Acute HIV-1 Infection: Paradigm and Singularity
Abstract
:1. Introduction
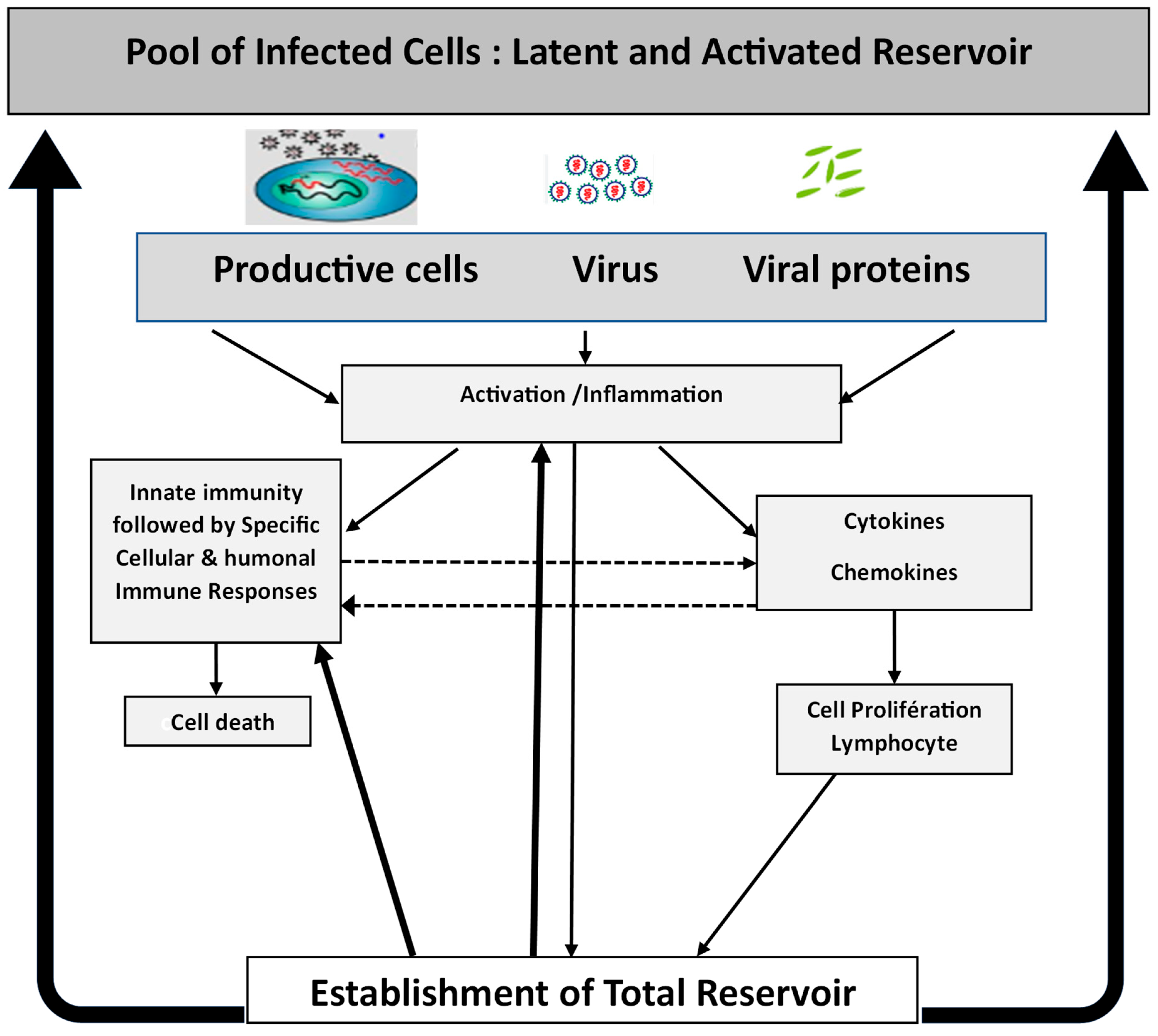
2. HIV-1 Infection and Viral Reservoir Establishment
3. Immune Response
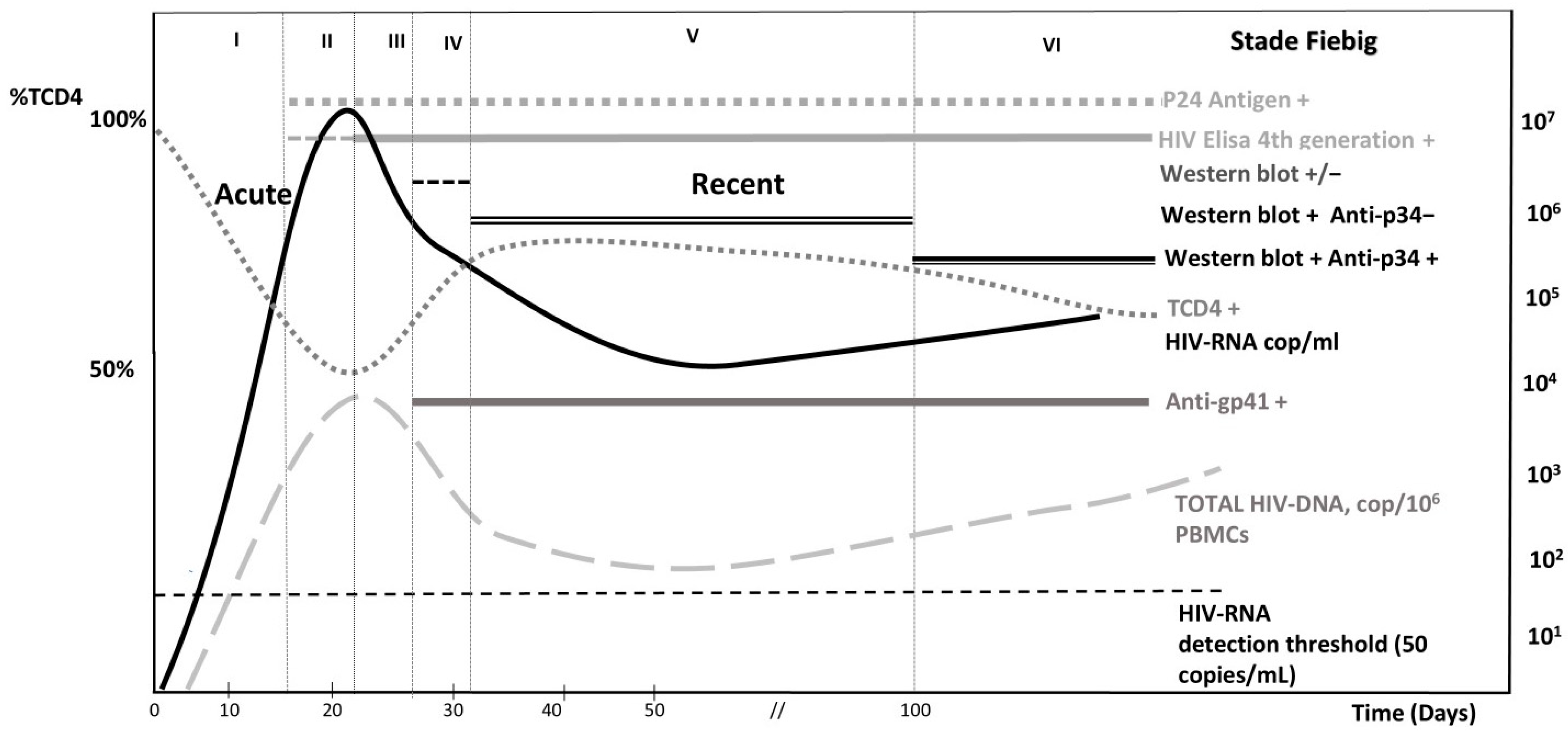
4. Diagnosing Acute HIV Infection: A Critical Issue
5. Key Virological Considerations in Acute HIV Treatment
6. Key Immunological Considerations in Acute HIV Treatment
7. Clinical Implications and Challenges in Acute HIV Treatment
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Joint United Nations Programme on HIV/AIDS (UNAIDS). Global HIV Statistics 2023 Fact Sheet. July 2024. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 2 March 2025).
- Hollingsworth, T.D.; Anderson, R.M.; Fraser, C. HIV-1 transmission, by stage of infection. J. Infect. Dis. 2008, 198, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Purcell, D.W.; Sansom, S.L.; Hayes, D.; Hall, H.I. Vital Signs: HIV Transmission Along the Continuum of Care United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Wawer, M.J.; Gray, R.H.; Sewankambo, N.K.; Serwadda, D.; Li, X.; Laeyendecker, O.; Kiwanuka, N.; Kigozi, G.; Kiddugavu, M.; Lutalo, T.; et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J. Infect. Dis. 2005, 191, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Pilcher, C.D.; Joaki, G.; Hoffman, I.F.; Martinson, F.E.; Mapanje, C.; Stewart, P.W.; Powers, K.A.; Galvin, S.; Chilongozi, D.; Gama, S.; et al. Amplified transmission of HIV-1: Comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS 2007, 21, 1723–1730. [Google Scholar] [CrossRef]
- Daar, E.S.; Pilcher, C.D.; Hecht, F.M. Clinical presentation and diagnosis of primary HIV-1 infection. Curr. Opin. HIV AIDS 2008, 3, 10–15. [Google Scholar] [CrossRef]
- Organisation, W.H. Consolidated Guidelines on HIV, Viral Hepatitis and STI Prevention, Diagnosis, Treatment and Care for Key Populations. 2022. Available online: https://www.who.int/publications/i/item/9789240052390 (accessed on 2 March 2025).
- Delobel, P. Initiation d’un Premier Traitement Antirétroviral Chez l’adulte Vivant avec le VIH. 2024. Available online: https://www.has-sante.fr/jcms/p_3545694/fr/initiation-d-un-premier-traitement-antiretroviral-chez-l-adulte-vivant-avec-le-vih (accessed on 2 March 2025).
- Panel on Antiretroviral Guidelines for Adults and Adolescents. US guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Dep. Health Hum. Serv. 2024, 6. [Google Scholar]
- EACS Guidelines for the Management of People Living with HIV in Europe. Available online: https://www.eacsociety.org/media/guidelines-12.0.pdf (accessed on 2 March 2025).
