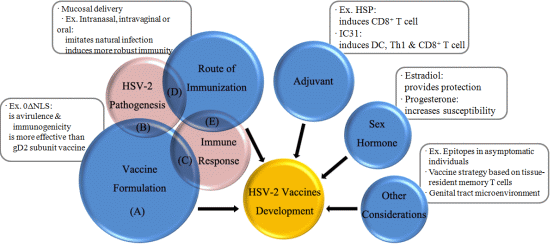
HSV-2 Vaccine: Current Status and Insight into Factors for Developing an Efficient Vaccine
Abstract
:1. Introduction
2. HSV-2 Pathogenesis

3. Immune Response to HSV-2
3.1. Innate Immune Response: An Immediate Nonspecific Protection
Cytokines: A Blessing or a Curse
3.2. Adaptive Immune Response
3.2.1. Cellular Immunity: Keeping a Balance between CD4+ T Cells and CD8+ T Cells
3.2.2. Humoral Immunity: A New Role of B Cells and Secreted Antibody
4. Vaccine Formulation
| Type | Formation | Adjuvant | Delivery route | Efficacy |
|---|---|---|---|---|
| Subunit vaccine [66] | gD2ΔTMR340-363 and ICP4383-766 | Matrix M-2 | s.c. (Scruff of the neck) | Induce humoral and cellular immune responses, reduce recurrent disease |
| Subunit vaccine [67] | mature form of gG2 | CpG | s.c. followed by i.n. | Low disease scores, survival rate: 73%, no neutralization capacity |
| Subunit vaccine [52] | gD-Fc fusion protein | CpG | i.n. | Induce strong mucosal and systematic immune responses, protection lasts for 6 months |
| Subunit vaccine [68] | gB, gD, or gB and gD | CpG | i.m. (tibialis anterior muscle) | Induce neutralizing antibody response and T cell response |
| Subunit vaccine [69] | gC2 and gD2 | CpG and alum | i.m. (calf muscle) | Induce neutralizing antibody response and CD4+ T cell response, better protection than gC2 or gD2 alone |
| Subunit vaccine [70] | liposome containing gD21-306-HD, phospholipid, and cholesterol | MPL | s.c. | Induce the level of IFN-γ, reduce disease burden, survival rate: 71% |
| Replication defective vaccine [71,72] | dl5-29 | - | s.c. (Scruff of the neck) [71] or i.m. [72] | Immunogenic and efficacious in vivo [71], greater immunogenicity and protection [72] |
| Replication defective vaccine [73] | ICP0− mutant-based dominant-negative recombinant virus | - | s.c. (left rear flank) | Safe, induce strong HSV-2-specific memory CD4+ and CD8+ Tcell responses |
| Replication defective vaccine [74] | ICP8- virus encoded B7-2 | - | s.c. (hind flank) | Induce more IFN-γ-producing CD4+ T cells, reduce diseases, suppress infection |
| Live attenuated vaccine/Replication defective vaccine [75] | gE2-del virus | - | Safety test: i.m. (gastrocnemius muscle) or i.v. (tail vein) or ivag immune test: i.m. | Safe, induce incomplete protection |
| Live attenuated vaccine [55,56] | ICP0− mutant virus | - | Ocular [55], s.c. (right rear footpads) [56] | Safe [55], 10–100 times better protection than gB subunit vaccine, survival rate: 99.1% [56] |
| DNA vaccine [76] | gD2 plasmid DNA encoding UL46 and UL47 | Vaxfectin | i.m. (rear leg) | Induce complete protection, reduce virus reactivation |
| DNA vaccine [77] | gD2 plasmid DNA | Vaxfectin | i.m. | Induce IgG, reduce virus copies in DRG, survival rate: 80% |
| DNA vaccine [78] | gD2 and gB2 CTL epitope plasmid DNA | - | i.m. (quadriceps muscle) | Induce serum IgG and Th1 immune response, survival rate: 90% |
| Inactivated vaccine [79] | whole formalin-inactivated virus | MPL and alum | i.m. | Provide nearly complete protection after challenge |
| Peptide vaccine [80] | gB T cell and B cell epitope-based peptides | - | i.m. and i.p. | T cell epitopes increase IFN-γ-producing CD8+ T cells, B cell epitopes induce high humoral response |
| Peptide vaccine [60] | 32 HSV-2 peptides with HSP70 | QS-21 saponin | i.d. | Induce cellular immune response, survival rate: 54% |
| Peptide vaccine [81] | gB CD8+ T cell epitope-based peptides extended by palmitic acid moiety | Self-adjuvant | ivag | Induce specific memory CD8+ cytotoxic T cells |
5. Route of Immunization
6. Adjuvant
7. Influence of Sex Hormone
8. Future Perspectives

Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Looker, K.J.; Garnett, G.P.; Schmid, G.P. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull. WHO 2008, 86, 805–812. [Google Scholar]
- Gupta, R.; Warren, T.; Wald, A. Genital herpes. Lancet 2007, 370, 2127–2137. [Google Scholar] [CrossRef]
- Mark, K.E.; Wald, A.; Magaret, A.S.; Selke, S.; Kuntz, S.; Huang, M.L.; Corey, L. Rapidly cleared episodes of oral and anogenital herpes simplex virus shedding in HIV-infected adults. J. Acquir. Immune Defic. Syndr. 2010, 54, 482–488. [Google Scholar] [CrossRef]
