La Jolla Virus: The Pathology and Transmission in Its Host Drosophila suzukii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drosophila suzukii Cultures Maintenance
2.2. Extraction and Quantification of La Jolla Virus in Flies
2.3. Transmission Assays
2.4. Egg to Adult Viability
2.5. The Feeding Behavior of Infected Flies
2.6. Statistical Analysis and Graph Design
3. Results
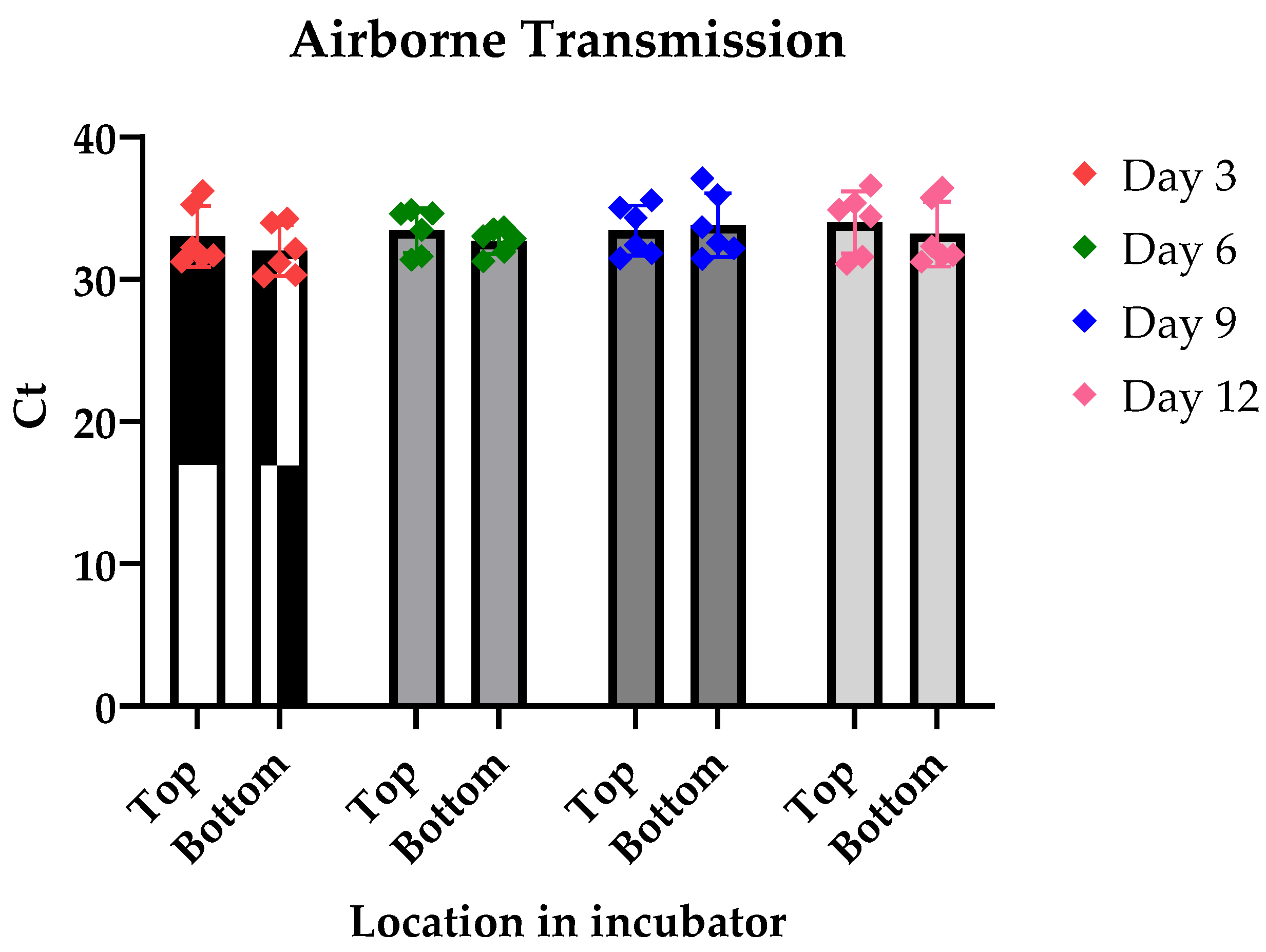
3.1. Airborne Transmission
3.2. Venereal Transmission
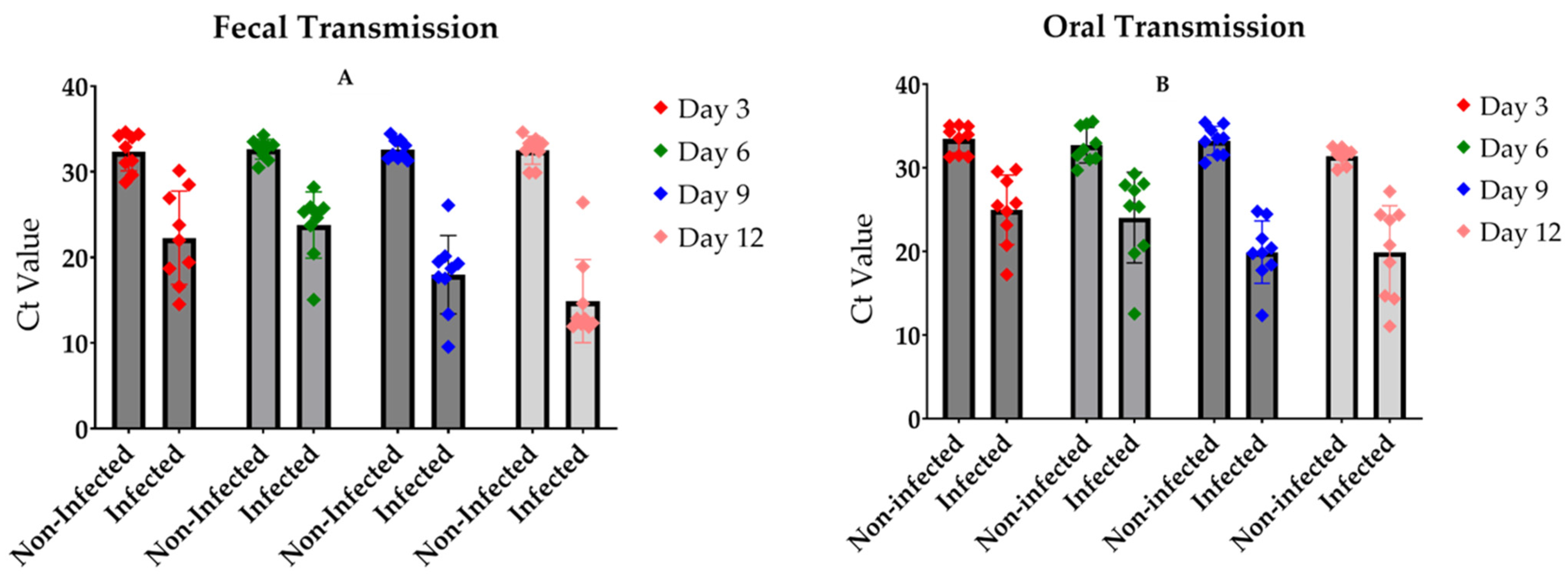
3.3. The Transmission of the Virus Occurs by Oral and Fecal Contamination
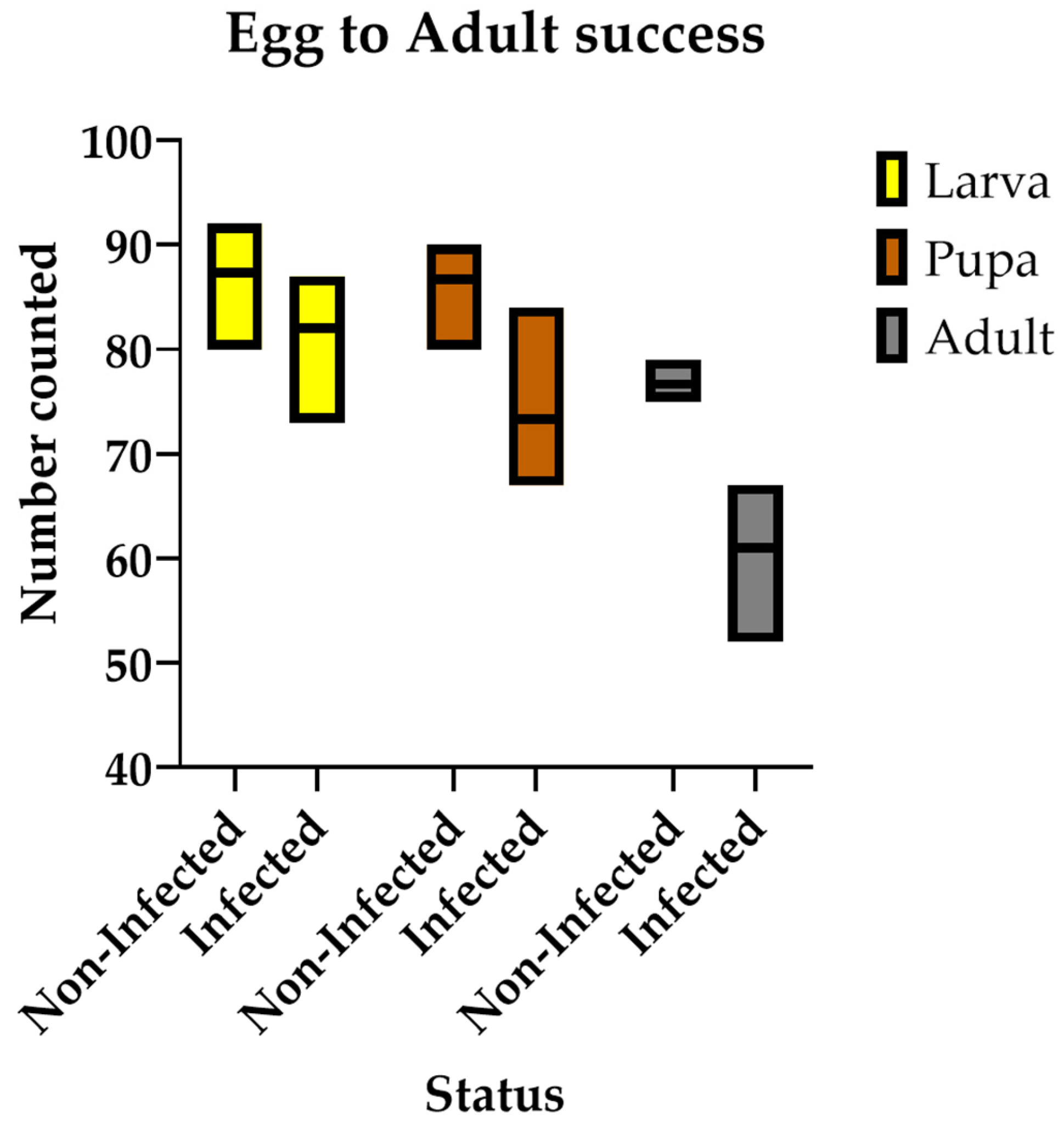
3.4. Chronic LJV Infection Decreases Egg-to-Adult Success Rate
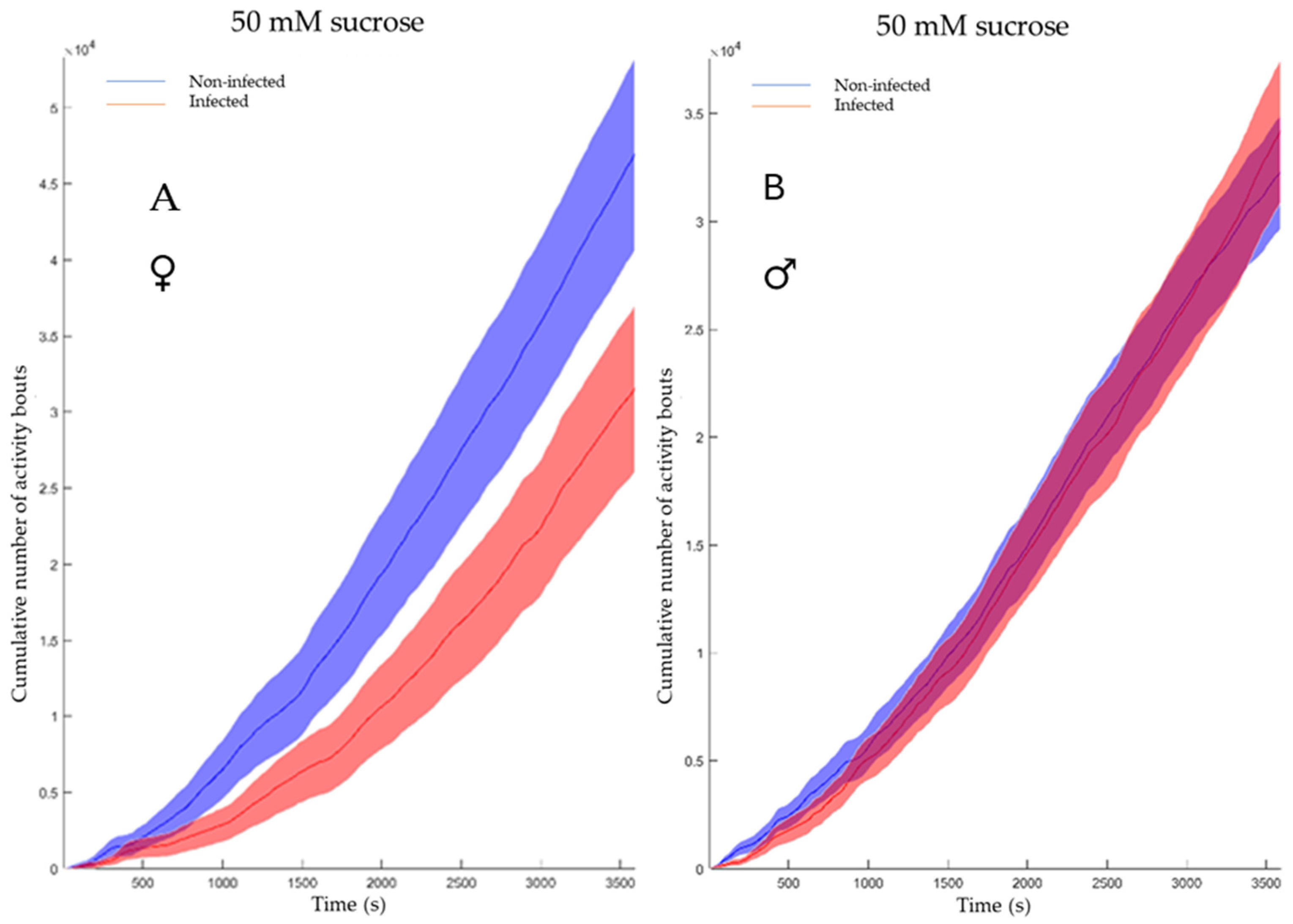
3.5. LJV Affects the Feeding Behavior of Female Flies
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LJV | La Jolla Virus |
| DWV | Deformed Wing Virus |
| DCV | Drosophila C Virus |
References
- Garcia, F.R.M.; Lasa, R.; Funes, C.F.; Buzzetti, K. Drosophila suzukii Management in Latin America: Current Status and Perspectives. J. Econ. Entomol. 2022, 115, 1008–1023. [Google Scholar] [CrossRef] [PubMed]
- Hauser, M. A historic account of the invasion of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in the continental United States, with remarks on their identification. Pest Manag. Sci. 2011, 67, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Deprá, M.; Poppe, J.L.; Schmitz, H.J.; De Toni, D.C.; Valente, V.L.S. The first records of the invasive pest Drosophila suzukii in the South American continent. J. Pest Sci. 2014, 87, 379–383. [Google Scholar] [CrossRef]
