Epidemiology and Emerging Trends of Zoonotic Viral Diseases of Pigs in India
Abstract
:1. Introduction
2. Major Zoonotic Viral Diseases of Pigs in India
2.1. Japanese Encephalitis Virus (JEV)
2.2. Hepatitis E Virus (HEV)
2.3. Swine Influenza Virus (SIV)
2.4. Rotavirus (RV)
3. Emerging and Sporadically Reported Zoonotic Viruses of Pigs in India
3.1. Chandipura Virus (CHPV)
3.2. Rabies Virus
3.3. Pseudorabies or ADV (SuHV-1)
3.4. Porcine Astrovirus (PAstV)
3.5. Torque Teno Sus Virus (TTSuV)
4. Other Emerging Viruses Not Reported in Pigs in India but Circulating in Neighboring Countries
4.1. Swine Acute Diarrhea Syndrome Coronavirus (SADS-CoV) and SARS-CoV-2
4.2. Nipah Virus (NiV)
5. Advancements in Molecular and Serological Tools for Zoonotic Virus Detection and Surveillance
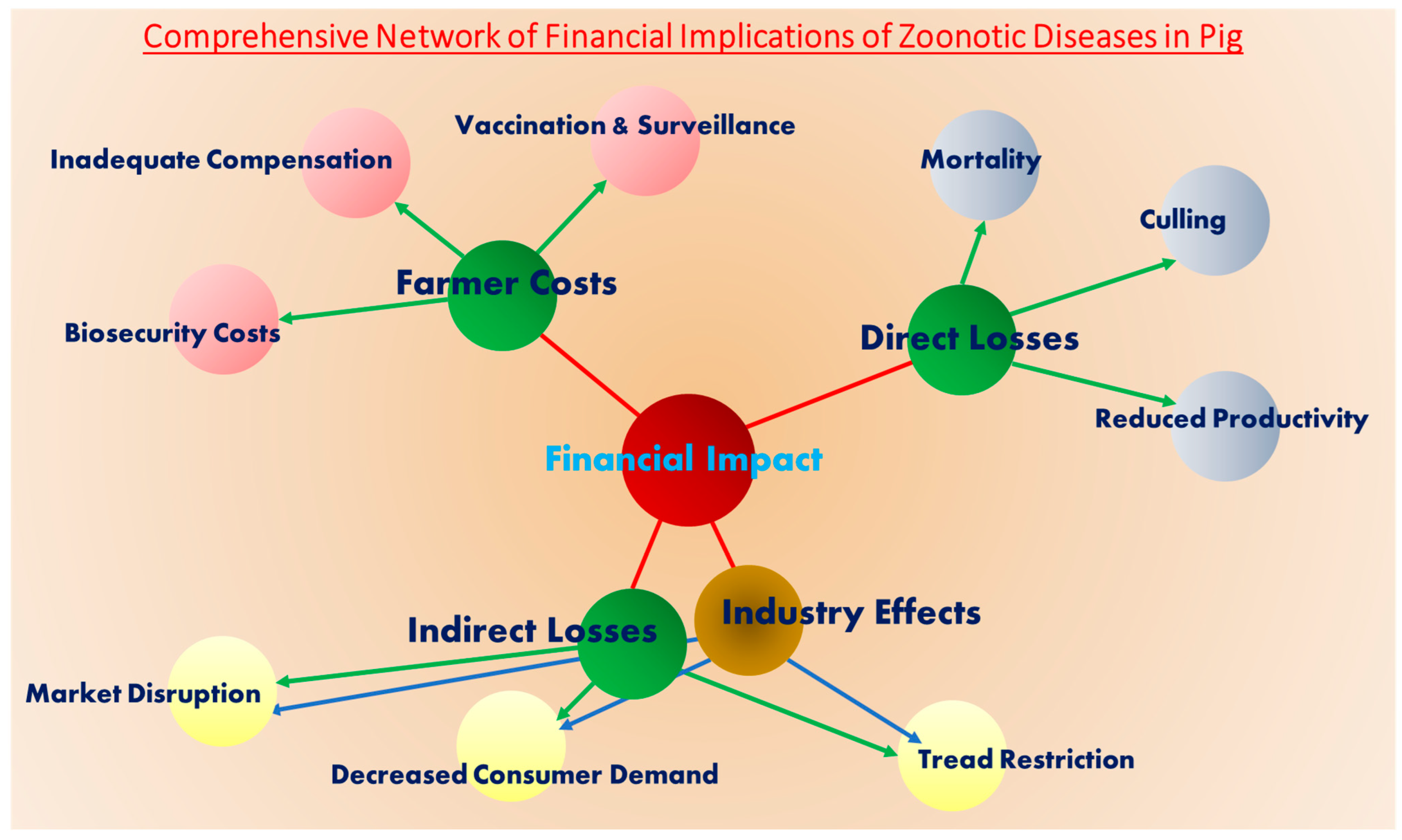
6. Financial Implications of Viral Zoonotic Diseases in Pig Farming
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Viruses and Diseases | |
| JEV | Japanese Encephalitis Virus |
| HEV | Hepatitis E Virus |
| NiV | Nipah Virus |
| SIV | Swine Influenza Virus |
| ADV | Aujeszky’s Disease Virus (also known as Pseudorabies Virus—PRV) |
| SuHV-1 | Suid Herpesvirus 1 |
| PAstV | Porcine Astrovirus |
| TTSuV | Torque Teno Sus Virus |
| CHPV | Chandipura Virus |
| SADS-CoV | Swine Acute Diarrhea Syndrome Coronavirus |
| PCVAD | Porcine Circovirus-Associated Diseases |
| COVID-19 | Coronavirus Disease 2019 |
| Laboratory and Diagnostic Techniques | |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| RNA-PAGE | RNA Polyacrylamide Gel Electrophoresis |
| FAT | Fluorescent Antibody Test |
| RFLP | Restriction Fragment Length Polymorphism |
| Immunochromatographic Assay | A rapid test technique for detecting specific antigens or antibodies |
| Scientific Institutions and Vaccines | |
| ICAR-IVRI | Indian Council of Agricultural Research—Indian Veterinary Research Institute |
| SA-14-14-2 | A live-attenuated Japanese Encephalitis vaccine strain |
| JENVAC | A Japanese Encephalitis Vaccine |
| Biological and Medical Terms | |
| RNA | Ribonucleic Acid |
| DNA | Deoxyribonucleic Acid |
| HA | Hemagglutinin (a surface protein of viruses, particularly influenza) |
| NA | Neuraminidase (an enzyme found on the surface of influenza viruses) |
| Epidemiology and Pathogenesis | |
| Amplifying Reservoirs | Host species that increase pathogen transmission |
| Dead-End Hosts | Hosts that do not contribute to further transmission of a pathogen |
| Immunocompromised | Individuals with weakened immune systems |
| Pandemic | A global outbreak of an infectious disease |
| Seroepidemiological | Relating to the study of disease patterns through serological (antibody-based) testing |
| Electropherotype Patterns | Distinct genetic patterns of viral RNA visualized through electrophoresis |
| Genomic diversity | Refers to the variations in DNA or RNA sequences among individuals, populations, or species, driven by mutations, recombination, horizontal gene transfer, and epigenetic modifications, influencing evolution, adaptability, and disease dynamics. |
| Torque Teno Virus Subtypes | |
| Iotatorquevirus | A genus within the Anelloviridae family of viruses |