- Freeman, E.E.; Weiss, H.A.; Glynn, J.R.; Cross, P.L.; Whitworth, J.A.; Hayes, R.J. Herpes simplex virus 2 infection increases HIV acquisition in men and women: Systematic review and meta-analysis of longitudinal studies. Aids 2006, 20, 73–83. [Google Scholar] [CrossRef]
- Horbul, J.E.; Schmechel, S.C.; Miller, B.R.L.; Rice, S.A.; Southern, P.J. Herpes simplex virus-induced epithelial damage and susceptibility to human immunodeficiency virus type 1 infection in human cervical organ culture. PLoS One 2011, 6, e22638. [Google Scholar]
- Barnabas, R.V.; Wasserheit, J.N.; Huang, Y.D.; Janes, H.; Morrow, R.; Fuchs, J.; Mark, K.E.; Casapia, M.; Mehrotra, D.V.; Buchbinder, S.P.; et al. Impact of herpes simplex virus type 2 on HIV-1 acquisition and progression in an HIV vaccine trial (the step study). J. Acquir. Immune Defic. Syndr. 2011, 57, 238–244. [Google Scholar] [CrossRef]
- Thurman, A.R.; Doncel, G.F. Herpes simplex virus and HIV: Genital infection synergy and novel approaches to dual prevention. Int. J. STD Aids 2012, 23, 613–619. [Google Scholar] [CrossRef]
- Martinelli, E.; Tharinger, H.; Frank, I.; Arthos, J.; Piatak, M.; Lifson, J.D.; Blanchard, J.; Gettie, A.; Robbiani, M. HSV-2 infection of dendritic cells amplifies a highly susceptible HIV-1 cell target. PLoS Pathol. 2011, 7, e1002109. [Google Scholar] [CrossRef]
- De Jong, M.; de Witte, L.; Taylor, M.E.; Geijtenbeek, T.B.H. Herpes simplex virus type 2 enhances HIV-1 susceptibility by affecting langerhans cell function. J. Immunol. 2010, 185, 1633–1641. [Google Scholar] [CrossRef]
- Johnston, C.; Koelle, D.M.; Wald, A. HSV-2: In pursuit of a vaccine. J. Clin. Invest. 2011, 121, 4600–4609. [Google Scholar] [CrossRef]
- Cohen, J. Immunology. Painful failure of promising genital herpes vaccine. Science 2010, 330, 304. [Google Scholar] [CrossRef]
- Spear, P.G.; Eisenberg, R.J.; Cohen, G.H. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 2000, 275, 1–8. [Google Scholar] [CrossRef]
- Kopp, S.J.; Storti, C.S.; Muller, W.J. Herpes simplex virus-2 glycoprotein interaction with HVEM influences virus-specific recall cellular responses at the mucosa. Clin. Dev. Immunol. 2012, 2012, 284104:1–284104:10. [Google Scholar]
- Yoon, M.; Kopp, S.J.; Taylor, J.M.; Storti, C.S.; Spear, P.G.; Muller, W.J. Functional interaction between herpes simplex virus type 2 gD and HVEM transiently dampens local chemokine production after murine mucosal infection. PLoS One 2011, 6, e1612. [Google Scholar]
- Stiles, K.M.; Whitbeck, J.C.; Lou, H.; Cohen, G.H.; Eisenberg, R.J.; Krummenacher, C. Herpes simplex virus glycoprotein D interferes with binding of herpesvirus entry mediator to its ligands through downregulation and direct competition. J. Virol. 2010, 84, 11646–11660. [Google Scholar] [CrossRef]
- Peng, T.; Zhu, J.; Klock, A.; Phasouk, K.; Huang, M.L.; Koelle, D.M.; Wald, A.; Corey, L. Evasion of the mucosal innate immune system by herpes simplex virus type 2. J. Virol. 2009, 83, 12559–12568. [Google Scholar]
- Fakioglu, E.; Wilson, S.S.; Mesquita, P.M.M.; Hazrati, E.; Cheshenko, N.; Blaho, J.A.; Herold, B.C. Herpes simplex virus downregulates secretory leukocyte protease inhibitor: A novel immune evasion mechanism. J. Virol. 2008, 82, 9337–9344. [Google Scholar] [CrossRef]
- Stefanidou, M.; Ramos, I.; Casullo, V.M.; Trepanier, J.B.; Rosenbaum, S.; Fernandez-Sesma, A.; Herold, B.C. Herpes simplex VIrus 2 (HSV-2) prevents dendritic cell maturation, induces apoptosis, and triggers release of proinflammatory cytokines: Potential links to HSV-HIV synergy. J. Virol. 2013, 87, 1443–1453. [Google Scholar] [CrossRef]
- Peretti, S.; Shaw, A.; Blanchard, J.; Bohm, R.; Morrow, G.; Lifson, J.D.; Gettie, A.; Pope, M. Immunomodulatory effects of HSV-2 infection on immature macaque dendritic cells modify innate and adaptive responses. Blood 2005, 106, 1305–1313. [Google Scholar] [CrossRef]
- Dervillez, X.; Gottimukkala, C.; Kabbara, K.W.; Nguyen, C.; Badakhshan, T.; Kim, S.M.; Nesburn, A.B.; Wechsler, S.L.; BenMohamed, L. Future of an “asymptomatic” T-cell epitope-based therapeutic herpes simplex vaccine. Future Virol. 2012, 7, 371–378. [Google Scholar] [CrossRef]
