Khafaga, T.; Loney, T.; Howarth, B.; Sher Shah, M.; Abou Tayoun, A.; Alsheikh-Ali, A.; Camp, J.V.; Nowotny, N. Potentially Zoonotic Viruses in Wild Rodents, United Arab Emirates, 2019-A Pilot Study. Viruses 2023, 15, 695. [Google Scholar] [CrossRef]
- Abdelwhab, E.M.; Mettenleiter, T.C. Zoonotic Animal Influenza Virus and Potential Mixing Vessel Hosts. Viruses 2023, 15, 980. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Jin, Q.; Li, P.; Xing, G.; Lu, Q.; Zhang, G. Potential cross-species transmission risks of emerging swine enteric coronavirus to human beings. J. Med. Virol. 2023, 95, e28919. [Google Scholar] [CrossRef]
- Brussow, H. Viral infections at the animal-human interface-Learning lessons from the SARS-CoV-2 pandemic. Microb. Biotechnol. 2023, 16, 1397–1411. [Google Scholar] [CrossRef]
- Begeman, L.; van Riel, D.; Koopmans, M.P.G.; Kuiken, T. The pathogenesis of zoonotic viral infections: Lessons learned by studying reservoir hosts. Front. Microbiol. 2023, 14, 1151524. [Google Scholar] [CrossRef]
- Bankar, N.J.; Tidake, A.A.; Bandre, G.R.; Ambad, R.; Makade, J.G.; Hawale, D.V. Emerging and Re-Emerging Viral Infections: An Indian Perspective. Cureus 2022, 14, e30062. [Google Scholar] [CrossRef]
- Majiwa, H.; Bukachi, S.A.; Omia, D.; Fevre, E.M. Knowledge, perceptions, and practices around zoonotic diseases among actors in the livestock trade in the Lake Victoria crescent ecosystem in East Africa. Front. Public Health 2023, 11, 1199664. [Google Scholar] [CrossRef]
- Layton, D.S.; Choudhary, A.; Bean, A.G.D. Breaking the chain of zoonoses through biosecurity in livestock. Vaccine 2017, 35, 5967–5973. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.S.; Jeggo, M. The One Health Approach-Why Is It So Important? Trop. Med. Infect. Dis. 2019, 4, 88. [Google Scholar] [CrossRef] [PubMed]
- Apandi, Y.; Nazni, W.A.; Azleen, Z.A.N.; Vythilingam, I.; Noorazian, M.Y.; Azahari, A.H.; Zainah, S.; Lee, H.L. The first isolation of chikungunya virus from nonhuman primates in Malaysia. J. Gen. Mol. Virol. 2009, 1, 035–039. [Google Scholar]
- Althouse, B.M.; Guerbois, M.; Cummings, D.A.T.; Diop, O.M.; Faye, O.; Faye, A.; Diallo, D.; Sadio, B.D.; Sow, A.; Faye, O.; et al. Role of monkeys in the sylvatic cycle of chikungunya virus in Senegal. Nat. Commun. 2018, 9, 1046. [Google Scholar] [CrossRef] [PubMed]
- Lounibos, L.P.; Kramer, L.D. Invasiveness of Aedes aegypti and Aedes albopictus and Vectorial Capacity for Chikungunya Virus. J. Infect. Dis. 2016, 214 (Suppl. S5), S453–S458. [Google Scholar] [CrossRef]
- Swanepoel, R.; Smit, S.B.; Rollin, P.E.; Formenty, P.; Leman, P.A.; Kemp, A.; Burt, F.J.; Grobbelaar, A.A.; Croft, J.; Bausch, D.G.; et al. Studies of reservoir hosts for Marburg virus. Emerg. Infect. Dis. 2007, 13, 1847–1851. [Google Scholar] [CrossRef]
- Monath, T.P. Ecology of Marburg and Ebola viruses: Speculations and directions for future research. J. Infect. Dis. 1999, 179, S127–S138. [Google Scholar] [CrossRef]
- Schuh, A.J.; Amman, B.R.; Towner, J.S. Filoviruses and bats. Microbiol. Aust. 2017, 38, 12–16. [Google Scholar] [CrossRef]
- Towner, J.S.; Pourrut, X.; Albarino, C.G.; Nkogue, C.N.; Bird, B.H.; Grard, G.; Ksiazek, T.G.; Gonzalez, J.P.; Nichol, S.T.; Leroy, E.M. Marburg virus infection detected in a common African bat. PLoS ONE 2007, 2, e764. [Google Scholar] [CrossRef]
- Huijbregts, B.; De Wachter, P.; Obiang, L.S.N.; Akou, M.E. Ebola and the decline of gorilla Gorilla gorilla and chimpanzee Pan troglodytes populations in Minkebe Forest, north-eastern Gabon. Oryx 2003, 37, 437–443. [Google Scholar] [CrossRef]
- Martini, G.A. Marburg virus disease. Postgrad. Med. J. 1973, 49, 542–546. [Google Scholar] [CrossRef]
- Childs, M.L.; Nova, N.; Colvin, J.; Mordecai, E.A. Mosquito and primate ecology predict human risk of yellow fever virus spillover in Brazil. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180335. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, W.H.R. The night-resting habits of monkeys in a small area on the edge of the semliki forest, uganda. a study in relation to the epidemiology of sylvan yellow fever. J. Anim. Ecol. 1951, 20, 11–30. [Google Scholar] [CrossRef]
- Monath, T.P. Yellow fever: An update. Lancet Infect. Dis. 2001, 1, 11–20. [Google Scholar] [CrossRef]
- Arita, I.; Henderson, D.A. Smallpox and monkeypox in non-human primates. Bull. World Health Organ. 1968, 39, 277–283. [Google Scholar] [PubMed]
- Cho, C.T.; Wenner, H.A. Monkey Pox. Bacteriol. Rev. 1973, 37, 1–18. [Google Scholar] [CrossRef]
- Di Giulio, D.B.; Eckburg, P.B. Human monkeypox: An emerging zoonosis. Lancet Infect. Dis. 2004, 4, 15–25. [Google Scholar] [CrossRef]
- Khodakevich, L.; Jezek, Z. Isolation of Monkeypox virus from wild squirrel infected in nature. Lancet 1986, 327, 98–99. [Google Scholar] [CrossRef]
- Nolen, L.D.; Osadebe, L.; Katomba, J.; Likofata, J.; Mukadi, D.; Monroe, B.; Doty, J.; Kalemba, L.; Malekani, J.; Kabamba, J.; et al. Introduction of Monkeypox into a Community and Household: Risk Factors and Zoonotic Reservoirs in the Democratic Republic of the Congo. Am. J. Trop. Med. Hyg. 2015, 93, 410–415. [Google Scholar] [CrossRef]
- Epstein, J.H.; Anthony, S.J.; Islam, A.; Kilpatrick, A.M.; Ali Khan, S.; Balkey, M.D.; Ross, N.; Smith, I.; Zambrana-Torrelio, C.; Tao, Y.; et al. Nipah virus dynamics in bats and implications for spillover to humans. Proc. Natl. Acad. Sci. USA 2020, 117, 29190–29201. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.B.; Lek Koh, C.; Hooi, P.S.; Wee, K.F.; Khong, J.H.; Chua, B.H.; Chan, Y.P.; Lim, M.E.; Lam, S.K. Isolation of Nipah virus from Malaysian Island flying-foxes. Microbes Infect. 2002, 4, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Bedi, J.S.; Vijay, D.; Dhaka, P. Nipah. In Textbook of Zoonoses; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 163–167. [Google Scholar]
- Faus-Cotino, J.; Reina, G.; Pueyo, J. Nipah Virus: A Multidimensional Update. Viruses 2024, 16, 179. [Google Scholar] [CrossRef]
- AbuBakar, S.; Chang, L.-Y.; Ali, A.R.M.; Sharifah, S.H.; Yusoff, K.; Zamrod, Z. Isolation and molecular identification of Nipah virus from pigs. Emerg. Infect. Dis. 2004, 10, 2228–2230. [Google Scholar] [CrossRef]
- Nikolay, B.; Salje, H.; Hossain, M.J.; Khan, A.; Sazzad, H.M.S.; Rahman, M.; Daszak, P.; Stroher, U.; Pulliam, J.R.C.; Kilpatrick, A.M.; et al. Transmission of Nipah Virus—14 Years of Investigations in Bangladesh. N. Engl. J. Med. 2019, 380, 1804–1814. [Google Scholar] [CrossRef]
- Karan, L.S.; Makenov, M.T.; Korneev, M.G.; Sacko, N.; Boumbaly, S.; Bayandin, R.B.; Gladysheva, A.V.; Kourouma, K.; Toure, A.H.; Kartashov, M.; et al. Lassa virus in the host rodent mastomys natalensis within urban areas of N’zerekore, guinea. bioRxiv 2019, 616466. [Google Scholar] [CrossRef]
- Olayemi, A.; Obadare, A.; Oyeyiola, A.; Fasogbon, S.; Igbokwe, J.; Igbahenah, F.; Ortsega, D.; Günther, S.; Verheyen, E.; Fichet-Calvet, E. Small mammal diversity and dynamics within Nigeria, with emphasis on reservoirs of the lassa virus. Syst. Biodivers. 2017, 16, 118–127. [Google Scholar] [CrossRef]
- Marien, J.; Lo Iacono, G.; Rieger, T.; Magassouba, N.; Gunther, S.; Fichet-Calvet, E. Households as hotspots of Lassa fever? Assessing the spatial distribution of Lassa virus-infected rodents in rural villages of Guinea. Emerg. Microbes Infect. 2020, 9, 1055–1064. [Google Scholar] [CrossRef]
- Ogbu, O.; Ajuluchukwu, E.; Uneke, C.J. Lassa fever in West African sub-region: An overview. J. Vector Borne Dis. 2007, 44, 1. [Google Scholar]
- Roger, M.; Beral, M.; Licciardi, S.; Soule, M.; Faharoudine, A.; Foray, C.; Olive, M.M.; Maquart, M.; Soulaimane, A.; Madi Kassim, A.; et al. Evidence for circulation of the rift valley fever virus among livestock in the union of Comoros. PLoS Negl. Trop. Dis. 2014, 8, e3045. [Google Scholar] [CrossRef]
- Eisa, M. Preliminary survey of domestic animals of the Sudan for precipitating antibodies to Rift Valley fever virus. J. Hyg. 1984, 93, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Rodrigue Simonet, P.N.; Alexandre Michel, N.N.; Abel, W.; Albert, E.; Martin Hermann, G.; Franziska, S. Diversity and Abundance of Potential Vectors of Rift Valley Fever Virus in the North Region of Cameroon. Insects 2020, 11, 814. [Google Scholar] [CrossRef]
- Nicolas, G.; Chevalier, V.; Tantely, L.M.; Fontenille, D.; Durand, B. A spatially explicit metapopulation model and cattle trade analysis suggests key determinants for the recurrent circulation of rift valley Fever virus in a pilot area of madagascar highlands. PLoS Negl. Trop. Dis. 2014, 8, e3346. [Google Scholar] [CrossRef]
- Linthicum, K.J.; Davies, F.G.; Kairo, A.; Bailey, C.L. Rift Valley fever virus (family Bunyaviridae, genus Phlebovirus). Isolations from Diptera collected during an inter-epizootic period in Kenya. J. Hyg. 1985, 95, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, R.K.; Kutikuppala, L.V.S.; Kandi, V.; Mishra, S.; Rabaan, A.A.; Costa, S.; Al-Qaim, Z.H.; Padhi, B.K.; Sah, R. Rift valley fever (RVF) viral zoonotic disease steadily circulates in the Mauritanian animals and humans: A narrative review. Health Sci. Rep. 2023, 6, e1384. [Google Scholar] [CrossRef]
- Michelitsch, A.; Wernike, K.; Klaus, C.; Dobler, G.; Beer, M. Exploring the Reservoir Hosts of Tick-Borne Encephalitis Virus. Viruses 2019, 11, 669. [Google Scholar] [CrossRef]
- Mansfield, K.L.; Johnson, N.; Phipps, L.P.; Stephenson, J.R.; Fooks, A.R.; Solomon, T. Tick-borne encephalitis virus—A review of an emerging zoonosis. J. Gen. Virol. 2009, 90 Pt 8, 1781–1794. [Google Scholar] [CrossRef]
- Pustijanac, E.; Bursic, M.; Talapko, J.; Skrlec, I.; Mestrovic, T.; Lisnjic, D. Tick-Borne Encephalitis Virus: A Comprehensive Review of Transmission, Pathogenesis, Epidemiology, Clinical Manifestations, Diagnosis, and Prevention. Microorganisms 2023, 11, 1634. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.; Wahaab, A.; Nawaz, M.; Khan, S.; Nazir, J.; Liu, K.; Wei, J.; Ma, Z. Potential Role of Birds in Japanese Encephalitis Virus Zoonotic Transmission and Genotype Shift. Viruses 2021, 13, 357. [Google Scholar] [CrossRef]
