Dysfunctional Senescent Herpes Simplex Virus-Specific CD57+CD8+ T Cells Are Associated with Symptomatic Recurrent Ocular Herpes in Humans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Study Population
2.2. HLA Typing
2.3. Peripheral Blood Mononuclear Cell (PBMC) Isolation
2.4. Flow Cytometry Analysis
2.5. CD107 Cytotoxicity Assay
2.6. Statistical Analyses
3. Results
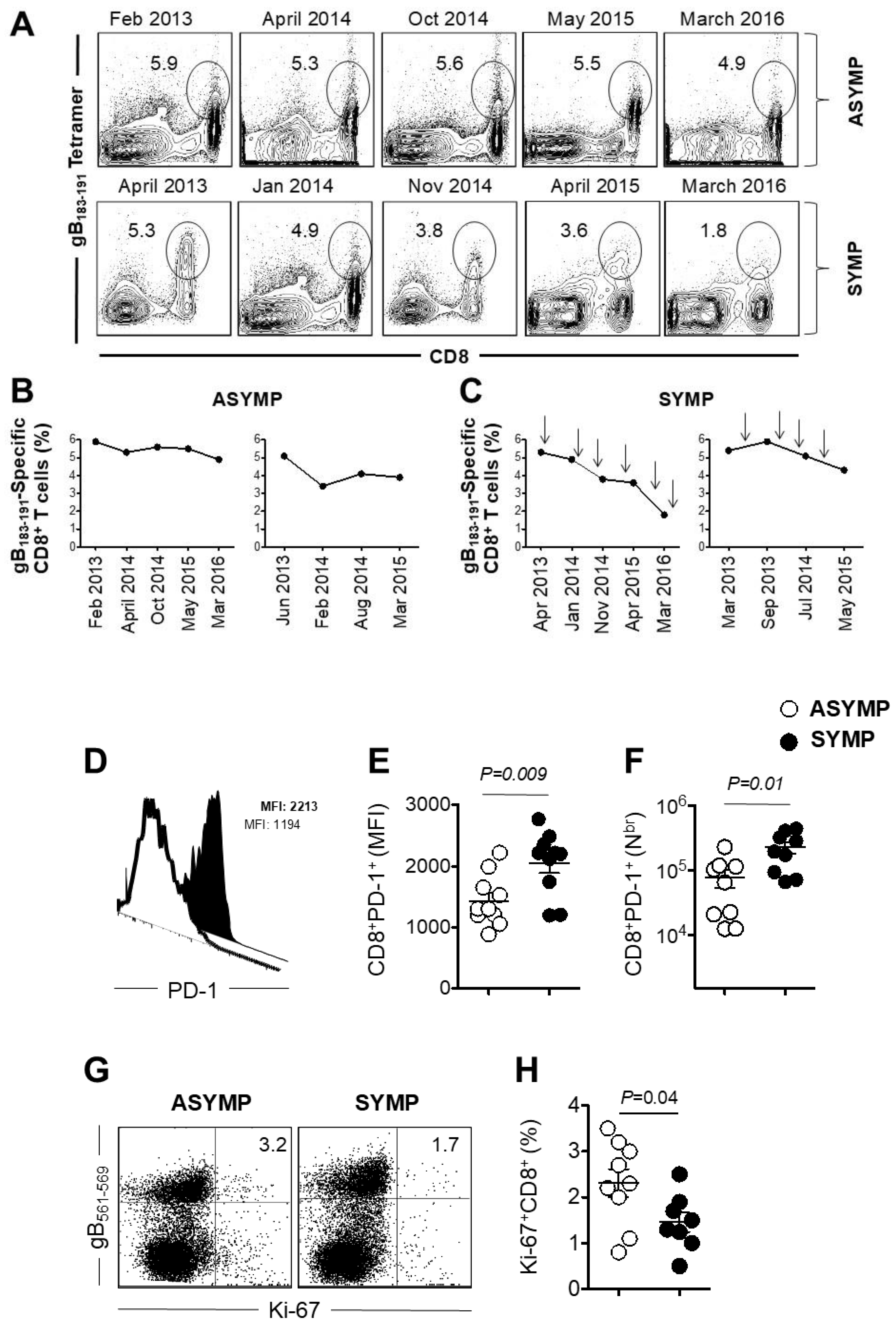
3.1. Decline of HSV-Specific CD8+ T Cells in Seropositive SYMP Individuals
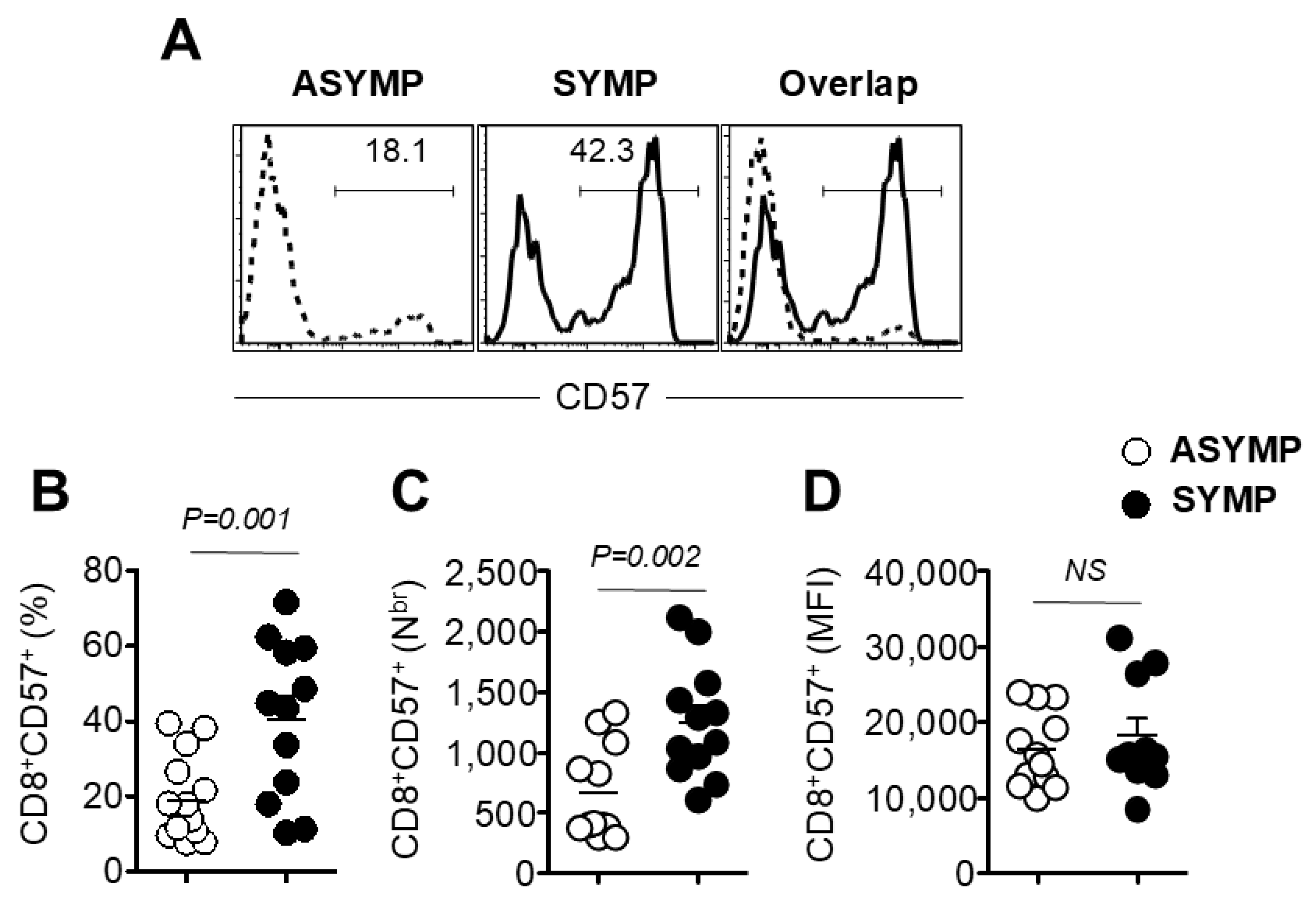
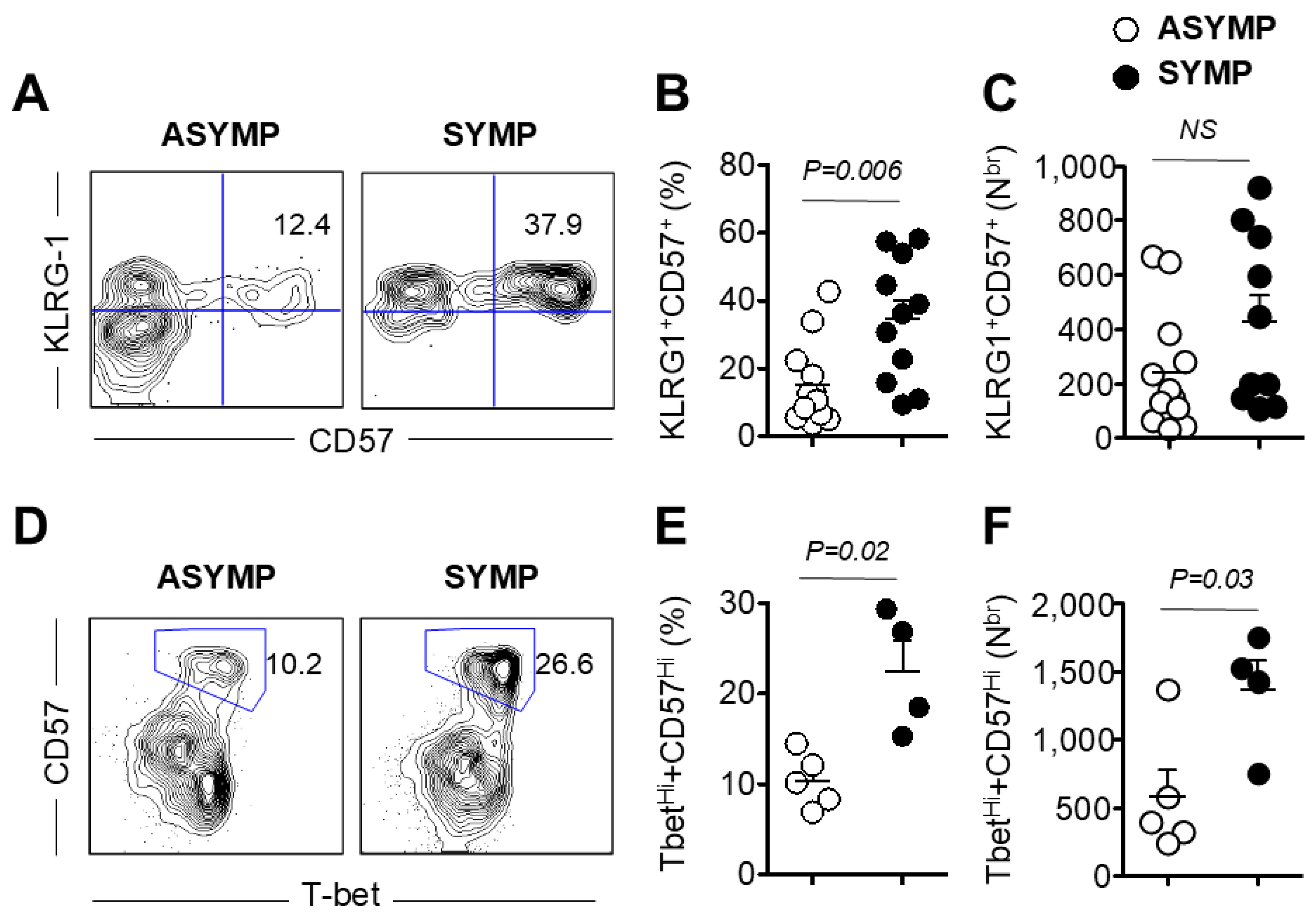
3.2. Increased Frequency of Senescent CD8+ T Cells in HSV-Seropositive SYMP Participants Compared to ASYMP Participants
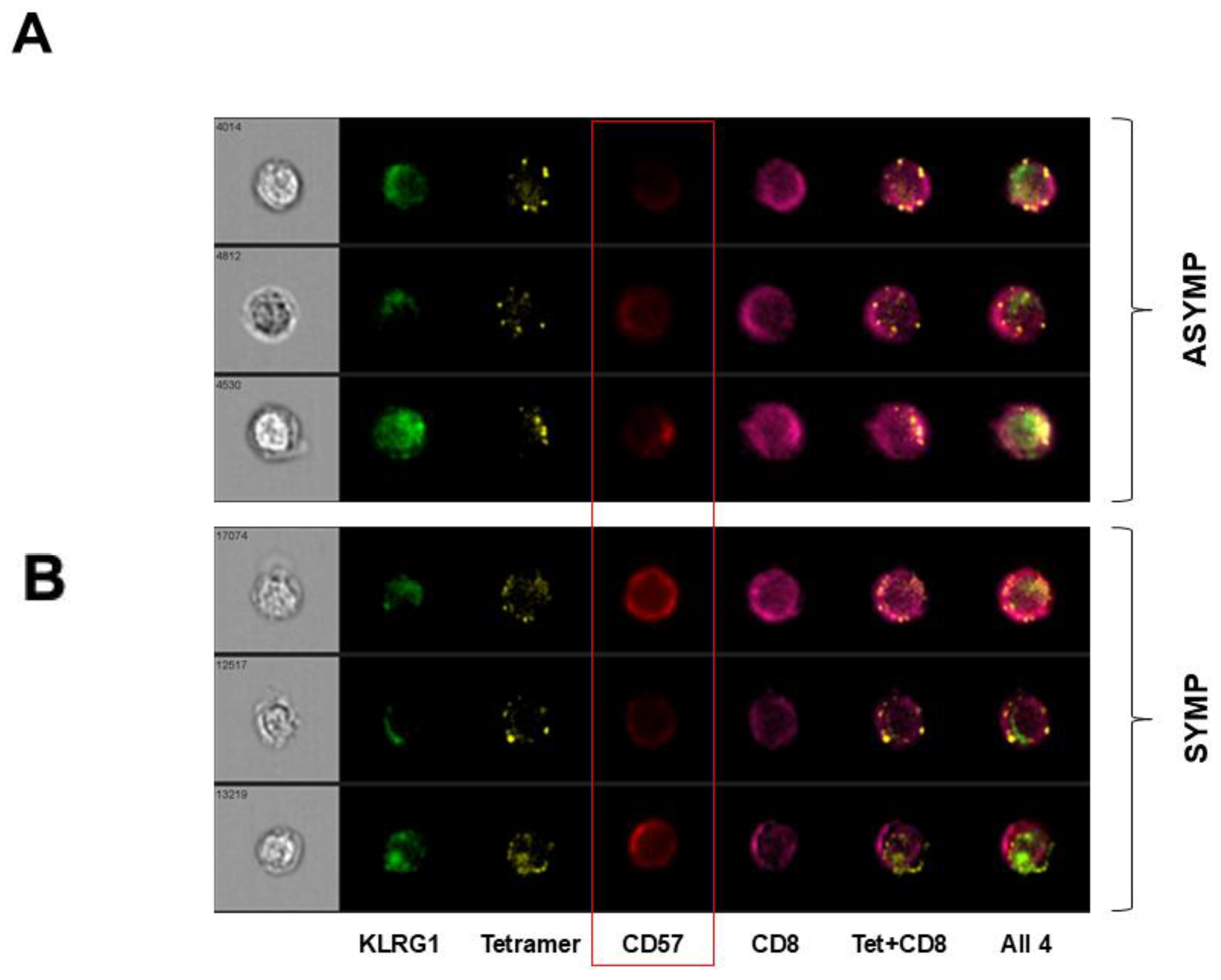
3.3. HSV-Specific CD8+ T Cells Isolated from SYMP Participants Were Terminally Differentiated
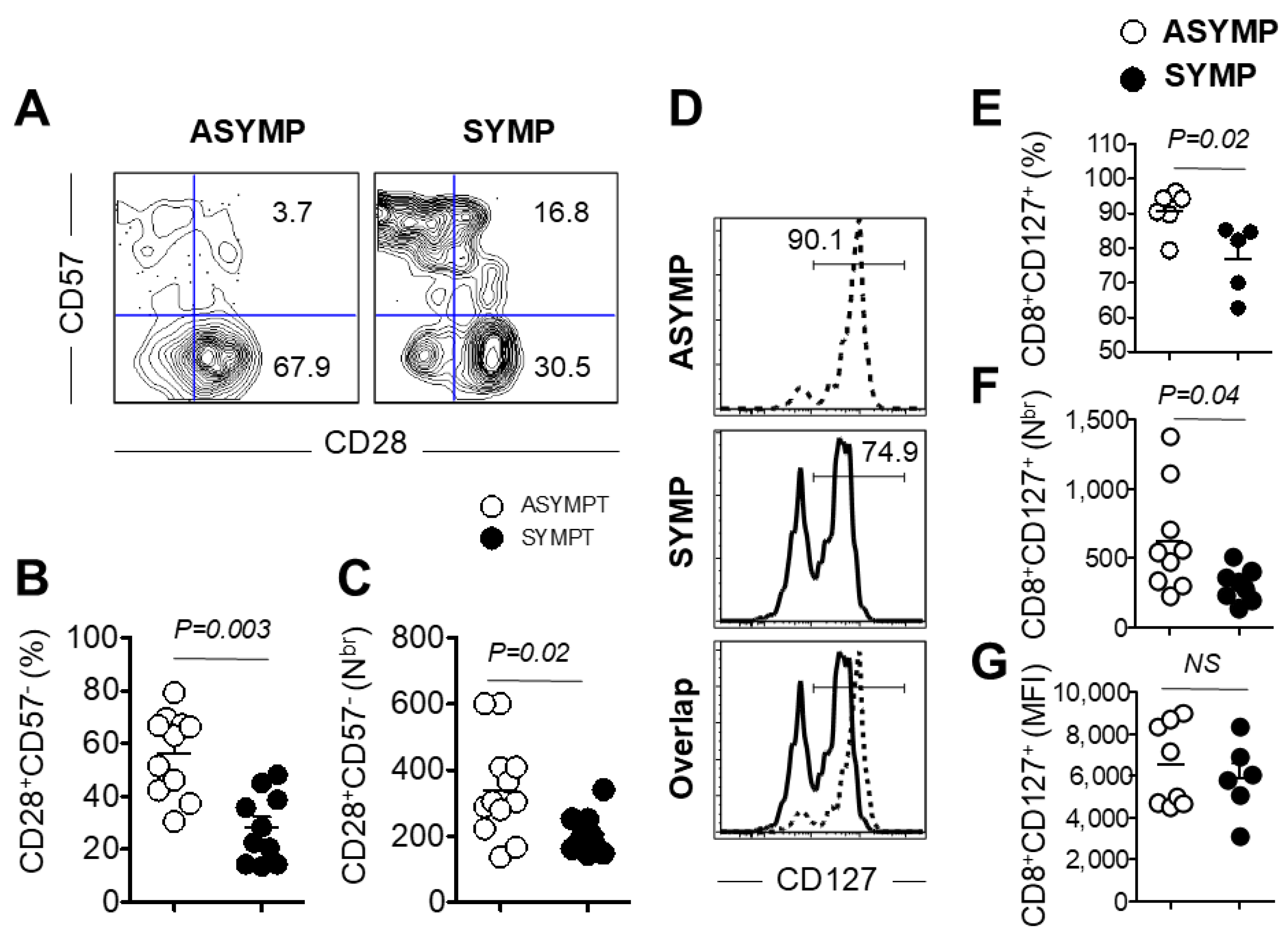
3.4. Decreased Expression of Co-Stimulatory Molecule CD28 and Survival Molecule CD127 on PBMC Isolated from HSV-Seropositive SYMP Participants
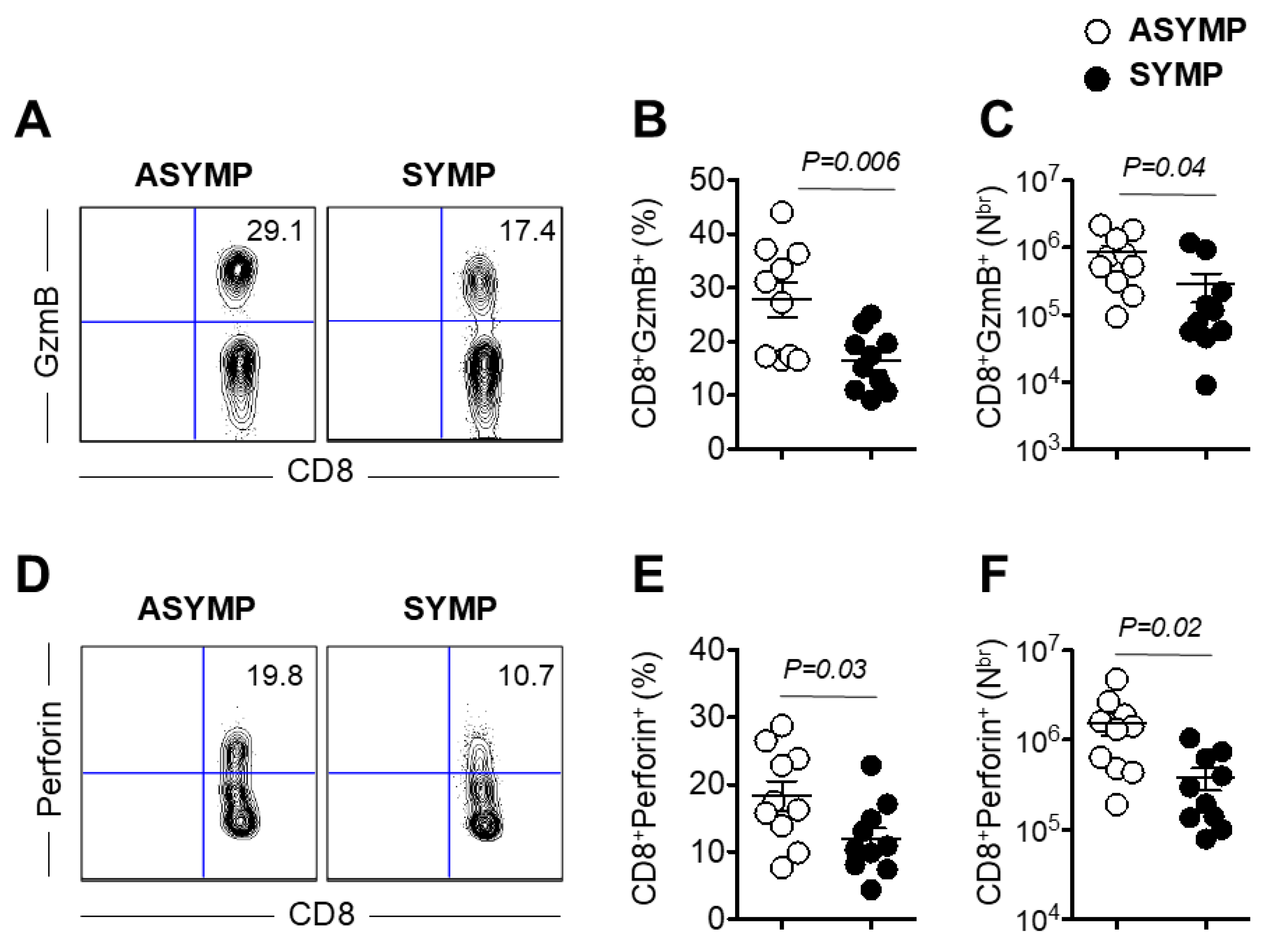
3.5. Decreased Production of Effector Molecules Granzyme B and Perforin by HSV-Specific CD8+ T Cells Isolated from HSV-1 Seropositive SYMP Individuals
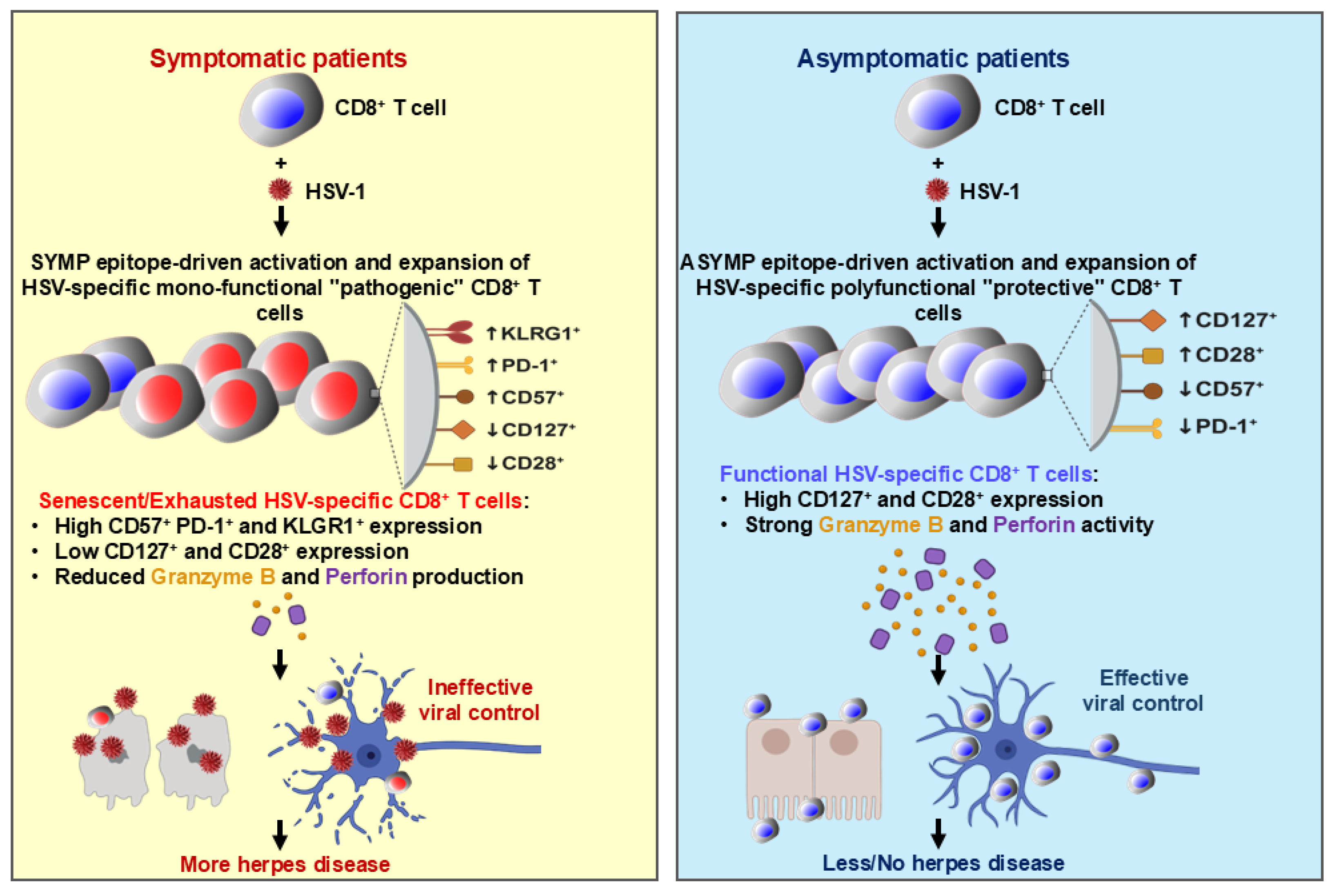
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuo, T.; Wang, C.; Badakhshan, T.; Chilukuri, S.; BenMohamed, L. The Challenges and Opportunities for the Development of a T-Cell Epitope-Based Herpes Simplex Vaccine. Vaccine 2014, 32, 6733–6745. [Google Scholar] [CrossRef] [PubMed]
- Samandary, S.; Kridane-Miledi, H.; Sandoval, J.S.; Choudhury, Z.; Langa-Vives, F.; Spencer, D.; Chentoufi, A.A.; Lemonnier, F.A.; BenMohamed, L. Associations of HLA-A, HLA-B and HLA-C Alleles Frequency with Prevalence of Herpes Simplex Virus Infections and Diseases Across Global Populations: Implication for the Development of an Universal CD8+ T-Cell Epitope-Based Vaccine. Hum. Immunol. 2014, 75, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dervillez, X.; Chentoufi, A.A.; Badakhshan, T.; Bettahi, I.; BenMohamed, L. Targeting the genital tract mucosa with a lipopeptide/recombinant adenovirus prime/boost vaccine induces potent and long-lasting CD8+ T cell immunity against herpes: Importance of MyD88. J. Immunol. 2012, 189, 4496–4509. [Google Scholar] [CrossRef] [PubMed]