- Chentoufi, A.A.; Binder, N.R.; Berka, N.; Durand, G.; Nguyen, A.; Bettahi, I.; Maillere, B.; BenMohamed, L. Asymptomatic human CD4+ cytotoxic T-cell epitopes identified from herpes simplex virus glycoprotein B. J. Virol. 2008, 82, 11792–11802. [Google Scholar] [CrossRef]
- Chentoufi, A.A.; BenMohamed, L. Future viral vectors for the delivery of asymptomatic herpes epitope-based immunotherapeutic vaccines. Future Virol. 2010, 5, 525–528. [Google Scholar] [CrossRef]
- Zhang, X.; Castelli, F.A.; Zhu, X.; Wu, M.; Maillere, B.; BenMohamed, L. Gender-dependent HLA-DR-restricted epitopes identified from herpes simplex virus type 1 glycoprotein D. Clin. Vaccine Immunol. 2008, 15, 1436–1449. [Google Scholar] [CrossRef]
- Chentoufi, A.A.; Zhang, X.; Lamberth, K.; Dasgupta, G.; Bettahi, I.; Nguyen, A.; Wu, M.; Zhu, X.; Mohebbi, A.; Buus, S.; et al. HLA-A*0201-restricted CD8+ cytotoxic T lymphocyte epitopes identified from herpes simplex virus glycoprotein D. J. Immunol. 2008, 180, 426–437. [Google Scholar]
- Mark, K.E.; Wald, A.; Magaret, A.S.; Selke, S.; Olin, L.; Huang, M.L.; Corey, L. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J. Infect. Dis. 2008, 198, 1141–1149. [Google Scholar] [CrossRef]
- Lund, J.M.; Linehan, M.M.; Iijima, N.; Iwasaki, A. Cutting edge: Plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J. Immunol. 2006, 177, 7510–7514. [Google Scholar]
- Zhang, S.-Y.; Jouanguy, E.; Ugolini, S.; Smahi, A.; Elain, G.; Romero, P.; Segal, D.; Sancho-Shimizu, V.; Lorenzo, L.; Puel, A.; et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science 2007, 317, 1522–1527. [Google Scholar] [CrossRef]
- Kurt-Jones, E.A.; Chan, M.; Zhou, S.; Wang, J.; Reed, G.; Bronson, R.; Arnold, M.M.; Knipe, D.M.; Finberg, R.W. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc. Natl. Acad. Sci. USA 2004, 101, 1315–1320. [Google Scholar] [CrossRef]
- Nazli, A.; Yao, X.D.; Smieja, M.; Rosenthal, K.L.; Ashkar, A.A.; Kaushic, C. Differential induction of innate anti-viral responses by TLR ligands against Herpes simplex virus, type 2, infection in primary genital epithelium of women. Antivir. Res. 2009, 81, 103–112. [Google Scholar] [CrossRef]
- Carr, D.J.J.; Tomanek, L.; Silverman, R.H.; Campbell, I.L.; Williams, B.R.G. RNA-dependent protein kinase is required for alpha-1 interferon transgene-induced resistance to genital herpes simplex virus type 2. J. Virol. 2005, 79, 9341–9345. [Google Scholar] [CrossRef]
- Duluc, D.; Gannevat, J.; Anguiano, E.; Zurawski, S.; Carley, M.; Boreham, M.; Stecher, J.; Dullaers, M.; Banchereau, J.; Oh, S. Functional diversity of human vaginal APC subsets in directing T-cell responses. Mucosal Immunol. 2013, 6, 626–638. [Google Scholar] [CrossRef]
- Conrady, C.D.; Halford, W.P.; Carr, D.J.J. Loss of the type I interferon pathway increases vulnerability of mice to genital herpes simplex virus 2 infection. J. Virol. 2011, 85, 1625–1633. [Google Scholar] [CrossRef]
- Lee, A.J.; Ashkar, A.A. Herpes simplex virus-2 in the genital mucosa: Insights into the mucosal host response and vaccine development. Curr. Opin. Infect. Dis. 2012, 25, 92–99. [Google Scholar] [CrossRef]
- Iversen, M.B.; Ank, N.; Melchjorsen, J.; Paludan, S.R. Expression of type III interferon (IFN) in the vaginal mucosa is mediated primarily by dendritic cells and displays stronger dependence on NF-kappaB than type I IFNs. J. Virol. 2010, 84, 4579–4586. [Google Scholar] [CrossRef]
- Kuwajima, S.; Sato, T.; Ishida, K.; Tada, H.; Tezuka, H.; Ohteki, T. Interleukin 15-dependent crosstalk between conventional and plasmacytoid dendritic cells is essential for CpG-induced immune activation. Nat. Immunol. 2006, 7, 740–746. [Google Scholar] [CrossRef]
- Ohteki, T.; Tada, H.; Ishida, K.; Sato, T.; Maki, C.; Yamada, T.; Hamuro, J.; Koyasu, S. Essential roles of DC-derived IL-15 as a mediator of inflammatory responses in vivo. J. Exp. Med. 2006, 203, 2329–2338. [Google Scholar] [CrossRef]
- Gill, N.; Rosenthal, K.L.; Ashkar, A.A. NK and NKT cell-independent contribution of interleukin-15 to innate protection against mucosal viral infection. J. Virol. 2005, 79, 4470–4478. [Google Scholar] [CrossRef]
- Thatte, A.; deWitte-Orr, S.J.; Lichty, B.; Mossman, K.L.; Ashkar, A.A. A critical role for IL-15 in TLR-mediated innate antiviral immunity against genital HSV-2 infection. Immunol. Cell Biol. 2011, 89, 663–669. [Google Scholar] [CrossRef]