- Cheret, A.; Bauer, R.; Meiffredy, V.; Lopez, P.; Ajana, F.; Lacombe, K.; Morlat, P.; Lascoux, C.; Reynes, J.; Calin, R.; et al. Once-daily dolutegravir versus darunavir plus cobicistat in adults at the time of primary HIV-1 infection: The OPTIPRIM2-ANRS 169 randomized, open-label, Phase 3 trial. J. Antimicrob. Chemother. 2022, 77, 2506–2515. [Google Scholar] [CrossRef]
- Robb, M.L.; Ananworanich, J. Lessons from acute HIV infection. Curr. Opin. HIV AIDS 2016, 11, 555–560. [Google Scholar] [CrossRef]
- Lore, K.; Smed-Sorensen, A.; Vasudevan, J.; Mascola, J.R.; Koup, R.A. Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. J. Exp. Med. 2005, 201, 2023–2033. [Google Scholar] [CrossRef]
- Russell, R.A.; Martin, N.; Mitar, I.; Jones, E.; Sattentau, Q.J. Multiple proviral integration events after virological synapse-mediated HIV-1 spread. Virology 2013, 443, 143–149. [Google Scholar] [CrossRef]
- Veenhuis, R.T.; Abreu, C.M.; Costa, P.A.G.; Ferreira, E.A.; Ratliff, J.; Pohlenz, L.; Shirk, E.N.; Rubin, L.H.; Blankson, J.N.; Gama, L.; et al. Monocyte-derived macrophages contain persistent latent HIV reservoirs. Nat. Microbiol. 2023, 8, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Altfeld, M.; Gale, M., Jr. Innate immunity against HIV-1 infection. Nat. Immunol. 2015, 16, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Gardner, M.B.; Miller, C.J. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 2000, 74, 6087–6095. [Google Scholar] [CrossRef] [PubMed]
- McMichael, A.J.; Borrow, P.; Tomaras, G.D.; Goonetilleke, N.; Haynes, B.F. The immune response during acute HIV-1 infection: Clues for vaccine development. Nat. Rev. Immunol. 2010, 10, 11–23. [Google Scholar] [CrossRef]
- Cohen, M.S.; Shaw, G.M.; McMichael, A.J.; Haynes, B.F. Acute HIV-1 Infection. N. Engl. J. Med. 2011, 364, 1943–1954. [Google Scholar] [CrossRef]
- Keele, B.F.; Giorgi, E.E.; Salazar-Gonzalez, J.F.; Decker, J.M.; Pham, K.T.; Salazar, M.G.; Sun, C.; Grayson, T.; Wang, S.; Li, H.; et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. USA 2008, 105, 7552–7557. [Google Scholar] [CrossRef]
- Long, E.M.; Rainwater, S.M.; Lavreys, L.; Mandaliya, K.; Overbaugh, J. HIV type 1 variants transmitted to women in Kenya require the CCR5 coreceptor for entry, regardless of the genetic complexity of the infecting virus. AIDS Res. Hum. Retroviruses 2002, 18, 567–576. [Google Scholar] [CrossRef]
- Shepherd, J.C.; Jacobson, L.P.; Qiao, W.; Jamieson, B.D.; Phair, J.P.; Piazza, P.; Quinn, T.C.; Margolick, J.B. Emergence and persistence of CXCR4-tropic HIV-1 in a population of men from the multicenter AIDS cohort study. J. Infect. Dis. 2008, 198, 1104–1112. [Google Scholar] [CrossRef]
- Sucupira, M.C.; Sanabani, S.; Cortes, R.M.; Giret, M.T.; Tomiyama, H.; Sauer, M.M.; Sabino, E.C.; Janini, L.M.; Kallas, E.G.; Diaz, R.S. Faster HIV-1 disease progression among Brazilian individuals recently infected with CXCR4-utilizing strains. PLoS ONE 2012, 7, e30292. [Google Scholar] [CrossRef]
- Moyle, G.J.; Wildfire, A.; Mandalia, S.; Mayer, H.; Goodrich, J.; Whitcomb, J.; Gazzard, B.G. Epidemiology and predictive factors for chemokine receptor use in HIV-1 infection. J. Infect. Dis. 2005, 191, 866–872. [Google Scholar] [CrossRef]
- Frange, P.; Meyer, L.; Deveau, C.; Tran, L.; Goujard, C.; Ghosn, J.; Girard, P.M.; Morlat, P.; Rouzioux, C.; Chaix, M.L. Recent HIV-1 infection contributes to the viral diffusion over the French territory with a recent increasing frequency. PLoS ONE 2012, 7, e31695. [Google Scholar] [CrossRef] [PubMed]
- Castor, D.; Low, A.; Evering, T.; Karmon, S.; Davis, B.; Figueroa, A.; LaMar, M.; Garmon, D.; Mehandru, S.; Markowitz, M. Transmitted drug resistance and phylogenetic relationships among acute and early HIV-1-infected individuals in New York City. J. Acquir. Immune Defic. Syndr. 2012, 61, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cheret, A.; Bacchus, C.; Avettand-Fenoel, V.; Nembot, G.; Melard, A.; Blanc, C.; Lascoux-Combe, C.; Slama, L.; Allegre, T.; Allavena, C.; et al. A single HIV-1 cluster and a skewed immune homeostasis drive the early spread of HIV among resting CD4+ cell subsets within one month post-infection. PLoS ONE 2013, 8, e64219. [Google Scholar]
- Li, H.; Bar, K.J.; Wang, S.; Decker, J.M.; Chen, Y.; Sun, C.; Salazar-Gonzalez, J.F.; Salazar, M.G.; Learn, G.H.; Morgan, C.J.; et al. High Multiplicity Infection by HIV-1 in Men Who Have Sex with Men. PLoS Pathog. 2010, 6, e1000890. [Google Scholar] [CrossRef]
- Tully, D.C.; Ogilvie, C.B.; Batorsky, R.E.; Bean, D.J.; Power, K.A.; Ghebremichael, M.; Bedard, H.E.; Gladden, A.D.; Seese, A.M.; Amero, M.A.; et al. Differences in the Selection Bottleneck between Modes of Sexual Transmission Influence the Genetic Composition of the HIV-1 Founder Virus. PLoS Pathog. 2016, 12, e1005619. [Google Scholar] [CrossRef]
- Cheret, A.; Bacchus-Souffan, C.; Avettand-Fenoel, V.; Melard, A.; Nembot, G.; Blanc, C.; Samri, A.; Saez-Cirion, A.; Hocqueloux, L.; Lascoux-Combe, C.; et al. Combined ART started during acute HIV infection protects central memory CD4+ T cells and can induce remission. J. Antimicrob. Chemother. 2015, 70, 2108–2120. [Google Scholar] [CrossRef]
- Kariuki, S.M.; Selhorst, P.; Arien, K.K.; Dorfman, J.R. The HIV-1 transmission bottleneck. Retrovirology 2017, 14, 22. [Google Scholar] [CrossRef]
- Miller, W.C.; Rosenberg, N.E.; Rutstein, S.E.; Powers, K.A. Role of acute and early HIV infection in the sexual transmission of HIV. Curr. Opin. HIV AIDS 2010, 5, 277–282. [Google Scholar] [CrossRef]
- Chan, P.; Spudich, S. Central Nervous System Effects of Early HIV Infection and Consequences of Antiretroviral Therapy Initiation during Acute HIV. Viruses 2024, 16, 1082. [Google Scholar] [CrossRef]
- Haase, A.T. Population biology of HIV-1 infection: Viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu. Rev. Immunol. 1999, 17, 625–656. [Google Scholar] [CrossRef]
- Robb, M.L.; Eller, L.A.; Kibuuka, H.; Rono, K.; Maganga, L.; Nitayaphan, S.; Kroon, E.; Sawe, F.K.; Sinei, S.; Sriplienchan, S.; et al. Prospective Study of Acute HIV-1 Infection in Adults in East Africa and Thailand. N. Engl. J. Med. 2016, 374, 2120–2130. [Google Scholar] [CrossRef] [PubMed]
- Kelley, C.F.; Hernandez-Ramos, I.; Franco-Paredes, C.; del Rio, C. Clinical, epidemiologic characteristics of foreign-born Latinos with HIV/AIDS at an urban HIV clinic. AIDS Read. 2007, 17, 73–74, 78–80, 85–88. [Google Scholar] [PubMed]
- Ndhlovu, Z.M.; Kamya, P.; Mewalal, N.; Kloverpris, H.N.; Nkosi, T.; Pretorius, K.; Laher, F.; Ogunshola, F.; Chopera, D.; Shekhar, K.; et al. Magnitude and Kinetics of CD8+ T Cell Activation during Hyperacute HIV Infection Impact Viral Set Point. Immunity 2015, 43, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Selhorst, P.; Combrinck, C.; Ndabambi, N.; Ismail, S.D.; Abrahams, M.R.; Lacerda, M.; Samsunder, N.; Garrett, N.; Abdool Karim, Q.; Abdool Karim, S.S.; et al. Replication Capacity of Viruses from Acute Infection Drives Hiv-1 Disease Progression. J. Virol. 2017, 91, 10–1128. [Google Scholar] [CrossRef]
- Abreu, C.M.; Veenhuis, R.T.; Avalos, C.R.; Graham, S.; Parrilla, D.R.; Ferreira, E.A.; Queen, S.E.; Shirk, E.N.; Bullock, B.T.; Li, M.; et al. Myeloid and CD4 T Cells Comprise the Latent Reservoir in Antiretroviral Therapy-Suppressed SIVmac251-Infected Macaques. mBio 2019, 10. [Google Scholar] [CrossRef]
- Ananworanich, J.; Sacdalan, C.P.; Pinyakorn, S.; Chomont, N.; de Souza, M.; Luekasemsuk, T.; Schuetz, A.; Krebs, S.J.; Dewar, R.; Jagodzinski, L.; et al. Virological and immunological characteristics of HIV-infected individuals at the earliest stage of infection. J. Virus Erad. 2016, 2, 43–48. [Google Scholar] [CrossRef]
- Eriksson, S.; Graf, E.H.; Dahl, V.; Strain, M.C.; Yukl, S.A.; Lysenko, E.S.; Bosch, R.J.; Lai, J.; Chioma, S.; Emad, F.; et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 2013, 9, e1003174. [Google Scholar] [CrossRef]
- Chun, T.W.; Engel, D.; Berrey, M.M.; Shea, T.; Corey, L.; Fauci, A.S. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc. Natl. Acad. Sci. USA 1998, 95, 8869–8873. [Google Scholar] [CrossRef]
- Avettand-Fenoel, V.; Hocqueloux, L.; Ghosn, J.; Cheret, A.; Frange, P.; Melard, A.; Viard, J.P.; Rouzioux, C. Total HIV-1 DNA, a Marker of Viral Reservoir Dynamics with Clinical Implications. Clin. Microbiol. Rev. 2016, 29, 859–880. [Google Scholar] [CrossRef]