- Chentoufi, A.A.; Dasgupta, G.; Christensen, N.D.; Hu, J.; Choudhury, Z.S.; Azeem, A.; Jester, J.V.; Nesburn, A.B.; Wechsler, S.L.; BenMohamed, L. A novel HLA (HLA-A*0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes. J. Immunol. 2010, 184, 2561–2571. [Google Scholar] [CrossRef]
- Chentoufi, A.A.; Zhang, X.; Lamberth, K.; Dasgupta, G.; Bettahi, I.; Nguyen, A.; Wu, M.; Zhu, X.; Mohebbi, A.; Buus, S.; et al. HLA-A*0201-restricted CD8+ cytotoxic T lymphocyte epitopes identified from herpes simplex virus glycoprotein D. J. Immunol. 2008, 180, 426–437. [Google Scholar] [CrossRef]
- Langenberg, A.G.; Corey, L.; Ashley, R.L.; Leong, W.P.; Straus, S.E. A prospective study of new infections with herpes simplex virus type 1 and type 2. Chiron HSV Vaccine Study Group. N. Engl. J. Med. 1999, 341, 1432–1438. [Google Scholar] [CrossRef]
- Dasgupta, G.; BenMohamed, L. Of mice and not humans: How reliable are animal models for evaluation of herpes CD8(+)-T cell-epitopes-based immunotherapeutic vaccine candidates? Vaccine 2011, 29, 5824–5836. [Google Scholar] [CrossRef]
- Chentoufi, A.A.; Binder, N.R.; Berka, N.; Durand, G.; Nguyen, A.; Bettahi, I.; Maillere, B.; BenMohamed, L. Asymptomatic human CD4+ cytotoxic T-cell epitopes identified from herpes simplex virus glycoprotein B. J. Virol. 2008, 82, 11792–11802. [Google Scholar] [CrossRef]
- BenMohamed, L.; Bertrand, G.; McNamara, C.D.; Gras-Masse, H.; Hammer, J.; Wechsler, S.L.; Nesburn, A.B. Identification of novel immunodominant CD4+ Th1-type T-cell peptide epitopes from herpes simplex virus glycoprotein D that confer protective immunity. J. Virol. 2003, 77, 9463–9473. [Google Scholar] [CrossRef]
- Zhang, X.; Issagholian, A.; Berg, E.A.; Fishman, J.B.; Nesburn, A.B.; BenMohamed, L. Th-cytotoxic T-lymphocyte chimeric epitopes extended by Nepsilon-palmitoyl lysines induce herpes simplex virus type 1-specific effector CD8+ Tc1 responses and protect against ocular infection. J. Virol. 2005, 79, 15289–15301. [Google Scholar] [CrossRef]
