Perinatal Lamb Model of Respiratory Syncytial Virus (RSV) Infection
Abstract
:1. Introduction
2. Perinatal and Adult Lung in Regards to RSV Infection
3. Lambs as a Model of RSV Infection of Infants



| Feature | Advantage of Model | References |
|---|---|---|
| Prenatal alveologenesis | Similar to infants | [43,44] |
| Airway branching pattern | Similar to infants | [42] |
| Submucosal glands in airways | Similar to infants | [43,45,46,47] |
| Number/development of Clara cells | Similar to infants | [27,64] |
| Number/development of type II cells | Similar to infants | [27,64,73] |
| Lung size | Similar to infants | Generally known |
| Can survive at 90% gestation | Similar to infants | [19] |
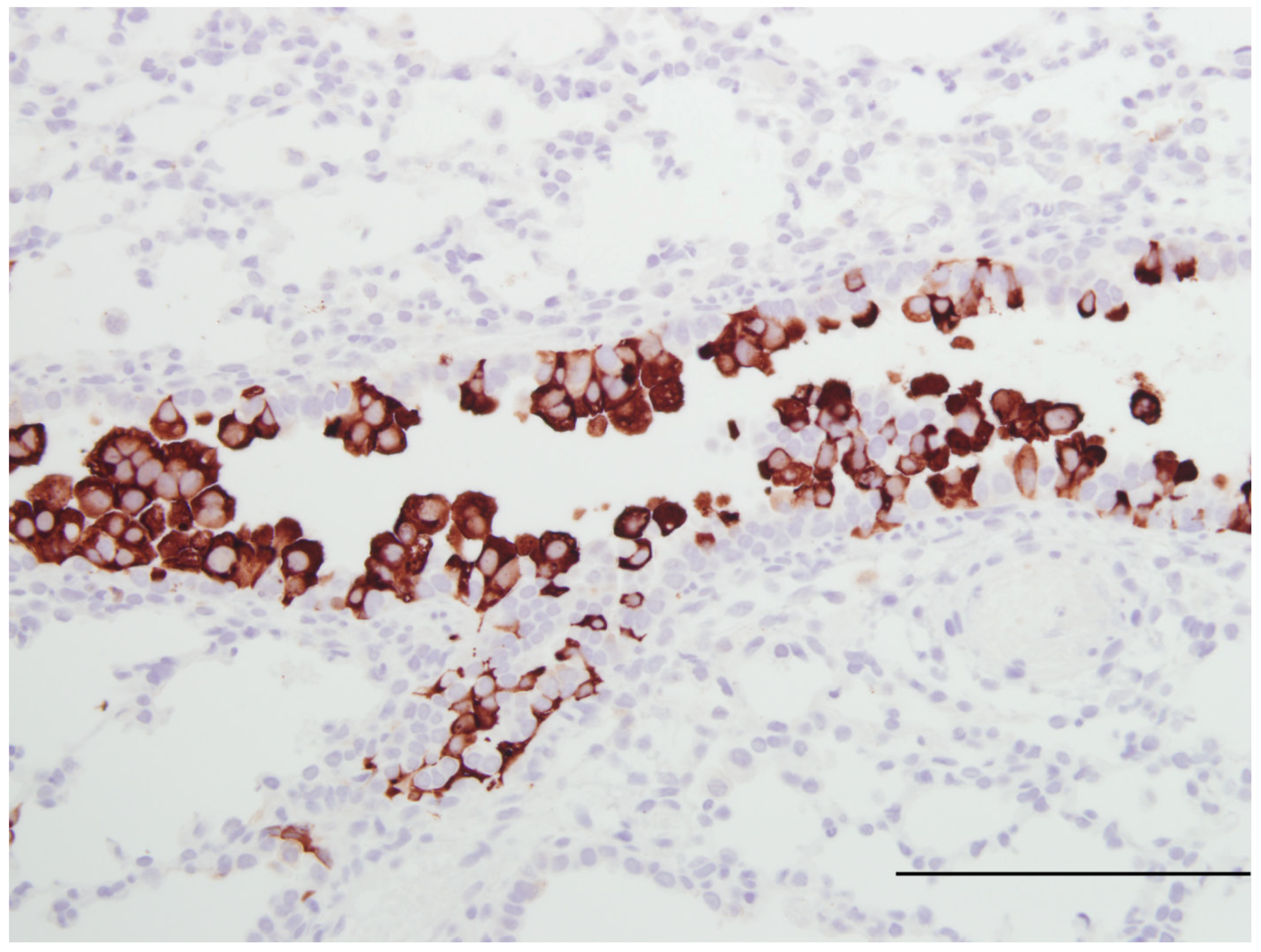
| Susceptible to human RSV strains | Similar to infants | [16,20] Figure 1 |
| Susceptible to bovine RSV strains | Permissible to RSV strains | [14,15,16,17,95] |
| Susceptible to ovine PI-3 | Paramyxovirus susceptibility | [18] |
| Bronchiolitis | Similar to infants | [15,18,21,82,85] |
| Syncytial cell formation | [19,20] Figure 1 | |
| Neutrophil infiltration | [15,18,19,20,82,85] | |
| CD4, CD8 T cells | [19,78] | |
| B cells and plasma cells | [19,78] | |
| Enhanced RSV disease severity in | Similar to infants | [19,20,74] |
| preterm and newborn | ||
| Reduced immune responses preterm | Similar to infants | [71,75,76,79] |
| Reduced neutrophils responses preterm | Similar to infants | [74] |
| Dendritic cell responses to RSV | Similar to infants | [75,76,77] |
| IL-8 gene expression | Similar to infants | [74,78] |
| Functional Duox/LPO system | Similar to human/infants | [62] |
| Innate immune responses | Similar to infants | [34,38,71,72,74,78,79] |
| Adaptive immune responses | Similar to infants | [34,38,42,78] |
| Outbred (genetic diversity) | Similar to infants | [34,38,42,78] |
| Newborn lamb can be deprived of maternal | Can vaccinate newborn | Generally known |
| immunoglobulin (Ig) | without interference by | |
| maternal Ig | ||
| Jugular vein large and accessible | Allows placement of | Generally known |
| catheter to deliver drugs | ||
| Synchronized birth | Allows groups of lambs | Generally known |
| of similar age | ||
| VEGF reduces RSV severity | Model can test anti-RSV | [82,85] |
| therapies and drugs | ||
| Fetal lambs exposed to ethanol in vivo have | Model can test drugs and | [89,90] |
| reduced SP-A production, lung | risk factors for lung | |
| development, HIF 1α, HIF 2α, VEGF, and | development and RSV | |
| VEGFR | susceptibility | |
| Enhanced lymphocytic responses following | Model can study FI-RSV | Manuscript in preparation |
| FI-RSV vaccination | pathogenesis, mechanisms | |
| and vaccines |
Acknowledgements
Conflict of Interest
References
- Nair, H.; Nokes, D.J.; Gessner, B.D.; Dherani, M.; Madhi, S.A.; Singleton, R.J.; O'Brien, K.L.; Roca, A.; Wright, P.F.; Bruce, N.; et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef]
- Chanock, R.; Roizman, B.; Myers, R. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). I. Isolation, properties and characterization. Am. J. Hyg. 1957, 66, 281–290. [Google Scholar]