- Lee, J.C.; Bruck, D.J.; Dreves, A.J.; Ioriatti, C.; Vogt, H.; Baufeld, P. In Focus: Spotted wing drosophila, Drosophila suzukii, across perspectives. Pest Manag. Sci. 2011, 67, 1349–1351. [Google Scholar] [CrossRef]
- Knapp, L.; Mazzi, D.; Finger, R. The economic impact of Drosophila suzukii: Perceived costs and revenue losses of Swiss cherry, plum and grape growers. Pest Manag. Sci. 2021, 77, 978–1000. [Google Scholar] [CrossRef]
- Fernández-Moreno, M.A.; Farr, C.L.; Kaguni, L.S.; Garesse, R. Drosophila melanogaster as a Model System to Study Mitochondrial Biology. In Mitochondria. Methods in Molecular Biology™; Leister, D., Herrmann, J.M., Eds.; Humana Press: Totowa, NJ, USA, 2007; Volume 372. [Google Scholar] [CrossRef]
- Bieńkowski, A.O.; Orlova-Bienkowskaja, M.J. Invasive agricultural pest Drosophila suzukii (Diptera, drosophilidae) appeared in the russian caucasus. Insects 2020, 11, 826. [Google Scholar] [CrossRef]
- Little, C.M.; Little, C.M.; Chapman, T.W.; Hillier, N.K. Plasticity Is Key to Success of Drosophila suzukii (Diptera: Drosophilidae) Invasion. J. Insect Sci. 2020, 20, 5. [Google Scholar] [CrossRef]
- Haviland, D.R.; Beers, E.H. Chemical control programs for Drosophila suzukii that comply with international limitations on pesticide residues for exported sweet cherries. J. Integr. Pest Manag. 2012, 3, F1–F6. [Google Scholar] [CrossRef]
- Connolly, C. The risk of insecticides to pollinating insects. Commun. Integr. Biol. 2013, 6, e25074. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1. [Google Scholar] [CrossRef]