- Pegu, S.R.; Das, P.J.; Sonowal, J.; Sengar, G.S.; Deb, R.; Yadav, A.K.; Rajkhowa, S.; Choudhury, M.; Gulati, B.R.; Gupta, V.K. Japanese Encephalitis Virus Genotype III Strains Detection and Genome Sequencing from Indian Pig and Mosquito Vector. Vaccines 2023, 11, 150. [Google Scholar] [CrossRef]
- Walsh, M.G.; Pattanaik, A.; Vyas, N.; Saxena, D.; Webb, C.; Sawleshwarkar, S.; Mukhopadhyay, C. High-risk landscapes of Japanese encephalitis virus outbreaks in India converge on wetlands, rain-fed agriculture, wild Ardeidae, and domestic pigs and chickens. Int. J. Epidemiol. 2022, 51, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- van den Hurk, A.F.; Skinner, E.; Ritchie, S.A.; Mackenzie, J.S. The Emergence of Japanese Encephalitis Virus in Australia in 2022: Existing Knowledge of Mosquito Vectors. Viruses 2022, 14, 1208. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wahaab, A.; Khan, S.; Nawaz, M.; Anwar, M.N.; Liu, K.; Wei, J.; Hameed, M.; Ma, Z. Recent Population Dynamics of Japanese Encephalitis Virus. Viruses 2023, 15, 1312. [Google Scholar] [CrossRef]
- Oliveira, A.R.S.; Cohnstaedt, L.W.; Strathe, E.; Hernandez, L.E.; McVey, D.S.; Piaggio, J.; Cernicchiaro, N. Meta-analyses of the proportion of Japanese encephalitis virus infection in vectors and vertebrate hosts. Parasit Vectors 2017, 10, 418. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.; L’Ambert, G.; Balanca, G.; Pradier, S.; Grosbois, V.; Balenghien, T.; Baldet, T.; Lecollinet, S.; Leblond, A.; Gaidet-Drapier, N. An Integrative Eco-Epidemiological Analysis of West Nile Virus Transmission. Ecohealth 2017, 14, 474–489. [Google Scholar] [CrossRef]
- Adelman, J.S.; Tokarz, R.E.; Euken, A.E.; Field, E.N.; Russell, M.C.; Smith, R.C. Relative Influence of Land Use, Mosquito Abundance, and Bird Communities in Defining West Nile Virus Infection Rates in Culex Mosquito Populations. Insects 2022, 13, 758. [Google Scholar] [CrossRef]
- Hayes, E.B.; Komar, N.; Nasci, R.S.; Montgomery, S.P.; O’Leary, D.R.; Campbell, G.L. Epidemiology and transmission dynamics of West Nile virus disease. Emerg. Infect. Dis. 2005, 11, 1167–1173. [Google Scholar] [CrossRef]
- de Freitas Costa, E.; Streng, K.; Avelino de Souza Santos, M.; Counotte, M.J. The effect of temperature on the boundary conditions of West Nile virus circulation in Europe. PLoS Negl. Trop. Dis. 2024, 18, e0012162. [Google Scholar] [CrossRef]
- Warner, B.M.; Stein, D.R.; Griffin, B.D.; Tierney, K.; Leung, A.; Sloan, A.; Kobasa, D.; Poliquin, G.; Kobinger, G.P.; Safronetz, D. Development and Characterization of a Sin Nombre Virus Transmission Model in Peromyscus maniculatus. Viruses 2019, 11, 183. [Google Scholar] [CrossRef]
- Ling, J.; Verner-Carlsson, J.; Eriksson, P.; Plyusnina, A.; Lohmus, M.; Jarhult, J.D.; van de Goot, F.; Plyusnin, A.; Lundkvist, A.; Sironen, T. Genetic analyses of Seoul hantavirus genome recovered from rats (Rattus norvegicus) in the Netherlands unveils diverse routes of spread into Europe. J. Med. Virol. 2019, 91, 724–730. [Google Scholar] [CrossRef]
- Schlohsarczyk, E.K.; Drewes, S.; Koteja, P.; Rohrs, S.; Ulrich, R.G.; Teifke, J.P.; Herden, C. Tropism of Puumala orthohantavirus and Endoparasite Coinfection in the Bank Vole Reservoir. Viruses 2023, 15, 612. [Google Scholar] [CrossRef] [PubMed]
- Lwande, O.W.; Mohamed, N.; Bucht, G.; Ahlm, C.; Olsson, G.; Evander, M. Seewis hantavirus in common shrew (Sorex araneus) in Sweden. Virol. J. 2020, 17, 198. [Google Scholar] [CrossRef]
- Gu, S.H.; Minarro, M.; Feliu, C.; Hugot, J.P.; Forrester, N.L.; Weaver, S.C.; Yanagihara, R. Multiple Lineages of Hantaviruses Harbored by the Iberian Mole (Talpa occidentalis) in Spain. Viruses 2023, 15, 1313. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, L.S.; Souza, L.C.; Langoni, H. Hantaviruses as emergent zoonoses. J. Venom. Anim. Toxins Incl. Trop. Dis. 2008, 14, 558–571. [Google Scholar] [CrossRef]
- Carver, S.; Mills, J.N.; Parmenter, C.A.; Parmenter, R.R.; Richardson, K.S.; Harris, R.L.; Douglass, R.J.; Kuenzi, A.J.; Luis, A.D. Toward a Mechanistic Understanding of Environmentally Forced Zoonotic Disease Emergence: Sin Nombre Hantavirus. Bioscience 2015, 65, 651–666. [Google Scholar] [CrossRef]
- Toledo, J.; Haby, M.M.; Reveiz, L.; Sosa Leon, L.; Angerami, R.; Aldighieri, S. Evidence for Human-to-Human Transmission of Hantavirus: A Systematic Review. J. Infect. Dis. 2022, 226, 1362–1371. [Google Scholar] [CrossRef] [PubMed]
- Ashique, S.; Sandhu, N.K.; Das, S.; Haque, S.N.; Koley, K. Global Comprehensive Outlook of Hantavirus Contagion on Humans: A Review. Infect. Disord. Drug Targets 2022, 22, e050122199975. [Google Scholar] [CrossRef]
- Martinez, V.P.; Bellomo, C.; San Juan, J.; Pinna, D.; Forlenza, R.; Elder, M.; Padula, P.J. Person-to-person transmission of Andes viru. Emerg. Infect. Dis. 2005, 11, 1848–1853. [Google Scholar] [CrossRef] [PubMed]
- Nurettin, C.; Engin, B.; Sukru, T.; Munir, A.; Zati, V.; Aykut, O. The Seroprevalence of Crimean-Congo Hemorrhagic Fever in Wild and Domestic Animals: An Epidemiological Update for Domestic Animals and First Seroevidence in Wild Animals from Turkiye. Vet. Sci. 2022, 9, 462. [Google Scholar] [CrossRef]
- Gargili, A.; Estrada-Pena, A.; Spengler, J.R.; Lukashev, A.; Nuttall, P.A.; Bente, D.A. The role of ticks in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus: A review of published field and laboratory studies. Antiviral Res. 2017, 144, 93–119. [Google Scholar] [CrossRef]
- Bernard, C.; Holzmuller, P.; Bah, M.T.; Bastien, M.; Combes, B.; Jori, F.; Grosbois, V.; Vial, L. Systematic Review on Crimean-Congo Hemorrhagic Fever Enzootic Cycle and Factors Favoring Virus Transmission: Special Focus on France, an Apparently Free-Disease Area in Europe. Front. Vet. Sci. 2022, 9, 932304. [Google Scholar] [CrossRef] [PubMed]
- Ergönül, Ö. Crimean-Congo haemorrhagic fever. Lancet Infect. Dis. 2006, 6, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Balinandi, S.; Whitmer, S.; Mulei, S.; Nyakarahuka, L.; Tumusiime, A.; Kyondo, J.; Baluku, J.; Mutyaba, J.; Mugisha, L.; Malmberg, M.; et al. Clinical and Molecular Epidemiology of Crimean-Congo Hemorrhagic Fever in Humans in Uganda, 2013–2019. Am. J. Trop. Med. Hyg. 2021, 106, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Tsergouli, K.; Karampatakis, T.; Haidich, A.B.; Metallidis, S.; Papa, A. Nosocomial infections caused by Crimean-Congo haemorrhagic fever virus. J. Hosp. Infect. 2020, 105, 43–52. [Google Scholar] [CrossRef]
- Osako, H.; Xu, Q.; Nabeshima, T.; Balingit, J.C.; Nwe, K.M.; Yu, F.; Inoue, S.; Hayasaka, D.; Ngwe Tun, M.M.; Morita, K.; et al. Clinical Factors Associated with SFTS Diagnosis and Severity in Cats. Viruses 2024, 16, 874. [Google Scholar] [CrossRef]
- Saga, Y.; Yoshida, T.; Yoshida, R.; Yazawa, S.; Shimada, T.; Inasaki, N.; Itamochi, M.; Yamazaki, E.; Oishi, K.; Tani, H. Long-Term Detection and Isolation of Severe Fever with Thrombocytopenia Syndrome (SFTS) Virus in Dog Urine. Viruses 2023, 15, 2228. [Google Scholar] [CrossRef]
- Chae, J.-B.; Rim, J.-M.; Han, S.-W.; Cho, Y.-K.; Kang, J.-G.; Chae, J.-S. Prevalence, Isolation, and Molecular Characterization of Severe Fever with Thrombocytopenia Syndrome Virus in Cattle from the Republic of Korea. Vector-Borne Zoonotic Dis. 2024, 24, 826–834. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Li, X.; Zhang, G.; Sun, S.; Wang, Z.; Zhao, J.; Xiu, L.; Jiang, N.; Zhang, H.; et al. Direct transmission of severe fever with thrombocytopenia syndrome virus from farm-raised fur animals to workers in Weihai, China. Virol. J. 2024, 21, 113. [Google Scholar] [CrossRef]
- Chen, C.; Li, P.; Li, K.F.; Wang, H.L.; Dai, Y.X.; Cheng, X.; Yan, J.B. Animals as amplification hosts in the spread of severe fever with thrombocytopenia syndrome virus: A systematic review and meta-analysis. Int. J. Infect. Dis. 2019, 79, 77–84. [Google Scholar] [CrossRef]
- Yun, S.M.; Lee, W.G.; Ryou, J.; Yang, S.C.; Park, S.W.; Roh, J.Y.; Lee, Y.J.; Park, C.; Han, M.G. Severe fever with thrombocytopenia syndrome virus in ticks collected from humans, South Korea, 2013. Emerg. Infect. Dis. 2014, 20, 1358–1361. [Google Scholar] [CrossRef]
- Yu, X.J.; Liang, M.F.; Zhang, S.Y.; Liu, Y.; Li, J.D.; Sun, Y.L.; Zhang, L.; Zhang, Q.F.; Popov, V.L.; Li, C.; et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 2011, 364, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, C.; Cheng, C.; Zhang, G.; Yu, T.; Lawrence, K.; Li, H.; Sun, J.; Yang, Z.; Ye, L.; et al. Rapid Spread of Severe Fever with Thrombocytopenia Syndrome Virus by Parthenogenetic Asian Longhorned Ticks. Emerg. Infect. Dis. 2022, 28, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Oshima, H.; Okumura, H.; Maeda, K.; Ishijima, K.; Yoshikawa, T.; Kurosu, T.; Fukushi, S.; Shimojima, M.; Saijo, M. A Patient with Severe Fever with Thrombocytopenia Syndrome (SFTS) Infected from a Sick Dog with SFTS Virus Infection. Jpn. J. Infect. Dis. 2022, 75, 423–426. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.; Hu, W.; Wu, J.; Wang, Y.; Mei, L.; Walker, D.H.; Ren, J.; Wang, Y.; Yu, X.-J. Person-to-Person Transmission of Severe Fever with Thrombocytopenia Syndrome Virus. Vector-Borne Zoonotic Dis. 2012, 12, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Fang, Y.; Cao, F.; Zhang, G.; Cheng, S.; Yu, Y.; Huang, R.; Ni, Z.; Li, J. A person-to-person transmission cluster of severe fever with thrombocytopenia syndrome characterized by mixed viral infections with familial and nosocomial clustering. Heliyon 2024, 10, e24502. [Google Scholar] [CrossRef]
- Munster, V.J.; Baas, C.; Lexmond, P.; Waldenström, J.; Wallensten, A.; Fransson, T.; Rimmelzwaan, G.F.; Beyer, W.E.; Schutten, M.; Olsen, B.; et al. Spatial, temporal, and species variation in prevalence of influenza A viruses in wild migratory birds. PLoS Pathog. 2007, 3, e61. [Google Scholar] [CrossRef]
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef]
- Millsapps, E.M.; Underwood, E.C.; Barr, K.L. Development and Application of Treatment for Chikungunya Fever. Res. Rep. Trop. Med. 2022, 13, 55–66. [Google Scholar] [CrossRef]
- U. S. Food & Drug Administration. FDA Approves First Vaccine to Prevent Disease Caused by Chikungunya Virus. 2023. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-vaccine-prevent-disease-caused-chikungunya-virus (accessed on 27 December 2024).