- Chanock, R.; Finberg, L. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). Ii. Epidemiologic aspects of infection in infants and young children. Am. J. Hyg. 1957, 66, 291–300. [Google Scholar]
- Empey, K.M.; Peebles, R.S., Jr.; Kolls, J.K. Pharmacologic advances in the treatment and prevention of respiratory syncytial virus. Clin. Infect. Dis. 2010, 50, 1258–1267. [Google Scholar] [CrossRef]
- Meert, K.L.; Sarnaik, A.P.; Gelmini, M.J.; Lieh-Lai, M.W. Aerosolized ribavirin in mechanically ventilated children with respiratory syncytial virus lower respiratory tract disease: A prospective, double-blind, randomized trial. Crit. Care Med. 1994, 22, 566–572. [Google Scholar] [CrossRef]
- Wu, H.; Pfrarr, D.S.; Losonsky, G.A.; Kiener, P.A. Immunoprophylaxis of RSV infection: Advancing from RSV-IGIV to palivizumab and motavizumab. Curr. Top Microbiol. Immunol. 2008, 317, 103–123. [Google Scholar]
- Olszweska, W.; Openshaw, P. Emerging drugs for respiratory syncytial virus infection. Expert Opin. Ermg. Drugs 2009, 14, 207–217. [Google Scholar] [CrossRef]
- Graham, B.S. Pathogenesis of respiratory syncytial virus vaccine-augmented pathology. Am. J. Respir. Crit. Care Med. 1995, 152, S63–S66. [Google Scholar]
- Collins, P.L.; Melero, J.A. Progress in understanding and controlling respiratory syncytial virus: Still crazy after all these years. Virus Res. 2011, 162, 80–99. [Google Scholar] [CrossRef]
- Hon, K.L.; Leung, T.F.; Cheng, W.Y.; Ko, N.M.; Tang, W.K.; Wong, W.W.; Yeung, W.H.; Chan, P.K. Respiratory syncytial virus morbidity, premorbid factors, seasonality, and implications for prophylaxis. J. Crit. Care 2012. ePub ahead of print. [Google Scholar]
- Sommer, C.; Resch, B.; Simoes, E.A. Risk factors for severe respiratory syncytial virus lower respiratory tract infection. J. Open Microbiol. 2012, 5, 144–154. [Google Scholar]
- Martin, J.A.; Hamilton, B.E.; Sutton, P.D.; Ventura, S.J.; Mathews, T.J.; Osterman, M.J. Births: Final data for 2008. Natl. Vital Stat. Rep. 2010, 59, 3–71. [Google Scholar]
- Lawn, J.E.; Gravett, M.G.; Nunes, T.M.; Rubens, C.E.; Stanton, C. Global Report on Preterm Birth and Stillbirth (1 of 7): Definitions, Description of the Burden and Opportunities to Improve Data; Report; BMC: London, UK, 2010. [Google Scholar]
- Belknap, E.B.; Ciszewski, D.K.; Baker, J.C. Experimental respiratory syncytial virus infection in calves and lambs. J. Vet. Diagn. Invest. 1995, 7, 285–298. [Google Scholar] [CrossRef]
- Cutlip, R.C.; Lehmkuhl, H.D. Lesions in lambs experimentally infected with bovine respiratory syncytial virus. Am. J. Vet. Res. 1979, 40, 1479–1482. [Google Scholar]
- Lapin, C.D.; Hiatt, P.W.; Langston, C.; Mason, E.; Piedra, P.T. A lamb model for human respiratory syncytial virus infection. Pediatr. Pulmonol. 1993, 15, 151–156. [Google Scholar] [CrossRef]
- Lehmkuhl, H.D.; Cutlip, R.C. Experimental respiratory syncytial virus infection in feeder-age lambs. Am. J. Vet. Res. 1979, 40, 1729–1730. [Google Scholar]
- Grubor, B.; Gallup, J.M.; Meyerholz, D.K.; Crouch, E.; Evans, R.B.; Brogden, K.A.; Lehmkuhl, H.D.; Ackermann, M.R. Enhanced surfactant protein and defensin mRNA levels and reduced viral replication during paramyxoviral pneumonia in neonatal lambs. Clin. Vaccine Immunol. 2004, 11, 599–607. [Google Scholar] [CrossRef]