- Farooq, M.; Pisante, M. Innovations in sustainable agriculture. In Innovations in Sustainable Agriculture; Springer Cham: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Schetelig, M.F.; Lee, K.Z.; Otto, S.; Talmann, L.; Stökl, J.; Degenkolb, T.; Halitschke, R. Environmentally sustainable pest control options for Drosophila suzukii. J. Appl. Entomol. 2017, 142, 3–17. [Google Scholar] [CrossRef]
- Nikhil Raj, M.; Samal, I.; Paschapur, A.; Subbanna, A.R.N.S. Entomopathogenic viruses and their potential role in sustainable pest management. In New and Future Developments in Microbial Biotechnology and Bioengineering: Sustainable Agriculture: Revitalization through Organic Products; Elsevier: Amsterdam, The Netherlands, 2022; pp. 47–72. [Google Scholar] [CrossRef]
- Lee, K.Z.; Vilcinskas, A. Analysis of virus susceptibility in the invasive insect pest Drosophila suzukii. J. Invertebr. Pathol. 2017, 148, 138–141. [Google Scholar] [CrossRef]
- Webster, C.L.; Waldron, F.M.; Robertson, S.; Crowson, D.; Ferrari, G.; Quintana, J.F.; Brouqui, J.M.; Bayne, E.H.; Longdon, B.; Buck, A.H.; et al. The discovery, distribution, and evolution of viruses associated with Drosophila melanogaster. PLoS Biol. 2015, 13, e1002210. [Google Scholar] [CrossRef]
- Carrau, T.; Hiebert, N.; Vilcinskas, A.; Lee, K.Z. Identification and characterization of natural viruses associated with the invasive insect pest Drosophila suzukii. J. Invertebr. Pathol. 2018, 154, 74–78. [Google Scholar] [CrossRef]
- Carrau, T.; Lamp, B.; Reuscher, C.M.; Vilcinskas, A.; Lee, K.Z. Organization of the structural protein region of la jolla virus isolated from the invasive pest insect Drosophila suzukii. Viruses 2021, 13, 740. [Google Scholar] [CrossRef]