- Roth, K.; Ferreira, V.H.; Kaushic, C. HSV-2 vaccine: Current state and insights into development of a vaccine that targets genital mucosal protection. Microb. Pathog. 2013, 58, 45–54. [Google Scholar] [CrossRef]
- Thapa, M.; Carr, D.J.J. Herpes simplex virus type 2-induced mortality following genital infection is blocked by anti-tumor necrosis factor alpha antibody in CXCL10-deficient mice. J. Virol. 2008, 82, 10295–10301. [Google Scholar]
- Zhu, J.; Koelle, D.M.; Cao, J.; Vazquez, J.; Huang, M.L.; Hladik, F.; Wald, A.; Corey, L. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J. Exp. Med. 2007, 204, 595–603. [Google Scholar] [CrossRef]
- Zhu, J.; Peng, T.; Johnston, C.; Phasouk, K.; Kask, A.S.; Klock, A.; Jin, L.; Diem, K.; Koelle, D.M.; Wald, A.; et al. Immune surveillance by CD8 alpha alpha(+) skin-resident T cells in human herpes virus infection. Nature 2013, 497, 494–497. [Google Scholar] [CrossRef]
- Tang, V.A.; Rosenthal, K.L. Intravaginal infection with herpes simplex virus type-2 (HSV-2) generates a functional effector memory T cell population that persists in the murine genital tract. J. Reprod. Immunol. 2010, 87, 39–44. [Google Scholar] [CrossRef]
- Shin, H.N.; Iwasaki, A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 2012, 491, 463–468. [Google Scholar] [CrossRef]
- Lund, J.M.; Hsing, L.; Pham, T.T.; Rudensky, A.Y. Coordination of early protective immunity to viral infection by regulatory T cells. Science 2008, 320, 1220–1224. [Google Scholar] [CrossRef]
- Corey, L.; Langenberg, A.G.; Ashley, R.; Sekulovich, R.E.; Izu, A.E.; Douglas, J.M., Jr.; Handsfield, H.H.; Warren, T.; Marr, L.; Tyring, S.; et al. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: Two randomized controlled trials. Chiron HSV Vaccine Study Group. JAMA 1999, 282, 331–340. [Google Scholar]
- Belshe, R.B.; Leone, P.A.; Bernstein, D.I.; Wald, A.; Levin, M.J.; Stapleton, J.T.; Gorfinkel, I.; Morrow, R.L.A.; Ewell, M.G.; Stokes-Riner, A.; et al. Efficacy results of a trial of a herpes simplex vaccine. N. Engl. J. Med. 2012, 366, 34–43. [Google Scholar] [CrossRef]
- Iijima, N.; Linehan, M.M.; Zamora, M.; Butkus, D.; Dunn, R.; Kehry, M.R.; Laufer, T.M.; Iwasaki, A. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. J. Exp. Med. 2008, 205, 3041–3052. [Google Scholar] [CrossRef]
- Halford, W.P.; Geltz, J.; Gershburg, E. Pan-HSV-2 IgG antibody in vaccinated mice and guinea pigs correlates with protection against herpes simplex virus 2. PLoS One 2013, 8, e65523. [Google Scholar] [CrossRef]
- Li, Z.L.; Palaniyandi, S.; Zeng, R.Y.; Tuo, W.B.; Roopenian, D.C.; Zhu, X.P. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc. Natl. Acad. Sci. USA 2011, 108, 4388–4393. [Google Scholar]
- Chu, C.F.; Meador, M.G.; Young, C.G.; Strasser, J.E.; Bourne, N.; Milligan, G.N. Antibody-mediated protection against genital herpes simplex virus type 2 disease in mice by Fc gamma receptor-dependent and -independent mechanisms. J. Reprod. Immunol. 2008, 78, 58–67. [Google Scholar] [CrossRef]
- Ye, L.L.; Zeng, R.Y.; Bai, Y.; Roopenian, D.C.; Zhu, X.P. Efficient mucosal vaccination mediated by the neonatal Fc receptor. Nat. Biotechnol. 2011, 29, 158–163. [Google Scholar] [CrossRef]
- Fening, S.W.; Esper, F.; Scholl, D.; Huang, Y.T. HSV IgG antibody inhibits virus detection in CSF. J. Clin. Virol. 2012, 55, 164–167. [Google Scholar] [CrossRef]
- Casanova, G.; Cancela, R.; Alonzo, L.; Benuto, R.; Magana Mdel, C.; Hurley, D.R.; Fishbein, E.; Lara, C.; Gonzalez, T.; Ponce, R.; et al. A double-blind study of the efficacy and safety of the ICP10deltaPK vaccine against recurrent genital HSV-2 infections. Cutis 2002, 70, 235–239. [Google Scholar]
- Halford, W.P.; Puschel, R.; Rakowski, B. Herpes simplex virus 2 ICP0(-) mutant viruses are avirulent and immunogenic: Implications for a genital herpes vaccine. PLoS One 2010, 5, e12251. [Google Scholar] [CrossRef]
- Halford, W.P.; Puschel, R.; Gershburg, E.; Wilber, A.; Gershburg, S.; Rakowski, B. A live-attenuated HSV-2 ICP0(-) virus elicits 10 to 100 times greater protection against genital herpes than a glycoprotein D subunit vaccine. PLoS One 2011, 6, e17748. [Google Scholar]