- Meyerholz, D.K.; Grubor, B.; Fach, S.J.; Sacco, R.E.; Lehmkuhl, H.D.; Gallup, J.M.; Ackermann, M.R. Reduced clearance of respiratory syncytial virus in a preterm lamb model. Microb. Infect. 2004, 6, 1312–1319. [Google Scholar] [CrossRef]
- Olivier, A.; Gallup, J.M.; de Macedo, M.M.; Varga, S.M.; Ackermann, M.R. Human respiratory syncytial virus A2 strain replicates and induces innate immune responses by respiratory epithelia of neonatal lambs. Int. J. Exp. Pathol. 2009, 90, 431–438. [Google Scholar] [CrossRef]
- Johnson, J.E.; Gonzales, R.A.; Olson, S.J.; Wright, P.F.; Graham, B.S. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod. Pathol. 2007, 20, 108–119. [Google Scholar] [CrossRef]
- Burri, P.H. Fetal and postnatal development of the lung. Annu. Rev. Physiol. 1984, 46, 617–628. [Google Scholar] [CrossRef]
- Jeffery, P.K.; Gaillard, D.; Moret, S. Human airway secretory cells during development and in mature airway epithelium. Eur. Respir. J. 1992, 5, 93–104. [Google Scholar]
- Lukacs, N.W.; Smitt, J.J.; Mukherjee, S.; Morris, S.B.; Nunez, G.; Lindell, D.M. Respiratory virus-induced TLR7 activation controls IL-17-associated increased mucus via IL-23 regulation. J. Immunol. 2010, 185, 2231–2239. [Google Scholar] [CrossRef]
- Barth, P.J.; Wolf, M.; Ramaswamy, A. Distribution and number of clara cells in the normal and disturbed development of the human fetal lung. Pediatr. Pathol. 1994, 14, 637–651. [Google Scholar] [CrossRef]
- Boers, J.E.; Ambergen, A.W.; Thunnissen, F.B. Number and proliferation of clara cells in normal human airway epithelium. Am. J. Respir. Crit. Care Med. 1999, 159, 1585–1591. [Google Scholar]
- Plopper, C.G.; Mariassy, A.T.; Hill, L.H. Ultrastructure of the nonciliated bronchiolar epithelial (clara) cell of mammalian lung: I. A comparison of rabbit, guinea pig, rat, hamster, and mouse. Exp. Lung. Res. 1980, 1, 139–154. [Google Scholar] [CrossRef]
- Bernard, A.; Thielemans, N.; Lauwerys, R.; Langhendries, J.P.; Van Lierde, M.; Freund, M.M. Clara cell protein in human amniotic fluid: a potential marker of fetal lung growth. Pediatr. Res. 1994, 36, 771–775. [Google Scholar] [CrossRef]
- Elizur, A.; Adair-Kirk, T.L.; Kelley, D.G.; Griffin, G.L.; de Mello, D.E.; Senior, R.M. Clara cells impact the pulmonary innate immune response to LPS. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 293, L383–L392. [Google Scholar] [CrossRef]
- Wang, S.Z.; Rosenberger, C.L.; Bao, Y.X.; Stark, J.M.; Harrod, K.S. Clara cell secretory protein modulates lung inflammatory and immune responses to respiratory syncytial virus infection. J. Immunol. 2003, 171, 1051–1060. [Google Scholar]
- You, D.; Becnel, D.; Wang, K.; Ripple, M.; Daly, M.; Cormier, S.A. Exposure of neonates to respiratory syncytial virus is critical in determining subsequent airway response in adults. Respir. Res. 2006, 7, 107–117. [Google Scholar] [CrossRef]
- Willems, F.; Vollstedt, S.; Suter, M. Phenotype and function of neonatal DC. Eur. J. Immunol. 2009, 39, 26–35. [Google Scholar] [CrossRef]
- Thornburg, N.J.; Shepherd, B.; Crowe, J.E., Jr. Transforming growth factor beta is a major regulator of human neonatal immune responses following respiratory syncytial virus infection. J. Virol. 2010, 84, 12895–12902. [Google Scholar] [CrossRef]