- Linscheid, Y.; Kessel, T.; Vilcinskas, A.; Lee, K.Z. Pathogenicity of La Jolla Virus in Drosophila suzukii following Oral Administration. Viruses 2022, 14, 2158. [Google Scholar] [CrossRef]
- Ferreira, Á.G.; Naylor, H.; Esteves, S.S.; Pais, I.S.; Martins, N.E.; Teixeira, L. The Toll-Dorsal Pathway Is Required for Resistance to Viral Oral Infection in Drosophila. PLoS Pathogens 2014, 10, e1004507. [Google Scholar] [CrossRef]
- Wong, Z.S.; Brownlie, J.C.; Johnson, K.N. Impact of ERK activation on fly survival and Wolbachia-mediated protection during virus infection. J. Gen. Virol. 2016, 97, 1446–1452. [Google Scholar] [CrossRef]
- Cory, J.S. Insect virus transmission: Different routes to persistence. Curr. Opin. Insect Sci. 2015, 8, 130–135. [Google Scholar] [CrossRef]
- Jakobs, R.; Gariepy, T.D.; Sinclair, B.J. Adult plasticity of cold tolerance in a continental-temperate population of Drosophila suzukii. J. Insect Physiol. 2015, 79, 1–9. [Google Scholar] [CrossRef]
- Itskov, P.M.; Moreira, J.M.; Vinnik, E.; Lopes, G.; Safarik, S.; Dickinson, M.H.; Ribeiro, C. Automated monitoring and quantitative analysis of feeding behaviour in Drosophila. Nat. Commun. 2014, 5, 4560. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Ge, S.; Jones, T.; Santosh, M.; Silva, L.F.; Cao, Y.; Oliveira, M.L.S.; Zhang, M.; BéruBé, K. The role of airborne particles and environmental considerations in the transmission of SARS-CoV-2. Geosci. Front. 2021, 12, 101189. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Wang, Y.; Li, W.; Wei, Z.; Tang, S.; Chen, R. Mechanisms, Techniques and Devices of Airborne Virus Detection: A Review. Int. J. Environ. Res. Public Health 2023, 20, 5471. [Google Scholar] [CrossRef] [PubMed]