- Wang, K.N.; Kappel, J.D.; Canders, C.; Davila, W.F.; Sayre, D.; Chavez, M.; Pesnicak, L.; Cohen, J.I. A herpes simplex virus 2 glycoprotein D mutant generated by bacterial artificial chromosome mutagenesis is severely impaired for infecting neuronal cells and infects only vero cells expressing exogenous HVEM. J. Virol. 2012, 86, 12891–12902. [Google Scholar] [CrossRef]
- De Bruyn, G.; Vargas-Cortez, M.; Warren, T.; Tyring, S.K.; Fife, K.H.; Lalezari, J.; Brady, R.C.; Shahmanesh, M.; Kinghorn, G.; Beutner, K.R.; et al. A randomized controlled trial of a replication defective (gH deletion) herpes simplex virus vaccine for the treatment of recurrent genital herpes among immunocompetent subjects. Vaccine 2006, 24, 914–920. [Google Scholar] [CrossRef]
- Stanberry, L.R.; Spruance, S.L.; Cunningham, A.L.; Bernstein, D.I.; Mindel, A.; Sacks, S.; Tyring, S.; Aoki, F.Y.; Slaoui, M.; Denis, M.; et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 2002, 347, 1652–1661. [Google Scholar] [CrossRef]
- Mo, A.; Musselli, C.; Chen, H.; Pappas, J.; LeClair, K.; Liu, A.; Chicz, R.M.; Truneh, A.; Monks, S.; Levey, D.L.; et al. A heat shock protein based polyvalent vaccine targeting HSV-2: CD4 (+) and CD8 (+) cellular immunity and protective efficacy. Vaccine 2011, 29, 8530–8541. [Google Scholar] [CrossRef]
- Wald, A.; Koelle, D.M.; Fife, K.; Warren, T.; Leclair, K.; Chicz, R.M.; Monks, S.; Levey, D.L.; Musselli, C.; Srivastava, P.K. Safety and immunogenicity of long HSV-2 peptides complexed with rhHsc70 in HSV-2 seropositive persons. Vaccine 2011, 29, 8520–8529. [Google Scholar] [CrossRef]
- Dasgupta, G.; Chentoufi, A.A.; Kalantari, M.; Falatoonzadeh, P.; Chun, S.; Lim, C.H.; Felgner, P.L.; Davies, D.H.; BenMohamed, L. Immunodominant “asymptomatic” herpes simplex virus 1 and 2 protein antigens identified by probing whole-ORFome microarrays with serum antibodies from seropositive asymptomatic versus symptomatic individuals. J. Virol. 2012, 86, 4358–4369. [Google Scholar] [CrossRef]
- Meseda, C.A.; Stout, R.R.; Weir, J.P. Evaluation of a needle-free delivery platform for prime-boost immunization with DNA and modified vaccinia virus Ankara vectors expressing herpes simplex virus 2 glycoprotein D. Viral Immunol. 2006, 19, 250–259. [Google Scholar] [CrossRef]
- Macmillan, L.; Ifere, G.O.; He, Q.; Igietseme, J.U.; Kellar, K.L.; Okenu, D.M.; Eko, F.O. A recombinant multivalent combination vaccine protects against Chlamydia and genital herpes. FEMS Immunol. Med. Microbiol. 2007, 49, 46–55. [Google Scholar] [CrossRef]
- Cattamanchi, A.; Posavad, C.M.; Wald, A.; Baine, Y.; Moses, J.; Higgins, T.J.; Ginsberg, R.; Ciccarelli, R.; Corey, L.; Koelle, D.M. Phase I study of a herpes simplex virus type 2 (HSV-2) DNA vaccine administered to healthy, HSV-2-seronegative adults by a needle-free injection system. Clin. Vaccine Immunol. 2008, 15, 1638–1643. [Google Scholar] [CrossRef]
- Skoberne, M.; Cardin, R.; Lee, A.; Kazimirova, A.; Zielinski, V.; Garvie, D.; Lundberg, A.; Larson, S.; Bravo, F.J.; Bernstein, D.I.; et al. An adjuvanted herpes simplex virus 2 subunit vaccine elicits a T cell response in mice and is an effective therapeutic vaccine in guinea pigs. J. Virol. 2013, 87, 3930–3942. [Google Scholar] [CrossRef]
- Gorander, S.; Harandi, A.M.; Lindqvist, M.; Bergstrom, T.; Liljeqvist, J.A. Glycoprotein G of herpes simplex virus 2 as a novel vaccine antigen for immunity to genital and neurological disease. J. Virol. 2012, 86, 7544–7553. [Google Scholar] [CrossRef]
- Khodai, T.; Chappell, D.; Christy, C.; Cockle, P.; Eyles, J.; Hammond, D.; Gore, K.; McCluskie, M.J.; Evans, D.M.; Lang, S.; et al. Single and combination herpes simplex virus type 2 glycoprotein vaccines adjuvanted with CpG oligodeoxynucleotides or monophosphoryl lipid a exhibit differential immunity that is not correlated to protection in animal models. Clin. Vaccine Immunol. 2011, 18, 1702–1709. [Google Scholar] [CrossRef]
- Awasthi, S.; Lubinski, J.M.; Shaw, C.E.; Barrett, S.M.; Cai, M.; Wang, F.S.; Betts, M.; Kingsley, S.; DiStefano, D.J.; Balliet, J.W.; et al. Immunization with a vaccine combining herpes simplex virus 2 (HSV-2) glycoprotein C (gC) and gD subunits improves the protection of dorsal root ganglia in mice and reduces the frequency of recurrent vaginal shedding of HSV-2 DNA in guinea pigs compared to immunization with gD alone. J. Virol. 2011, 85, 10472–10486. [Google Scholar] [CrossRef]