- Taki, E.; Ghanavati, R.; Navidifar, T.; Dashtbin, S.; Heidary, M.; Moghadamnia, M. Ebanga™: The most recent FDA-approved drug for treating Ebola. Front. Pharmacol. 2023, 14, 1083429. [Google Scholar] [CrossRef]
- Saxena, D.; Kaul, G.; Dasgupta, A.; Chopra, S. Atoltivimab/maftivimab/odesivimab (Inmazeb) combination to treat infection caused by Zaire ebolavirus. Drugs Today 2021, 57, 483–490. [Google Scholar] [CrossRef]
- U. S. Food & Drug Administration. First FDA-Approved Vaccine for the Prevention of Ebola Virus Disease, Marking a Critical Milestone in Public Health Preparedness and Response. 2019. Available online: https://www.fda.gov/news-events/press-announcements/first-fda-approved-vaccine-prevention-ebola-virus-disease-marking-critical-milestone-public-health (accessed on 10 December 2024).
- U. S. Food & Drug Administration. YF-Vax. 2019. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/yf-vax (accessed on 10 December 2024).
- U. S. Centers for Disease Control and Prevention. Tecovirimat (TPOXX) for Treatment of Mpox. 2025. Available online: https://www.cdc.gov/mpox/hcp/clinical-care/tecovirimat.html (accessed on 20 February 2025).
- Garcia-Atutxa, I.; Mondragon-Teran, P.; Huerta-Saquero, A.; Villanueva-Flores, F. Advancements in monkeypox vaccines development: A critical review of emerging technologies. Front. Immunol. 2024, 15, 1456060. [Google Scholar] [CrossRef]
- Chong, H.T.; Kamarulzaman, A.; Tan, C.T.; Goh, K.J.; Thayaparan, T.; Kunjapan, S.R.; Chew, N.K.; Chua, K.B.; Lam, S.K. Treatment of acute Nipah encephalitis with ribavirin. Ann. Neurol. 2001, 49, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Playford, E.G.; Munro, T.; Mahler, S.M.; Elliott, S.; Gerometta, M.; Hoger, K.L.; Jones, M.L.; Griffin, P.; Lynch, K.D.; Carroll, H.; et al. Safety, tolerability, pharmacokinetics, and immunogenicity of a human monoclonal antibody targeting the G glycoprotein of henipaviruses in healthy adults: A first-in-human, randomised, controlled, phase 1 study. Lancet Infect. Dis. 2020, 20, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, L.; Cross, R.W.; Woolsey, C.; West, B.R.; Borisevich, V.; Agans, K.N.; Prasad, A.N.; Deer, D.J.; Stuart, L.; McCavitt-Malvido, M.; et al. Therapeutic administration of a cross-reactive mAb targeting the fusion glycoprotein of Nipah virus protects nonhuman primates. Sci. Transl. Med. 2024, 16, eadl2055. [Google Scholar] [CrossRef] [PubMed]
- Sulis, G.; Peebles, A.; Basta, N.E. Lassa fever vaccine candidates: A scoping review of vaccine clinical trials. Trop. Med. Int. Health 2023, 28, 420–431. [Google Scholar] [CrossRef]
- Alkan, C.; Jurado-Cobena, E.; Ikegami, T. Advancements in Rift Valley fever vaccines: A historical overview and prospects for next generation candidates. Npj Vaccines 2023, 8, 171. [Google Scholar] [CrossRef]
- Hills, S.L.; Poehling, K.A.; Chen, W.H.; Staples, J.E. Tick-Borne Encephalitis Vaccine: Recommendations of the Advisory Committee on Immunization Practices, United States, 2023. MMWR Recomm. Rep. 2023, 72, 1–29. [Google Scholar] [CrossRef]
- World Health Organization. Japanese Encephalitis Vaccines: WHO position paper. Wkly. Epidemiol. Rec. 2015, 90, 69–88. [Google Scholar]
- Biedenbender, R.; Bevilacqua, J.; Gregg, A.M.; Watson, M.; Dayan, G. Phase II, randomized, double-blind, placebo-controlled, multicenter study to investigate the immunogenicity and safety of a West Nile virus vaccine in healthy adults. J. Infect. Dis. 2011, 203, 75–84. [Google Scholar] [CrossRef]
- Woods, C.W.; Sanchez, A.M.; Swamy, G.K.; McClain, M.T.; Harrington, L.; Freeman, D.; Poore, E.A.; Slifka, D.K.; Poer DeRaad, D.E.; Amanna, I.J.; et al. An observer blinded, randomized, placebo-controlled, phase I dose escalation trial to evaluate the safety and immunogenicity of an inactivated West Nile virus Vaccine, HydroVax-001, in healthy adults. Vaccine 2019, 37, 4222–4230. [Google Scholar] [CrossRef]
- Durbin, A.P.; Wright, P.F.; Cox, A.; Kagucia, W.; Elwood, D.; Henderson, S.; Wanionek, K.; Speicher, J.; Whitehead, S.S.; Pletnev, A.G. The live attenuated chimeric vaccine rWN/DEN4Δ30 is well-tolerated and immunogenic in healthy flavivirus-naïve adult volunteers. Vaccine 2013, 31, 5772–5777. [Google Scholar] [CrossRef]
- Liu, R.; Ma, H.; Shu, J.; Zhang, Q.; Han, M.; Liu, Z.; Jin, X.; Zhang, F.; Wu, X. Vaccines and Therapeutics Against Hantaviruses. Front. Microbiol. 2019, 10, 2989. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Lu, Q.B.; Yao, W.S.; Zhao, J.; Zhang, X.A.; Cui, N.; Yuan, C.; Yang, T.; Peng, X.F.; Lv, S.M.; et al. Clinical efficacy and safety evaluation of favipiravir in treating patients with severe fever with thrombocytopenia syndrome. EBioMedicine 2021, 72, 103591. [Google Scholar] [CrossRef] [PubMed]
- U. S. Food & Drug Administration. Influenza (Flu) Antiviral Drugs and Related Information. 2024. Available online: https://www.fda.gov/drugs/information-drug-class/influenza-flu-antiviral-drugs-and-related-information (accessed on 21 February 2025).
- U. S. Food & Drug Administration. Vaccines Licensed for Use in the United States. 2024. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states?utm_source=chatgpt.com (accessed on 21 February 2025).
- U. S. Centers for Disease Control and Prevention Influenza (Flu) Vaccine Safety. 2024. Available online: https://www.cdc.gov/vaccine-safety/vaccines/flu.html?utm_source=chatgpt.com (accessed on 21 February 2025).
- Bettis, A.A.; L’Azou Jackson, M.; Yoon, I.K.; Breugelmans, J.G.; Goios, A.; Gubler, D.J.; Powers, A.M. The global epidemiology of chikungunya from 1999 to 2020: A systematic literature review to inform the development and introduction of vaccines. PLoS Negl. Trop. Dis. 2022, 16, e0010069. [Google Scholar] [CrossRef]
- Rama, K.; de Roo, A.M.; Louwsma, T.; Hofstra, H.S.; Gurgel do Amaral, G.S.; Vondeling, G.T.; Postma, M.J.; Freriks, R.D. Clinical outcomes of chikungunya: A systematic literature review and meta-analysis. PLoS Negl. Trop. Dis. 2024, 18, e0012254. [Google Scholar] [CrossRef] [PubMed]
- de Lima Cavalcanti, T.Y.V.; Pereira, M.R.; de Paula, S.O.; Franca, R.F.O. A Review on Chikungunya Virus Epidemiology, Pathogenesis and Current Vaccine Development. Viruses 2022, 14, 969. [Google Scholar] [CrossRef]
- Barker, D.; Han, X.; Wang, E.; Dagley, A.; Anderson, D.M.; Jha, A.; Weaver, S.C.; Julander, J.; Nykiforuk, C.; Kodihalli, S. Equine Polyclonal Antibodies Prevent Acute Chikungunya Virus Infection in Mice. Viruses 2023, 15, 1479. [Google Scholar] [CrossRef]
- Guo, M.; Du, S.; Lai, L.; Wu, W.; Huang, X.; Li, A.; Li, H.; Li, C.; Wang, Q.; Sun, L.; et al. Development and evaluation of recombinant E2 protein based IgM capture enzyme-linked immunosorbent assay (ELISA) and double antigen sandwich ELISA for detection of antibodies to Chikungunya virus. PLoS Negl. Trop. Dis. 2022, 16, e0010829. [Google Scholar] [CrossRef]
- Schneider, M.; Narciso-Abraham, M.; Hadl, S.; McMahon, R.; Toepfer, S.; Fuchs, U.; Hochreiter, R.; Bitzer, A.; Kosulin, K.; Larcher-Senn, J.; et al. Safety and immunogenicity of a single-shot live-attenuated chikungunya vaccine: A double-blind, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2023, 401, 2138–2147. [Google Scholar] [CrossRef]
- Valneva. Valneva Reports High Sustained Immune Response in Adolescents One Year After Single Vaccination with Its Chikungunya Vaccine. 2025. Available online: https://valneva.com/press-release/valneva-reports-high-sustained-immune-response-in-adolescents-one-year-after-single-vaccination-with-its-chikungunya-vaccine/ (accessed on 21 February 2025).
- Valneva. Valneva Reports Positive Phase 2 Results in Children for its Chikungunya Vaccine and Announces Phase 3 Dose Decision. 2025. Available online: https://valneva.com/press-release/valneva-reports-positive-phase-2-results-in-children-for-its-chikungunya-vaccine-and-announces-phase-3-dose-decision/ (accessed on 21 February 2025).