- Nesburn, A.B.; Bettahi, I.; Zhang, X.; Zhu, X.; Chamberlain, W.; Afifi, R.E.; Wechsler, S.L.; BenMohamed, L. Topical/mucosal delivery of sub-unit vaccines that stimulate the ocular mucosal immune system. Ocul. Surf. 2006, 4, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Pawelec, G. Human T cell aging and the impact of persistent viral infections. Front. Immunol. 2013, 4, 271. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Sinclair, E.; Hatano, H.; Hsue, P.Y.; Epling, L.; Hecht, F.M.; Bangsberg, D.R.; Martin, J.N.; McCune, J.M.; Deeks, S.G.; et al. Impact of HIV on CD8+ T cell CD57 expression is distinct from that of CMV and aging. PLoS ONE 2014, 9, e89444. [Google Scholar] [CrossRef]
- Saeidi, A.; Chong, Y.K.; Yong, Y.K.; Tan, H.Y.; Barathan, M.; Rajarajeswaran, J.; Sabet, N.S.; Sekaran, S.D.; Ponnampalavanar, S.; Che, K.F.; et al. Concurrent loss of co-stimulatory molecules and functional cytokine secretion attributes leads to proliferative senescence of CD8(+) T cells in HIV/TB co-infection. Cell. Immunol. 2015, 297, 19–32. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jeong, I.; Joh, J.S.; Jung, Y.W.; Sim, S.Y.; Choi, B.; Jee, H.G.; Lim, D.G. Differential expression of CD57 in antigen-reactive CD4+ T cells between active and latent tuberculosis infection. Clin. Immunol. 2015, 159, 37–46. [Google Scholar] [CrossRef]
- Focosi, D.; Bestagno, M.; Burrone, O.; Petrini, M. CD57+ T lymphocytes and functional immune deficiency. J. Leukoc. Biol. 2010, 87, 107–116. [Google Scholar] [CrossRef]
- Simonetta, F.; Hua, S.; Lecuroux, C.; Gerard, S.; Boufassa, F.; Saez-Cirion, A.; Pancino, G.; Goujard, C.; Lambotte, O.; Venet, A.; et al. High eomesodermin expression among CD57+ CD8+ T cells identifies a CD8+ T cell subset associated with viral control during chronic human immunodeficiency virus infection. J. Virol. 2014, 88, 11861–11871. [Google Scholar] [CrossRef] [PubMed]