- Ottati, S.; Persico, A.; Rossi, M.; Bosco, D.; Vallino, M.; Abbà, S.; Molinatto, G.; Palmano, S.; Balestrini, R.; Galetto, L.; et al. Biological characterization of Euscelidius variegatus iflavirus 1. J Invertebr Pathol. 2020, 173, 107370. [Google Scholar] [CrossRef]
- Habayeb, M.S.; Cantera, R.; Casanova, G.; Ekström, J.O.; Albright, S.; Hultmark, D. The Drosophila Nora virus is an enteric virus; transmitted via feces. J. Invertebr. Pathol. 2009, 101, 29–33. [Google Scholar] [CrossRef]
- de Miranda, J.R.; Genersch, E. Deformed wing virus. J. Invertebr. Pathol. 2010, 103 (Suppl. 1), S48–S61. [Google Scholar] [CrossRef]
- Lanzi, G.; de Miranda, J.R.; Boniotti, M.B.; Cameron, C.E.; Lavazza, A.; Capucci, L.; Camazine, S.M.; Rossi, C. Molecular and Biological Characterization of Deformed Wing Virus of Honeybees (Apis mellifera L.). J. Virol. 2006, 80, 4998–5009. [Google Scholar] [CrossRef]
- Heinig-Hartberger, M.; Hellhammer, F.; Zöller, D.D.J.A.; Dornbusch, S.; Bergmann, S.; Vocadlova, K.; Junglen, S.; Stern, M.; Lee, K.-Z.; Becker, S.C. Culex Y Virus: A Native Virus of Culex Species Characterized In Vivo. Viruses 2023, 15, 235. [Google Scholar] [CrossRef]
- Ovenden, J.R.; Mahon, R.J. Venereal transmission of Sindbis virus between individuals of Aedes australis (Diptera: Culicidae). J. Med. Entomol. 1984, 21, 292–295. [Google Scholar] [CrossRef]
- Shroyer, D.A. Venereal transmission of St. Louis encephalitis virus by Culex quinquefasciatus males (Diptera: Culicidae). J. Med. Entomol. 1990, 27, 334–337. [Google Scholar] [CrossRef]