- Levy, O.; Martin, S.; Eichenwald, E.; Ganz, T.; Valore, E.; Carroll, S.F.; Lee, K.; Goldmann, D.; Thorne, G.M. Impaired innate immunity in the newborn: Newborn neutrophils are deficient in bactericidal/permeability-increasing protein. Pediatrics 1999, 104, 1327–1333. [Google Scholar] [CrossRef]
- Ambruso, D.R.; Bentwood, B.; Henson, P.M.; Johnston, R.B., Jr. Oxidative metabolism of cord blood neutrophils: Relationship to content and degranulation of cytoplasmic granules. Pediatr. Res. 1984, 18, 1148–1153. [Google Scholar] [CrossRef]
- Qing, G.; Rajaraman, K.; Bortolussi, R. Diminished priming of neonatal polymorphonuclear leukocytes by lipopolysaccharide is associated with reduced CD14 expression. Infect. Immun. 1995, 63, 248–252. [Google Scholar]
- Remijsen, Q.; Kuijpers, T.W.; Wirawan, E.; Lippens, S.; Vandenabeele, P.; Vanden Berghe, T. Dying for a cause: Netosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011, 18, 581–588. [Google Scholar] [CrossRef]
- Levy, O. Innate immunity of the newborn: Basic mechanisms and clinical correlates. Nat. Rev. Immunol. 2007, 7, 379–390. [Google Scholar] [CrossRef]
- Bem, R.A.; Domachowske, J.B.; Rosenberg, H.F. Animal models of human respiratory syncytial virus disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 301, L148–L156. [Google Scholar] [CrossRef]
- Thomas, L.H.; Cook, R.S.; Howard, C.J.; Gaddum, R.M.; Taylor, G. Influence of selective T-lymphocyte depletion on the lung pathology of gnotobiotic calves and the distribution of different T-lymphocyte subsets following challenge with bovine respiratory syncytial virus. Res. Vet. Sci. 1996, 61, 38–44. [Google Scholar] [CrossRef]
- Kalina, W.V.; Woolums, A.R.; Berghous, R.D.; Gerswin, L.J. Formalin-inactivated bovine RSV vaccine enhances a Th2 mediated immune response in infected cattle. Vaccine 2004, 22, 1465–1474. [Google Scholar] [CrossRef]
- Scheerlinck, J.P.; Snibson, K.J.; Bowles, V.M.; Sutton, P. Biomedical applications of sheep models: From asthma to vaccines. Trends Biotechnol. 2008, 26, 259–266. [Google Scholar] [CrossRef]
- Alcorn, D.G.; Adamson, T.M.; Maloney, J.E.; Robinson, P.M. A morphologic and morphometric analysis of fetal lung development in the sheep. Anat. Rec. 1981, 201, 655–667. [Google Scholar] [CrossRef]
- Flecknoe, S.L.; Wallace, M.J.; Cock, M.L.; Harding, R.; Hooper, S.B. Changes in alveolar epithelial cell proportions during fetal and postnatal development in sheep. Am. J. Physiol. Lung. Cell Mol. Physiol. 2003, 285, L664–L679. [Google Scholar]
- Plopper, C.G.; Mariassy, A.T.; Lollini, L.O. Structure as revealed by airway dissection. A comparison of mammalian lungs. Am. Rev. Respir. Dis. 1983, 128, S4–S7. [Google Scholar]
- Smith, L.J.; McKay, K.O.; van Asperen, P.P.; Selvadurai, H.; Fitzgerald, D.A. Normal development of the lung and premature birth. Paediatr. Respir. Rev. 2010, 11, 135–142. [Google Scholar] [CrossRef]
- Borthwick, D.W.; West, J.D.; Keighren, M.A.; Flockhart, J.H.; Innes, B.A.; Dorin, J.R. Murine submucosal glands are clonally derived and show a cystic fibrosis gene-dependent distribution pattern. Am J Resp Cell Mol Biol 1999, 20, 1181–1189. [Google Scholar]