- Duttagupta, P.A.; Boesteanu, A.C.; Katsikis, P.D. Costimulation signals for memory CD8+ T cells during viral infections. Crit. Rev. Immunol. 2009, 29, 469–486. [Google Scholar] [CrossRef]
- Weng, N.P.; Akbar, A.N.; Goronzy, J. CD28(-) T cells: Their role in the age-associated decline of immune function. Trends Immunol. 2009, 30, 306–312. [Google Scholar] [CrossRef]
- Palmer, B.E.; Blyveis, N.; Fontenot, A.P.; Wilson, C.C. Functional and phenotypic characterization of CD57+CD4+ T cells and their association with HIV-1-induced T cell dysfunction. J. Immunol. 2005, 175, 8415–8423. [Google Scholar] [CrossRef]
- Henson, S.M.; Franzese, O.; Macaulay, R.; Libri, V.; Azevedo, R.I.; Kiani-Alikhan, S.; Plunkett, F.J.; Masters, J.E.; Jackson, S.; Griffiths, S.J.; et al. KLRG1 signaling induces defective Akt (ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood 2009, 113, 6619–6628. [Google Scholar] [CrossRef] [PubMed]
- Ibegbu, C.C.; Xu, Y.X.; Harris, W.; Maggio, D.; Miller, J.D.; Kourtis, A.P. Expression of killer cell lectin-like receptor G1 on antigen-specific human CD8+ T lymphocytes during active, latent, and resolved infection and its relation with CD57. J. Immunol. 2005, 174, 6088–6094. [Google Scholar] [CrossRef] [PubMed]
- Dervillez, X.; Qureshi, H.; Chentoufi, A.A.; Khan, A.A.; Kritzer, E.; Yu, D.C.; Diaz, O.R.; Gottimukkala, C.; Kalantari, M.; Villacres, M.C.; et al. Asymptomatic HLA-A*02:01-restricted epitopes from herpes simplex virus glycoprotein B preferentially recall polyfunctional CD8+ T cells from seropositive asymptomatic individuals and protect HLA transgenic mice against ocular herpes. J. Immunol. 2013, 191, 5124–5138. [Google Scholar] [CrossRef] [PubMed]
- Betts, M.R.; Brenchley, J.M.; Price, D.A.; De Rosa, S.C.; Douek, D.C.; Roederer, M.; Koup, R.A. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 2003, 281, 65–78. [Google Scholar] [CrossRef]
- Zhang, X.; Chentoufi, A.A.; Dasgupta, G.; Nesburn, A.B.; Wu, M.; Zhu, X.; Carpenter, D.; Wechsler, S.L.; You, S.; BenMohamed, L. A genital tract peptide epitope vaccine targeting TLR-2 efficiently induces local and systemic CD8+ T cells and protects against herpes simplex virus type 2 challenge. Mucosal Immunol. 2009, 2, 129–143. [Google Scholar] [CrossRef]