- de Miranda, J.R.; Fries, I. Venereal and vertical transmission of deformed wing virus in honeybees (Apis mellifera L.). J. Invertebr. Pathol. 2008, 98, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafiz, I.; Gerth, S.; Claussen, J.; Weule, M.; Hufnagel, E.; Vilcinskas, A.; Lee, K.Z. Radioactivity and GMO-Free Sterile Insect Technology for the Sustainable Control of the Invasive Pest Drosophila suzukii. Adv. Biol. 2024, 8, e2400100. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.G.; Olea-Popelka, F.J.; Eisen, L.; Moore, C.G.; Blair, C.D. Transmission dynamics of an insect-specific flavivirus in a naturally infected Culex pipiens laboratory colony and effects of co-infection on vector competence for West Nile virus. Virology 2012, 427, 90–97. [Google Scholar] [CrossRef]
- Hernández-Pelegrín, L.; Huditz, H.-I.; García-Castillo, P.; de Ruijter, N.C.A.; van Oers, M.M.; Herrero, S.; Ros, V.I.D. Covert RNA viruses in medflies differ in their mode of transmission and tissue tropism. J. Virol. 2024, 98, e0010824. [Google Scholar] [CrossRef]
- Geng, P.; Li, W.; de Miranda, J.R.; Qian, Z.; An, L.; Terenius, O. Studies on the transmission and tissue distribution of Antheraea pernyi iflavirus in the Chinese oak silkmoth Antheraea pernyi. Virology 2017, 502, 171–175. [Google Scholar] [CrossRef]
- Virto, C.; Navarro, D.; Tellez, M.M.; Herrero, S.; Williams, T.; Murillo, R.; Caballero, P. Natural populations of Spodoptera exigua are infected by multiple viruses that are transmitted to their offspring. J. Invertebr. Pathol. 2014, 122, 22–27. [Google Scholar] [CrossRef]
