Hantavirus Reservoirs: Current Status with an Emphasis on Data from Brazil
Abstract
:1. Introduction
- (i)
- geographical distribution of the host to map the maximum area where the hantavirus species is distributed and, consequently, where the hantavirus species that colonizes this host may occur;
- (ii)
- abundance of the reservoir species in the natural environment, which is considered a strong indicator of greater risk for human infection based on the fact that hantaviruses are zoonosis whose transmission depends on host population density;
- (iii)
- how the agent interacts with the host reservoir, which is a critical factor for understanding the spread and maintenance of hantaviruses in the environment. Lack of illness in the reservoir and the establishment of prolonged infection that may persist for the entire lifetime of the reservoir animal ensure that the hantavirus will remain in the environment throughout the lifespan of the reservoir animals and ensures transmission among rodents and;
- (iv)
- host/reservoir habits in natural and anthropic environments. For example, the tendency of rodents to enter homes is an important factor in hantavirus epidemiology because this occurrence allows these reservoirs to come into close contact with humans.
2. Hantavirus Hosts
2.1. Rodents
2.1.1. Taxonomical Characterization
2.1.2. Sigmodontinae Subfamily
2.1.3. Infection
2.1.4. Geographic Distribution of Hantavirus Infections in Old World Rodent Hosts
2.1.5. Geographic Distribution of Hantavirus Infection in New World Rodent Hosts
- (1)
- (2)
- (3)
- (4)
- (5)
- (6)
- rodents of the genus Holochilus, which are reservoirs of the viruses Rio Mearim in Brazil and Alto Paraguay in Paraguay (See Supplementary Table 2).
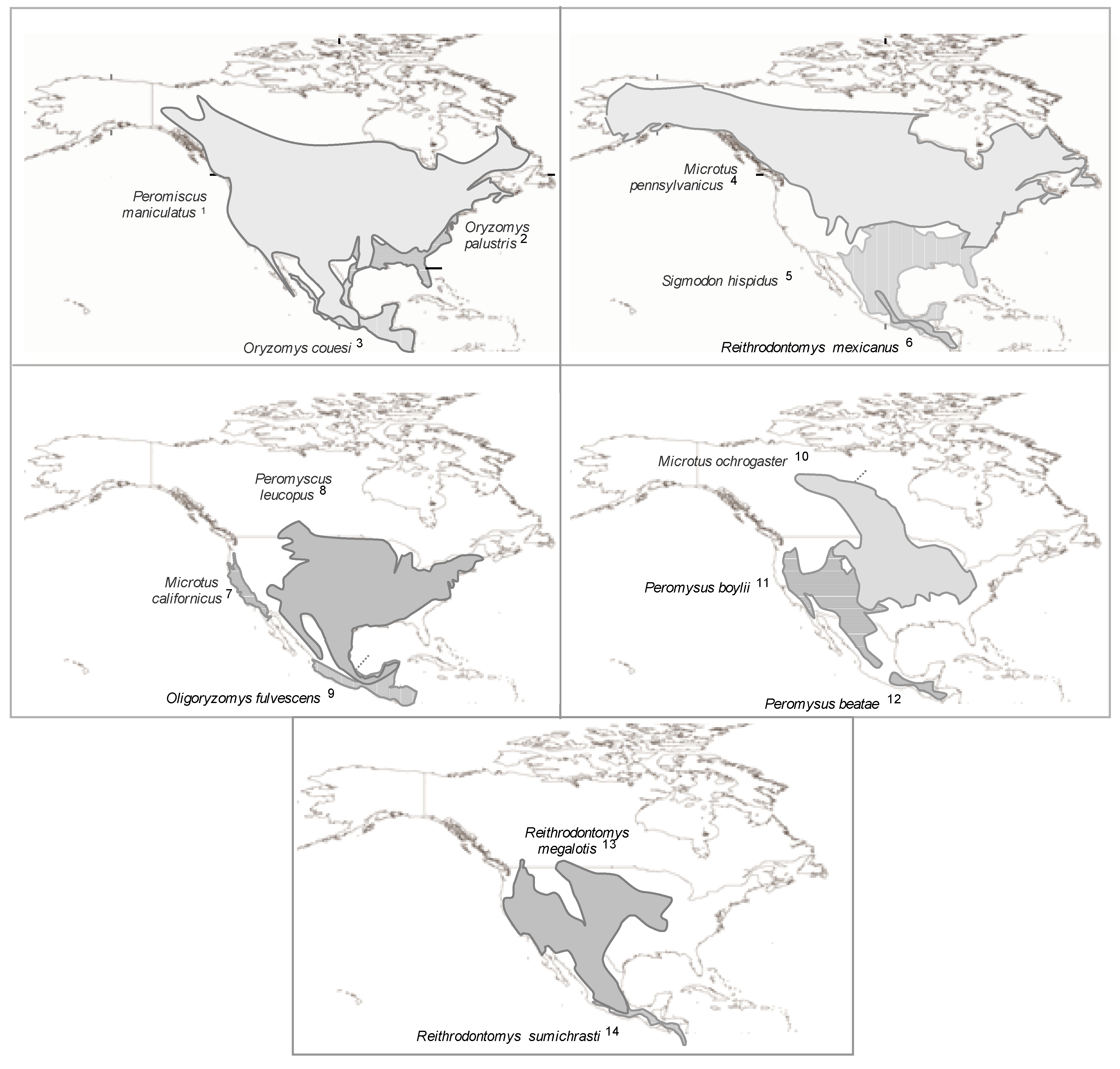

2.1.5.1. Rodent Reservoirs in Brazil


2.2. New Reservoirs
2.2.1. Insectivores
2.2.2. Bats
3. Risk Factors for Hantavirus Transmission to Humans
- (1)
- environmental regulators (climate and resource availability) that modulate transmission rates through effects on reproductive success and population density;
- (2)
- anthropogenic factors such as environmental disruptions that affect ecosystem complexity and favor opportunist or generalist species that can act as hantavirus reservoirs;
- (3)
- genetic factors that can affect transmission;
- (4)
- behavioral factors such as intra- or inter-specific aggression;
- (5)
- physiological factors that control the response to and duration of infection in the host.