- Tedeschi, V.; Paldino, G.; Kunkl, M.; Paroli, M.; Sorrentino, R.; Tuosto, L.; Fiorillo, M.T. CD8(+) T Cell Senescence: Lights and Shadows in Viral Infections, Autoimmune Disorders and Cancer. Int. J. Mol. Sci. 2022, 23, 3374. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar]
- Deeks, S.G.; Kitchen, C.M.; Liu, L.; Guo, H.; Gascon, R.; Narvaez, A.B.; Hunt, P.; Martin, J.N.; Kahn, J.O.; Levy, J.; et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood 2004, 104, 942–947. [Google Scholar] [CrossRef]
- Golden-Mason, L.; Palmer, B.; Klarquist, J.; Mengshol, J.A.; Castelblanco, N.; Rosen, H.R. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J. Virol. 2007, 81, 9249–9258. [Google Scholar] [CrossRef]
- Wedemeyer, H.; He, X.S.; Nascimbeni, M.; Davis, A.R.; Greenberg, H.B.; Hoofnagle, J.H.; Liang, T.J.; Alter, H.; Rehermann, B. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J. Immunol. 2002, 169, 3447–3458. [Google Scholar] [CrossRef]
- Ramello, M.C.; Núñez, N.G.; Tosello Boari, J.; Bossio, S.N.; Canale, F.P.; Abrate, C.; Ponce, N.; Del Castillo, A.; Ledesma, M.; Viel, S.; et al. Polyfunctional KLRG-1(+)CD57(+) Senescent CD4(+) T Cells Infiltrate Tumors and Are Expanded in Peripheral Blood From Breast Cancer Patients. Front. Immunol. 2021, 12, 713132. [Google Scholar] [CrossRef] [PubMed]
- Hay, Z.L.Z.; Slansky, J.E. Granzymes: The Molecular Executors of Immune-Mediated Cytotoxicity. Int. J. Mol. Sci. 2022, 23, 1833. [Google Scholar] [CrossRef] [PubMed]
- Seoane, R.; Vidal, S.; Bouzaher, Y.H.; El Motiam, A.; Rivas, C. The Interaction of Viruses with the Cellular Senescence Response. Biology 2020, 9, 455. [Google Scholar] [CrossRef]
- Reyes, A.; Ortiz, G.; Duarte, L.F.; Fernández, C.; Hernández-Armengol, R.; Palacios, P.A.; Prado, Y.; Andrade, C.A.; Rodriguez-Guilarte, L.; Kalergis, A.M.; et al. Contribution of viral and bacterial infections to senescence and immunosenescence. Front. Cell Infect. Microbiol. 2023, 13, 1229098. [Google Scholar] [CrossRef]