| Kappatorquevirus | Another genus of the Anelloviridae family |
References
- Rahman, M.T.; Sobur, M.A.; Islam, M.S.; Ievy, S.; Hossain, M.J.; El Zowalaty, M.E.; Rahman, A.T.; Ashour, H.M. Zoonotic Diseases: Etiology, Impact and Control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef]
- Glud, H.A.; George, S.; Skovgaard, K.; Larsen, L.E. Zoonotic and reverse zoonotic transmission of viruses between humans and pigs. APMIS 2021, 129, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Lyon, C.J.; Ying, B.; Hu, T. Climate change, its impact on emerging infectious diseases and new technologies to combat the challenge. Emerg. Microbes Infect. 2024, 13, 2356143. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.I.; Lee, Y.M. Japanese encephalitis: The virus and vaccines. Hum. Vaccin. Immunother. 2014, 10, 263–279. [Google Scholar] [CrossRef]
- Chakravarti, S.K.; Sarkar, J.K.; Chakravarty, M.S.; Mukherjee, M.K.; Mukherjee, K.K.; Das, B.C.; Hati, A.K. The first epidemic of Japanese encephalitis studies in India-virological studies. Indian J. Med. Res. 1975, 63, 77–82. [Google Scholar]
- Pearce, J.C.; Learoyd, T.P.; Langendorf, B.J.; Logan, J.G. Japanese encephalitis: The vectors, ecology and potential for expansion. J. Travel. Med. 2018, 25, S16–S26. [Google Scholar] [CrossRef]
- Hameed, M.; Wahaab, A.; Nawaz, M.; Khan, S.; Nazir, J.; Liu, K.; Wei, J.; Ma, Z. Potential Role of Birds in Japanese Encephalitis Virus Zoonotic Transmission and Genotype Shift. Viruses 2021, 13, 357. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Japanese Encephalitis. Available online: https://www.who.int/news-room/fact-sheets/detail/japanese-encephalitis (accessed on 6 August 2024).
- Kulkarni, R.; Sapkal, G.N.; Kaushal, H.; Mourya, D.T. Japanese Encephalitis: A Brief Review on Indian Perspectives. Open Virol. J. 2018, 12, 121–130. [Google Scholar] [CrossRef]
- Pegu, S.R.; Das, P.J.; Sonowal, J.; Sengar, G.S.; Deb, R.; Yadav, A.K.; Rajkhowa, S.; Choudhury, M.; Gulati, B.R.; Gupta, V.K. Japanese Encephalitis Virus Genotype III Strains Detection and Genome Sequencing from Indian Pig and Mosquito Vector. Vaccines 2023, 11, 150. [Google Scholar] [CrossRef]
- Hick, P.M.; Finlaison, D.S.; Parrish, K.; Gu, X.; Hayton, P.; O’Connor, T.; Read, A.; Zhang, J.; Spiers, Z.B.; Pinczowski, P.; et al. Experimental Infections of Pigs with Japanese Encephalitis Virus Genotype 4. Microorganisms 2024, 12, 2163. [Google Scholar] [CrossRef]
- Augustyniak, A.; Pomorska-Mól, M. An Update in Knowledge of Pigs as the Source of Zoonotic Pathogens. Animals 2023, 13, 3281. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, K.L.; Hernández-Triana, L.M.; Banyard, A.C.; Fooks, A.R.; Johnson, N. Japanese encephalitis virus infection, diagnosis and control in domestic animals. Vet. Microbiol. 2017, 201, 85–92. [Google Scholar] [CrossRef]
- Rajaiah, P.; Kumar, A. Japanese encephalitis virus in India: An update on virus genotypes. Indian J. Med. Res. 2022, 156, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Indian Veterinary Research Institute (IVRI). Vaccine Developed. “Vero Cell Based Inactivated JE Vaccine Candidate for Pigs”. 2022. Available online: https://www.ivri.nic.in/Technologies/Vaccines.aspx (accessed on 2 March 2025).