- Olson, K.; Macias, P.; Hutton, S.; Ernst, W.A.; Fujii, G.; Adler-Moore, J.P. Liposomal gD ectodomain (gD(1–306)) vaccine protects against HSV2 genital or rectal infection of female and male mice. Vaccine 2009, 28, 548–560. [Google Scholar] [CrossRef]
- Mundle, S.T.; Hernandez, H.; Hamberger, J.; Catalan, J.; Zhou, C.; Stegalkina, S.; Tiffany, A.; Kleanthous, H.; Delagrave, S.; Anderson, S.F. High-purity preparation of HSV-2 vaccine candidate ACAM529 is immunogenic and efficacious in vivo. PLoS One 2013, 8, e57224. [Google Scholar]
- Delagrave, S.; Hernandez, H.; Zhou, C.H.; Hamberger, J.F.; Mundle, S.T.; Catalan, J.; Baloglu, S.; Anderson, S.F.; DiNapoli, J.M.; Londono-Hayes, P.; et al. Immunogenicity and efficacy of intramuscular replication-defective and subunit vaccines against herpes simplex virus type 2 in the mouse genital model. PLoS One 2012, 7, e46714. [Google Scholar] [CrossRef]
- Akhrameyeva, N.V.; Zhang, P.W.; Sugiyama, N.; Behar, S.M.; Yao, F. Development of a glycoprotein D-expressing dominant-negative and replication-defective herpes simplex virus 2 (HSV-2) recombinant viral vaccine against HSV-2 infection in mice. J. Virol. 2011, 85, 5036–5047. [Google Scholar] [CrossRef]
- Vagvala, S.P.; Thebeau, L.G.; Wilson, S.R.; Morrison, L.A. Virus-encoded B7–2 costimulation molecules enhance the protective capacity of a replication-defective herpes simplex virus type 2 vaccine in immunocompetent mice. J. Virol. 2009, 83, 953–960. [Google Scholar] [CrossRef]
- Awasthi, S.; Zumbrun, E.E.; Si, H.X.; Wang, F.S.; Shaw, C.E.; Cai, M.; Lubinski, J.M.; Barrett, S.M.; Balliet, J.W.; Flynn, J.A.; et al. Live attenuated herpes simplex virus 2 glycoprotein E deletion mutant as a vaccine candidate defective in neuronal spread. J. Virol. 2012, 86, 4586–4598. [Google Scholar] [CrossRef]
- Veselenak, R.L.; Shlapobersky, M.; Pyles, R.B.; Wei, Q.; Sullivan, S.M.; Bourne, N. A vaxfectin (R)-adjuvanted HSV-2 plasmid DNA vaccine is effective for prophylactic and therapeutic use in the guinea pig model of genital herpes. Vaccine 2012, 30, 7046–7051. [Google Scholar] [CrossRef]
- Shlapobersky, M.; Marshak, J.O.; Dong, L.C.; Huang, M.I.; Wei, Q.; Chu, A.; Rolland, A.; Sullivan, S.; Koelle, D.M. Vaxfectin-adjuvanted plasmid DNA vaccine improves protection and immunogenicity in a murine model of genital herpes infection. J. Gen. Virol. 2012, 93, 1305–1315. [Google Scholar] [CrossRef]
- Huilan, Y.; Cui, Z.; Jianyong, F.; Lei, G.; Wei, Q. Construction of, and T-helper (Th)1/Th2 immune responses to, a herpes simplex virus type 2 glycoprotein D-cytotoxic T-lymphocyte epitope DNA vaccine. Clin. Exp. Dermatol. 2010, 35, 537–542. [Google Scholar]
- Morello, C.S.; Kraynyak, K.A.; Levinson, M.S.; Chen, Z.J.; Lee, K.F.; Spector, D.H. Inactivated HSV-2 in MPL/alum adjuvant provides nearly complete protection against genital infection and shedding following long term challenge and rechallenge. Vaccine 2012, 30, 6541–6550. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, D.Y.; Zhang, L.Y.; Yao, Z.D.; Chen, Z.W.; Yu, S.K.; Wang, X.L. Identification of B- and T-cell epitopes from glycoprotein B of herpes simplex virus 2 and evaluation of their immunogenicity and protection efficacy. Vaccine 2012, 30, 3034–3041. [Google Scholar] [CrossRef]
- Zhang, X.; Chentoufi, A.A.; Dasgupta, G.; Nesburn, A.B.; Wu, M.; Zhu, X.; Carpenter, D.; Wechsler, S.L.; You, S.; BenMohamed, L. A genital tract peptide epitope vaccine targeting TLR-2 efficiently induces local and systemic CD8(+) T cells and protects against herpes simplex virus type 2 challenge. Mucosal Immunol. 2009, 2, 129–143. [Google Scholar] [CrossRef]
- Jazayeri, M.; Soleimanjahi, H.; Fotouhi, F.; Pakravan, N. Comparison of intramuscular and footpad subcutaneous immunization with DNA vaccine encoding HSV-gD2 in mice. Comp. Immunol. Microbiol. Infect. Dis. 2009, 32, 453–461. [Google Scholar] [CrossRef]
- Iwasaki, A. Mucosal dendritic cells. Annu. Rev. Immunol. 2007, 25, 381–418. [Google Scholar] [CrossRef]