- Roques, P.; Fritzer, A.; Dereuddre-Bosquet, N.; Wressnigg, N.; Hochreiter, R.; Bossevot, L.; Pascal, Q.; Guehenneux, F.; Bitzer, A.; Corbic Ramljak, I.; et al. Effectiveness of CHIKV vaccine VLA1553 demonstrated by passive transfer of human sera. JCI Insight 2022, 7, e160173. [Google Scholar] [CrossRef]
- Schmidt, C.; Schnierle, B.S. Chikungunya Vaccine Candidates: Current Landscape and Future Prospects. Drug Des. Dev. Ther. 2022, 16, 3663–3673. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.L.; Coates, E.E.; Plummer, S.H.; Carter, C.A.; Berkowitz, N.; Conan-Cibotti, M.; Cox, J.H.; Beck, A.; O’Callahan, M.; Andrews, C.; et al. Effect of a Chikungunya Virus-Like Particle Vaccine on Safety and Tolerability Outcomes: A Randomized Clinical Trial. JAMA 2020, 323, 1369–1377. [Google Scholar] [CrossRef]
- Shaw, C.A.; August, A.; Bart, S.; Booth, P.J.; Knightly, C.; Brasel, T.; Weaver, S.C.; Zhou, H.; Panther, L. A phase 1, randomized, placebo-controlled, dose-ranging study to evaluate the safety and immunogenicity of an mRNA-based chikungunya virus vaccine in healthy adults. Vaccine 2023, 41, 3898–3906. [Google Scholar] [CrossRef] [PubMed]
- Varikkodan, M.M.; Kunnathodi, F.; Azmi, S.; Wu, T.Y. An Overview of Indian Biomedical Research on the Chikungunya Virus with Particular Reference to Its Vaccine, an Unmet Medical Need. Vaccines 2023, 11, 1102. [Google Scholar] [CrossRef] [PubMed]
- Akoi Bore, J.; Timothy, J.W.S.; Tipton, T.; Kekoura, I.; Hall, Y.; Hood, G.; Longet, S.; Fornace, K.; Lucien, M.S.; Fehling, S.K.; et al. Serological evidence of zoonotic filovirus exposure among bushmeat hunters in Guinea. Nat. Commun. 2024, 15, 4171. [Google Scholar] [CrossRef]
- Makenov, M.T.; Boumbaly, S.; Tolno, F.R.; Sacko, N.; N’Fatoma, L.T.; Mansare, O.; Kolie, B.; Stukolova, O.A.; Morozkin, E.S.; Kholodilov, I.S.; et al. Marburg virus in Egyptian Rousettus bats in Guinea: Investigation of Marburg virus outbreak origin in 2021. PLoS Negl. Trop. Dis. 2023, 17, e0011279. [Google Scholar] [CrossRef]
- Jayaprakash, A.D.; Ronk, A.J.; Prasad, A.N.; Covington, M.F.; Stein, K.R.; Schwarz, T.M.; Hekmaty, S.; Fenton, K.A.; Geisbert, T.W.; Basler, C.F.; et al. Marburg and Ebola Virus Infections Elicit a Complex, Muted Inflammatory State in Bats. Viruses 2023, 15, 350. [Google Scholar] [CrossRef]
- Simon, J.K.; Kennedy, S.B.; Mahon, B.E.; Dubey, S.A.; Grant-Klein, R.J.; Liu, K.; Hartzel, J.; Coller, B.G.; Welebob, C.; Hanson, M.E.; et al. Immunogenicity of rVSVDeltaG-ZEBOV-GP Ebola vaccine (ERVEBO(R)) in African clinical trial participants by age, sex, and baseline GP-ELISA titer: A post hoc analysis of three Phase 2/3 trials. Vaccine 2022, 40, 6599–6606. [Google Scholar] [CrossRef]
- Lee, A.W.; Liu, K.; Lhomme, E.; Blie, J.; McCullough, J.; Onorato, M.T.; Connor, L.; Simon, J.K.; Dubey, S.; VanRheenen, S.; et al. Immunogenicity and Vaccine Shedding After 1 or 2 Doses of rVSVDeltaG-ZEBOV-GP Ebola Vaccine (ERVEBO(R)): Results From a Phase 2, Randomized, Placebo-controlled Trial in Children and Adults. Clin. Infect. Dis. 2024, 78, 870–879. [Google Scholar] [CrossRef]
- Woolsey, C.; Cross, R.W.; Agans, K.N.; Borisevich, V.; Deer, D.J.; Geisbert, J.B.; Gerardi, C.; Latham, T.E.; Fenton, K.A.; Egan, M.A.; et al. A highly attenuated Vesiculovax vaccine rapidly protects nonhuman primates against lethal Marburg virus challenge. PLoS Negl. Trop. Dis. 2022, 16, e0010433. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, G.; Cao, W.; He, S.; Leung, A.; Stroher, U.; Fairchild, M.J.; Nichols, R.; Crowell, J.; Fusco, J.; et al. A Cloned Recombinant Vesicular Stomatitis Virus-Vectored Marburg Vaccine, PHV01, Protects Guinea Pigs from Lethal Marburg Virus Disease. Vaccines 2022, 10, 1004. [Google Scholar] [CrossRef] [PubMed]
- Flaxman, A.; Sebastian, S.; Appelberg, S.; Cha, K.M.; Ulaszewska, M.; Purushotham, J.; Gilbride, C.; Sharpe, H.; Spencer, A.J.; Bibi, S.; et al. Potent immunogenicity and protective efficacy of a multi-pathogen vaccination targeting Ebola, Sudan, Marburg and Lassa viruse. PLoS Pathog. 2024, 20, e1012262. [Google Scholar] [CrossRef]
- Cross, R.W.; Longini, I.M.; Becker, S.; Bok, K.; Boucher, D.; Carroll, M.W.; Diaz, J.V.; Dowling, W.E.; Draghia-Akli, R.; Duworko, J.T.; et al. An introduction to the Marburg virus vaccine consortium, MARVAC. PLoS Pathog. 2022, 18, e1010805. [Google Scholar] [CrossRef] [PubMed]
- Parish, L.A.; Stavale, E.J.; Houchens, C.R.; Wolfe, D.N. Developing Vaccines to Improve Preparedness for Filovirus Outbreaks: The Perspective of the USA Biomedical Advanced Research and Development Authority (BARDA). Vaccines 2023, 11, 1120. [Google Scholar] [CrossRef]
- Remaining Patients Discharged in Uganda’s Ebola Sudan Outbreak. 2025. Available online: https://www.cidrap.umn.edu/ebola/remaining-patients-discharged-ugandas-ebola-sudan-outbreak (accessed on 21 February 2025).
- Tests Under Way in Suspected Ebola Outbreak in DR Congo. 2025. Available online: https://www.cidrap.umn.edu/ebola/tests-under-way-suspected-ebola-outbreak-dr-congo (accessed on 21 February 2025).
- Rwanda Declares End of Marburg Virus Disease Outbreak. 2025. Available online: https://www.moh.gov.rw/news-detail/article-9 (accessed on 21 February 2025).
- World Health Organization. Marburg Virus Disease–United Republic of Tanzania. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2025-DON554 (accessed on 21 February 2025).
- 100% Marburg Case Fatality Rate in Tanzania—Vax-Before-Travel. 2025. Available online: https://www.vax-before-travel.com/100-marburg-case-fatality-rate-tanzania-2025-02-14 (accessed on 21 February 2025).
- Gruber, M.F.; Rubin, S.; Krause, P.R. Approaches to demonstrating the effectiveness of filovirus vaccines: Lessons from Ebola and COVID-19. Front. Immunol. 2023, 14, 1109486. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Kishore, S.; Nag, S.; Dhasmana, A.; Preetam, S.; Mitra, O.; Leon-Figueroa, D.A.; Mohanty, A.; Chattu, V.K.; Assefi, M.; et al. Ebola Virus Disease Vaccines: Development, Current Perspectives & Challenges. Vaccines 2023, 11, 268. [Google Scholar] [CrossRef]
- Silva, N.I.O.; Albery, G.F.; Arruda, M.S.; Oliveira, G.F.G.; Costa, T.A.; de Mello, É.M.; Moreira, G.D.; Reis, E.V.; Silva, S.A.D.; Silva, M.C.; et al. Ecological drivers of sustained enzootic yellow fever virus transmission in Brazil, 2017–2021. PLoS Negl. Trop. Dis. 2023, 17, e0011407. [Google Scholar] [CrossRef]
- de Oliveira, C.H.; Andrade, M.S.; Campos, F.S.; da C. Cardos, J.; Goncalves-Dos-Santos, M.E.; Oliveira, R.S.; Aquino-Teixeira, S.M.; Campos, A.A.; Almeida, M.A.; Simonini-Teixeira, D.; et al. Yellow Fever Virus Maintained by Sabethes Mosquitoes during the Dry Season in Cerrado, a Semiarid Region of Brazil, in 2021. Viruses 2023, 15, 757. [Google Scholar]
- Ribeiro, I.P.; Delatorre, E.; de Abreu, F.V.S.; Dos Santos, A.A.C.; Furtado, N.D.; Ferreira-de-Brito, A.; de Pina-Costa, A.; Neves, M.; de Castro, M.G.; Motta, M.A.; et al. Ecological, Genetic, and Phylogenetic Aspects of YFV 2017–2019 Spread in Rio de Janeiro State. Viruses 2023, 15, 437. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Sousa, J.R.; Bezerra Junior, P.S.; Cerqueira, V.D.; Oliveira Junior, C.A.; Rivero, G.R.C.; Castro, P.H.G.; Silva, G.A.; Muniz, J.; da Silva, E.V.P.; et al. Experimental Yellow Fever in Squirrel Monkey: Characterization of Liver In Situ Immune Response. Viruses 2023, 15, 551. [Google Scholar] [CrossRef]
- Furtado, N.D.; de Mello, I.S.; de Godoy, A.S.; Noske, G.D.; Oliva, G.; Canard, B.; Decroly, E.; Bonaldo, M.C. Amino Acid Polymorphisms on the Brazilian Strain of Yellow Fever Virus Methyltransferase Are Related to the Host’s Immune Evasion Mediated by Type I Interferon. Viruses 2023, 15, 191. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Good, S.S.; Julander, J.G.; Weight, A.E.; Moussa, A.; Sommadossi, J.P. AT-752, a double prodrug of a guanosine nucleotide analog, inhibits yellow fever virus in a hamster model. PLoS Negl. Trop. Dis. 2022, 16, e0009937. [Google Scholar] [CrossRef]
- de Freitas, C.S.; Higa, L.M.; Sacramento, C.Q.; Ferreira, A.C.; Reis, P.A.; Delvecchio, R.; Monteiro, F.L.; Barbosa-Lima, G.; James Westgarth, H.; Vieira, Y.R.; et al. Yellow fever virus is susceptible to sofosbuvir both in vitro and in vivo. PLoS Negl. Trop. Dis. 2019, 13, e0007072. [Google Scholar] [CrossRef]
- Doyle, M.P.; Genualdi, J.R.; Bailey, A.L.; Kose, N.; Gainza, C.; Rodriguez, J.; Reeder, K.M.; Nelson, C.A.; Jethva, P.N.; Sutton, R.E.; et al. solation of a potently neutralizing and protective human monoclonal antibody targeting Yellow fever virus. mBio 2022, 13, e0051222. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.A.; Barrett, A.D.T. The Present and Future of Yellow Fever Vaccines. Pharmaceuticals 2021, 14, 891. [Google Scholar] [CrossRef]
- Juan-Giner, A.; Hombach, J. The life-long protective immunity of yellow fever vaccination: Time to review? Lancet Glob. Health 2024, 12, e352–e353. [Google Scholar] [CrossRef] [PubMed]
- Montalvo Zurbia-Flores, G.; Rollier, C.S.; Reyes-Sandoval, A. Re-thinking yellow fever vaccines: Fighting old foes with new generation vaccines. Hum. Vaccin. Immunother. 2022, 18, 1895644. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Anil, K.; Potey, A.V.; Sindhu, Y.; Grappi, S.; Lapini, G.; Manney, S.; Tyagi, P.; Montomoli, E.; Poonawalla, C.S.; et al. A phase I clinical study to assess safety and immunogenicity of yellow fever vaccine. NPJ Vaccines 2022, 7, 170. [Google Scholar] [CrossRef]
- Sandberg, J.T.; Lofling, M.; Varnaite, R.; Emgard, J.; Al-Tawil, N.; Lindquist, L.; Gredmark-Russ, S.; Klingstrom, J.; Lore, K.; Blom, K.; et al. Safety and immunogenicity following co-administration of Yellow fever vaccine with Tick-borne encephalitis or Japanese encephalitis vaccines: Results from an open label, non-randomized clinical trial. PLoS Negl. Trop. Dis. 2023, 17, e0010616. [Google Scholar] [CrossRef]
- Yu, C.; Zuo, L.; Miao, J.; Mao, L.; Selekon, B.; Gonofio, E.; Nakoune, E.; Berthet, N.; Wong, G. Development of a Novel Loop-Mediated Isothermal Amplification Method for the Rapid Detection of Monkeypox Virus Infections. Viruses 2022, 15, 84. [Google Scholar] [CrossRef]
- Gong, L.; Chen, X.; Wang, Y.; Liang, J.; Liu, X.; Wang, Y. Rapid, sensitive, and highly specific detection of monkeypox virus by CRISPR-based diagnostic platform. Front. Public Health 2023, 11, 1137968. [Google Scholar] [CrossRef]
- World Health Organization. Mpox. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/mpox (accessed on 27 November 2024).