- Arankalle, V.A.; Chobe, L.P.; Joshi, M.V.; Chadha, M.S.; Kundu, B.; Walimbe, A.M. Human and swine hepatitis E viruses from Western India belong to different genotypes. J. Hepatol. 2002, 36, 417–425. [Google Scholar] [CrossRef]
- Aslan, A.T.; Balaban, H.Y. Hepatitis E virus: Epidemiology, diagnosis, clinical manifestations, and treatment. World J. Gastroenterol. 2020, 26, 5543. [Google Scholar] [CrossRef]
- Purdy, M.A.; Harrison, T.J.; Jameel, S.; Meng, X.J.; Okamoto, H.; Van der Poel, W.H.M.; Smith, D.B.; ICTV Report Consortium. ICTV virus taxonomy profile: Hepeviridae. J. General. Virol. 2017, 98, 2645–2646. [Google Scholar] [CrossRef]
- Songtanin, B.; Molehin, A.J.; Brittan, K.; Manatsathit, W.; Nugent, K. Hepatitis E Virus Infections: Epidemiology, Genetic Diversity, and Clinical Considerations. Viruses 2023, 15, 1389. [Google Scholar] [CrossRef]
- Meng, X.J. Hepatitis E virus: Animal reservoirs and zoonotic risk. Vet. Microbiol. 2010, 140, 256–265. [Google Scholar] [CrossRef]
- Kasorndorkbua, C.; Guenette, D.K.; Huang, F.F.; Thomas, P.J.; Meng, X.J.; Halbur, P.G. Routes of transmission of swine hepatitis E virus in pigs. J. Clin. Microbiol. 2004, 42, 5047–5052. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wu, X.; Xia, J. Hepatitis E virus infection during pregnancy. Virol. J. 2020, 17, 73. [Google Scholar] [CrossRef]
- Milton, A.A.P.; Das, S.; Ghatak, S.; Srinivas, K.; Angappan, M.; Prasad, M.C.B.; Wahlang, L.; Priya, G.B.; Khan, S.; Sailo, B.; et al. First Seroepidemiological Investigation of Hepatitis E Virus Infection in Backyard Pigs from Northeastern India: Prevalence and Associated Risk Factors. Food Environ. Virol. 2023, 15, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Bansal, M.; Kaur, S.; Deka, D.; Singh, R.; Gill, J.P.S. Seroepidemiology and molecular characterization of hepatitis E virus infection in swine and occupationally exposed workers in Punjab, India. Zoonoses Public Health 2017, 64, 662–672. [Google Scholar] [CrossRef]
- Arankalle, V.A.; Joshi, M.V.; Kulkarni, A.M.; Gandhe, S.S.; Chobe, L.P.; Rautmare, S.S.; Mishra, A.C.; Padbidri, V.S. Prevalence of anti-hepatitis E virus antibodies in different Indian animal species. J. Viral Hepat. 2001, 8, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Chauhan, U.K.; Naik, S.; Anderson, D.; Aggarwal, R. Hepatitis E virus infection among animals in northern India: An unlikely source of human disease. J. Viral Hepat. 2007, 14, 310–317. [Google Scholar] [CrossRef]
- Galwankar, S.; Clem, A. Swine influenza A (H1N1) strikes a potential for global disaster. J. Emerg. Trauma. Shock. 2009, 2, 99–105. [Google Scholar]
- Bakre, A.A.; Jones, L.P.; Kyriakis, C.S.; Hanson, J.M.; Bobbitt, D.E.; Bennett, H.K.; Todd, K.V.; Orr-Burks, N.; Murray, J.; Zhang, M.; et al. Molecular epidemiology and glycomics of swine influenza viruses circulating in commercial swine farms in the southeastern and midwest United States. Vet. Microbiol. 2020, 251, 108914. [Google Scholar] [CrossRef]
- Nagarajan, K.; Saikumar, G.; Arya, R.S.; Gupta, A.; Somvanshi, R.; Pattnaik, B. Influenza A H1N1 virus in Indian pigs & its genetic relatedness with pandemic human influenza A 2009 H1N1. Indian J. Med. Res. 2010, 132, 160–167. [Google Scholar]
- Senthilkumar, D.; Kulkarni, D.D.; Venkatesh, G.; Gupta, V.; Patel, P.; Dixit, M.; Singh, B.; Bhatia, S.; Tosh, C.; Dubey, S.C.; et al. Widespread Prevalence of Antibodies Against Swine Influenza A (pdm H1N1 09) Virus in Pigs of Eastern Uttar Pradesh, India. Curr. Microbiol. 2021, 78, 2753–2761. [Google Scholar] [CrossRef]
- Pawaiya, R.V.S.; Dhama, K.; Mahendran, M.; Tripathi, B.N. Swine flu and the current influenza A (H1N1) pandemic in humans: A review. Indian J. Vet. Pathol. 2009, 33, 1–17. [Google Scholar]
- Pegu, S.R.; Sarma, D.K.; Rajkhowa, S.; Choudhury, M. Sero-prevelance and pathology of important viral pathogens causing reproductive problems in domestic pigs of NE India. J. Entomol. Zool. Stud. 2017, 5, 1816–1818. [Google Scholar]
- Greenberg, H.B.; Estes, M.K. Rotaviruses: From pathogenesis to vaccination. Gastroenterology 2009, 136, 1939–1951. [Google Scholar] [CrossRef]