- Barathan, M.; Mohamed, R.; Saeidi, A.; Vadivelu, J.; Chang, L.Y.; Gopal, K.; Ram, M.R.; Ansari, A.W.; Kamarulzaman, A.; Velu, V.; et al. Increased frequency of late-senescent T cells lacking CD127 in chronic hepatitis C disease. Eur. J. Clin. Investig. 2015, 45, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Luciano, A.A.; Lederman, M.M.; Valentin-Torres, A.; Bazdar, D.A.; Sieg, S.F. Impaired induction of CD27 and CD28 predicts naive CD4 T cell proliferation defects in HIV disease. J. Immunol. 2007, 179, 3543–3549. [Google Scholar] [CrossRef]
- Kudryavtsev, I.; Zinchenko, Y.; Serebriakova, M.; Akisheva, T.; Rubinstein, A.; Savchenko, A.; Borisov, A.; Belenjuk, V.; Malkova, A.; Yablonskiy, P.; et al. A Key Role of CD8+ T Cells in Controlling of Tuberculosis Infection. Diagnostics 2023, 13, 2961. [Google Scholar] [CrossRef]
- De Biasi, S.; Meschiari, M.; Gibellini, L.; Bellinazzi, C.; Borella, R.; Fidanza, L.; Gozzi, L.; Iannone, A.; Lo Tartaro, D.; Mattioli, M.; et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat. Commun. 2020, 11, 3434. [Google Scholar] [CrossRef]
- Evangelou, K.; Veroutis, D.; Paschalaki, K.; Foukas, P.G.; Lagopati, N.; Dimitriou, M.; Papaspyropoulos, A.; Konda, B.; Hazapis, O.; Polyzou, A.; et al. Pulmonary infection by SARS-CoV-2 induces senescence accompanied by an inflammatory phenotype in severe COVID-19: Possible implications for viral mutagenesis. Eur. Respir. J. 2022, 60, 2102951. [Google Scholar] [CrossRef]
- Kombe Kombe, A.J.; Biteghe, F.A.N.; Ndoutoume, Z.N.; Jin, T. CD8(+) T-cell immune escape by SARS-CoV-2 variants of concern. Front. Immunol. 2022, 13, 962079. [Google Scholar] [CrossRef]
- Alcaide, M.L.; Parmigiani, A.; Pallikkuth, S.; Roach, M.; Freguja, R.; Della Negra, M.; Bolivar, H.; Fischl, M.A.; Pahwa, S. Immune activation in HIV-infected aging women on antiretrovirals--implications for age-associated comorbidities: A cross-sectional pilot study. PLoS ONE 2013, 8, e63804. [Google Scholar]