- Kim, C.J.; McKinnon, L.R.; Kovacs, C.; Kandel, G.; Huibner, S.; Chege, D.; Shahabi, K.; Benko, E.; Loutfy, M.; Ostrowski, M.; et al. Mucosal Th17 cell function is altered during HIV infection and is an independent predictor of systemic immune activation. J. Immunol. 2013, 191, 2164–2173. [Google Scholar] [CrossRef]
- Yukl, S.A.; Shergill, A.K.; Ho, T.; Killian, M.; Girling, V.; Epling, L.; Li, P.; Wong, L.K.; Crouch, P.; Deeks, S.G.; et al. The distribution of HIV DNA and RNA in cell subsets differs in gut and blood of HIV-positive patients on ART: Implications for viral persistence. J. Infect. Dis. 2013, 208, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Valcour, V.; Chalermchai, T.; Sailasuta, N.; Marovich, M.; Lerdlum, S.; Suttichom, D.; Suwanwela, N.C.; Jagodzinski, L.; Michael, N.; Spudich, S.; et al. Central nervous system viral invasion and inflammation during acute HIV infection. J. Infect. Dis. 2012, 206, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Cheret, A.; Durier, C.; Melard, A.; Ploquin, M.; Heitzmann, J.; Lecuroux, C.; Avettand-Fenoel, V.; David, L.; Pialoux, G.; Chennebault, J.M.; et al. Impact of early cART on HIV blood and semen compartments at the time of primary infection. PLoS ONE 2017, 12, e0180191. [Google Scholar] [CrossRef] [PubMed]
- Mariaggi, A.A.; Bauer, R.; Charre, C.; Gardiennet, E.; Meiffredy, V.; Ajana, F.; Lacombe, K.; Pialoux, G.; Cua, E.; Rouzioux, C.; et al. HIV-1-RNA and total HIV-1-DNA loads in the genital compartment in men receiving dolutegravir- versus darunavir-based combined ART (cART) regimens during primary HIV infection. J. Antimicrob. Chemother. 2022, 77, 735–739. [Google Scholar] [CrossRef]
- Goujard, C.; Bonarek, M.; Meyer, L.; Bonnet, F.; Chaix, M.L.; Deveau, C.; Sinet, M.; Galimand, J.; Delfraissy, J.F.; Venet, A.; et al. CD4 cell count and HIV DNA level are independent predictors of disease progression after primary HIV type 1 infection in untreated patients. Clin. Infect. Dis. 2006, 42, 709–715. [Google Scholar] [CrossRef]
- Tremeaux, P.; Lenfant, T.; Boufassa, F.; Essat, A.; Melard, A.; Gousset, M.; Delelis, O.; Viard, J.P.; Bary, M.; Goujard, C.; et al. Increasing contribution of integrated forms to total HIV DNA in blood during HIV disease progression from primary infection. EBioMedicine 2019, 41, 455–464. [Google Scholar] [CrossRef]
- Sallusto, F.; Geginat, J.; Lanzavecchia, A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu. Rev. Immunol. 2004, 22, 745–763. [Google Scholar] [CrossRef]
- Appay, V.; Douek, D.C.; Price, D.A. CD8+ T cell efficacy in vaccination and disease. Nat. Med. 2008, 14, 623–628. [Google Scholar] [CrossRef]
- Ganesan, A.; Chattopadhyay, P.K.; Brodie, T.M.; Qin, J.; Gu, W.; Mascola, J.R.; Michael, N.L.; Follmann, D.A.; Roederer, M. Immunologic and virologic events in early HIV infection predict subsequent rate of progression. J. Infect. Dis. 2010, 201, 272–284. [Google Scholar] [CrossRef]
- Buzon, M.J.; Sun, H.; Li, C.; Shaw, A.; Seiss, K.; Ouyang, Z.; Martin-Gayo, E.; Leng, J.; Henrich, T.J.; Li, J.Z.; et al. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat. Med. 2014, 20, 139–142. [Google Scholar] [CrossRef]
- Gattinoni, L.; Speiser, D.E.; Lichterfeld, M.; Bonini, C. T memory stem cells in health and disease. Nat. Med. 2017, 23, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Chomont, N.; El-Far, M.; Ancuta, P.; Trautmann, L.; Procopio, F.A.; Yassine-Diab, B.; Boucher, G.; Boulassel, M.R.; Ghattas, G.; Brenchley, J.M.; et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 2009, 15, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.; Maldarelli, F.; Wiegand, A.; Bernstein, B.; Hanna, G.J.; Brun, S.C.; Kempf, D.J.; Mellors, J.W.; Coffin, J.M.; King, M.S. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc. Natl. Acad. Sci. USA 2008, 105, 3879–3884. [Google Scholar] [CrossRef] [PubMed]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef]
- Rustagi, A.; Gale, M., Jr. Innate antiviral immune signaling, viral evasion and modulation by HIV-1. J. Mol. Biol. 2014, 426, 1161–1177. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Roberts, L.; Passmore, J.A.; Williamson, C.; Little, F.; Bebell, L.M.; Mlisana, K.; Burgers, W.A.; van Loggerenberg, F.; Walzl, G.; Djoba Siawaya, J.F.; et al. Plasma cytokine levels during acute HIV-1 infection predict HIV disease progression. AIDS 2010, 24, 819–831. [Google Scholar] [CrossRef]
- Stacey, A.R.; Norris, P.J.; Qin, L.; Haygreen, E.A.; Taylor, E.; Heitman, J.; Lebedeva, M.; DeCamp, A.; Li, D.; Grove, D.; et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J. Virol. 2009, 83, 3719–3733. [Google Scholar] [CrossRef]
- Naranbhai, V.; Abdool Karim, S.S.; Altfeld, M.; Samsunder, N.; Durgiah, R.; Sibeko, S.; Abdool Karim, Q.; Carr, W.H. Innate immune activation enhances hiv acquisition in women, diminishing the effectiveness of tenofovir microbicide gel. J. Infect. Dis. 2012, 206, 993–1001. [Google Scholar] [CrossRef]
- Scott-Algara, D.; Truong, L.X.; Versmisse, P.; David, A.; Luong, T.T.; Nguyen, N.V.; Theodorou, I.; Barre-Sinoussi, F.; Pancino, G. Cutting edge: Increased NK cell activity in HIV-1-exposed but uninfected Vietnamese intravascular drug users. J. Immunol. 2003, 171, 5663–5667. [Google Scholar] [CrossRef]
- Borrow, P. Innate immunity in acute HIV-1 infection. Curr. Opin. HIV AIDS 2011, 6, 353–363. [Google Scholar] [CrossRef]
- Doisne, J.M.; Urrutia, A.; Lacabaratz-Porret, C.; Goujard, C.; Meyer, L.; Chaix, M.L.; Sinet, M.; Venet, A. CD8+ T cells specific for EBV, cytomegalovirus, and influenza virus are activated during primary HIV infection. J. Immunol. 2004, 173, 2410–2418. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.H.; Tudor-Williams, G.; Banda, N.K.; Cotton, M.F.; Curiel, T.; Monks, C.; Baba, T.W.; Ruprecht, R.M.; Kupfer, A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat. Med. 1995, 1, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Ouyang, J.; Isnard, S.; Lin, J.; Fombuena, B.; Zhu, B.; Routy, J.P. Sharing CD4+ T Cell Loss: When COVID-19 and HIV Collide on Immune System. Front. Immunol. 2020, 11, 596631. [Google Scholar] [CrossRef] [PubMed]
- Brenchley, J.M.; Price, D.A.; Douek, D.C. HIV disease: Fallout from a mucosal catastrophe? Nat. Immunol. 2006, 7, 235–239. [Google Scholar] [CrossRef]
- Lafeuillade, A.; Poggi, C.; Tamalet, C.; Profizi, N. Human immunodeficiency virus type 1 dynamics in different lymphoid tissue compartments. J. Infect. Dis. 1997, 176, 804–806. [Google Scholar] [CrossRef]
- Estes, J.D. Pathobiology of HIV/SIV-associated changes in secondary lymphoid tissues. Immunol. Rev. 2013, 254, 65–77. [Google Scholar] [CrossRef]
- Chevalier, M.F.; Didier, C.; Girard, P.M.; Manea, M.E.; Campa, P.; Barre-Sinoussi, F.; Scott-Algara, D.; Weiss, L. CD4 T-Cell Responses in Primary HIV Infection: Interrelationship with Immune Activation and Virus Burden. Front. Immunol. 2016, 7, 395. [Google Scholar] [CrossRef]
- Klatt, N.R.; Funderburg, N.T.; Brenchley, J.M. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 2013, 21, 6–13. [Google Scholar] [CrossRef]
- Hoenigl, M.; Perez-Santiago, J.; Nakazawa, M.; de Oliveira, M.F.; Zhang, Y.; Finkelman, M.A.; Letendre, S.; Smith, D.; Gianella, S. (1-->3)-beta-d-Glucan: A Biomarker for Microbial Translocation in Individuals with Acute or Early HIV Infection? Front. Immunol. 2016, 7, 404. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, H.; Feng, J.; Ying, L.; Ji, M.; Wei, S.; Ma, Q. Time-restricted feeding improves metabolic syndrome by activating thermogenesis in brown adipose tissue and reducing inflammatory markers. Front. Immunol. 2025, 16, 1501850. [Google Scholar] [CrossRef] [PubMed]
- Koup, R.A.; Safrit, J.T.; Cao, Y.; Andrews, C.A.; McLeod, G.; Borkowsky, W.; Farthing, C.; Ho, D.D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 1994, 68, 4650–4655. [Google Scholar] [CrossRef] [PubMed]
- Pantaleo, G.; Demarest, J.F.; Soudeyns, H.; Graziosi, C.; Denis, F.; Adelsberger, J.W.; Borrow, P.; Saag, M.S.; Shaw, G.M.; Sekaly, R.P.; et al. Major expansion of CD8+ T cells with a predominant V beta usage during the primary immune response to HIV. Nature 1994, 370, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Goonetilleke, N.; Liu, M.K.; Salazar-Gonzalez, J.F.; Ferrari, G.; Giorgi, E.; Ganusov, V.V.; Keele, B.F.; Learn, G.H.; Turnbull, E.L.; Salazar, M.G.; et al. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J. Exp. Med. 2009, 206, 1253–1272. [Google Scholar] [CrossRef]