- Sadiq, A.; Khan, J. Rotavirus in developing countries: Molecular diversity, epidemiological insights, and strategies for effective vaccination. Front. Microbiol. 2024, 14, 1297269. [Google Scholar] [CrossRef] [PubMed]
- Ball, L.A. The universal taxonomy of viruses in theory and practice. In Virus Taxonomy, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 3–8. [Google Scholar]
- Matthijnssens, J.; Ciarlet, M.; McDonald, S.M.; Attoui, H.; Bányai, K.; Brister, J.R.; Buesa, J.; Esona, M.D.; Estes, M.K.; Gentsch, J.R.; et al. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG). Arch. Virol. 2011, 156, 1397–1413. [Google Scholar] [CrossRef] [PubMed]
- Estes, M.K.; Greenberg, H.A. Rotaviruses. In Fields Virology, 5th ed.; Knipe, D.M., Howley, P.M., Griffin, D.E., Lamb, R.A., Martin, M.A., Roizman, B., Straus, S.E., Eds.; Kluwer Health/Lippincott, Williams and Wilkins: Philadelphia, PA, USA, 2007; pp. 1917–1974. [Google Scholar]
- Malik, Y.S.; Kumar, N.; Sharma, K.; Sharma, R.; Kumar, H.B.; Anupamlal, K.; Chandrashekar, K.M. Epidemiology and genetic diversity of rotavirus strains associated with acute gastroenteritis in bovine, porcine, poultry and human population of Madhya Pradesh, Central India 2004–2008. Adv. Anim. Vet. Sci. 2013, 1, 111–115. [Google Scholar]
- Das, S.; Das, P.J.; Handique, P.J. Molecular characterization of porcine group A rotavirus to contain piglet diarrhea for productivity enhancement in North East India. Virusdisease 2021, 32, 314–319. [Google Scholar] [CrossRef]
- Barman, N.; Sarma, D.; Penseart, M. Detection of swine rotavirus and transmissible gastroenteritis virus in piglets with diarrhoea by sandwich ELISA. Indian J. Anim. Sci. 1998, 68, 21266. [Google Scholar]
- Barman, N.N.; Barman, B.; Sarma, D.K.; Pensaert, M.B. Prevalence of rotavirus, transmissible gastroenteritis virus and porcine epidemic diarrhoea virus antibodies in pigs of Assam, India. Indian J. Anim. Sci. 2003, 73, 576–578. [Google Scholar]
- Bora, D.; Barman, N.; Bhattacharyya, D. Isolation of rotavirus in MA 104 cell line from diarrhoeic piglets of Assam. Indian J. Virol. 2007, 18, 40–43. [Google Scholar]
- Dubal, Z.B.; Bhilegaonkar, K.N.; Barbuddhe, S.B.; Kolhe, R.P.; Kaur, S.; Rawat, S.; Nambiar, P.; Karunakaran, M. Prevalence and genotypic (G and P) determination of porcine group A rotaviruses from different regions of India. Trop. Anim. Health Prod. 2013, 45, 609–615. [Google Scholar] [CrossRef]
- Kattoor, J.J.; Saurabh, S.; Sircar, S.; Vinodhkumar, O.R.; De, U.K.; Dhama, K.; Ghosh, S.; Singh, R.K.; Malik, Y.S. Frequency distribution of porcine rotavirus—A and capsid protein gene based sequence and phylogenetic analysis indicating marked heterogeneity among prevailing strains, India. Virusdisease 2018, 29, 96–102. [Google Scholar] [CrossRef]
- Kusumakar, A.L.; Savita; Malik, Y.S.; Minakshi; Prasad, G. Genomic diversity among group A rotaviruses from diarrheic children, piglets, buffalo and cow calves of Madhya Pradesh. Indian J. Microbiol. 2010, 50, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Bora, D.P.; Chakraborty, P.; Das, S.; Barman, N.N. Circulation of group A rotaviruses among neonates of human, cow and pig: Study from Assam, a north eastern state of India. Indian J. Virol. 2013, 24, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.P.; Bora, D.P.; Hazarika, R.A.; Tamuly, S.; Deka, N.K.; Gogoi, A.; Malik, Y.S. Detection and Genotypic Characterisation of Rotavirus from Piglets and In-Contact Children of Assam, a North-Eastern State of India. J. Immunol. Immunopathol. 2017, 19, 82–91. [Google Scholar] [CrossRef]
- Chakraborty, P.; Barman, N.N.; Sharma, I. Restriction fragment length polymorphism analysis of rotavirus VP7-encoding gene from humans and animals of Northeast India: A relative study of Indian and global isolates. Epidemiol. Infect. 2015, 143, 2503–2511. [Google Scholar] [CrossRef]
- Bhatt, P.N.; Rodrigues, F. Chandipura: A new Arbovirus isolated in India from patients with febrile illness. Indian J. Med. Res. 1967, 55, 1295–1305. [Google Scholar]
- Sudeep, A.B.; Bondre, V.P.; Gurav, Y.K.; Gokhale, M.D.; Sapkal, G.N.; Mavale, M.S.; George, R.P.; Mishra, A.C. Isolation of Chandipura virus (Vesiculovirus: Rhabdoviridae) from Sergentomyia species of sandflies from Nagpur, Maharashtra, India. Indian J. Med. Res. 2014, 139, 769–772. [Google Scholar]