- Zhang, S.Y.; Zhang, Z.; Fu, J.L.; Kang, F.B.; Xu, X.S.; Nie, W.M.; Zhou, C.B.; Zhao, M.; Wang, F.S. Progressive CD127 down-regulation correlates with increased apoptosis of CD8 T cells during chronic HIV-1 infection. Eur. J. Immunol. 2009, 39, 1425–1434. [Google Scholar] [CrossRef]
- Brenchley, J.M.; Karandikar, N.J.; Betts, M.R.; Ambrozak, D.R.; Hill, B.J.; Crotty, L.E.; Casazza, J.P.; Kuruppu, J.; Migueles, S.A.; Connors, M.; et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 2003, 101, 2711–2720. [Google Scholar] [CrossRef]
- Voehringer, D.; Koschella, M.; Pircher, H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1). Blood 2002, 100, 3698–3702. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.R.; Li, Q.; Odunsi, K.; Shrikant, P.A. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity 2010, 32, 67–78. [Google Scholar] [CrossRef]
- Dolfi, D.V.; Mansfield, K.D.; Polley, A.M.; Doyle, S.A.; Freeman, G.J.; Pircher, H.; Schmader, K.E.; Wherry, E.J. Increased T-bet is associated with senescence of influenza virus-specific CD8 T cells in aged humans. J. Leukoc. Biol. 2013, 93, 825–836. [Google Scholar] [CrossRef]
- Hersperger, A.R.; Martin, J.N.; Shin, L.Y.; Sheth, P.M.; Kovacs, C.M.; Cosma, G.L.; Makedonas, G.; Pereyra, F.; Walker, B.D.; Kaul, R.; et al. Increased HIV-specific CD8+ T-cell cytotoxic potential in HIV elite controllers is associated with T-bet expression. Blood 2011, 117, 3799–3808. [Google Scholar] [CrossRef] [PubMed]
- Chentoufi, A.A.; Kritzer, E.; Tran, M.V.; Dasgupta, G.; Lim, C.H.; Yu, D.C.; Afifi, R.E.; Jiang, X.; Carpenter, D.; Osorio, N.; et al. The herpes simplex virus 1 latency-associated transcript promotes functional exhaustion of virus-specific CD8+ T cells in latently infected trigeminal ganglia: A novel immune evasion mechanism. J. Virol. 2011, 85, 9127–9138. [Google Scholar] [CrossRef]
- Srivastava, R.; Dervillez, X.; Khan, A.A.; Chentoufi, A.A.; Chilukuri, S.; Shukr, N.; Fazli, Y.; Ong, N.N.; Afifi, R.E.; Osorio, N.; et al. The Herpes Simplex Virus Latency-Associated Transcript Gene Is Associated with a Broader Repertoire of Virus-Specific Exhausted CD8+ T Cells Retained within the Trigeminal Ganglia of Latently Infected HLA Transgenic Rabbits. J. Virol. 2016, 90, 3913–3928. [Google Scholar] [CrossRef]