- Jakubowska, A.K.; Murillo, R.; Carballo, A.; Williams, T.; van Lent, J.W.; Caballero, P.; Herrero, S. Iflavirus increases its infectivity and physical stability in association with baculovirus. PeerJ 2016, 4, e1687. [Google Scholar] [CrossRef]
- Habayeb, M.S.; Ekengren, S.K.; Hultmark, D. Nora virus, a persistent virus in Drosophila, defines a new picorna-like virus family. J. Gen. Virol. 2006, 87, 3045–3051. [Google Scholar] [CrossRef]
- Chen, Y.P.; Becnel, J.J.; Valles, S.M. RNA Viruses Infecting Pest Insects. In Insect Pathology, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012. [Google Scholar] [CrossRef]


| Description | Sequence | Product Length (bp) |
|---|---|---|
| LJV specific probe * | 5′-ACTCGGCGTTATCGTTACAACCGCACATATC-3′ | |
| LJV forward primer | 5′-CAACACGTTGTGCTGCCTGA-3′ | 128 |
| LJV reverse primer | 5′-TCCATCCAAACTCCACCTCC-3′ | 128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelhafiz, I.; Kessel, T.; Vilcinskas, A.; Lee, K.-Z. La Jolla Virus: The Pathology and Transmission in Its Host Drosophila suzukii. Viruses 2025, 17, 408. https://doi.org/10.3390/v17030408
Abdelhafiz I, Kessel T, Vilcinskas A, Lee K-Z. La Jolla Virus: The Pathology and Transmission in Its Host Drosophila suzukii. Viruses. 2025; 17(3):408. https://doi.org/10.3390/v17030408
Chicago/Turabian StyleAbdelhafiz, Ibrahim, Tobias Kessel, Andreas Vilcinskas, and Kwang-Zin Lee. 2025. "La Jolla Virus: The Pathology and Transmission in Its Host Drosophila suzukii" Viruses 17, no. 3: 408. https://doi.org/10.3390/v17030408
APA StyleAbdelhafiz, I., Kessel, T., Vilcinskas, A., & Lee, K.-Z. (2025). La Jolla Virus: The Pathology and Transmission in Its Host Drosophila suzukii. Viruses, 17(3), 408. https://doi.org/10.3390/v17030408