- Memariani, M.; Memariani, H. Global Re-emergence of Monkeypox: A Synoptic Review. Ibnosina J. Med. Biomed. Sci. 2024, 16, 049–056. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Alasiri, N.A.; Aljeldah, M.; Alshukairiis, A.N.; AlMusa, Z.; Alfouzan, W.A.; Abuzaid, A.A.; Alamri, A.A.; Al-Afghani, H.M.; Al-Baghli, N.; et al. An Updated Review on Monkeypox Viral Disease: Emphasis on Genomic Diversity. Biomedicines 2023, 11, 1832. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Haider, F.; Khan, S.; Ahmad, I.; Ahmed, N.; Imran, M.; Rashid, S.; Ren, Z.-G.; Khattak, S.; Ji, X.-Y. Clinical Manifestation, Transmission, Pathogenesis, and Diagnosis of Monkeypox Virus: A Comprehensive Review. Life 2023, 13, 522. [Google Scholar] [CrossRef]
- Brüssow, H. Pandemic potential of poxviruses: From an ancient killer causing smallpox to the surge of monkeypox. Microb. Biotechnol. 2023, 16, 1723–1735. [Google Scholar] [CrossRef]
- Almehmadi, M.; Allahyani, M.; Alsaiari, A.A.; Alshammari, M.K.; Alharbi, A.S.; Hussain, K.H.; Alsubaihi, L.I.; Kamal, M.; Alotaibi, S.S.; Alotaibi, A.N.; et al. A Glance at the Development and Patent Literature of Tecovirimat: The First-in-Class Therapy for Emerging Monkeypox Outbreak. Viruses 2022, 14, 1870. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, A.; Mahmood, A.; Hayat, C.; Hakami, M.A.; Alotaibi, B.S.; Umair, M.; Abdalla, A.N.; Li, P.; He, P.; Wadood, A.; et al. Computer-assisted drug repurposing for thymidylate kinase drug target in monkeypox virus. Front. Cell Infect. Microbiol. 2023, 13, 1159389. [Google Scholar] [CrossRef]
- Srivastava, V.; Naik, B.; Godara, P.; Das, D.; Mattaparthi, V.S.K.; Prusty, D. Identification of FDA-approved drugs with triple targeting mode of action for the treatment of monkeypox: A high throughput virtual screening study. Mol. Divers. 2024, 28, 1093–1107. [Google Scholar] [CrossRef]
- Turtle, L.; Subramaniam, K. Modified vaccinia Ankara-Bavarian Nordic vaccine against mpox in children. Lancet Infect. Dis. 2023, 23, 989–990. [Google Scholar] [CrossRef]
- Larkin, H.D. FDA Authorizes Intradermal Vaccine, Streamlines Rules to Increase Monkeypox Treatment Access. JAMA 2022, 328, 819. [Google Scholar] [CrossRef]
- Zhang, F.; Chai, Z.; Wang, X.; Zhang, Z.; Yang, Z.; Liu, W.; Ren, H.; Jin, Y.; Yue, J. Monkeypox: Can we count on the current smallpox immunization? Virology 2024, 592, 109994. [Google Scholar] [CrossRef] [PubMed]
- Zuiani, A.; Dulberger, C.L.; De Silva, N.S.; Marquette, M.; Lu, Y.-J.; Palowitch, G.M.; Dokic, A.; Sanchez-Velazquez, R.; Schlatterer, K.; Sarkar, S.; et al. A multivalent mRNA monkeypox virus vaccine (BNT166) protects mice and macaques from orthopoxvirus disease. Cell 2024, 187, 1363–1373.e12. [Google Scholar] [CrossRef] [PubMed]
- Harapan, H.; Ophinni, Y.; Megawati, D.; Frediansyah, A.; Mamada, S.S.; Salampe, M.; Bin Emran, T.; Winardi, W.; Fathima, R.; Sirinam, S.; et al. Monkeypox: A Comprehensive Review. Viruses 2022, 14, 2155. [Google Scholar] [CrossRef]
- Jiang, R.M.; Zheng, Y.J.; Zhou, L.; Feng, L.Z.; Ma, L.; Xu, B.P.; Xu, H.M.; Liu, W.; Xie, Z.D.; Deng, J.K.; et al. Diagnosis, treatment, and prevention of monkeypox in children: An experts’ consensus statement. World J. Pediatr. 2023, 19, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.; Maisner, A. Nipah Virus Impairs Autocrine IFN Signaling by Sequestering STAT1 and STAT2 into Inclusion Bodies. Viruses 2023, 15, 554. [Google Scholar] [CrossRef]
- Pollak, N.M.; Olsson, M.; Marsh, G.A.; Macdonald, J.; McMillan, D. Evaluation of three rapid low-resource molecular tests for Nipah virus. Front. Microbiol. 2022, 13, 1101914. [Google Scholar] [CrossRef]
- Kirichenko, A.; Bryushkova, E.; Dedkov, V.; Dolgova, A. A Novel DNAzyme-Based Fluorescent Biosensor for Detection of RNA-Containing Nipah Henipavirus. Biosensors 2023, 13, 252. [Google Scholar] [CrossRef]
- Fan, P.; Sun, M.; Zhang, X.; Zhang, H.; Liu, Y.; Yao, Y.; Li, M.; Fang, T.; Sun, B.; Chen, Z.; et al. A potent Henipavirus cross-neutralizing antibody reveals a dynamic fusion-triggering pattern of the G-tetramer. Nat. Commun. 2024, 15, 4330. [Google Scholar] [CrossRef]
- Chen, L.; Sun, M.; Zhang, H.; Zhang, X.; Yao, Y.; Li, M.; Li, K.; Fan, P.; Zhang, H.; Qin, Y.; et al. Potent human neutralizing antibodies against Nipah virus derived from two ancestral antibody heavy chains. Nat. Commun. 2024, 15, 2987. [Google Scholar] [CrossRef]
- Woolsey, C.; Borisevich, V.; Fears, A.C.; Agans, K.N.; Deer, D.J.; Prasad, A.N.; O’Toole, R.; Foster, S.L.; Dobias, N.S.; Geisbert, J.B.; et al. Recombinant vesicular stomatitis virus-vectored vaccine induces long-lasting immunity against Nipah virus disease. J. Clin. Investig. 2023, 133, e164946. [Google Scholar] [CrossRef]
- Monath, T.P.; Nichols, R.; Feldmann, F.; Griffin, A.; Haddock, E.; Callison, J.; Meade-White, K.; Okumura, A.; Lovaglio, J.; Hanley, P.W.; et al. Immunological correlates of protection afforded by PHV02 live, attenuated recombinant vesicular stomatitis virus vector vaccine against Nipah virus disease. Front. Immunol. 2023, 14, 1216225. [Google Scholar] [CrossRef]
- Tan, F.H.; Sukri, A.; Idris, N.; Ong, K.C.; Schee, J.P.; Tan, C.T.; Tan, S.H.; Wong, K.T.; Wong, L.P.; Tee, K.K.; et al. A systematic review on Nipah virus: Global molecular epidemiology and medical countermeasures development. Virus Evol. 2024, 10, veae048. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Mohanty, A.; Shah, A.; Kumar Padhi, B.; Sah, R. Outbreak of an emerging zoonotic Nipah virus: An emerging concern. J. Biosaf. Biosecur. 2023, 5, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.A.; Mehr, A.J.; Ostrowsky, J.T.; Ulrich, A.K.; Moua, N.M.; Fay, P.C.; Hart, P.J.; Golding, J.P.; Benassi, V.; Preziosi, M.P.; et al. Measures to prevent and treat Nipah virus disease: Research priorities for 2024–2029. Lancet Infect. Dis. 2024, 24, e707–e717. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.L.; Zhang, X.D.; Li, B.J.; Qin, T.; Cao, Z.J.; Fan, Q.J.; Yang, J.; Jin, D.; Lu, S.; Zheng, Y.Y.; et al. Comparative evaluation of standard RT-PCR assays and commercial Real-Time RT-PCR kits for detection of Lassa Virus. Microbiol. Spectr. 2023, 11, e0501122. [Google Scholar] [CrossRef]
- Deschambault, Y.; Soule, G.; Klassen, L.; Sloan, A.; Audet, J.; Azaransky, K.; Musa, A.S.; Ahmad, A.; Akinpelu, A.M.; Mba, N.; et al. An Outbred Guinea Pig Disease Model for Lassa Fever Using a Host-Adapted Clade III Nigerian Lassa Virus. Viruses 2023, 15, 769. [Google Scholar] [CrossRef]
- Scher, G.; Yankowski, C.; Kurup, D.; Josleyn, N.M.; Wilkinson, E.R.; Wells, J.; Steffens, J.; Lynn, G.; Vantongeren, S.; Zeng, X.; et al. Inactivated rabies-based Lassa fever virus vaccine candidate LASSARAB protects nonhuman primates from lethal disease. npj Vaccines 2024, 9, 143. [Google Scholar] [CrossRef]
- Mateo, M.; Reynard, S.; Pietrosemoli, N.; Perthame, E.; Journeaux, A.; Noy, K.; Germain, C.; Carnec, X.; Picard, C.; Borges-Cardoso, V.; et al. Rapid protection induced by a single-shot Lassa vaccine in male cynomolgus monkeys. Nat. Commun. 2023, 14, 1352. [Google Scholar] [CrossRef]
- Ugwu, C.; Olumade, T.; Nwakpakpa, E.; Onyia, V.; Odeh, E.; Duruiheoma, R.O.; Ojide, C.K.; Eke, M.A.; Nwafor, I.E.; Chika-Igwenyi, N.; et al. Humoral and cellular immune responses to Lassa fever virus in Lassa fever survivors and their exposed contacts in Southern Nigeria. Sci. Rep. 2022, 12, 22330. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, Y.; Jia, X.; Zhou, M.; Mao, W.; Dong, S.; Zhang, Y.; Xiao, G.; Wang, W. Screening and Identification of Lassa Virus Entry Inhibitors from a Fragment-Based Drug Discovery Library. Viruses 2022, 14, 2649. [Google Scholar] [CrossRef]
- Aloke, C.; Obasi, N.A.; Aja, P.M.; Emelike, C.U.; Egwu, C.O.; Jeje, O.; Edeogu, C.O.; Onisuru, O.O.; Orji, O.U.; Achilonu, I. Combating Lassa Fever in West African Sub-Region: Progress, Challenges, and Future Perspectives. Viruses 2023, 15, 146. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Méndez, E.; Angelino, P.; Keulen, L.; Water, S.; Rockx, B.; Pijlman, G.P.; Ciuffi, A.; Kortekaas, J.; Schreur, P.J.W. Transcriptomic profiling reveals intense host-pathogen dispute compromising homeostasis during acute Rift Valley fever virus infection. J. Virol. 2023, 97, e0041523. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yuan, Y.; Liu, Y.; Zhang, L. Arm race between Rift Valley fever virus and host. Front. Immunol. 2022, 13, 1084230. [Google Scholar] [CrossRef] [PubMed]
- Michaely, L.M.; Rissmann, M.; Armando, F.; von Arnim, F.; Keller, M.; Eiden, M.; Konig, R.; Gutjahr, B.; Baumgartner, W.; Groschup, M.H.; et al. Rift Valley Fever Virus Non-Structural Protein S Is Associated with Nuclear Translocation of Active Caspase-3 and Inclusion Body Formation. Viruses 2022, 14, 2487. [Google Scholar] [CrossRef]
- Nyakarahuka, L.; Whitmer, S.; Klena, J.; Balinandi, S.; Talundzic, E.; Tumusiime, A.; Kyondo, J.; Mulei, S.; Patel, K.; Baluku, J.; et al. Detection of Sporadic Outbreaks of Rift Valley Fever in Uganda through the National Viral Hemorrhagic Fever Surveillance System, 2017–2020. Am. J. Trop. Med. Hyg. 2023, 108, 995–1002. [Google Scholar] [CrossRef]
- Juma, J.; Konongoi, S.L.; Nsengimana, I.; Mwangi, R.; Akoko, J.; Nyamota, R.; Muli, C.; Dobi, P.O.; Kiritu, E.; Osiany, S.; et al. Using Multiplex Amplicon PCR Technology to Efficiently and Timely Generate Rift Valley Fever Virus Sequence Data for Genomic Surveillance. Viruses 2023, 15, 477. [Google Scholar] [CrossRef]
- Hao, M.; Bian, T.; Fu, G.; Chen, Y.; Fang, T.; Zhao, C.; Liu, S.; Yu, C.; Li, J.; Chen, W. An adenovirus-vectored RVF vaccine confers complete protection against lethal RVFV challenge in A129 mice. Front. Microbiol. 2023, 14, 1114226. [Google Scholar] [CrossRef]
- Bian, T.; Wang, B.; Fu, G.; Hao, M.; Chen, Y.; Fang, T.; Liu, S.; Yu, C.; Li, J.; Chen, W. Single-dose of a replication-competent adenovirus-vectored vaccine provides sterilizing protection against Rift Valley fever virus challenge. Front. Immunol. 2022, 13, 907675. [Google Scholar] [CrossRef]
- Lacote, S.; Tamietti, C.; Chabert, M.; Confort, M.P.; Conquet, L.; Pulido, C.; Aurine, N.; Baquerre, C.; Thiesson, A.; Pain, B.; et al. Intranasal Exposure to Rift Valley Fever Virus Live-Attenuated Strains Leads to High Mortality Rate in Immunocompetent Mice. Viruses 2022, 14, 2470. [Google Scholar] [CrossRef]