| Subject-Level Characteristic | All Subjects (n = 781) |
|---|---|
| Gender [no. (%)]: | |
| Female | 395 (51%) |
| Male | 386 (49%) |
| Race [no. (%)]: | |
| White | 543 (69%) |
| Nonwhite | 238 (31%) |
| Age [median (range) year]: | 30 (21–67 year) |
| HSV status [no. (%)] | |
| HSV-1-positive | 283 (36%) |
| HSV-2-positive | 366 (47%) |
| HSV-1-& 2-positive | 31 (4%) |
| HSV-negative | 92 (12%) |
| HLA [no. (%)] | |
| HLA-A*02:01-positive | 411 (53%) |
| HLA-A*02:01-negative | 370 (447%) |
| Herpes Disease Status [no. (%)] | |
| ASYMPTOMATIC (ASYMP) | 711 (91%) |
| SYMPTOMATIC (SYMP) | 70 (9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chentoufi, A.A.; Khan, A.A.; Srivastava, R.; Karan, S.; Lekbach, Y.; Vahed, H.; BenMohamed, L. Dysfunctional Senescent Herpes Simplex Virus-Specific CD57+CD8+ T Cells Are Associated with Symptomatic Recurrent Ocular Herpes in Humans. Viruses 2025, 17, 606. https://doi.org/10.3390/v17050606
Chentoufi AA, Khan AA, Srivastava R, Karan S, Lekbach Y, Vahed H, BenMohamed L. Dysfunctional Senescent Herpes Simplex Virus-Specific CD57+CD8+ T Cells Are Associated with Symptomatic Recurrent Ocular Herpes in Humans. Viruses. 2025; 17(5):606. https://doi.org/10.3390/v17050606
Chicago/Turabian StyleChentoufi, Aziz A., Arif A. Khan, Ruchi Srivastava, Sweta Karan, Yassir Lekbach, Hawa Vahed, and Lbachir BenMohamed. 2025. "Dysfunctional Senescent Herpes Simplex Virus-Specific CD57+CD8+ T Cells Are Associated with Symptomatic Recurrent Ocular Herpes in Humans" Viruses 17, no. 5: 606. https://doi.org/10.3390/v17050606
APA StyleChentoufi, A. A., Khan, A. A., Srivastava, R., Karan, S., Lekbach, Y., Vahed, H., & BenMohamed, L. (2025). Dysfunctional Senescent Herpes Simplex Virus-Specific CD57+CD8+ T Cells Are Associated with Symptomatic Recurrent Ocular Herpes in Humans. Viruses, 17(5), 606. https://doi.org/10.3390/v17050606