- Saez-Cirion, A.; Lacabaratz, C.; Lambotte, O.; Versmisse, P.; Urrutia, A.; Boufassa, F.; Barre-Sinoussi, F.; Delfraissy, J.F.; Sinet, M.; Pancino, G.; et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc. Natl. Acad. Sci. USA 2007, 104, 6776–6781. [Google Scholar] [CrossRef]
- Cocchi, F.; DeVico, A.L.; Garzino-Demo, A.; Arya, S.K.; Gallo, R.C.; Lusso, P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 1995, 270, 1811–1815. [Google Scholar] [CrossRef]
- Walker, B.D.; Yu, X.G. Unravelling the mechanisms of durable control of HIV-1. Nat. Rev. Immunol. 2013, 13, 487–498. [Google Scholar] [CrossRef]
- Emu, B.; Sinclair, E.; Hatano, H.; Ferre, A.; Shacklett, B.; Martin, J.N.; McCune, J.M.; Deeks, S.G. HLA class I-restricted T-cell responses may contribute to the control of human immunodeficiency virus infection, but such responses are not always necessary for long-term virus control. J. Virol. 2008, 82, 5398–5407. [Google Scholar] [CrossRef]
- Altfeld, M.; Kalife, E.T.; Qi, Y.; Streeck, H.; Lichterfeld, M.; Johnston, M.N.; Burgett, N.; Swartz, M.E.; Yang, A.; Alter, G.; et al. HLA Alleles Associated with Delayed Progression to AIDS Contribute Strongly to the Initial CD8(+) T Cell Response against HIV-1. PLoS Med. 2006, 3, e403. [Google Scholar] [CrossRef]
- Demers, K.R.; Makedonas, G.; Buggert, M.; Eller, M.A.; Ratcliffe, S.J.; Goonetilleke, N.; Li, C.K.; Eller, L.A.; Rono, K.; Maganga, L.; et al. Temporal Dynamics of CD8+ T Cell Effector Responses during Primary HIV Infection. PLoS Pathog. 2016, 12, e1005805. [Google Scholar] [CrossRef]
- Roberts, E.R.; Carnathan, D.G.; Li, H.; Shaw, G.M.; Silvestri, G.; Betts, M.R. Collapse of Cytolytic Potential in SIV-Specific CD8+ T Cells Following Acute SIV Infection in Rhesus Macaques. PLoS Pathog. 2016, 12, e1006135. [Google Scholar] [CrossRef] [PubMed]
- Florez-Alvarez, L.; Hernandez, J.C.; Zapata, W. NK Cells in HIV-1 Infection: From Basic Science to Vaccine Strategies. Front. Immunol. 2018, 9, 2290. [Google Scholar] [CrossRef] [PubMed]
- Pohlmeyer, C.W.; Gonzalez, V.D.; Irrinki, A.; Ramirez, R.N.; Li, L.; Mulato, A.; Murry, J.P.; Arvey, A.; Hoh, R.; Deeks, S.G.; et al. Identification of NK Cell Subpopulations That Differentiate HIV-Infected Subject Cohorts with Diverse Levels of Virus Control. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- Sant, A.J.; McMichael, A. Revealing the role of CD4(+) T cells in viral immunity. J. Exp. Med. 2012, 209, 1391–1395. [Google Scholar] [CrossRef]
- Schuetz, A.; Deleage, C.; Sereti, I.; Rerknimitr, R.; Phanuphak, N.; Phuang-Ngern, Y.; Estes, J.D.; Sandler, N.G.; Sukhumvittaya, S.; Marovich, M.; et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. Pathogens 2014, 10, e1004543. [Google Scholar] [CrossRef]
- Mattapallil, J.J.; Douek, D.C.; Hill, B.; Nishimura, Y.; Martin, M.; Roederer, M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 2005, 434, 1093–1097. [Google Scholar] [CrossRef]
- Schieffer, M.; Jessen, H.K.; Oster, A.F.; Pissani, F.; Soghoian, D.Z.; Lu, R.; Jessen, A.B.; Zedlack, C.; Schultz, B.T.; Davis, I.; et al. Induction of Gag-specific CD4 T cell responses during acute HIV infection is associated with improved viral control. J. Virol. 2014, 88, 7357–7366. [Google Scholar] [CrossRef]
- Soghoian, D.Z.; Jessen, H.; Flanders, M.; Sierra-Davidson, K.; Cutler, S.; Pertel, T.; Ranasinghe, S.; Lindqvist, M.; Davis, I.; Lane, K.; et al. HIV-specific cytolytic CD4 T cell responses during acute HIV infection predict disease outcome. Sci. Transl. Med. 2012, 4, 123ra25. [Google Scholar] [CrossRef]
- Frater, J.; Ewings, F.; Hurst, J.; Brown, H.; Robinson, N.; Fidler, S.; Babiker, A.; Weber, J.; Porter, K.; Phillips, R.E. HIV-1-specific CD4(+) responses in primary HIV-1 infection predict disease progression. AIDS 2014, 28, 699–708. [Google Scholar] [CrossRef]
- Potter, S.J.; Lacabaratz, C.; Lambotte, O.; Perez-Patrigeon, S.; Vingert, B.; Sinet, M.; Colle, J.H.; Urrutia, A.; Scott-Algara, D.; Boufassa, F.; et al. Preserved central memory and activated effector memory CD4+ T-cell subsets in human immunodeficiency virus controllers: An ANRS EP36 study. J. Virol. 2007, 81, 13904–13915. [Google Scholar] [CrossRef]
- Zaunders, J.; van Bockel, D. Innate and Adaptive Immunity in Long-Term Non-Progression in HIV Disease. Front. Immunol. 2013, 4, 95. [Google Scholar] [CrossRef] [PubMed]
- Hellerstein, M.K.; Hoh, R.A.; Hanley, M.B.; Cesar, D.; Lee, D.; Neese, R.A.; McCune, J.M. Subpopulations of long-lived and short-lived T cells in advanced HIV-1 infection. J. Clin. Investig. 2003, 112, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Margolick, J.B.; Gange, S.J.; Detels, R.; O’Gorman, M.R.; Rinaldo, C.R., Jr.; Lai, S. Impact of inversion of the CD4/CD8 ratio on the natural history of HIV-1 infection. J. Acquir. Immune Defic. Syndr. 2006, 42, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Chapon, M.; Randriamampita, C.; Maubec, E.; Badoual, C.; Fouquet, S.; Wang, S.F.; Marinho, E.; Farhi, D.; Garcette, M.; Jacobelli, S.; et al. Progressive upregulation of PD-1 in primary and metastatic melanomas associated with blunted TCR signaling in infiltrating T lymphocytes. J. Investig. Dermatol. 2011, 131, 1300–1307. [Google Scholar] [CrossRef]
- Palmer, B.E.; Neff, C.P.; Lecureux, J.; Ehler, A.; Dsouza, M.; Remling-Mulder, L.; Korman, A.J.; Fontenot, A.P.; Akkina, R. In vivo blockade of the PD-1 receptor suppresses HIV-1 viral loads and improves CD4+ T cell levels in humanized mice. J. Immunol. 2013, 190, 211–219. [Google Scholar] [CrossRef]
- Autran, B.; Hadida, F.; Haas, G. Evolution and plasticity of CTL responses against HIV. Curr. Opin. Immunol. 1996, 8, 546–553. [Google Scholar] [CrossRef]
- Moir, S.; Buckner, C.M.; Ho, J.; Wang, W.; Chen, J.; Waldner, A.J.; Posada, J.G.; Kardava, L.; O’Shea, M.A.; Kottilil, S.; et al. B cells in early and chronic HIV infection: Evidence for preservation of immune function associated with early initiation of antiretroviral therapy. Blood 2010, 116, 5571–5579. [Google Scholar] [CrossRef]
- Levesque, M.C.; Moody, M.A.; Hwang, K.K.; Marshall, D.J.; Whitesides, J.F.; Amos, J.D.; Gurley, T.C.; Allgood, S.; Haynes, B.B.; Vandergrift, N.A.; et al. Polyclonal B cell differentiation and loss of gastrointestinal tract germinal centers in the earliest stages of HIV-1 infection. PLoS Med. 2009, 6, e1000107. [Google Scholar] [CrossRef]
- Lane, H.C.; Masur, H.; Edgar, L.C.; Whalen, G.; Rook, A.H.; Fauci, A.S. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 1983, 309, 453–458. [Google Scholar] [CrossRef]
- Hart, M.; Steel, A.; Clark, S.A.; Moyle, G.; Nelson, M.; Henderson, D.C.; Wilson, R.; Gotch, F.; Gazzard, B.; Kelleher, P. Loss of discrete memory B cell subsets is associated with impaired immunization responses in HIV-1 infection and may be a risk factor for invasive pneumococcal disease. J. Immunol. 2007, 178, 8212–8220. [Google Scholar] [CrossRef]
- Moir, S.; Fauci, A.S. B cells in HIV infection and disease. Nat. Rev. Immunol. 2009, 9, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Decker, J.M.; Wang, S.; Hui, H.; Kappes, J.C.; Wu, X.; Salazar-Gonzalez, J.F.; Salazar, M.G.; Kilby, J.M.; Saag, M.S.; et al. Antibody neutralization and escape by HIV-1. Nature 2003, 422, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Klatt, N.R.; Chomont, N.; Douek, D.C.; Deeks, S.G. Immune activation and HIV persistence: Implications for curative approaches to HIV infection. Immunol. Rev. 2013, 254, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.L.; Kouyos, R.D.; Balmer, B.; Grube, C.; Weber, R.; Gunthard, H.F. Frequency and Spectrum of Unexpected Clinical Manifestations of Primary HIV-1 Infection. Clin. Infect. Dis. 2015, 61, 1013–1021. [Google Scholar] [CrossRef]
- Schacker, T.; Collier, A.C.; Hughes, J.; Shea, T.; Corey, L. Clinical and epidemiologic features of primary HIV infection. Ann. Intern. Med. 1996, 125, 257–264. [Google Scholar] [CrossRef]
- Lievre, L.; Deveau, C.; Gerbe, J.; Enel, P.; Tran, L.; De Castro, N.; Costagliola, D.; Meyer, L.; Primo Study, G.; Clinical Epidemieology, G. Yearly number of patients diagnosed with primary HIV-1 infection in France estimated by a capture-recapture approach. AIDS 2006, 20, 2392–2395. [Google Scholar] [CrossRef]