- Joshi, M.V.; Patil, D.R.; Tupe, C.D.; Umarani, U.B.; Ayachit, V.M.; Geevarghese, G.; Mishra, A.C. Incidence of neutralizing antibodies to Chandipura virus in domestic animals from Karimnagar and Warangal Districts of Andhra Pradesh, India. Acta Virol. 2005, 49, 69–71. [Google Scholar]
- Sudeep, A.B.; Gurav, Y.K.; Bondre, V.P. Changing clinical scenario in Chandipura virus infection. Indian J. Med. Res. 2016, 143, 712–721. [Google Scholar] [CrossRef]
- Fisher, C.R.; Streicker, D.G.; Schnell, M.J. The spread and evolution of rabies virus: Conquering new frontiers. Nat. Rev. Microbiol. 2018, 16, 241–255. [Google Scholar] [CrossRef]
- Preethi, D.; Julie, B.; Bhadra, P. Rabies in domestic pig-first report from south India. J. Indian Vet. Assoc. Kerala JIVA 2020, 18, 123–127. [Google Scholar]
- Boro, P.K.; Dutta, J.B.; Choudhury, R.S.; Das, A.; Das, T.; Dilip, L.; Isloor, S. Rabies in pig—A case report. Indian J. Comp. Microbiol. Immunol. Infect. Dis. 2023, 44, 160–162. [Google Scholar] [CrossRef]
- Kinge, K.V.; Supe, A.C. Epidemiology of animal bite cases reported to anti-rabies vaccination OPD at a tertiary-care hospital, Nagpur. Int. J. Med. Sci. Public Health 2016, 5, 1579–1582. [Google Scholar] [CrossRef]
- Nair, R.P.; Jayson, E.J. Wild pig rabies—A case study from Pathippara, Malappuram, Kerala. Int. J. Res. Med. Basic Sci. 2020, 6, 1–5. [Google Scholar]
- Ye, G.; Liu, H.; Zhou, Q.; Liu, X.; Huang, L.; Weng, C. A Tug of War: Pseudorabies Virus and Host Antiviral Innate Immunity. Viruses 2022, 14, 547. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.H.; Fu, P.F.; Chen, H.Y.; Wang, Z.Y. Pseudorabies Virus: From Pathogenesis to Prevention Strategies. Viruses 2022, 14, 1638. [Google Scholar] [CrossRef]
- Sehl, J.; Teifke, J.P. Comparative Pathology of Pseudorabies in Different Naturally and Experimentally Infected Species—A Review. Pathogens 2020, 9, 633. [Google Scholar] [CrossRef]
- Vanamayya, P.R.; PATTNAIK, B.; Pateriya, A.K.; Desai, G.S.; Rajukumar, K. Genomic detection methods for detection of pseudorabies virus infection in Indian pigs. Indian J. Anim. Sci. 2009, 79, 1200–1204. [Google Scholar]
- Bhattacharyya, H.; Mukherjee, S.; Das, S. An outbreak of pseudorabies in piglets. Indian J. Anim. Health 1973, 12, 17–19. [Google Scholar]
- Vanamayya, P.R. Annual Report; Indian Veterinary Research Institute: Bareilly, India, 2002. [Google Scholar]
- Tao, J.; Li, B.; Cheng, J.; Shi, Y.; Qiao, C.; Lin, Z.; Liu, H. Genomic Divergence Characterization and Quantitative Proteomics Exploration of Type 4 Porcine Astrovirus. Viruses 2022, 14, 1383. [Google Scholar] [CrossRef]
- Xiao, C.T.; Luo, Z.; Lv, S.L.; Opriessnig, T.; Li, R.C.; Yu, X.L. Identification and characterization of multiple porcine astrovirus genotypes in Hunan province, China. Arch. Virol. 2017, 162, 943–952. [Google Scholar] [CrossRef]
- Sawant, P.M.; Waghchaure, R.B.; Shinde, P.A.; Palikondawar, A.P.; Lavania, M. Detection and Molecular Characterization of Animal Adenovirus and Astrovirus from Western Maharashtra, India. Viruses 2023, 15, 1679. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, J.J.; Malik, Y.S.; Saurabh, S.; Sircar, S.; Vinodhkumar, O.R.; Bora, D.P.; Dhama, K.; Ghosh, S.; Banyai, K.; Touil, N.; et al. First report and genetic characterization of porcine astroviruses of lineage 4 and 2 in diarrhoeic pigs in India. Transbound. Emerg. Dis. 2019, 66, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Kour, R.; Kumar, P.; Jindal, N.; Kumari Minhas, S.; Kumar, R.; Gupta, A.K.; Malik, A. Molecular detection and characterization reveals circulation of multiple genotypes of porcine astrovirus in Haryana, India. Arch. Virol. 2021, 166, 2847–2852. [Google Scholar] [CrossRef]
- Kaur, R.; Kumar, P.; Jindal, N.; Minhas, S.K.; Panghal, R.; Sheoran, D.; Kakker, N.K.; Prakash, A.; Joshi, V.G. Porcine astrovirus detection and characterization in healthy and diarrheic pigs from Haryana, India. Indian J. Anim. Res. 2023, 57, 1351–1357. [Google Scholar] [CrossRef]