- Saegerman, C.; Humblet, M.F.; Leandri, M.; Gonzalez, G.; Heyman, P.; Sprong, H.; L’Hostis, M.; Moutailler, S.; Bonnet, S.I.; Haddad, N.; et al. First Expert Elicitation of Knowledge on Possible Drivers of Observed Increasing Human Cases of Tick-Borne Encephalitis in Europe. Viruses 2023, 15, 791. [Google Scholar] [CrossRef]
- Beicht, J.; Kubinski, M.; Zdora, I.; Puff, C.; Biermann, J.; Gerlach, T.; Baumgartner, W.; Sutter, G.; Osterhaus, A.; Prajeeth, C.K.; et al. Induction of humoral and cell-mediated immunity to the NS1 protein of TBEV with recombinant Influenza virus and MVA affords partial protection against lethal TBEV infection in mice. Front. Immunol. 2023, 14, 1177324. [Google Scholar] [CrossRef] [PubMed]
- Bestehorn-Willmann, M.; Girl, P.; Greiner, F.; Mackenstedt, U.; Dobler, G.; Lang, D. Increased Vaccination Diversity Leads to Higher and Less-Variable Neutralization of TBE Viruses of the European Subtype. Vaccines 2023, 11, 1044. [Google Scholar] [CrossRef] [PubMed]
- Kubinski, M.; Beicht, J.; Zdora, I.; Biermann, J.; Puff, C.; Gerlach, T.; Tscherne, A.; Baumgartner, W.; Osterhaus, A.; Sutter, G.; et al. A recombinant Modified Vaccinia virus Ankara expressing prME of tick-borne encephalitis virus affords mice full protection against TBEV infection. Front. Immunol. 2023, 14, 1182963. [Google Scholar] [CrossRef]
- Matveev, A.; Khlusevich, Y.; Kozlova, I.; Matveev, L.; Emelyanova, L.; Tikunov, A.; Baykov, I.; Tikunova, N. New Neutralizing Epitope Exposed on the Domain II of Tick-Borne Encephalitis Virus Envelope Glycoprotein E. Viruses 2023, 15, 1256. [Google Scholar] [CrossRef] [PubMed]
- Stone, E.T.; Pinto, A.K. T Cells in Tick-Borne Flavivirus Encephalitis: A Review of Current Paradigms in Protection and Disease Pathology. Viruses 2023, 15, 958. [Google Scholar] [CrossRef]
- Hoornweg, T.E.; Godeke, G.J.; Hoogerwerf, M.N.; van Kasteren, P.B.; de Vries, A.; Sprong, H.; Verjans, G.; van Riel, D.; Reimerink, J.H.J.; Rockx, B.; et al. Rescue and in vitro characterization of a divergent TBEV-Eu strain from the Netherlands. Sci. Rep. 2023, 13, 2872. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Williams, D.T.; van den Hurk, A.F.; Smith, D.W.; Currie, B.J. Japanese Encephalitis Virus: The Emergence of Genotype IV in Australia and Its Potential Endemicity. Viruses 2022, 14, 2480. [Google Scholar] [CrossRef]
- Martin, G.E.; Tran, T.; Papadakis, G.; Kinsella, P.; Druce, J.; Caly, L.; Williamson, D.A.; Lim, C.K. A real-time PCR assay for Japanese encephalitis virus (JEV) genotype IV as a public health laboratory response to an emerging outbreak in Australia. Pathology 2023, 55, 869–870. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.; Wang, Z.; Wang, G.; Fu, S.; Li, F.; Yang, L.; Yuan, Y.; Shen, K.; Wang, H.; et al. Peripheral nerve injury induced by Japanese Encephalitis virus in C57BL/6 mouse. J. Virol. 2023, 97, e0165822. [Google Scholar] [CrossRef]
- Yadav, P.; Chakraborty, P.; Jha, N.K.; Dewanjee, S.; Jha, A.K.; Panda, S.P.; Mishra, P.C.; Dey, A.; Jha, S.K. Molecular Mechanism and Role of Japanese Encephalitis Virus Infection in Central Nervous System-Mediated Diseases. Viruses 2022, 14, 2686. [Google Scholar] [CrossRef]
- Zhao, G.; Gao, Y.; Zhang, J.; Zhang, H.; Xie, C.; Nan, F.; Feng, S.; Ha, Z.; Li, C.; Zhu, X.; et al. Toll-like receptor 2 signaling pathway activation contributes to a highly efficient inflammatory response in Japanese encephalitis virus-infected mouse microglial cells by proteomics. Front. Microbiol. 2022, 13, 989183. [Google Scholar] [CrossRef] [PubMed]
- Shukla, M.; Garg, A.; Dhole, T.N.; Chaturvedi, R. Exaggerated levels of some specific TLRs, cytokines and chemokines in Japanese encephalitis infected BV2 and neuro 2A cell lines associated with worst outcome. Virol. J. 2023, 20, 16. [Google Scholar] [CrossRef]
- Kakoti, G.; Dutta, P.; Ram Das, B.; Borah, J.; Mahanta, J. Clinical profile and outcome of Japanese encephalitis in children admitted with acute encephalitis syndrome. Biomed. Res. Int. 2013, 2013, 152656. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Tripathi, P.; Singh, S.; Bannerji, G. Clinical Features in Children Hospitalized during the 2005 Epidemic of Japanese Encephalitis in Uttar Pradesh, India. Clin. Infect. Dis. 2006, 43, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Taraphdar, D.; Mukhopadhyay, B.B.; Kumar, M.; Mukhopadhyay, S.K.; Chatterjee, S. Influence of socio-economic status and environmental factors on serologically diagnosed Japanese encephalitis cases in the state of West Bengal, India during 2005–2010. Health 2012, 4, 6–12. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chattopadhyay, D.; Bhattacharya, M.K.; Mukherjee, B. Serosurveillance for Japanese encephalitis in children in several districts of West Bengal, India. Acta Paediatr. 2004, 93, 390–393. [Google Scholar] [CrossRef]
- Srivastava, N.; Deval, H.; Mittal, M.; Deoshatwar, A.; Bondre, V.P.; Kant, R.; Yadav, R. Extent of disability among paediatric Japanese encephalitis survivors and predictors of poor outcome: A retrospective cohort study in North India. BMJ Open 2022, 12, e060795. [Google Scholar] [CrossRef]
- Qi, Z.; Zhao, J.; Li, Y.; Zhang, B.; Hu, S.; Chen, Y.; Ma, J.; Shu, Y.; Wang, Y.; Cheng, P. Live-attenuated Japanese encephalitis virus inhibits glioblastoma growth and elicits potent antitumor immunity. Front. Immunol. 2023, 14, 982180. [Google Scholar] [CrossRef]
- Ndione, M.H.D.; Ndiaye, E.H.; Faye, M.; Diagne, M.M.; Diallo, D.; Diallo, A.; Sall, A.A.; Loucoubar, C.; Faye, O.; Diallo, M.; et al. Re-Introduction of West Nile Virus Lineage 1 in Senegal from Europe and Subsequent Circulation in Human and Mosquito Populations between 2012 and 2021. Viruses 2022, 14, 2720. [Google Scholar] [CrossRef]
- Ruiz-López, M.J.; Aguilera-Sepúlveda, P.; Cebrián-Camisón, S.; Figuerola, J.; Magallanes, S.; Varona, S.; Cuesta, I.; Cano-Gómez, C.; Sánchez-Mora, P.; Camacho, J.; et al. Re-Emergence of a West Nile Virus (WNV) Variant in South Spain with Rapid Spread Capacity. Viruses 2023, 15, 2372. [Google Scholar] [CrossRef]
- Reemtsma, H.; Holicki, C.M.; Fast, C.; Bergmann, F.; Eiden, M.; Groschup, M.H.; Ziegler, U. Pathogenesis of West Nile Virus Lineage 2 in Domestic Geese after Experimental Infection. Viruses 2022, 14, 1319. [Google Scholar] [CrossRef] [PubMed]
- Benzarti, E.; Murray, K.O.; Ronca, S.E. Interleukins, Chemokines, and Tumor Necrosis Factor Superfamily Ligands in the Pathogenesis of West Nile Virus Infection. Viruses 2023, 15, 806. [Google Scholar] [CrossRef] [PubMed]
- Hruskovicova, J.; Bhide, K.; Petrouskova, P.; Tkacova, Z.; Mochnacova, E.; Curlik, J.; Bhide, M.; Kulkarni, A. Engineering the Single Domain Antibodies Targeting Receptor Binding Motifs Within the Domain III of West Nile Virus Envelope Glycoprotein. Front. Microbiol. 2022, 13, 801466. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Khatib, M.N.; Ballal, S.; Kaur, M.; Nathiya, D.; Sharma, S.; Prasad, G.V.S.; Sinha, A.; Gaidhane, A.M.; Mohapatra, P.; et al. West Nile Virus in a changing climate: Epidemiology, pathology, advances in diagnosis and treatment, vaccine designing and control strategies, emerging public health challenges—A comprehensive review. Emerg. Microbes Infect. 2025, 14, 2437244. [Google Scholar] [CrossRef]
- Kaiser, J.A.; Barrett, A.D.T. Twenty Years of Progress Toward West Nile Virus Vaccine Development. Viruses 2019, 11, 823. [Google Scholar] [CrossRef]
- Ulbert, S. West Nile virus vaccines—Current situation and future directions. Hum. Vaccines Immunother. 2019, 15, 2337–2342. [Google Scholar] [CrossRef]
- Weiß, R.; Issmail, L.; Rockstroh, A.; Grunwald, T.; Fertey, J.; Ulbert, S. Immunization with different recombinant West Nile virus envelope proteins induces varying levels of serological cross-reactivity and protection from infection. Front. Cell Infect. Microbiol. 2023, 13, 1279147. [Google Scholar] [CrossRef]
- Sun, H.; Acharya, D.; Paul, A.M.; Lai, H.; He, J.; Bai, F.; Chen, Q. Antibody-Dependent Enhancement Activity of a Plant-Made Vaccine against West Nile Virus. Vaccines 2023, 11, 197. [Google Scholar] [CrossRef]
- Pattnaik, A.; Sahoo, B.R.; Struble, L.R.; Borgstahl, G.E.O.; Zhou, Y.; Franco, R.; Barletta, R.G.; Osorio, F.A.; Petro, T.M.; Pattnaik, A.K. A Ferritin Nanoparticle-Based Zika Virus Vaccine Candidate Induces Robust Humoral and Cellular Immune Responses and Protects Mice from Lethal Virus Challenge. Vaccines 2023, 11, 821. [Google Scholar] [CrossRef]
- Nazneen, F.; Thompson, E.A.; Blackwell, C.; Bai, J.S.; Huang, F.; Bai, F. An effective live-attenuated Zika vaccine candidate with a modified 5’ untranslated region. NPJ Vaccines 2023, 8, 50. [Google Scholar] [CrossRef]
- Stass, R.; Engdahl, T.B.; Chapman, N.S.; Wolters, R.M.; Handal, L.S.; Diaz, S.M.; Crowe, J.E., Jr.; Bowden, T.A. Mechanistic basis for potent neutralization of Sin Nombre hantavirus by a human monoclonal antibody. Nat. Microbiol. 2023, 8, 1293–1303. [Google Scholar] [CrossRef]
- Aviziniene, A.; Kucinskaite-Kodze, I.; Petraityte-Burneikiene, R.; Zvirbliene, A.; Mertens, M.L.; Schmidt, S.; Schlegel, M.; Lattwein, E.; Koellner, B.; Ulrich, R.G. Characterization of a Panel of Cross-Reactive Hantavirus Nucleocapsid Protein-Specific Monoclonal Antibodies. Viruses 2023, 15, 532. [Google Scholar] [CrossRef]
- Hooper, J.; Paolino, K.M.; Mills, K.; Kwilas, S.; Josleyn, M.; Cohen, M.; Somerville, B.; Wisniewski, M.; Norris, S.; Hill, B.; et al. A Phase 2a Randomized, Double-Blind, Dose-Optimizing Study to Evaluate the Immunogenicity and Safety of a Bivalent DNA Vaccine for Hemorrhagic Fever with Renal Syndrome Delivered by Intramuscular Electroporation. Vaccines 2020, 8, 377. [Google Scholar] [CrossRef]
- Noor, F.; Ashfaq, U.A.; Asif, M.; Adeel, M.M.; Alshammari, A.; Alharbi, M. Comprehensive computational analysis reveals YXXPhi[I/L/M/F/V] motif and YXXPhi-like tetrapeptides across HFRS causing Hantaviruses and their association with viral pathogenesis and host immune regulation. Front. Immunol. 2022, 13, 1031608. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.; Ahmad, S.; Anwar, Z.; Hussain, Z.; Safdar, M.; Rizwan, M.; Waseem, M.; Hussain, A.; Akhlaq, M.; et al. HantavirusesDB: Vaccinomics and RNA-based therapeutics database for the potentially emerging human respiratory pandemic agents. Microb. Pathog. 2021, 160, 105161. [Google Scholar] [CrossRef]