- Kahn, J.O.; Walker, B.D. Acute human immunodeficiency virus type 1 infection. N. Engl. J. Med. 1998, 339, 33–39. [Google Scholar] [CrossRef]
- Boufassa, F.; Bachmeyer, C.; Carre, N.; Deveau, C.; Persoz, A.; Jadand, C.; Sereni, D.; Bucquet, D. Influence of neurologic manifestations of primary human immunodeficiency virus infection on disease progression. SEROCO Study Group. J. Infect. Dis. 1995, 171, 1190–1195. [Google Scholar] [CrossRef]
- Crowell, T.A.; Colby, D.J.; Pinyakorn, S.; Fletcher, J.L.K.; Kroon, E.; Schuetz, A.; Krebs, S.J.; Slike, B.M.; Leyre, L.; Chomont, N.; et al. Acute Retroviral Syndrome Is Associated With High Viral Burden, CD4 Depletion, and Immune Activation in Systemic and Tissue Compartments. Clin. Infect. Dis. 2018, 66, 1540–1549. [Google Scholar] [CrossRef]
- Hoenigl, M.; Green, N.; Camacho, M.; Gianella, S.; Mehta, S.R.; Smith, D.M.; Little, S.J. Signs or Symptoms of Acute HIV Infection in a Cohort Undergoing Community-Based Screening. Emerg. Infect. Dis. 2016, 22, 532–534. [Google Scholar] [CrossRef]
- Kelley, C.F.; Barbour, J.D.; Hecht, F.M. The relation between symptoms, viral load, and viral load set point in primary HIV infection. J. Acquir. Immune Defic. Syndr. 2007, 45, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Ghosn, J.; Deveau, C.; Chaix, M.L.; Goujard, C.; Galimand, J.; Zitoun, Y.; Allegre, T.; Delfraissy, J.F.; Meyer, L.; Rouzioux, C. Despite being highly diverse, immunovirological status strongly correlates with clinical symptoms during primary HIV-1 infection: A cross-sectional study based on 674 patients enrolled in the ANRS CO 06 PRIMO cohort. J. Antimicrob. Chemother. 2010, 65, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Abdel Babiker, S.D.; Daniela De Angelis, D.E. Kholoud Porter, Time from HIV-1 seroconversion to AIDS and death before widespread use of highly-active antiretroviral therapy: A collaborative re-analysis. Collaborative Group on AIDS Incubation and HIV Survival including the CASCADE EU Concerted Action. Concerted Action on SeroConversion to AIDS and Death in Europe. Lancet 2000, 355, 1131–1137. [Google Scholar]
- Braun, D.L.; Kouyos, R.; Oberle, C.; Grube, C.; Joos, B.; Fellay, J.; McLaren, P.J.; Kuster, H.; Gunthard, H.F. A novel Acute Retroviral Syndrome Severity Score predicts the key surrogate markers for HIV-1 disease progression. PLoS ONE 2014, 9, e114111. [Google Scholar] [CrossRef]
- Avettand-Fenoel, V.; Chaix, M.L.; Blanche, S.; Burgard, M.; Floch, C.; Toure, K.; Allemon, M.C.; Warszawski, J.; Rouzioux, C. LTR real-time PCR for HIV-1 DNA quantitation in blood cells for early diagnosis in infants born to seropositive mothers treated in HAART area (ANRS CO 01). J. Med. Virol. 2009, 81, 217–223. [Google Scholar]
- Fiebig, E.W.; Wright, D.J.; Rawal, B.D.; Garrett, P.E.; Schumacher, R.T.; Peddada, L.; Heldebrant, C.; Smith, R.; Conrad, A.; Kleinman, S.H.; et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: Implications for diagnosis and staging of primary HIV infection. AIDS 2003, 17, 1871–1879. [Google Scholar] [CrossRef]
- Ananworanich, J.; Fletcher, J.L.; Pinyakorn, S.; van Griensven, F.; Vandergeeten, C.; Schuetz, A.; Pankam, T.; Trichavaroj, R.; Akapirat, S.; Chomchey, N.; et al. A novel acute HIV infection staging system based on 4th generation immunoassay. Retrovirology 2013, 10, 56. [Google Scholar] [CrossRef]
- Morlat, P. Prise en Charge Médicale des Personnes Vivant avec le VIH, Recommandations du Groupe d’Experts sous la Direction du Professeur P. Morlat; Conseil National du Sida et des Hépatites Virales: Paris, France, 2013; 476p. [Google Scholar]
- Mariaggi, A.A.; Gardiennet, E.; Stefic, K.; Essat, A.; Cheret, A.; Goujard, C.; Meyer, L.; Barin, F.; Avettand-Fenoel, V.; Cohort, A.P. Immunoblots may not be effective in confirming the recency of HIV-1 infection. J. Virol. Methods 2021, 290, 114074. [Google Scholar] [CrossRef]
- Pavie, J.; Rachline, A.; Loze, B.; Niedbalski, L.; Delaugerre, C.; Laforgerie, E.; Plantier, J.C.; Rozenbaum, W.; Chevret, S.; Molina, J.M.; et al. Sensitivity of five rapid HIV tests on oral fluid or finger-stick whole blood: A real-time comparison in a healthcare setting. PLoS ONE 2010, 5, e11581. [Google Scholar] [CrossRef]
- Stekler, J.D.; Tapia, K.; Maenza, J.; Stevens, C.E.; Ure, G.A.; O’Neal, J.D.; Lane, A.; Mullins, J.I.; Coombs, R.W.; Holte, S.; et al. No Time to Delay! Fiebig Stages and Referral in Acute HIV infection: Seattle Primary Infection Program Experience. AIDS Res. Hum. Retroviruses 2018, 34, 657–666. [Google Scholar] [CrossRef]
- Powers, K.A.; Ghani, A.C.; Miller, W.C.; Hoffman, I.F.; Pettifor, A.E.; Kamanga, G.; Martinson, F.E.; Cohen, M.S. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: A modelling study. Lancet 2011, 378, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Seng, R.; Rolland, M.; Beck-Wirth, G.; Souala, F.; Deveau, C.; Delfraissy, J.F.; Goujard, C.; Meyer, L. Trends in unsafe sex and influence of viral load among patients followed since primary HIV infection, 2000–2009. AIDS 2011, 25, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Cheret, A.; Nembot, G.; Melard, A.; Lascoux, C.; Slama, L.; Miailhes, P.; Yeni, P.; Abel, S.; Avettand-Fenoel, V.; Venet, A.; et al. Intensive five-drug antiretroviral therapy regimen versus standard triple-drug therapy during primary HIV-1 infection (OPTIPRIM-ANRS 147): A randomised, open-label, phase 3 trial. Lancet Infect. Dis. 2015, 15, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Fidler, S.; Porter, K.; Ewings, F.; Frater, J.; Ramjee, G.; Cooper, D.; Rees, H.; Fisher, M.; Schechter, M.; Kaleebu, P.; et al. Short-course antiretroviral therapy in primary HIV infection. N. Engl. J. Med. 2013, 368, 207–217. [Google Scholar]
- Yerly, S.; Kaiser, L.; Perneger, T.V.; Cone, R.W.; Opravil, M.; Chave, J.P.; Furrer, H.; Hirschel, B.; Perrin, L. Time of initiation of antiretroviral therapy: Impact on HIV-1 viraemia. The Swiss HIV Cohort Study. AIDS 2000, 14, 243–249. [Google Scholar] [CrossRef]
- Grijsen, M.L.; Steingrover, R.; Wit, F.W.; Jurriaans, S.; Verbon, A.; Brinkman, K.; van der Ende, M.E.; Soetekouw, R.; de Wolf, F.; Lange, J.M.; et al. No treatment versus 24 or 60 weeks of antiretroviral treatment during primary HIV infection: The randomized Primo-SHM trial. PLoS Med. 2012, 9, e1001196. [Google Scholar] [CrossRef]
- Yerly, S.; Perneger, T.V.; Vora, S.; Hirschel, B.; Perrin, L. Decay of cell-associated HIV-1 DNA correlates with residual replication in patients treated during acute HIV-1 infection. AIDS 2000, 14, 2805–2812. [Google Scholar] [CrossRef]
- Ananworanich, J.; Schuetz, A.; Vandergeeten, C.; Sereti, I.; de Souza, M.; Rerknimitr, R.; Dewar, R.; Marovich, M.; van Griensven, F.; Sekaly, R.; et al. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS ONE 2012, 7, e33948. [Google Scholar] [CrossRef]
- Lampinen, T.M.; Critchlow, C.W.; Kuypers, J.M.; Hurt, C.S.; Nelson, P.J.; Hawes, S.E.; Coombs, R.W.; Holmes, K.K.; Kiviat, N.B. Association of antiretroviral therapy with detection of HIV-1 RNA and DNA in the anorectal mucosa of homosexual men. AIDS 2000, 14, F69–F75. [Google Scholar] [CrossRef]
- Belmonte, L.; Olmos, M.; Fanin, A.; Parodi, C.; Bare, P.; Concetti, H.; Perez, H.; de Bracco, M.M.; Cahn, P. The intestinal mucosa as a reservoir of HIV-1 infection after successful HAART. AIDS 2007, 21, 2106–2108. [Google Scholar] [CrossRef]
- Laanani, M.; Ghosn, J.; Essat, A.; Melard, A.; Seng, R.; Gousset, M.; Panjo, H.; Mortier, E.; Girard, P.M.; Goujard, C.; et al. Impact of the Timing of Initiation of Antiretroviral Therapy During Primary HIV-1 Infection on the Decay of Cell-Associated HIV-DNA. Clin Infect Dis 2015, 60, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Mexas, A.M.; Graf, E.H.; Pace, M.J.; Yu, J.J.; Papasavvas, E.; Azzoni, L.; Busch, M.P.; Di Mascio, M.; Foulkes, A.S.; Migueles, S.A.; et al. Concurrent measures of total and integrated HIV DNA monitor reservoirs and ongoing replication in eradication trials. AIDS 2012, 26, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Z.; Gandhi, R.T. The sooner, the better: More evidence that early antiretroviral therapy lowers viral reservoirs in HIV-infected infants. J. Infect. Dis. 2014, 210, 1519–1522. [Google Scholar] [CrossRef] [PubMed]