- Banfi, B. A novel host defense system of airways is defective in cystic fibrosis: Update. Am. J. Resp. Crit. Care Med. 2007, 175, 967. [Google Scholar]
- Conner, G.E.; Salathe, M.; Forteza, R. Lactoperoxidase and hydrogen peroxide metabolism in the airway. Am. J. Respir. Crit. Care Med. 2002, 166, S57–S61. [Google Scholar] [CrossRef]
- Conner, G.E.; Wijkstrom-Frei, C.; Randell, S.H.; Fernandez, V.E.; Salathe, M. The lactoperoxidase system links anion transport to host defense in cystic fibrosis. FEBS Lett. 2007, 581, 271–278. [Google Scholar] [CrossRef]
- Fischer, H. Mechanism and function of DUOX in epithelia of the lung. Antioxid. Redox. Signal 2009, 11, 2453–2465. [Google Scholar] [CrossRef]
- Ampuero, S.; Luchsinger, V.; Tapia, L.; Palomino, M.A.; Larranaga, C.E. Sp-A1, SP-A2 and SP-D gene polymorphisms in severe acute respiratory syncytial infection in chilean infants. Infect. Genet. Evol. 2011, 11, 1368–1377. [Google Scholar] [CrossRef]
- Forteza, R.; Salathe, M.; Miot, F.; Forteza, R.; Conner, G.E. Regulated hydrogen peroxide production by Duox in human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2005, 32, 462–469. [Google Scholar] [CrossRef]
- Fragoso, M.A.; Fernandez, V.; Forteza, R.; Randell, S.H.; Salathe, M.; Conner, G.E. Transcellular thiocyanate transport by human airway epithelia. J. Physiol. 2004, 561, 183–194. [Google Scholar] [CrossRef]
- Furtmüller, P.G.; Jantschko, W.; Regelsberger, G.; Jakopitsch, C.; Arnhold, J.; Obinger, C. Reaction of lactoperoxidase compound I with halides and thiocyanate. Biochemistry 2002, 41, 11895–11900. [Google Scholar]
- Pedemonte, N.; Caci, E.; Sondo, E.; Caputo, A.; Rhoden, K.; Pfeffer, U.; Di Candia, M.; Bandettini, R.; Ravazzolo, R.; Zegarra-Moran, O.; et al. Thiocyanate transport in resting and IL-4-stimulated human bronchial epithelial cells: role of pendrin and anion channels. J. Immunol. 2007, 178, 5144–5153. [Google Scholar]
- Wijkstrom-Frei, C.; El-Chemaly, S.; Ali-Rachedi, R.; Gerson, C.; Cobas, M.A.; Forteza, R.; Salathe, M.; Conner, G.E. Lactoperoxidase and human airway host defense. Am. J. Respir. Cell Mol. Biol. 2003, 29, 206–212. [Google Scholar] [CrossRef]
- Fischer, A.J.; Lennemann, N.J.; Krishnamurthy, S.; Pocza, P.; Durairaj, L.; Launspack, J.L.; Rhein, B.A.; Wohlford-Lenane, C.; Lorentzen, D.; Banfi, B.; et al. Enhancement of respiratory mucosal antiviral defenses by the oxidation of iodide. Am. J. Respir. Cell Mol. Biol. 2011, 45, 874–881. [Google Scholar] [CrossRef]
- Geiszt, M.; Witta, J.; Baffi, J.; Lekstrom, K.; Leto, T.L. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003, 17, 1502–1504. [Google Scholar]
- Moskwa, P.; Lorentzen, D.; Excoffon, K.J.D.A.; Zabner, J.; McCray, P.B., Jr.; Nauseef, W.M.; Dupuy, C.; Banfi, B. A novel host defense system of airways is defective in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2007, 175, 174–183. [Google Scholar]
- Fischer, H.; Gonzales, L.K.; Kolla, V.; Schwarzer, C.; Miot, F.; Illek, B.; Ballard, P.L. Developmental regulation of Duox1 expression and function in human fetal lung epithelial cells. Am. J. Physiol. Lung. Cell Mol. Physiol. 2007, 292, L1506–L1514. [Google Scholar] [CrossRef]