- Vaishali; Gupta, R.; Kumar, M.; Bansal, N.; Vivek; Kumar, P.; Kumar, P.; Jindal, N. Coinfection of porcine astrovirus and other porcine viruses in diarrheic pigs in Haryana, India. Arch. Virol. 2023, 168, 246. [Google Scholar] [CrossRef]
- Roach, S.N.; Langlois, R.A. Intra- and Cross-Species Transmission of Astroviruses. Viruses 2021, 13, 1127. [Google Scholar] [CrossRef]
- Mei, M.; Zhu, L.; Xu, Z.; Zhao, L.; Zhou, Y.; Wu, Y.; Li, S.; Wei, H.; Guo, W. Molecular investigation of Torque teno sus virus in geographically distinct porcine breeding herds of Sichuan, China. Virol. J. 2013, 10, 161. [Google Scholar] [CrossRef]
- Nieto, D.; Aramouni, M.; Grau-Roma, L.; Segalés, J.; Kekarainen, T. Dynamics of Torque teno sus virus 1 (TTSuV1) and 2 (TTSuV2) DNA loads in serum of healthy and postweaning multisystemic wasting syndrome (PMWS) affected pigs. Vet. Microbiol. 2011, 152, 284–290. [Google Scholar] [CrossRef]
- Subramanyam, V.; Hemadri, D.; Kashyap, S.P.; Hiremath, J.; Barman, N.N.; Ralte, E.L.; Patil, S.S.; Suresh, K.P.; Rahaman, H. Detection of torque teno sus virus infection in Indian pigs. Vet. World 2019, 12, 1467–1471. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, Y.; Yan, X.; Tang, X.; Li, Q.; Tan, Y.; Lan, T.; Ma, J. Swine acute diarrhea syndrome coronavirus (SADS-CoV) antagonizes interferon-β production via blocking IPS-1 and RIG-I. Virus Res. 2020, 278, 197843. [Google Scholar] [CrossRef]
- Turlewicz-Podbielska, H.; Pomorska-Mól, M. Porcine Coronaviruses: Overview of the State of the Art. Virol. Sin. 2021, 36, 833–851. [Google Scholar] [CrossRef]
- Singh, R.K.; Dhama, K.; Chakraborty, S.; Tiwari, R.; Natesan, S.; Khandia, R.; Munjal, A.; Vora, K.S.; Latheef, S.K.; Karthik, K.; et al. Nipah virus: Epidemiology, pathology, immunobiology and advances in diagnosis, vaccine designing and control strategies—A comprehensive review. Vet. Q. 2019, 39, 26–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, D.; Yang, M.; Chai, S.; Du, H.; Jiang, H. Nipah virus: Epidemiology, pathogenesis, treatment, and prevention. Front. Med. 2024, 18, 969–987. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Mitra, S.; Halder, S.; Deb, J.; Patra, A.; Sarkar, G.N. A clinico-epidemiological study of the first outbreak of Nipah virus in India–report from ground zero. Int. J. Med. Res. Review 2020, 8, 252–258. [Google Scholar] [CrossRef]
- Thomas, B.; Chandran, P.; Lilabi, M.P.; George, B.; Sivakumar, C.P.; Jayadev, V.K.; Bindu, V.; Rajasi, R.S.; Vijayan, B.; Mohandas, A.; et al. Nipah Virus Infection in Kozhikode, Kerala, South India, in 2018: Epidemiology of an Outbreak of an Emerging Disease. Indian J. Community Med. 2019, 44, 383–387. [Google Scholar]
- Arunkumar, G.; Chandni, R.; Mourya, D.T.; Singh, S.K.; Sadanandan, R.; Sudan, P.; Bhargava, B. Outbreak Investigation of Nipah Virus Disease in Kerala, India, 2018. J. Infect. Dis. 2019, 219, 1867–1878. [Google Scholar] [CrossRef]
- Kulkarni, D.D.; Tosh, C.; Venkatesh, G.; Senthil Kumar, D. Nipah virus infection: Current scenario. Indian J. Virol. 2013, 24, 398–408. [Google Scholar] [CrossRef]
- Himani, D.; Kumar, H.C.; Bhilegaonkar, K.N.; Kumar, A. Japanese encephalitis: A veterinary perspective. J. Foodborne Zoon. Dis. 2014, 2, 59–67. [Google Scholar]
- Arankalle, V.A. Hepatitis E in India. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2012, 82, 43–53. [Google Scholar] [CrossRef]
- Begum, N.; Polipalli, S.K.; Husain, S.A.; Kar, P. Molecular analysis of swine Hepatitis E virus from north India. Indian J. Med. Res. 2010, 132, 504–508. [Google Scholar] [CrossRef]
- Dubey, S.C.; Venkatesh, G.; Kulkarni, D.D. Epidemiological update on swine influenza (H1N1) in pigs. Indian J. Microbiol. 2009, 49, 324–331. [Google Scholar] [CrossRef]
- Sharma, V.; Chaudhry, D.; Kaushik, S. Evaluation of clinical applicability of reverse transcription-loop-mediated isothermal amplification assay for detection and subtyping of Influenza A viruses. J. Virol. Methods 2018, 253, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.R.; Dhanze, H.; Pantwane, P.; Sivakumar, M.; Gulati, B.R.; Kumar, A. Latex agglutination test for rapid on-site serodiagnosis of Japanese encephalitis in pigs using recombinant NS1 antigen. J. Vector Borne Dis. 2019, 56, 105–110. [Google Scholar]