- Gantner, P.; Barnig, C.; Partisani, M.; Lee, G.Q.; Beck-Wirth, G.; Faller, J.P.; Martinot, M.; Mosheni-Zadeh, M.; Cheneau, C.; Batard, M.L.; et al. Distribution and reduction magnitude of HIV-DNA burden in CD4+ T cell subsets depend on art initiation timing. AIDS 2018, 32, 921–926. [Google Scholar] [CrossRef]
- Murray, J.M.; Zaunders, J.; Emery, S.; Cooper, D.A.; Hey-Nguyen, W.J.; Koelsch, K.K.; Kelleher, A.D. HIV dynamics linked to memory CD4+ T cell homeostasis. PLoS ONE 2017, 12, e0186101. [Google Scholar] [CrossRef]
- McHantaf, G.; Cheret, A.; Melard, A.; Essat, A.; Gardiennet, E.; Bauer, R.; Charre, C.; Meiffredy, V.; Piroth, L.; Goujard, C.; et al. The build-up of stock of stable integrated proviruses overtime explains the difficulty in reducing HIV-1 DNA levels when treatment is initiated at the chronic stage of the infection. J. Virus Erad. 2023, 9, 100357. [Google Scholar] [CrossRef]
- Lisziewicz, J.; Rosenberg, E.; Lieberman, J.; Jessen, H.; Lopalco, L.; Siliciano, R.; Walker, B.; Lori, F. Control of HIV despite the discontinuation of antiretroviral therapy. N. Engl. J. Med. 1999, 340, 1683–1684. [Google Scholar] [CrossRef]
- Hocqueloux, L.; Prazuck, T.; Avettand-Fenoel, V.; Lafeuillade, A.; Cardon, B.; Viard, J.P.; Rouzioux, C. Long-term immunovirologic control following antiretroviral therapy interruption in patients treated at the time of primary HIV-1 infection. Aids 2010, 24, 1598–1601. [Google Scholar] [CrossRef]
- Goujard, C.; Girault, I.; Rouzioux, C.; Lecuroux, C.; Deveau, C.; Chaix, M.L.; Jacomet, C.; Talamali, A.; Delfraissy, J.F.; Venet, A.; et al. HIV-1 control after transient antiretroviral treatment initiated in primary infection: Role of patient characteristics and effect of therapy. Antivir. Ther. 2012, 17, 1001–1009. [Google Scholar] [CrossRef]
- Saez-Cirion, A.; Bacchus, C.; Hocqueloux, L.; Avettand-Fenoel, V.; Girault, I.; Lecuroux, C.; Potard, V.; Versmisse, P.; Melard, A.; Prazuck, T.; et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013, 9, e1003211. [Google Scholar] [CrossRef]
- Steingrover, R.; Pogany, K.; Fernandez Garcia, E.; Jurriaans, S.; Brinkman, K.; Schuitemaker, H.; Miedema, F.; Lange, J.M.; Prins, J.M. HIV-1 viral rebound dynamics after a single treatment interruption depends on time of initiation of highly active antiretroviral therapy. AIDS 2008, 22, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Steingrover, R.; Garcia, E.F.; van Valkengoed, I.G.; Bekker, V.; Bezemer, D.; Kroon, F.P.; Dekker, L.; Prins, M.; de Wolf, F.; Lange, J.M.; et al. Transient lowering of the viral set point after temporary antiretroviral therapy of primary HIV type 1 infection. AIDS Res. Hum. Retroviruses 2010, 26, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.P.; Hurst, J.; Stohr, W.; Robinson, N.; Brown, H.; Fisher, M.; Kinloch, S.; Cooper, D.; Schechter, M.; Tambussi, G.; et al. HIV-1 DNA predicts disease progression and post-treatment virological control. Elife 2014, e03821. [Google Scholar] [CrossRef] [PubMed]
- Gunst, J.D.; Gohil, J.; Li, J.Z.; Bosch, R.J.; White Catherine Seamon, A.; Chun, T.W.; Mothe, B.; Gittens, K.; Praiss, L.; De Scheerder, M.A.; et al. Time to HIV viral rebound and frequency of post-treatment control after analytical interruption of antiretroviral therapy: An individual data-based meta-analysis of 24 prospective studies. Nat. Commun. 2025, 16, 906. [Google Scholar] [CrossRef]
- Lambotte, O.; Boufassa, F.; Madec, Y.; Nguyen, A.; Goujard, C.; Meyer, L.; Rouzioux, C.; Venet, A.; Delfraissy, J.F. HIV controllers: A homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2005, 41, 1053–1056. [Google Scholar] [CrossRef]
- Dalmasso, C.; Carpentier, W.; Meyer, L.; Rouzioux, C.; Goujard, C.; Chaix, M.L.; Lambotte, O.; Avettand-Fenoel, V.; Le Clerc, S.; de Senneville, L.D.; et al. Distinct genetic loci control plasma HIV-RNA and cellular HIV-DNA levels in HIV-1 infection: The ANRS Genome Wide Association 01 study. PLoS ONE 2008, 3, e3907. [Google Scholar] [CrossRef]
- Sodora, D.L.; Ross, T.M. Simian immunodeficiency virus pathogenesis. Curr. HIV Res. 2009, 7, 1. [Google Scholar] [CrossRef]
- Nchinda, N.; Elangovan, R.; Yun, J.; Dickson-Tetteh, L.; Kirtley, S.; Hemelaar, J.; WHO-UNAIDS Network for HIV Isolation and Characterisation. Characterisation, Global associations of key populations with HIV-1 recombinants: A systematic review, global survey, and individual participant data meta-analysis. Front. Public Health 2023, 11, 1153638. [Google Scholar] [CrossRef]
- Bimela, J.S.; Nanfack, A.J.; Yang, P.; Dai, S.; Kong, X.P.; Torimiro, J.N.; Duerr, R. Antiretroviral Imprints and Genomic Plasticity of HIV-1 pol in Non-clade B: Implications for Treatment. Front. Microbiol. 2021, 12, 812391. [Google Scholar] [CrossRef]
- Visseaux, B.; Assoumou, L.; Mahjoub, N.; Grude, M.; Trabaud, M.A.; Raymond, S.; Wirden, M.; Morand-Joubert, L.; Roussel, C.; Montes, B.; et al. Surveillance of HIV-1 primary infections in France from 2014 to 2016: Toward stable resistance, but higher diversity, clustering and virulence? J. Antimicrob. Chemother. 2020, 75, 183–193. [Google Scholar] [CrossRef]
- Haggblom, A.; Svedhem, V.; Singh, K.; Sonnerborg, A.; Neogi, U. Virological failure in patients with HIV-1 subtype C receiving antiretroviral therapy: An analysis of a prospective national cohort in Sweden. Lancet HIV 2016, 3, e166–e174. [Google Scholar] [CrossRef] [PubMed]
- Tremeaux, P.; Lemoine, F.; Melard, A.; Gousset, M.; Boufassa, F.; Orr, S.; Monceaux, V.; Gascuel, O.; Lambotte, O.; Hocqueloux, L.; et al. In-Depth Characterization of Full-Length Archived Viral Genomes after Nine Years of Posttreatment HIV Control. Microbiol. Spectr. 2023, 11, e0326722. [Google Scholar] [CrossRef] [PubMed]
- Evering, T.H.; Mehandru, S.; Racz, P.; Tenner-Racz, K.; Poles, M.A.; Figueroa, A.; Mohri, H.; Markowitz, M. Absence of HIV-1 evolution in the gut-associated lymphoid tissue from patients on combination antiviral therapy initiated during primary infection. PLoS Pathog. 2012, 8, e1002506. [Google Scholar] [CrossRef] [PubMed]
- Imamichi, H.; Dewar, R.L.; Adelsberger, J.W.; Rehm, C.A.; O’Doherty, U.; Paxinos, E.E.; Fauci, A.S.; Lane, H.C. Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. Proc. Natl. Acad. Sci. USA 2016, 113, 8783–8788. [Google Scholar] [CrossRef]
- Baxter, A.E.; O’Doherty, U.; Kaufmann, D.E. Beyond the replication-competent HIV reservoir: Transcription and translation-competent reservoirs. Retrovirology 2018, 15, 18. [Google Scholar] [CrossRef]
- Hurst, J.; Hoffmann, M.; Pace, M.; Williams, J.P.; Thornhill, J.; Hamlyn, E.; Meyerowitz, J.; Willberg, C.; Koelsch, K.K.; Robinson, N.; et al. Immunological biomarkers predict HIV-1 viral rebound after treatment interruption. Nat. Commun. 2015, 6, 8495. [Google Scholar] [CrossRef]
- Haissman, J.M.; Vestergaard, L.S.; Sembuche, S.; Erikstrup, C.; Mmbando, B.; Mtullu, S.; Lemnge, M.M.; Gerstoft, J.; Ullum, H. Plasma cytokine levels in Tanzanian HIV-1-infected adults and the effect of antiretroviral treatment. J. Acquir. Immune Defic. Syndr. 2009, 52, 493–497. [Google Scholar] [CrossRef]
- Muema, D.M.; Akilimali, N.A.; Ndumnego, O.C.; Rasehlo, S.S.; Durgiah, R.; Ojwach, D.B.A.; Ismail, N.; Dong, M.; Moodley, A.; Dong, K.L.; et al. Association between the cytokine storm, immune cell dynamics, and viral replicative capacity in hyperacute HIV infection. BMC Med. 2020, 18, 81. [Google Scholar] [CrossRef]
- Allers, K.; Puyskens, A.; Epple, H.J.; Schurmann, D.; Hofmann, J.; Moos, V.; Schneider, T. The effect of timing of antiretroviral therapy on CD4 T-cell reconstitution in the intestine of HIV-infected patients. Mucosal. Immunol. 2015, 9, 265–274. [Google Scholar] [CrossRef]
- Krebs, S.J.; Ananworanich, J. Immune activation during acute HIV infection and the impact of early antiretroviral therapy. Curr. Opin. HIV AIDS 2016, 11, 163–172. [Google Scholar] [CrossRef]