- Tengvall, S.; O’Hagan, D.; Harandi, A.M. Rectal immunization generates protective immunity in the female genital tract against herpes simplex virus type 2 infection: Relative importance of myeloid differentiation factor 88. Antivir. Res. 2008, 78, 202–214. [Google Scholar] [CrossRef]
- Binder, R.J.; Srivastava, P.K. Peptides chaperoned by heat-shock proteins are a necessary and sufficient source of antigen in the cross-priming of CD8+ T cells. Nat. Immunol. 2005, 6, 593–599. [Google Scholar] [CrossRef]
- Koelle, D.M.; Magaret, A.; McClurkan, C.L.; Remington, M.L.; Warren, T.; Teofilovici, F.; Wald, A. Phase I dose-escalation study of a monovalent heat shock protein 70-herpes simplex virus type 2 (HSV-2) peptide-based vaccine designed to prime or boost CD8 T-Cell responses in HSV-naive and HSV-2-infected subjects. Clin. Vaccine Immunol. 2008, 15, 773–782. [Google Scholar] [CrossRef]
- Schellack, C.; Prinz, K.; Egyed, A.; Fritz, J.H.; Wittmann, B.; Ginzler, M.; Swatosch, G.; Zauner, W.; Kast, C.; Akira, S.; et al. IC31, a novel adjuvant signaling via TLR9, induces potent cellular and humoral immune responses. Vaccine 2006, 24, 5461–5472. [Google Scholar] [CrossRef]
- Lingnau, K.; Riedl, K.; von Gabain, A. IC31 and IC30, novel types of vaccine adjuvant based on peptide delivery systems. Expert Rev. Vaccines 2007, 6, 741–746. [Google Scholar] [CrossRef]
- Wizel, B.; Persson, J.; Thorn, K.; Nagy, E.; Harandi, A.M. Nasal and skin delivery of IC31 (R)-adjuvanted recombinant HSV-2 gD protein confers protection against genital herpes. Vaccine 2012, 30, 4361–4368. [Google Scholar] [CrossRef]
- Wegmann, F.; Gartlan, K.H.; Harandi, A.M.; Brinckmann, S.A.; Coccia, M.; Hillson, W.R.; Kok, W.L.; Cole, S.; Ho, L.P.; Lambe, T.; et al. Polyethyleneimine is a potent mucosal adjuvant for viral glycoprotein antigens. Nat. Biotechnol. 2012, 30, 883–888. [Google Scholar] [CrossRef]
- Ma, Y.F.; Yang, Y.W. Delivery of DNA-based cancer vaccine with polyethylenimine. Eur. J. Pharm. Sci. 2010, 40, 75–83. [Google Scholar] [CrossRef]
- Hu, K.; Dou, J.; Yu, F.; He, X.; Yuan, X.; Wang, Y.; Liu, C.; Gu, N. An ocular mucosal administration of nanoparticles containing DNA vaccine pRSC-gD-IL-21 confers protection against mucosal challenge with herpes simplex virus type 1 in mice. Vaccine 2011, 29, 1455–1462. [Google Scholar] [CrossRef]
- Bernstein, D.I.; Cardin, R.D.; Bravo, F.J.; Strasser, J.E.; Farley, N.; Chalk, C.; Lay, M.; Fairman, J. Potent adjuvant activity of cationic liposome-DNA complexes for genital herpes vaccines. Clin. Vaccine Immunol. 2009, 16, 699–705. [Google Scholar] [CrossRef]
- Bernstein, D.I.; Farley, N.; Bravo, F.J.; Earwood, J.; McNeal, M.; Fairman, J.; Cardin, R. The adjuvant CLDC increases protection of a herpes simplex type 2 glycoprotein D vaccine in guinea pigs. Vaccine 2010, 28, 3748–3753. [Google Scholar] [CrossRef]
- Smith, L.R.; Wloch, M.K.; Ye, M.; Reyes, L.R.; Boutsaboualoy, S.; Dunne, C.E.; Chaplin, J.A.; Rusalov, D.; Rolland, A.P.; Fisher, C.L.; et al. Phase 1 clinical trials of the safety and immunogenicity of adjuvanted plasmid DNA vaccines encoding influenza A virus H5 hemagglutinin. Vaccine 2010, 28, 2565–2572. [Google Scholar] [CrossRef]
- Quenelle, D.C.; Collins, D.J.; Rice, T.L.; Prichard, M.N.; Marciani, D.J.; Kern, E.R. Effect of an immune enhancer, GPI-0100, on vaccination with live attenuated herpes simplex virus (HSV) type 2 or glycoprotein D on genital HSV-2 infections of guinea pigs. Antivir. Res. 2008, 80, 223–224. [Google Scholar] [CrossRef]
- Bourne, N.; Milligan, G.N.; Stanberry, L.R.; Stegall, R.; Pyles, R.B. Impact of immunization with glycoprotein D2/AS04 on herpes simplex virus type 2 shedding into the genital tract in guinea pigs that become infected. J. Infect. Dis. 2005, 192, 2117–2123. [Google Scholar] [CrossRef]
- Kim, S.B.; Han, Y.W.; Rahman, M.M.; Kim, S.J.; Yoo, D.J.; Kang, S.H.; Kim, K.; Eo, S.K. Modulation of protective immunity against herpes simplex virus via mucosal genetic co-transfer of DNA vaccine with beta(2)-adrenergic agonist. Exp. Mol. Med. 2009, 41, 812–823. [Google Scholar] [CrossRef]