- Gerson, C.; Sabater, J.; Scuri, M.; Torbati, A.; Coffey, R.; Abraham, J.W.; Lauredo, I.; Forteza, R.; Wanner, A.; Salathe, M.; et al. The lactoperoxidase system function in bacterial clearance of airways. Am. J. Resir. Cell Mol. Biol. 2000, 22, 665–671. [Google Scholar]
- Barth, P.J.; Koch, S.; Muller, B.; Unterstab, F.; von Wichert, P.; Moll, R. Proliferation and number of clara cell 10-kda protein (CC10)-reactive epithelial cells and basal cells in normal, hyperplastic and metaplastic bronchial mucosa. Virchows. Arch. 2000, 437, 648–655. [Google Scholar] [CrossRef]
- Plopper, C.G.; Hill, L.H.; Mariassy, A.T. Ultrastructure of the nonciliated bronchiolar epithelial (clara) cell of mammalian lung. A study of man with comparison of 15 mammalian species. Exp. Lung. Res. 1980, 1, 171–180. [Google Scholar] [CrossRef]
- Khoor, A.; Gray, M.E.; Singh, G.; Stahlman, M.T. Ontogeny of clara cell-specific protein and its mRNA: Their association with neuroepithelial bodies in human fetal lung and in bronchopulmonary dysplasia. J. Histochem. Cytochem. 1996, 44, 1429–1438. [Google Scholar] [CrossRef]
- Li, X.; Castleman, W.L. Effects of 4-ipomeanol on bovine parainfluenza type 3 virus-induced pneumonia in calves. Vet. Pathol. 1991, 28, 428–437. [Google Scholar] [CrossRef]
- Haynes, L.M.; Moore, D.D.; Kurt-Jones, E.A.; Finberg, R.W.; Anderson, L.J.; Tripp, R.A. Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J. Virol. 2001, 75, 10730–10737. [Google Scholar] [CrossRef]
- Liu, P.; Jamaluddin, M.; Li, K.; Garofalo, R.P.; Casola, A.; Brasier, A.R. Retinoic acid-inducible gene I mediates early antiviral response and toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J. Virol. 2007, 81, 1401–1411. [Google Scholar]
- Scagnolari, C.; Midulla, F.; Pierangeli, A.; Moretti, C.; Bonci, E.; Berardi, R.; De Angelis, D.; Selvaggi, C.; Di Marco, P.; Girardi, E.; et al. Gene expression of nucleic acid-sensing pattern recognition receptors in children hospitalized for respiratory syncytial virus-associated acute bronchiolitis. Clin. Vaccine Immunol. 2009, 16, 816–823. [Google Scholar] [CrossRef]
- Vareille, M.; Kieninger, E.; Edwards, M.R.; Regamey, N. The airway epithelium: soldier in the fight against respiratory viruses. Clin. Microbiol. Rev. 2011, 24, 210–229. [Google Scholar] [CrossRef]
- Sow, F.B.; Gallup, J.M.; Derscheid, R.; Krishnan, S.; Ackermann, M.R. Ontogeny of the immune response in the ovine lung. Immunol. Invest. 2012, 41, 304–316. [Google Scholar] [CrossRef]
- Kawashima, K.; Meyerholz, D.K.; Gallup, J.M.; Grubor, B.; Lazic, T.; Lehmkuhl, H.D.; Ackermann, M.R. Differential expression of ovine innate immune genes by preterm and neonatal lung epithelia infected with respiratory syncytial virus. Viral Immunol. 2006, 19, 316–323. [Google Scholar] [CrossRef]
- Meyerholz, D.K.; DeGraaff, J.A.; Gallup, J.M.; Ackermann, M.R. Depletion of alveolar glycogen corresponds with immunohistochemical development of CD208 antigen expression in perinatal lamb lung. J. Histochem. Cytochem. 2006, 54, 1247–1253. [Google Scholar] [CrossRef]
- Sow, F.B.; Gallup, J.M.; Krishnan, S.; Patera, A.C.; Suzich, J.; Ackermann, M.R. Respiratory syncytial virus infection is associated with an altered innate immunity and a heightened pro-inflammatory response in the lungs of preterm lambs. Resp. Res. 2011, 12, 106. [Google Scholar] [CrossRef]