- Ipp, H.; Zemlin, A.E.; Erasmus, R.T.; Glashoff, R.H. Role of inflammation in HIV-1 disease progression and prognosis. Crit. Rev. Clin. Lab. Sci. 2014, 51, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Herout, S.; Mandorfer, M.; Breitenecker, F.; Reiberger, T.; Grabmeier-Pfistershammer, K.; Rieger, A.; Aichelburg, M.C. Impact of Early Initiation of Antiretroviral Therapy in Patients with Acute HIV Infection in Vienna, Austria. PLoS ONE 2016, 11, e0152910. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.J.; Silveira, E.; Amancha, P.K.; Byrareddy, S.N.; Gumber, S.; Chang, K.T.; Ansari, A.A.; Villinger, F. Early initiation of antiretroviral treatment postSIV infection does not resolve lymphoid tissue activation. AIDS 2017, 31, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Lederman, M.M.; Funderburg, N.T.; Sekaly, R.P.; Klatt, N.R.; Hunt, P.W. Residual Immune Dysregulation Syndrome in Treated HIV infection. Adv. Immunol. 2013, 119, 51–83. [Google Scholar]
- Novelli, S.; Lecuroux, C.; Goujard, C.; Reynes, J.; Villemant, A.; Blum, L.; Essat, A.; Avettand-Fenoel, V.; Launay, O.; Molina, J.M.; et al. Persistence of monocyte activation under treatment in people followed since acute HIV-1 infection relative to participants at high or low risk of HIV infection. EBioMedicine 2020, 62, 103129. [Google Scholar] [CrossRef]
- Wang, S.X.; Ho, E.L.; Grill, M.; Lee, E.; Peterson, J.; Robertson, K.; Fuchs, D.; Sinclair, E.; Price, R.W.; Spudich, S. Peripheral neuropathy in primary HIV infection associates with systemic and central nervous system immune activation. J. Acquir. Immune Defic. Syndr. 2014, 66, 303–310. [Google Scholar] [CrossRef]
- Grijsen, M.; Koster, G.; van Vonderen, M.; van Kasteren, M.; Kootstra, G.; Steingrover, R.; de Wolf, F.; Prins, J.; Nieuwkerk, P. Temporary antiretroviral treatment during primary HIV-1 infection has a positive impact on health-related quality of life: Data from the Primo-SHM cohort study. HIV Med. 2012, 13, 630–635. [Google Scholar] [CrossRef]
- Autran, B.; Carcelain, G.; Li, T.S.; Blanc, C.; Mathez, D.; Tubiana, R.; Katlama, C.; Debre, P.; Leibowitch, J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science 1997, 277, 112–116. [Google Scholar] [CrossRef]
- Rosenberg, E.S.; Billingsley, J.M.; Caliendo, A.M.; Boswell, S.L.; Sax, P.E.; Kalams, S.A.; Walker, B.D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 1997, 278, 1447–1450. [Google Scholar] [CrossRef]
- Hecht, F.M.; Wang, L.; Collier, A.; Little, S.; Markowitz, M.; Margolick, J.; Kilby, J.M.; Daar, E.; Conway, B.; Holte, S. A multicenter observational study of the potential benefits of initiating combination antiretroviral therapy during acute HIV infection. J. Infect. Dis. 2006, 194, 725–733. [Google Scholar] [CrossRef]
- Oxenius, A.; Price, D.A.; Easterbrook, P.J.; O’Callaghan, C.A.; Kelleher, A.D.; Whelan, J.A.; Sontag, G.; Sewell, A.K.; Phillips, R.E. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc. Natl. Acad. Sci. USA 2000, 97, 3382–3387. [Google Scholar] [CrossRef] [PubMed]
- Deleage, C.; Schuetz, A.; Alvord, W.G.; Johnston, L.; Hao, X.P.; Morcock, D.R.; Rerknimitr, R.; Fletcher, J.L.; Puttamaswin, S.; Phanuphak, N.; et al. Impact of early cART in the gut during acute HIV infection. JCI Insight 2016, 1, e87065. [Google Scholar] [CrossRef] [PubMed]
- Cellerai, C.; Harari, A.; Stauss, H.; Yerly, S.; Geretti, A.M.; Carroll, A.; Yee, T.; Ainsworth, J.; Williams, I.; Sweeney, J.; et al. Early and prolonged antiretroviral therapy is associated with an HIV-1-specific T-cell profile comparable to that of long-term non-progressors. PLoS ONE 2011, 6, e18164. [Google Scholar] [CrossRef]
- Lecuroux, C.; Girault, I.; Boutboul, F.; Urrutia, A.; Goujard, C.; Meyer, L.; Lambotte, O.; Chaix, M.L.; Martinez, V.; Autran, B.; et al. Antiretroviral therapy initiation during primary HIV infection enhances both CD127 expression and the proliferative capacity of HIV-specific CD8+ T cells. AIDS 2009, 23, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Ndhlovu, Z.M.; Kazer, S.W.; Nkosi, T.; Ogunshola, F.; Muema, D.M.; Anmole, G.; Swann, S.A.; Moodley, A.; Dong, K.; Reddy, T.; et al. Augmentation of HIV-specific T cell function by immediate treatment of hyperacute HIV-1 infection. Sci. Transl. Med. 2019, 11, eaau0528. [Google Scholar] [CrossRef]
- Hansen, S.G.; Piatak, M., Jr.; Ventura, A.B.; Hughes, C.M.; Gilbride, R.M.; Ford, J.C.; Oswald, K.; Shoemaker, R.; Li, Y.; Lewis, M.S.; et al. Immune clearance of highly pathogenic SIV infection. Nature 2013, 502, 100–104. [Google Scholar] [CrossRef]
- Passaes, C.; Desjardins, D.; Chapel, A.; Monceaux, V.; Lemaitre, J.; Melard, A.; Perdomo-Celis, F.; Planchais, C.; Gourves, M.; Dimant, N.; et al. Early antiretroviral therapy favors post-treatment SIV control associated with the expansion of enhanced memory CD8(+) T-cells. Nat. Commun. 2024, 15, 178. [Google Scholar] [CrossRef]
- Thornhill, J.; Inshaw, J.; Oomeer, S.; Kaleebu, P.; Cooper, D.; Ramjee, G.; Schechter, M.; Tambussi, G.; Fox, J.; Miro, J.M.; et al. Enhanced normalisation of CD4/CD8 ratio with early antiretroviral therapy in primary HIV infection. J. Int. AIDS Soc. 2014, 17 (Suppl. 3), 19480. [Google Scholar] [CrossRef]
- Cao, W.; Mehraj, V.; Trottier, B.; Baril, J.G.; Leblanc, R.; Lebouche, B.; Cox, J.; Tremblay, C.; Lu, W.; Singer, J.; et al. Early Initiation Rather Than Prolonged Duration of Antiretroviral Therapy in HIV Infection Contributes to the Normalization of CD8 T-Cell Counts. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 62, 250–257. [Google Scholar] [CrossRef]
- Sereti, I.; Krebs, S.J.; Phanuphak, N.; Fletcher, J.L.; Slike, B.; Pinyakorn, S.; O’Connell, R.J.; Rupert, A.; Chomont, N.; Valcour, V.; et al. Persistent, Albeit Reduced, Chronic Inflammation in Persons Starting Antiretroviral Therapy in Acute HIV Infection. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2017, 64, 124–131. [Google Scholar] [CrossRef]
- Vasan, S.; Poles, M.A.; Horowitz, A.; Siladji, E.E.; Markowitz, M.; Tsuji, M. Function of NKT cells, potential anti-HIV effector cells, are improved by beginning HAART during acute HIV-1 infection. Int. Immunol. 2007, 19, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Killian, M.S.; Fujimura, S.H.; Hecht, F.M.; Levy, J.A. Similar changes in plasmacytoid dendritic cell and CD4 T-cell counts during primary HIV-1 infection and treatment. AIDS 2006, 20, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Planchais, C.; Hocqueloux, L.; Ibanez, C.; Gallien, S.; Copie, C.; Surenaud, M.; Kok, A.; Lorin, V.; Fusaro, M.; Delfau-Larue, M.H.; et al. Early Antiretroviral Therapy Preserves Functional Follicular Helper T and HIV-Specific B Cells in the Gut Mucosa of HIV-1-Infected Individuals. J. Immunol. 2018, 200, 3519–3529. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.S.; Pinyakorn, S.; Akapirat, S.; Pattanachaiwit, S.; Fletcher, J.L.; Chomchey, N.; Kroon, E.D.; Ubolyam, S.; Michael, N.L.; Robb, M.L.; et al. Initiation of Antiretroviral Therapy During Acute HIV-1 Infection Leads to a High Rate of Nonreactive HIV Serology. Clin. Infect. Dis. 2016, 63, 555–561. [Google Scholar] [CrossRef]
- Colby, D.J.; Trautmann, L.; Pinyakorn, S.; Leyre, L.; Pagliuzza, A.; Kroon, E.; Rolland, M.; Takata, H.; Buranapraditkun, S.; Intasan, J.; et al. Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat. Med. 2018, 24, 923–926. [Google Scholar] [CrossRef]
- Pantazis, N.; Touloumi, G.; Vanhems, P.; Gill, J.; Bucher, H.C.; Porter, K. The effect of antiretroviral treatment of different durations in primary HIV infection. AIDS 2008, 22, 2441–2450. [Google Scholar] [CrossRef]
- Novelli, S.; Delobel, P.; Bouchaud, O.; Avettand-Fenoel, V.; Fialaire, P.; Cabie, A.; Souala, F.; Raffi, F.; Catalan, P.; Weiss, L.; et al. Enhanced immunovirological response in women compared to men after antiretroviral therapy initiation during acute and early HIV-1 infection: Results from a longitudinal study in the French ANRS Primo cohort. J. Int. AIDS Soc. 2020, 23, e25485. [Google Scholar] [CrossRef]
- Imamichi, H.; Smith, M.; Adelsberger, J.W.; Izumi, T.; Scrimieri, F.; Sherman, B.T.; Rehm, C.A.; Imamichi, T.; Pau, A.; Catalfamo, M.; et al. Defective HIV-1 proviruses produce viral proteins. Proc. Natl. Acad. Sci. USA 2020, 117, 3704–3710. [Google Scholar] [CrossRef]
- Ambrosioni, J.; Junier, T.; Delhumeau, C.; Calmy, A.; Hirschel, B.; Zdobnov, E.; Kaiser, L.; Yerly, S. Impact of highly active antiretroviral therapy on the molecular epidemiology of newly diagnosed HIV infections. AIDS 2012, 26, 2079–2086. [Google Scholar] [CrossRef]