- Mocanu, A.; Cajvan, A.M.; Lazaruc, T.I.; Lupu, V.V.; Florescu, L.; Lupu, A.; Bogos, R.A.; Ioniuc, I.; Scurtu, G.; Dragan, F.; et al. Hantavirus Infection in Children-A Pilot Study of Single Regional Center. Viruses 2023, 15, 872. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Ma, Y.; Tang, K.; Zhang, C.; Wang, M.; Zhang, X.; Xue, M.; Jia, X.; Hu, H.; et al. Increased CD4+CD8+ Double Positive T Cells during Hantaan Virus Infection. Viruses 2022, 14, 2243. [Google Scholar] [CrossRef]
- Glass, G.E. Forecasting Outbreaks of Hantaviral Disease: Future Directions in Geospatial Modeling. Viruses 2023, 15, 1461. [Google Scholar] [CrossRef]
- Whitehouse, C.A. Crimean–Congo hemorrhagic fever. Antivir. Res. 2004, 64, 145–160. [Google Scholar] [CrossRef]
- Frank, M.G.; Weaver, G.; Raabe, V. Crimean-Congo Hemorrhagic Fever Virus for Clinicians-Epidemiology, Clinical Manifestations, and Prevention. Emerg. Infect. Dis. 2024, 30, 854–863. [Google Scholar] [CrossRef]
- Atim, S.A.; Niebel, M.; Ashraf, S.; Vudriko, P.; Odongo, S.; Balinandi, S.; Aber, P.; Bameka, R.; Ademun, A.R.; Masembe, C.; et al. Prevalence of Crimean-Congo haemorrhagic fever in livestock following a confirmed human case in Lyantonde district, Uganda. Parasit Vectors 2023, 16, 7. [Google Scholar] [CrossRef]
- Febrer-Sendra, B.; Fernandez-Soto, P.; Garcia-Bernalt Diego, J.; Crego-Vicente, B.; Negredo, A.; Munor-Bellido, J.L.; Belhassen-Garcia, M.; Sanchez-Seco, M.P.; Muro, A. A Novel RT-LAMP for the Detection of Different Genotypes of Crimean-Congo Haemorrhagic Fever Virus in Patients from Spain. Int. J. Mol. Sci. 2023, 24, 6411. [Google Scholar] [CrossRef]
- Hoste, A.C.R.; Djadjovski, I.; Jiménez-Clavero, M.Á.; Rueda, P.; Barr, J.N.; Sastre, P. Multiplex assay for simultaneous detection of antibodies against Crimean-Congo Hemorrhagic fever virus nucleocapsid protein and glycoproteins in ruminants. Microbiol. Spectr. 2023, 11, e0260022. [Google Scholar] [CrossRef]
- Saunders, J.E.; Gilbride, C.; Dowall, S.; Morris, S.; Ulaszewska, M.; Spencer, A.J.; Rayner, E.; Graham, V.A.; Kennedy, E.; Thomas, K.; et al. Adenoviral vectored vaccination protects against Crimean-Congo Haemorrhagic Fever disease in a lethal challenge model. EBioMedicine 2023, 90, 104523. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, P.; Jiang, L.; Wang, S.; Zhang, S.; Li, Y. Evaluation of the immunogenicity of vaccine candidates developed using a baculovirus surface display system for Crimean-Congo hemorrhagic fever virus in mice. Front. Microbiol. 2023, 14, 1107874. [Google Scholar] [CrossRef]
- Karaaslan, E.; Sorvillo, T.E.; Scholte, F.E.M.; O’Neal, T.J.; Welch, S.R.; Davies, K.A.; Coleman-McCray, J.D.; Harmon, J.R.; Ritter, J.M.; Pegan, S.D.; et al. Crimean Congo hemorrhagic fever virus nucleoprotein and GP38 subunit vaccine combination prevents morbidity in mice. npj Vaccines 2024, 9, 148. [Google Scholar] [CrossRef]
- Hawman, D.W.; Leventhal, S.S.; Meade-White, K.; Khandhar, A.; Murray, J.; Lovaglio, J.; Shaia, C.; Saturday, G.; Hinkley, T.; Erasmus, J.; et al. A replicating RNA vaccine confers protection in a rhesus macaque model of Crimean-Congo hemorrhagic fever. npj Vaccines 2024, 9, 86. [Google Scholar] [CrossRef]
- Alam, R.; Samad, A.; Ahammad, F.; Nur, S.M.; Alsaiari, A.A.; Imon, R.R.; Talukder, M.E.K.; Nain, Z.; Rahman, M.M.; Mohammad, F.; et al. In silico formulation of a next-generation multiepitope vaccine for use as a prophylactic candidate against Crimean-Congo hemorrhagic fever. BMC Med. 2023, 21, 36. [Google Scholar] [CrossRef]
- Shah, S.Z.; Jabbar, B.; Mirza, M.U.; Waqas, M.; Aziz, S.; Halim, S.A.; Ali, A.; Rafique, S.; Idrees, M.; Khalid, A.; et al. An Immunoinformatics Approach to Design a Potent Multi-Epitope Vaccine against Asia-1 Genotype of Crimean-Congo Haemorrhagic Fever Virus Using the Structural Glycoproteins as a Target. Vaccines 2022, 11, 61. [Google Scholar] [CrossRef]
- Igan, H.; Hanci, H. The six-year prevalence of Crimean-Congo Hemorrhagic Fever (CCHF) in Erzurum, Turkey. J. Vector Borne Dis. 2024. [Google Scholar] [CrossRef]
- Keşkek Türk, Y.; Kacı, F.N. Crimean-Congo Haemorrhagic Fever Virus: From Genomic Insights to Control Strategies. J. Inst. Sci. Technol. 2024, 14, 650–667. [Google Scholar] [CrossRef]
- Quan, C.; Liu, Q.; Yu, L.; Li, C.; Nie, K.; Ding, G.; Zhou, H.; Wang, X.; Sun, W.; Wang, H.; et al. SFTSV infection is associated with transient overproliferation of monoclonal lambda-type plasma cells. iScience 2023, 26, 106799. [Google Scholar] [CrossRef]
- Dai, Y.; Pu, Q.; Hu, N.; Zhu, J.; Han, Y.; Shi, P.; Li, J.; Jin, K. The dose-response relationship between smoking and the risk factor for invasive pulmonary aspergillosis in patients with severe fever with thrombocytopenia syndrome. Front. Microbiol. 2023, 14, 1209705. [Google Scholar] [CrossRef]
- Yoshikawa, R.; Kawakami, M.; Yasuda, J. The NSs protein of severe fever with thrombocytopenia syndrome virus differentially inhibits the type 1 interferon response among animal species. J. Biol. Chem. 2023, 299, 104819. [Google Scholar] [CrossRef]
- Li, Y.H.; Huang, W.W.; He, W.Q.; He, X.Y.; Wang, X.H.; Lin, Y.L.; Zhao, Z.J.; Zheng, Y.T.; Pang, W. Longitudinal analysis of immunocyte responses and inflammatory cytokine profiles in SFTSV-infected rhesus macaques. Front. Immunol. 2023, 14, 1143796. [Google Scholar] [CrossRef]
- Li, A.; Dai, X.; Chen, L.; Liu, L.; Li, C.; Liu, Y.; Wu, W.; Huang, X.; Li, J.; Wang, S.; et al. Immunogenicity and protective efficacy of an inactivated SFTS vaccine candidate in mice. Biosaf. Health 2022, 4, 45–52. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, Y.; Jiang, J.; Zheng, A. Two Point Mutations in the Glycoprotein of SFTSV Enhance the Propagation Recombinant Vesicular Stomatitis Virus Vectors at Assembly Step. Viruses 2023, 15, 800. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jeon, K.; Hong, J.J.; Park, S.I.; Cho, H.; Park, H.J.; Kwak, H.W.; Park, H.J.; Bang, Y.J.; Lee, Y.S.; et al. Heterologous vaccination utilizing viral vector and protein platforms confers complete protection against SFTSV. Sci. Rep. 2023, 13, 8189. [Google Scholar] [CrossRef]
- Park, J.Y.; Hewawaduge, C.; Sivasankar, C.; Lloren, K.K.S.; Oh, B.; So, M.Y.; Lee, J.H. An mRNA-Based Multiple Antigenic Gene Expression System Delivered by Engineered Salmonella for Severe Fever with Thrombocytopenia Syndrome and Assessment of Its Immunogenicity and Protection Using a Human DC-SIGN-Transduced Mouse Model. Pharmaceutics 2023, 15, 1339. [Google Scholar] [CrossRef]
- Raza, M.A.; Ashraf, M.A.; Amjad, M.N.; Din, G.U.; Shen, B.; Hu, Y. The peculiar characteristics and advancement in diagnostic methodologies of influenza A virus. Front. Microbiol. 2024, 15, 1435384. [Google Scholar] [CrossRef]
- Fu, X.; Wang, Q.; Ma, B.; Zhang, B.; Sun, K.; Yu, X.; Ye, Z.; Zhang, M. Advances in Detection Techniques for the H5N1 Avian Influenza Virus. Int. J. Mol. Sci. 2023, 24, 17157. [Google Scholar] [CrossRef]
- Chan, K.K.P.; Hui, D.S.C. Antiviral therapies for influenza. Curr. Opin. Infect. Dis. 2023, 36, 124–131. [Google Scholar] [CrossRef]
- Alrizg, S.A.S.; Ali Alsalem, A.S.; Alyami, H.A.; al Shaybah, M.H.S.; Alyami, A.S.; Al Hammam, M.A.; Almeel, Z.H.J.; Alslom, M.M.A.A.; Al Yami, G.H.M.; Alhassan, A.S.O. Emerging Strategies and Therapeutic Advances in Influenza Treatment: A Comprehensive Review. J. Ecohumanism 2024, 3, 936–944. [Google Scholar] [CrossRef]
- Ghosh, S.; Chowdhury, S.R.; Rahaman, M.; Basu, B.; Prajapati, B. Revolutionizing Influenza Treatment: A Deep Dive into Targeted Drug Delivery Systems. Curr. Pharm. Biotechnol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Greco, D. Influenza. In Global Health Essentials; Raviglione, M.C.B., Tediosi, F., Villa, S., Casamitjana, N., Plasència, A., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 129–132. [Google Scholar]
- Nachbagauer, R.; Feser, J.; Naficy, A.; Bernstein, D.I.; Guptill, J.; Walter, E.B.; Berlanda-Scorza, F.; Stadlbauer, D.; Wilson, P.C.; Aydillo, T.; et al. A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nat. Med. 2021, 27, 106–114. [Google Scholar] [CrossRef]
- Harris, E. NIH Launches Phase 1 Trial of Broader “Universal” Flu Vaccine. JAMA 2023, 330, 1421. [Google Scholar] [CrossRef]
- Widge, A.; Hofstetter, A.; Houser, K.; Awan, S.; Chen, G.; Florez, M.; Berkowitz, N.; Mendoza, F.; Hendel, C.; Holman, L.; et al. An influenza hemagglutinin stem nanoparticle vaccine induces cross-group 1 neutralizing antibodies in healthy adults. Sci. Transl. Med. 2023, 15, eade4790. [Google Scholar] [CrossRef] [PubMed]
- Uno, N.; Ross, T.M. Multivalent next generation influenza virus vaccines protect against seasonal and pre-pandemic viruses. Sci. Rep. 2024, 14, 1440. [Google Scholar] [CrossRef]
- Fatima, M.; Park, P.G.; Hong, K.J. Clinical advancements in mRNA vaccines against viral infections. Clin. Immunol. 2025, 271, 110424. [Google Scholar] [CrossRef]
- Cheng, Z.; Ma, J.; Zhao, C. Advantages of Broad-Spectrum Influenza mRNA Vaccines and Their Impact on Pulmonary Influenza. Vaccines 2024, 12, 1382. [Google Scholar] [CrossRef]
- van de Ven, K.; Lanfermeijer, J.; van Dijken, H.; Muramatsu, H.; Vilas Boas de Melo, C.; Lenz, S.; Peters, F.; Beattie, M.B.; Lin, P.J.C.; Ferreira, J.A.; et al. A universal influenza mRNA vaccine candidate boosts T cell responses and reduces zoonotic influenza virus disease in ferrets. Sci. Adv. 2022, 8, eadc9937. [Google Scholar] [CrossRef] [PubMed]
- Ananworanich, J.; Lee, I.T.; Ensz, D.; Carmona, L.; Schaefers, K.; Avanesov, A.; Stadlbauer, D.; Choi, A.; Pucci, A.; McGrath, S.; et al. Safety and Immunogenicity of mRNA-1010, an Investigational Seasonal Influenza Vaccine, in Healthy Adults: Final Results From a Phase 1/2 Randomized Trial. J. Infect. Dis. 2025, 231, e113–e122. [Google Scholar] [CrossRef]
- Gupta, D.; Mohan, S. Influenza vaccine: A review on current scenario and future prospects. J. Genet. Eng. Biotechnol. 2023, 21, 154. [Google Scholar] [CrossRef]
- Viboud, C.; Gostic, K.; Nelson, M.I.; Price, G.E.; Perofsky, A.; Sun, K.; Sequeira Trovao, N.; Cowling, B.J.; Epstein, S.L.; Spiro, D.J. Beyond clinical trials: Evolutionary and epidemiological considerations for development of a universal influenza vaccine. PLoS Pathog. 2020, 16, e1008583. [Google Scholar] [CrossRef] [PubMed]
- World Health Organiztion. Zoonotic Disease: Emerging Public Health Threats in the Region. Available online: https://www.emro.who.int/about-who/rc61/zoonotic-diseases.html (accessed on 23 February 2025).
- Protecting People and Animals from Disease Threats. 2023. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/078e6cc3-60d9-412d-9a88-a4444a2dc037/content (accessed on 27 December 2024).