- Fach, S.J.; Olivier, A.; Gallup, J.M.; Waters, T.E.; Ackermann, M.R.; Lehmkuhl, H.D.; Sacco, R.E. Differential expression of cytokine transcripts in neonatal and adult ovine alveolar macrophages in response to respiratory syncytial virus or toll-like receptor ligation. Vet. Immunol. Immunopathol. 2010, 136, 55–64. [Google Scholar] [CrossRef]
- Fach, S.J.; Brockmeier, S.L.; Hobbs, L.A.; Lehmkuhl, H.D.; Sacco, S.E. Pulmonary dendritic cells isolated from neonatal and adult ovine lung tissue. Vet. Immunol. Immun. Pathol. 2007, 112, 171–182. [Google Scholar]
- Fach, S.J.; Meyerholz, D.K.; Gallup, J.M.; Ackermann, M.R.; Lehmkuhl, H.D.; Sacco, R.E. Neonatal ovine pulmonary dendritic cells support bovine respiratory syncytial virus replication with enhanced interleukin (IL)-4 and IL-10 gene transcripts. Viral Immunol. 2007, 20, 119–130. [Google Scholar] [CrossRef]
- Sow, F.B.; Gallup, J.M.; Olivier, A.; Krishnan, S.; Patera, A.C.; Suzich, J.; Ackermann, M.R. Respiratory syncytial virus is associated with an inflammatory response in lungs and architectural remodeling of lung-draining lymph nodes of newborn lambs. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2011, 300, L12–L24. [Google Scholar] [CrossRef]
- Meyerholz, D.K.; Kawashima, K.; Gallup, J.; Grubor, B.; Ackermann, M.R. Expression of innate immune genes (SP-AD, SBD-1, TLR4) by respiratory epithelia at preterm gestation is less than full-term. Dev. Comp. Immunol. 2006, 30, 1060–1069. [Google Scholar] [CrossRef]
- Kurt-Jones, E.A.; Popova, L.; Kwinn, L.; Haynes, L.M.; Jones, L.P.; Tripp, R.A.; Walsh, E.E.; Freeman, M.W.; Golenbock, D.T.; Anderson, L.J.; et al. Pattern recognition receptors tlr4 and cd14 mediate response to respiratory syncytial virus. Nat. Immunol. 2000, 1, 398–401. [Google Scholar] [CrossRef]
- Delgado, M.F.; Coviello, S.; Monsalvo, A.C.; Melendi, G.A.; Hernandez, J.Z.; Batalle, J.P.; Diaz, L.; Trento, A.; Chang, H.Y.; Mitzner, W.; et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat. Med. 2009, 15, 34–41. [Google Scholar] [CrossRef]
- Olivier, A.; Gallup, J.M.; van Geelen, A.; Ackermann, M.R. Exogenous administration of vascular endothelial growth factor prior to human respiratory syncytial virus A2 infection reduces pulmonary pathology in neonatal lambs and alters epithelial innate immune responses. Exp. Lung. Res. 2011, 37, 131–143. [Google Scholar] [CrossRef]
- Neilson, K.A.; Yunis, E.J. Demonstration of respiratory syncytial virus in an autopsy series. Pediatr. Pathol. 1990, 10, 491–502. [Google Scholar] [CrossRef]
- Simoes, E.A.; Carbonell-Estrany, X.; Rieger, C.H.; Mitchell, I.; Fredrick, L.; Groothuis, J.R. The effect of respiratory syncytial virus on subsequent recurrent wheezing in atopic and nonatopic children. J. Allergy Clin. Immunol. 2010, 126, 256–262. [Google Scholar] [CrossRef]
- Meyerholz, D.K.; Gallup, J.M.; Lazic, T.; de Macedo, M.M.A.; Lehmkuhl, H.D.; Ackermann, M.R. Pretreatment with recombinant human vascular endothelial growth factor reduces virus replication and inflammation in a perinatal lamb model of RSV infection. Viral. Immunology 2007, 20, 188–196. [Google Scholar] [CrossRef]