- Brenner, B.G.; Roger, M.; Routy, J.P.; Moisi, D.; Ntemgwa, M.; Matte, C.; Baril, J.G.; Thomas, R.; Rouleau, D.; Bruneau, J.; et al. High rates of forward transmission events after acute/early HIV-1 infection. J. Infect. Dis. 2007, 195, 951–959. [Google Scholar] [CrossRef]
- O’Brien, M.; Markowitz, M. Should we treat acute HIV infection? Curr. HIV/AIDS Rep. 2012, 9, 101–110. [Google Scholar] [CrossRef]
- Baeten, J.M.; Kahle, E.; Lingappa, J.R.; Coombs, R.W.; Delany-Moretlwe, S.; Nakku-Joloba, E.; Mugo, N.R.; Wald, A.; Corey, L.; Donnell, D.; et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci. Transl. Med. 2011, 3, 77ra29. [Google Scholar] [CrossRef] [PubMed]
- Pilcher, C.D.; Tien, H.C.; Eron, J.J., Jr.; Vernazza, P.L.; Leu, S.Y.; Stewart, P.W.; Goh, L.E.; Cohen, M.S. Brief but efficient: Acute HIV infection and the sexual transmission of HIV. J. Infect. Dis. 2004, 189, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Pullium, J.K.; Adams, D.R.; Jackson, E.; Kim, C.N.; Smith, D.K.; Janssen, R.; Gould, K.; Folks, T.M.; Butera, S.; Otten, R.A. Pig-tailed macaques infected with human immunodeficiency virus (HIV) type 2GB122 or simian/HIV89.6p express virus in semen during primary infection: New model for genital tract shedding and transmission. J. Infect. Dis. 2001, 183, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Curtis, T.J.; Rodger, A.J.; Burns, F.; Nardone, A.; Copas, A.; Wayal, S. Patterns of sexualised recreational drug use and its association with risk behaviours and sexual health outcomes in men who have sex with men in London, UK: A comparison of cross-sectional studies conducted in 2013 and 2016. Sex Transm. Infect. 2020, 96, 197–203. [Google Scholar] [CrossRef]
- Hegazi, A.; Lee, M.J.; Whittaker, W.; Green, S.; Simms, R.; Cutts, R.; Nagington, M.; Nathan, B.; Pakianathan, M.R. Chemsex and the city: Sexualised substance use in gay bisexual and other men who have sex with men attending sexual health clinics. Int. J. STD AIDS 2017, 28, 362–366. [Google Scholar] [CrossRef]
- Maxwell, S.; Shahmanesh, M.; Gafos, M. Chemsex behaviours among men who have sex with men: A systematic review of the literature. Int. J. Drug Policy 2019, 63, 74–89. [Google Scholar] [CrossRef]
- Gonzalez-Baeza, A.; Dolengevich-Segal, H.; Perez-Valero, I.; Cabello, A.; Tellez, M.J.; Sanz, J.; Perez-Latorre, L.; Bernardino, J.I.; Troya, J.; De La Fuente, S.; et al. Sexualized Drug Use (Chemsex) Is Associated with High-Risk Sexual Behaviors and Sexually Transmitted Infections in HIV-Positive Men Who Have Sex with Men: Data from the U-SEX GESIDA 9416 Study. AIDS Patient Care STDS 2018, 32, 112–118. [Google Scholar] [CrossRef]
- Pufall, E.L.; Kall, M.; Shahmanesh, M.; Nardone, A.; Gilson, R.; Delpech, V.; Ward, H.; Positive Voices Study Group; Hart, G.; Anderson, J.; et al. Sexualized drug use (‘chemsex’) and high-risk sexual behaviours in HIV-positive men who have sex with men. HIV Med. 2018, 19, 261–270. [Google Scholar] [CrossRef]
- Molina, J.M.; Capitant, C.; Spire, B.; Pialoux, G.; Cotte, L.; Charreau, I.; Tremblay, C.; Le Gall, J.M.; Cua, E.; Pasquet, A.; et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. N. Engl. J. Med. 2015, 373, 2237–2246. [Google Scholar] [CrossRef]
- Liu, A.Y.; Cohen, S.E.; Vittinghoff, E.; Anderson, P.L.; Doblecki-Lewis, S.; Bacon, O.; Chege, W.; Postle, B.S.; Matheson, T.; Amico, K.R.; et al. Preexposure Prophylaxis for HIV Infection Integrated With Municipal- and Community-Based Sexual Health Services. JAMA Intern. Med. 2016, 176, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Baltes, V.; de Boissieu, P.; Champenois, K.; Luan, L.; Seng, R.; Essat, A.; Novelli, S.; Spire, B.; Molina, J.M.; Goujard, C.; et al. Sexual behaviour and STIs among MSM living with HIV in the PrEP era: The French ANRS PRIMO cohort study. J. Int. AIDS Soc. 2024, 27, e26226. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Calabrese, C. Socio-behavioral factors related to PrEP non-adherence among gay male PrEP users living in California and New York: A behavioral theory informed approach. J. Behav. Med. 2022, 45, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.; Van Dijck, C.; Florence, E. Facing increased sexually transmitted infection incidence in HIV preexposure prophylaxis cohorts: What are the underlying determinants and what can be done? Curr. Opin. Infect. Dis. 2020, 33, 51–58. [Google Scholar] [CrossRef]
- Methy, N.; Meyer, L.; Bajos, N.; Velter, A. Generational analysis of trends in unprotected sex in France among men who have sex with men: The major role of context-driven evolving patterns. PLoS ONE 2017, 12, e0171493. [Google Scholar]
- Moschese, D.; Lazzarin, S.; Colombo, M.L.; Caruso, F.; Giacomelli, A.; Antinori, S.; Gori, A. Breakthrough Acute HIV Infections among Pre-Exposure Prophylaxis Users with High Adherence: A Narrative Review. Viruses 2024, 16, 951. [Google Scholar] [CrossRef]
- Wyl, V.; Gianella, S.; Fischer, M.; Niederoest, B.; Kuster, H.; Battegay, M.; Bernasconi, E.; Cavassini, M.; Rauch, A.; Hirschel, B.; et al. Early antiretroviral therapy during primary HIV-1 infection results in a transient reduction of the viral setpoint upon treatment interruption. PLoS ONE 2011, 6, e27463. [Google Scholar]
- Sugiyama, F.H.C.; Dietz, L.L.; Sogaard, O.S. Utilizing immunotherapy towards achieving a functional cure for HIV-1. Curr. Opin. HIV AIDS 2024, 19, 187–193. [Google Scholar] [CrossRef]
- Program, U.M.H.R. Safety and Efficacy of Neutralizing Antibodies and Vaccination for Induction of HIV Remission (RV582). Available online: https://clinicaltrials.gov/study/NCT05769569?cond=acute%20hiv&intr=bNAbs&rank=3 (accessed on 2 March 2025).
- NIAID. Evaluation of Safety, Immunogenicity and Efficacy of a Triple Immune Regimen in Adults Initiated on ART During Acute HIV-1. Available online: https://clinicaltrials.gov/study/NCT06071767?cond=acute%20hiv&intr=bNAbs&rank=2 (accessed on 2 March 2025).
- ANRS. Phase II Trial of ART + Dual bNAbs vs. ART + Placebo During Primary HIV-1 Infection-impact on Post-ART Control (RHIEVIERA-02). Available online: https://clinicaltrials.gov/study/NCT05300035?cond=hiv&intr=bNAbs&rank=3 (accessed on 2 March 2025).
| Patient treated at the time of acute infection | Patient treated at the chronic phase |
| Transmission is typically homogeneous, involving a single virion with dominant CCR5 viral tropism | Both R5 and X4 variants emerging during infection and associated with rapid disease progression |
| Preventing escape mutations and limiting viral diversity Less defective proviral gene insertions, limiting immune activation | Large viral diversity |
| Lower virologic set point | |
| Best reduction of residual viremia | |
| The treatment quickly restores and protects the immune system: Protection of the different polyfunctionalities of TCD4+ and TCD8+, limit apoptosis by reducing activation and viral diversity | More limited immune restoration: From the surviving TCD4+ and TCD8+ clones to achieve the different polyfunctionalities |
| Major difference in the quantity and quality of the reservoir | |
| Short Half-Life Reservoir: TTM infection and TCM protection Better reduction in total HIV-1 DNA and integrated HIV-DNA Prevents the establishment of the stable form of integrated proviral HIV-1 DNA that is less prone to elimination | Long Half-Life Reservoir: TCM Infection Blood reservoirs are mainly composed of stable proviruses in long-lived quiescent cells and are unaffected by ART |
| Control of replication in post-treatment, VISCONTI: A preserved immune system that allows effective control of replication and suppression of the viral reservoir after treatment discontinuation. | No control of replication: An immune system unable to control replication after treatment discontinuation. |
| Optimal conditions for future cure trials | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chéret, A. Acute HIV-1 Infection: Paradigm and Singularity. Viruses 2025, 17, 366. https://doi.org/10.3390/v17030366
Chéret A. Acute HIV-1 Infection: Paradigm and Singularity. Viruses. 2025; 17(3):366. https://doi.org/10.3390/v17030366
Chicago/Turabian StyleChéret, Antoine. 2025. "Acute HIV-1 Infection: Paradigm and Singularity" Viruses 17, no. 3: 366. https://doi.org/10.3390/v17030366
APA StyleChéret, A. (2025). Acute HIV-1 Infection: Paradigm and Singularity. Viruses, 17(3), 366. https://doi.org/10.3390/v17030366