- Lindqvist, M.; Persson, J.; Thorn, K.; Harandi, A.M. The mucosal adjuvant effect of alpha-galactosylceramide for induction of protective immunity to sexually transmitted viral infection. J. Immunol. 2009, 182, 6435–6443. [Google Scholar] [CrossRef]
- Del Campo, J.; Lindqvist, M.; Cuello, M.; Backstrom, M.; Cabrerra, O.; Persson, J.; Perez, O.; Harandi, A.M. Intranasal immunization with a proteoliposome-derived cochleate containing recombinant gD protein confers protective immunity against genital herpes in mice. Vaccine 2010, 28, 1193–1200. [Google Scholar] [CrossRef]
- Rose, W.A.; McGowin, C.L.; Pyles, R.B. FSL-1, a bacterial-derived toll-like receptor 2/6 agonist, enhances resistance to experimental HSV-2 infection. Virol. J. 2009, 6, 195–205. [Google Scholar] [CrossRef]
- Brabin, L. Interactions of the female hormonal environment, susceptibility to viral infections, and disease progression. AIDS Patient Care STDs 2002, 16, 211–221. [Google Scholar] [CrossRef]
- Kaushic, C.; Roth, K.L.; Anipindi, V.; Xiu, F.M. Increased prevalence of sexually transmitted viral infections in women: The role of female sex hormones in regulating susceptibility and immune responses. J. Reprod. Immunol. 2011, 88, 204–209. [Google Scholar] [CrossRef]
- Gillgrass, A.E.; Ashkar, A.A.; Rosenthal, K.L.; Kaushic, C. Prolonged exposure to progesterone prevents induction of protective mucosal responses following intravaginal immunization with attenuated herpes simplex virus type 2. J. Virol. 2003, 77, 9845–9851. [Google Scholar] [CrossRef]
- Kaushic, C.; Ashkar, A.A.; Reid, L.A.; Rosenthal, K.L. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J. Virol. 2003, 77, 4558–4565. [Google Scholar] [CrossRef]
- Gillgrass, A.E.; Fernandez, S.A.; Rosenthal, K.L.; Kaushic, C. Estradiol regulates susceptibility following primary exposure to genital herpes simplex virus type 2, while progesterone induces inflammation. J. Virol. 2005, 79, 3107–3116. [Google Scholar] [CrossRef]
- Gillgrass, A.E.; Tang, V.A.; Towarnicki, K.M.; Rosenthal, K.L.; Kaushic, C. Protection against genital herpes infection in mice immunized under different hormonal conditions correlates with induction of vagina-associated lymphoid tissue. J. Virol. 2005, 79, 3117–3126. [Google Scholar] [CrossRef]
- Bhavanam, S.; Snider, D.R.; Kaushic, C. Intranasal and subcutaneous immunization under the effect of estradiol leads to better protection against genital HSV-2 challenge compared to progesterone. Vaccine 2008, 26, 6165–6172. [Google Scholar] [CrossRef]
- Pennock, J.W.; Stegall, R.; Bell, B.; Vargas, G.; Motamedi, M.; Milligan, G.; Bourne, N. Estradiol improves genital herpes vaccine efficacy in mice. Vaccine 2009, 27, 5830–5836. [Google Scholar] [CrossRef]
- Shust, G.F.; Cho, S.; Kim, M.; Madan, R.P.; Guzman, E.M.; Pollack, M.; Epstein, J.; Cohen, H.W.; Keller, M.J.; Herold, B.C. Female genital tract secretions inhibit herpes simplex virus infection: Correlation with soluble mucosal immune mediators and impact of hormonal contraception. Am. J. Reprod. Immunol. 2010, 63, 110–119. [Google Scholar]
- Roth, K.L.; Bhavanam, S.; Jiang, H.; Gillgrass, A.; Ho, K.; Ferreira, V.H.; Kaushic, C. Delayed but effective induction of mucosal memory immune responses against genital HSV-2 in the absence of secondary lymphoid organs. Mucosal Immunol. 2013, 6, 56–68. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhu, X.-P.; Muhammad, Z.S.; Wang, J.-G.; Lin, W.; Guo, S.-K.; Zhang, W. HSV-2 Vaccine: Current Status and Insight into Factors for Developing an Efficient Vaccine. Viruses 2014, 6, 371-390. https://doi.org/10.3390/v6020371
Zhu X-P, Muhammad ZS, Wang J-G, Lin W, Guo S-K, Zhang W. HSV-2 Vaccine: Current Status and Insight into Factors for Developing an Efficient Vaccine. Viruses. 2014; 6(2):371-390. https://doi.org/10.3390/v6020371
Chicago/Turabian StyleZhu, Xiao-Peng, Zaka S. Muhammad, Jian-Guang Wang, Wu Lin, Shi-Kun Guo, and Wei Zhang. 2014. "HSV-2 Vaccine: Current Status and Insight into Factors for Developing an Efficient Vaccine" Viruses 6, no. 2: 371-390. https://doi.org/10.3390/v6020371
APA StyleZhu, X.-P., Muhammad, Z. S., Wang, J.-G., Lin, W., Guo, S.-K., & Zhang, W. (2014). HSV-2 Vaccine: Current Status and Insight into Factors for Developing an Efficient Vaccine. Viruses, 6(2), 371-390. https://doi.org/10.3390/v6020371