- Kumar, H.C.; Dhanze, H.; Bhilegaonkar, K.N.; Chakurkar, E.B.; Kumar, A.; Yathish, H.M. Serological evidence of Japanese encephalitis virus infection in pigs in a low human incidence state, Goa, India. Prev. Vet. Med. 2020, 175, 104882. [Google Scholar] [CrossRef] [PubMed]
- Dhanze, H.; Singh, B.B.; Walsh, M.; Kumar, M.S.; Kumar, A.; Bhilegaonkar, K.N.; Brookes, V.J. Spatio-temporal epidemiology of Japanese encephalitis virus infection in pig populations of eastern Uttar Pradesh, India, 2013–2022. Zoonoses Public Health 2024, 71, 429–441. [Google Scholar] [CrossRef]
- Roberts, A.; Prakashan, D.; Dhanze, H.; Gandham, R.K.; Gandhi, S.; Sharma, G.T. Immuno-chromatic probe based lateral flow assay for point-of-care detection of Japanese encephalitis virus NS1 protein biomarker in clinical samples using a smartphone-based approach. Nanoscale Adv. 2022, 4, 3966–3977. [Google Scholar] [CrossRef] [PubMed]
- Dhanze, H.; Bhilegaonkar, K.N.; Kumar, C.; Kumar, M.S.; Singh, P.; Kumar, A. Development and evaluation of lateral flow assay for sero-diagnosis of Japanese encephalitis in swine. Anim. Biotechnol. 2020, 31, 350–356. [Google Scholar] [CrossRef]
- Sarika Tiwari, S.T.; Singh, R.K.; Ruchi Tiwari, R.T.; Dhole, T.N. Japanese encephalitis: A review of the Indian perspective. Braz. J. Infect. Dis. 2012, 16, 564–573. [Google Scholar] [CrossRef]
- Park, W.J.; Park, B.J.; Ahn, H.S.; Lee, J.B.; Park, S.Y.; Song, C.S.; Lee, S.W.; Yoo, H.S.; Choi, I.S. Hepatitis E virus as an emerging zoonotic pathogen. J. Vet. Sci. 2016, 17, 1–11. [Google Scholar] [CrossRef]
- McLean, R.K.; Graham, S.P. The pig as an amplifying host for new and emerging zoonotic viruses. One Health 2022, 14, 100384. [Google Scholar] [CrossRef]
- Mulvey, P.; Duong, V.; Boyer, S.; Burgess, G.; Williams, D.T.; Dussart, P.; Horwood, P.F. The Ecology and Evolution of Japanese Encephalitis Virus. Pathogens 2021, 10, 1534. [Google Scholar] [CrossRef] [PubMed]
- Ferri, G.; Vergara, A. Hepatitis E Virus in the Food of Animal Origin: A Review. Foodborne Pathog. Dis. 2021, 18, 368–377. [Google Scholar] [CrossRef]
- Petro-Turnquist, E.; Pekarek, M.J.; Weaver, E.A. Swine influenza A virus: Challenges and novel vaccine strategies. Front. Cell Infect. Microbiol. 2024, 14, 1336013. [Google Scholar] [CrossRef] [PubMed]
- Verberk, J.D.M.; van Dongen, J.A.P.; van de Kassteele, J.; Andrews, N.J.; van Gaalen, R.D.; Hahné, S.J.M.; Vennema, H.; Ramsay, M.; Braeckman, T.; Ladhani, S.; et al. Impact analysis of rotavirus vaccination in various geographic regions in Western Europe. Vaccine 2021, 39, 6671–6681. [Google Scholar] [CrossRef]
- Ghonaim, A.H.; Rouby, S.R.; Nageeb, W.M.; Elgendy, A.A.; Xu, R.; Jiang, C.; Ghonaim, N.H.; He, Q.; Li, W. Insights into recent advancements in human and animal rotavirus vaccines: Exploring new Frontiers. Virol. Sin. 2024, in press. [CrossRef]
- Yang, D.K.; Kim, H.H.; Lee, K.W.; Song, J.Y. The present and future of rabies vaccine in animals. Clin. Exp. Vaccine Res. 2013, 2, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.M.; Sun, X.D.; Jin, Z.; Wu, H.R.; Li, M.T.; Sun, G.Q.; Pei, X.; Wu, Y.T.; Liu, P.; Li, L.; et al. Dynamic analysis of rabies transmission and elimination in mainland China. One Health 2023, 17, 100615. [Google Scholar] [CrossRef]
- Takahashi-Omoe, H.; Omoe, K.; Okabe, N. Regulatory systems for prevention and control of rabies, Japan. Emerg. Infect. Dis. 2008, 14, 1368–1374. [Google Scholar] [CrossRef]
- Brisse, M.E.; Ly, H. Chandipura Virus Causing Large Viral Encephalitis Outbreaks in India. Pathogens 2024, 13, 1110. [Google Scholar] [CrossRef]
- Pomeranz, L.E.; Reynolds, A.E.; Hengartner, C.J. Molecular biology of pseudorabies virus: Impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. 2005, 69, 462–500. [Google Scholar] [CrossRef]
- Fang, Q.; Wang, C.; Liu, H.; Wu, Q.; Liang, S.; Cen, M.; Dong, Q.; Wei, Y.; Chen, Y.; Ouyang, K.; et al. Pathogenic Characteristics of a Porcine Astrovirus Strain Isolated in China. Viruses 2019, 11, 1156. [Google Scholar] [CrossRef] [PubMed]
- Kekarainen, T. What We Know About Torque Teno Sus Viruses? 2013. Available online: https://www.pig333.com/ (accessed on 25 December 2024).