- DeVincenzo, J.P.; Wilkinson, T.; Vaishnaw, A.; Cehelsky, J.; Meyers, R.; Nochur, S.; Harrison, L.; Meeking, P.; Mann, A.; Moane, E.; et al. Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am. J. Respir. Crit. Care Med. 2010, 182, 1305–1314. [Google Scholar] [CrossRef]
- Kwilas, S.; Liesman, R.M.; Zhang, L.; Walsh, E.; Pickles, R.J.; Peeples, M.E. Respiratory syncytial virus grown in vero cells contains a truncated attachment protein that alters its infectivity and dependence on glycosaminoglycans. J. Virol. 2009, 83, 10710–10718. [Google Scholar] [CrossRef]
- Sow, F.B.; Gallup, J.M.; Meyerholz, D.K.; Ackermann, M.R. Gene profiling studies in the neonatal ovine lung show enhanced effects of VEGF on the immune response. Dev. Comp. Immunol. 2009, 33, 761–771. [Google Scholar] [CrossRef]
- Castillow, E.M.; Varga, S.M. Overcoming T cell-mediated immunopathology to achieve safe RSV vaccination. Future Virol. 2008, 3, 445–454. [Google Scholar] [CrossRef]
- Jones, B.G.; Sealy, R.E.; Rudraraju, R.; Traina-Dorge, V.L.; Finneryfrock, B.; Cook, A.; Takimoto, T.; Portner, A.; Hurwitz, J.L. Sendai virus-based RSV vaccine protects African green monkeys from RSV infection. Vaccine 2012, 30, 959–968. [Google Scholar] [CrossRef]
- Lazic, T.; Sow, F.B.; Van Geelen, A.; Meyerholz, D.K.; Gallup, J.M.; Ackermann, M.R. Exposure to ethanol during the last trimester of pregnancy alters the maturation and immunity of the fetal lung. Alcohol 2011, 45, 673–680. [Google Scholar] [CrossRef]
- Lazic, T.; Wyatt, T.A.; Matic, M.; Meyerholz, D.K.; Grubor, B.; Gallup, J.M.; Kersting, K.W.; Imerman, P.M.; Almeida-De-Macedo, M.; Ackermann, M.R. Maternal alcohol ingestion reduces surfactant protein A expression by preterm fetal lung epithelia. Alcohol 2007, 41, 347–355. [Google Scholar] [CrossRef]
- LeVine, A.M.; Gwozdz, J.; Stark, J.; Bruno, M.; Whitsett, J.; Korfhagen, T. Surfactant protein-A enhances respiratory syncytial virus clearance in vivo. J. Clin. Invest. 1999, 103, 1015–1021. [Google Scholar] [CrossRef]
- LeVine, A.M.; Elliott, J.; Whitsett, J.A.; Srikiatkhachorn, A.; Crouch, E.; DeSilva, N.; Korfhagen, T. Surfactant protein-D enhances phagocytosis and pulmonary clearance of respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 2001, 31, 193–199. [Google Scholar]
- Meehan, J.T.; Cutlip, R.C.; Lehmkuhl, H.D.; Kluge, J.P.; Ackermann, M.R. Infected cell types in ovine lung following exposure to bovine respiratory syncytial virus. Vet. Pathol. 1994, 31, 229–236. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Derscheid, R.J.; Ackermann, M.R. Perinatal Lamb Model of Respiratory Syncytial Virus (RSV) Infection. Viruses 2012, 4, 2359-2378. https://doi.org/10.3390/v4102359
Derscheid RJ, Ackermann MR. Perinatal Lamb Model of Respiratory Syncytial Virus (RSV) Infection. Viruses. 2012; 4(10):2359-2378. https://doi.org/10.3390/v4102359
Chicago/Turabian StyleDerscheid, Rachel J., and Mark R. Ackermann. 2012. "Perinatal Lamb Model of Respiratory Syncytial Virus (RSV) Infection" Viruses 4, no. 10: 2359-2378. https://doi.org/10.3390/v4102359
APA StyleDerscheid, R. J., & Ackermann, M. R. (2012). Perinatal Lamb Model of Respiratory Syncytial Virus (RSV) Infection. Viruses, 4(10), 2359-2378. https://doi.org/10.3390/v4102359