- Berger, K.; Garbuglia, A.R. Editorial: Diagnosis of zoonoses: Relevance of BSL3 and BSL4 facilities. Front. Cell Infect. Microbiol. 2022, 12, 1052082. [Google Scholar] [CrossRef] [PubMed]
- Al-Eitan, L.; Khair, I.; Shakhatreh, Z.; Almahdawi, D.; Alahmad, S. Epidemiology, biosafety, and biosecurity of Avian Influenza: Insights from the East Mediterranean region. Rev. Med. Virol. 2024, 34, e2559. [Google Scholar] [CrossRef]
- Al-Eitan, L.N.; Khiarah, R.A.A.; Almahdawi, D.L. A targeted vaccination strategy: Integrating vaccines into biosafety, biosecurity, and one health initiatives. J. Biosaf. Biosecurity 2025, 7, 9–27. [Google Scholar] [CrossRef]
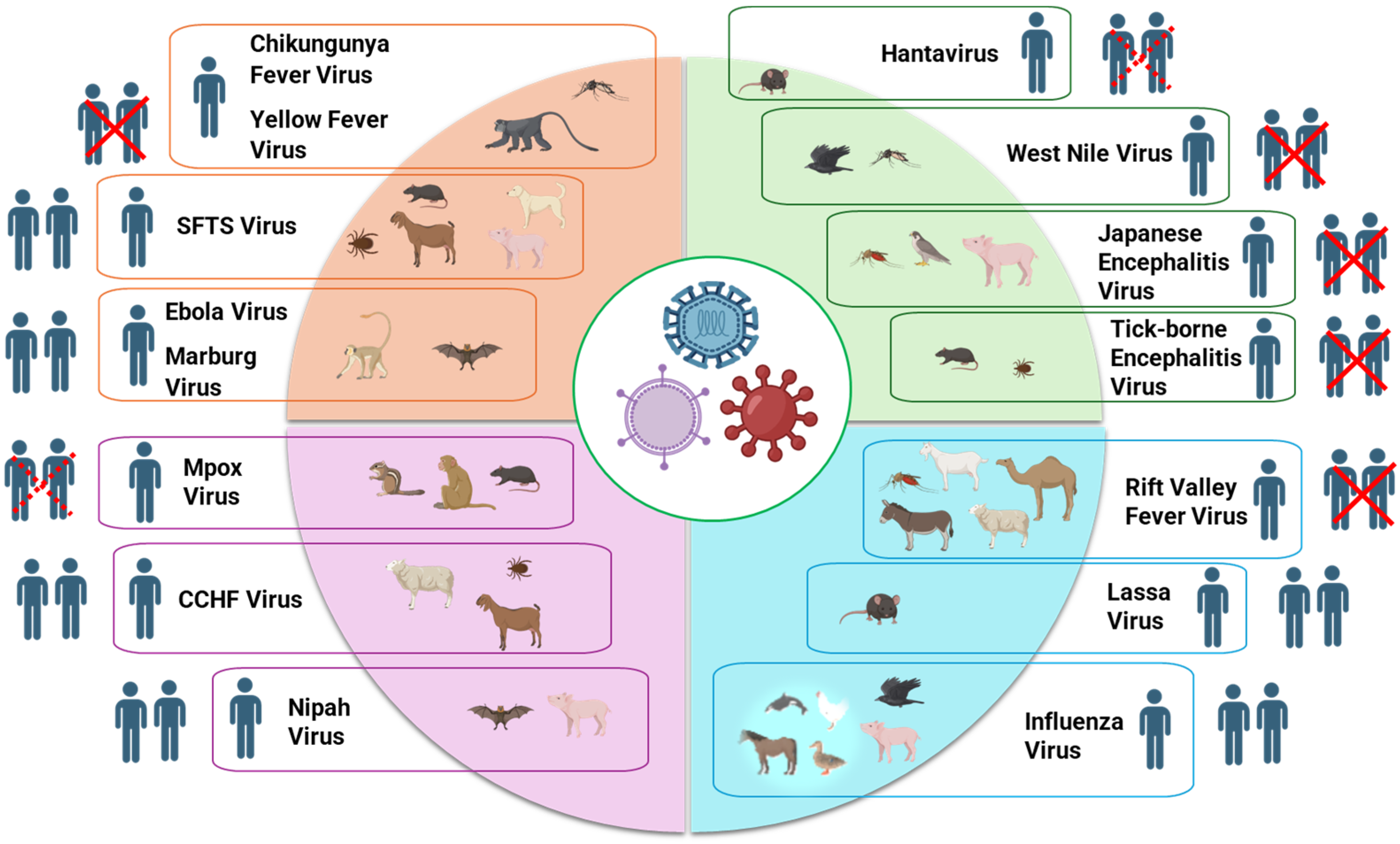
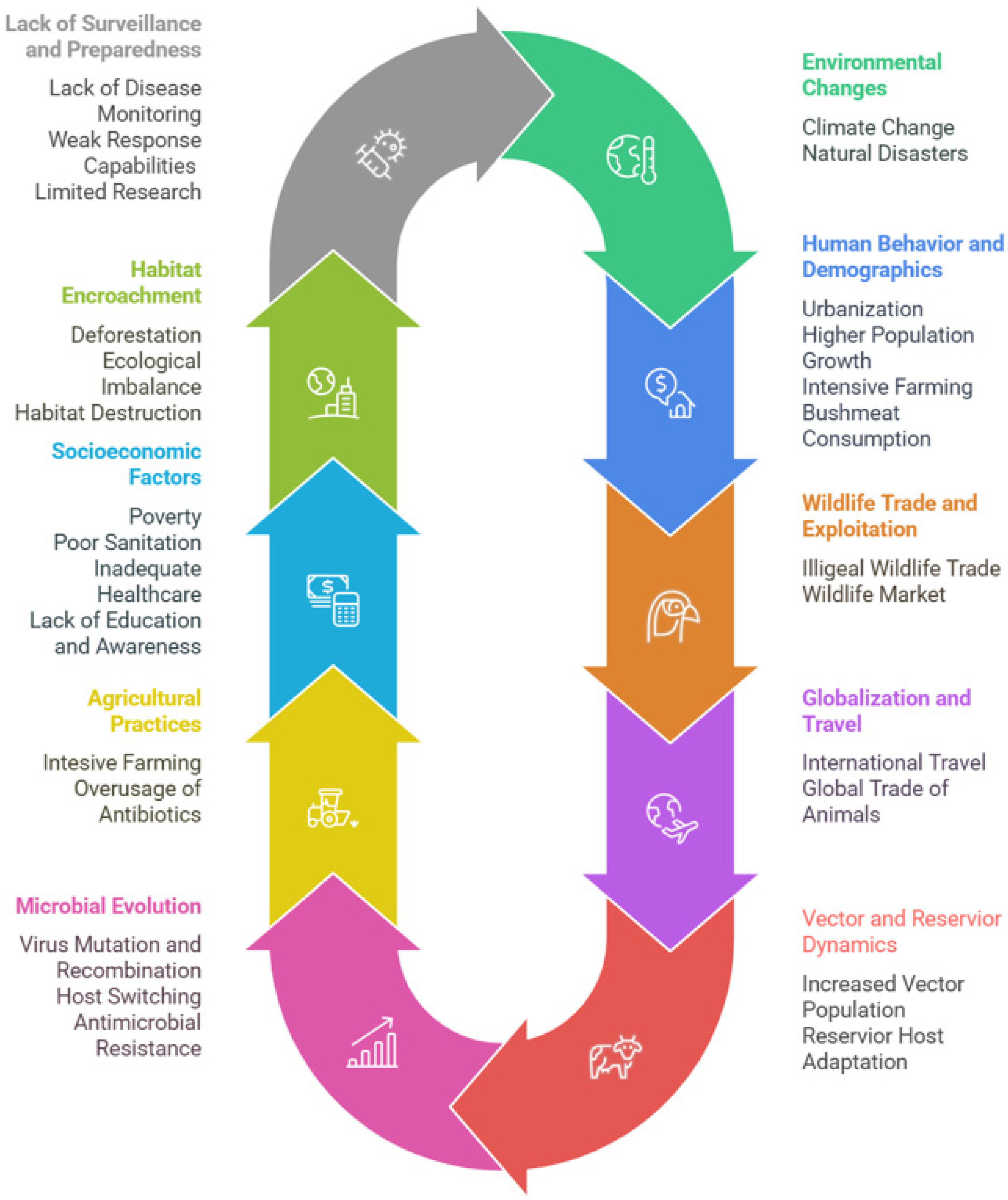
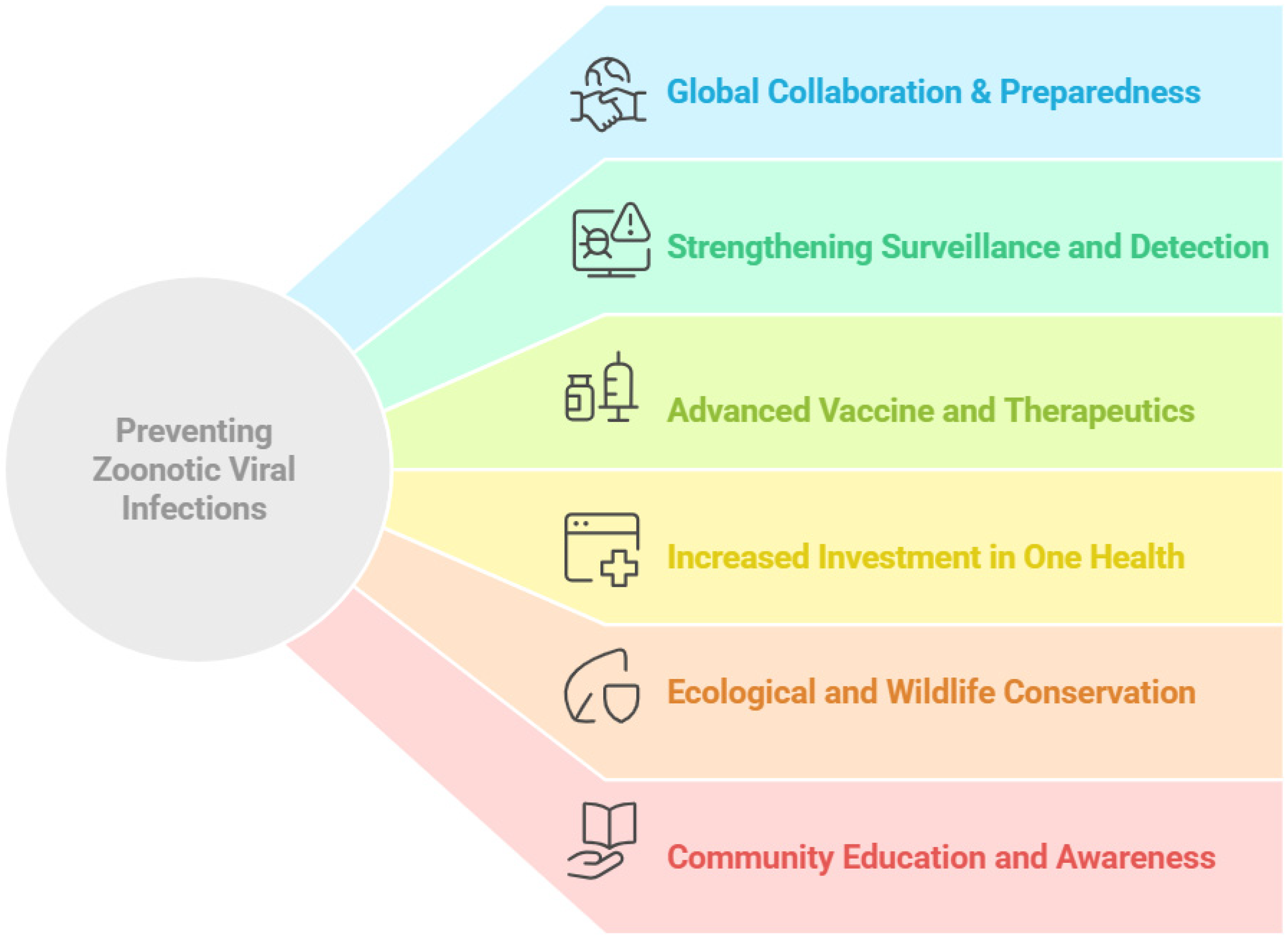
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatima, M.; An, T.; Park, P.-G.; Hong, K.-J. Advancements and Challenges in Addressing Zoonotic Viral Infections with Epidemic and Pandemic Threats. Viruses 2025, 17, 352. https://doi.org/10.3390/v17030352
Fatima M, An T, Park P-G, Hong K-J. Advancements and Challenges in Addressing Zoonotic Viral Infections with Epidemic and Pandemic Threats. Viruses. 2025; 17(3):352. https://doi.org/10.3390/v17030352
Chicago/Turabian StyleFatima, Munazza, Timothy An, Pil-Gu Park, and Kee-Jong Hong. 2025. "Advancements and Challenges in Addressing Zoonotic Viral Infections with Epidemic and Pandemic Threats" Viruses 17, no. 3: 352. https://doi.org/10.3390/v17030352
APA StyleFatima, M., An, T., Park, P.-G., & Hong, K.-J. (2025). Advancements and Challenges in Addressing Zoonotic Viral Infections with Epidemic and Pandemic Threats. Viruses, 17(3), 352. https://doi.org/10.3390/v17030352