| Pathogen | Etiology | Common Symptoms in Pigs | Route of Entry | Incubation Period | Transmission Cycle | Risk in Human |
|---|---|---|---|---|---|---|
| Japanese encephalitis virus (JEV) | Flavivirus | Fever, anorexia, neurological signs, reproductive failure | Mosquito bites (Culex spp.) | 4–14 days | Enzootic cycle between mosquitoes and pigs, with humans as dead-end hosts | High in rural areas; severe neurological disease; zoonotic |
| Hepatitis E virus (HEV) | Orthohepevirus A | Subclinical infection, liver damage, reproductive issues | Fecal–oral route | 2–8 weeks | Pigs act as reservoirs; humans infected via contaminated water or undercooked pork | High in regions with poor sanitation; causes hepatitis |
| Swine influenza virus (SIV) | Influenza A virus | Fever, cough, nasal discharge, lethargy | Inhalation | 1–3 days | Direct pig-to-pig transmission; aerosolized respiratory secretions | Moderate; influenza-like illness, potential pandemic risks |
| Rotavirus (RV) | Rotavirus A, B, or C | Diarrhea, dehydration, weight loss | Fecal–oral route | 1–3 days | Direct contact with feces; contamination of feed, water, or surfaces | Low; primarily affects children with gastroenteritis |
| Rabies virus | Lyssavirus | Neurological signs, aggression, paralysis | Bite wounds | 2 weeks to 3 months | Animal bites or scratches; rare cases through aerosol transmission | High; fatal encephalitis in humans if untreated |
| Chandipura virus (CHPV) | Vesiculovirus | Fever, vesicular lesions, neurological signs | Bite from sandflies | 3–7 days | Sandflies to pigs; potential spillover to humans | High; causes severe febrile illness and neurological symptoms |
| Pseudorabies/ADV (SuHV-1) | Suid alpha herpesvirus 1 | Respiratory distress, neurological signs, reproductive failure | Inhalation or ingestion | 3–7 days | Direct contact with infected secretions or contaminated feed | Low; humans are not natural hosts |
| Porcine astrovirus (PAstV) | Astrovirus | Mild diarrhea in piglets | Fecal–oral route | 2–6 days | Direct fecal contamination of feed and water | Low; rare cases of human infection |
| Torque teno sus virus (TTSuV) | Anellovirus | Subclinical infection, potential immunosuppression | Fecal route and also found in feces, nasal excretions, sera, and liver of infected pigs | Unknown | Persistent infection; transmitted via bodily fluids | Low; no confirmed zoonotic cases |
| Nipah virus (NiV) | Henipavirus | Fever, respiratory distress, neurological signs | Ingestion, inhalation | 4–14 days | Fruit bats to pigs; direct contact with infected pigs or contaminated materials; spillover to humans | High; severe encephalitis and respiratory illness |
| Pathogen | India: Current Status and Challenges | Important Global Perspective (Examples of Successful Management) | References |
|---|---|---|---|
| Japanese encephalitis virus (JEV) | Endemic with seasonal outbreaks; pigs act as amplifying hosts; challenges include limited vaccination coverage and vector control. | Countries like Japan and South Korea have effectively controlled JE through widespread vaccination campaigns and robust mosquito control measures. | [96] |
| Hepatitis E virus (HEV) | Widespread, particularly in areas with inadequate sanitation; transmission linked to consumption of undercooked pork and contaminated water. | Improved sanitation, public awareness, and stringent food safety regulations have reduced HEV incidence in developed nations. | [97] |
| Swine influenza virus (SIV) | Regular occurrences, often underreported; lacks a structured surveillance system and vaccination strategy. | The USA and European countries manage SIV through continuous surveillance and routine vaccination of swine herds. | [98] |
| Rotavirus (RV) | High prevalence in piglets, leading to significant morbidity; vaccination programs are not widely implemented. | European countries and the USA have reduced piglet mortality through effective vaccination strategies and improved farm hygiene practices. | [99,100] |
| Rabies virus | Rare in pigs; sporadic cases reported, often due to bites from rabid animals; limited awareness and vaccination in pig populations. | Rabies control in livestock is achieved through vaccination of domestic animals and control of wildlife reservoirs in countries like the USA. | [101,102,103] |
| Chandipura virus (CHPV) | Occasional outbreaks with high fatality rates, primarily affecting children; pigs’ role in transmission is not well-established. | Limited global data; control measures focus on vector control and public health awareness in affected regions. | [104] |
| Pseudorabies (Aujeszky’s disease virus—SuHV-1) | Endemic in certain regions with sporadic outbreaks; control measures are limited and inconsistent. | Successfully eradicated in the USA and several European countries through comprehensive vaccination programs and stringent biosecurity protocols. | [59,105] |
| Porcine astrovirus (PAstV) | Presence in pig populations with unclear clinical significance; lack of routine surveillance and research. | Research in countries like the USA focuses on understanding pathogenicity and developing diagnostic tools. | [64,106] |
| Torque teno sus virus (TTSuV) | Commonly detected in pigs; clinical impact remains uncertain; no control measures in place. | In countries like the USA and parts of Europe, focuses on its co-infection dynamics, improved diagnostic methods, and potential immunization strategies, though no specific control measures are currently in place. | [107] |
| Nipah virus (NiV) | Occasional outbreaks with high mortality rates in human; surveillance in pig populations is inadequate. | Malaysia eliminated NiV from pig farms following the 1998–1999 outbreak by culling infected animals and implementing strict biosecurity measures. | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajkhowa, S.; Sonowal, J.; Pegu, S.R.; Deb, R.; Gupta, V.K. Epidemiology and Emerging Trends of Zoonotic Viral Diseases of Pigs in India. Viruses 2025, 17, 381. https://doi.org/10.3390/v17030381
Rajkhowa S, Sonowal J, Pegu SR, Deb R, Gupta VK. Epidemiology and Emerging Trends of Zoonotic Viral Diseases of Pigs in India. Viruses. 2025; 17(3):381. https://doi.org/10.3390/v17030381
Chicago/Turabian StyleRajkhowa, Swaraj, Joyshikh Sonowal, Seema Rani Pegu, Rajib Deb, and Vivek Kumar Gupta. 2025. "Epidemiology and Emerging Trends of Zoonotic Viral Diseases of Pigs in India" Viruses 17, no. 3: 381. https://doi.org/10.3390/v17030381
APA StyleRajkhowa, S., Sonowal, J., Pegu, S. R., Deb, R., & Gupta, V. K. (2025). Epidemiology and Emerging Trends of Zoonotic Viral Diseases of Pigs in India. Viruses, 17(3), 381. https://doi.org/10.3390/v17030381