- (1)
- agriculture profile, as found in most cases in the southern states that involve corn fields that border gallery forests;
- (2)
- an association with the construction of stockpiles or other attachments that permit the entry of rodents and consequently direct access to the stored food or grain;
- (3)
- managing corn fields when using “direct seeding” and when keeping part of the harvest (cobs or bags of shelled corn) at the planting site, thereby allowing access by wild rodents that leave their droppings in the corn;
- (4)
- significant ecological imbalances such as deforestation combined with the near extinction of natural predators for rodents (snakes, hawks, owls, etc.), leading to a population increase and subsequent invasion of dwellings and attachments in rural areas when the food supply becomes exhausted;
- (5)
- areas reforested with pine and eucalyptus, where the wild rodent population is adapted to the new habitat, consequently increasing contact with humans during the extraction of timber, as observed in Southern Brazil;
- (6)
- urbanization of rural areas, where neighborhoods are established near forests or rural areas. The most common example occurred in the southeastern part of the State of Minas Gerais, where there was an invasion of rural dwellings by rats from an inundated floodplain during a period of torrential rains, in addition to other factors that have not been fully investigated, highlighting the great complexity of HPS epidemiology in Brazil.
4. Conclusions
Supplementary Files
Author Contributions
Conflicts of Interest
References and Notes
- Lee, H.W.; Lee, P.W.; Johnson, K.M. Isolation of the etiologic agent of Korean hemorrhagic fever. J. Infect. Dis. 1978, 137, 298–308. [Google Scholar] [CrossRef]
- Lee, P.W.; Gajdusek, D.C.; Gibbs, C.J.; Xu, Z.Y. Aetiological relation between Korean haemorrhagic fever with renal syndrome in People’s Republic of China. Lancet 1980, 1, 819–820. [Google Scholar]
- Brummer-Korvenkontio, M.; Vaheri, A.; Hovi, T.; von Bonsdorff, C.H.; Vuorimies, J.; Manni, T.; Penttinen, K.; Oker-Blom, N.; Lähdevirta, J. Nephropathia epidemica: Detection of antigen in bank voles and serologic diagnosis of human infection. J. Infect. Dis. 1980, 141, 131–134. [Google Scholar] [CrossRef]
- Lee, P.W.; Goldgaber, D.; Gibbs, C.J.; Gajdusek, D.C.; Yanagihara, R.; Svedmyr, A.; Hlaca, D.; Vesenjak-Hirjan, J.; Gligic, A. Other serotypes of hemorrhagic fever with renal syndrome viruses in Europe. Lancet 1982, 2, 1405–1406. [Google Scholar]
- Avsic-Zupanc, T.; Xiao, S.Y.; Stojanovic, R.; Gligic, A.; van der Groen, G.; LeDuc, J.W. Characterization of Dobrava virus: A Hantavirus from Slovenia, Yugoslavia. J. Med. Virol. 1992, 38, 132–137. [Google Scholar] [CrossRef]
- Lee, P.W.; Amyx, H.L.; Yanagihara, R.; Gajdusek, D.C.; Goldgaber, D.; Gibbs, C.J. Partial characterization of Prospect Hill virus isolated from meadow voles in the United States. J. Infect. Dis. 1985, 152, 826–829. [Google Scholar] [CrossRef]
- LeDuc, J.W.; Smith, G.A.; Pinheiro, F.P.; Vasconcelos, P.F.; Rosa, E.S.; Maiztegui, J.I. Isolation of a Hantaan-related virus from Brazilian rats and serologic evidence of its widespread distribution in South America. Am. J. Trop. Med. Hyg. 1985, 34, 810–815. [Google Scholar]
- Nichol, S.T.; Spiropoulou, C.F.; Morzunov, S.; Rollin, P.E.; Ksiazek, T.G.; Feldmann, H.; Sanchez, A.; Childs, J.; Zaki, S.; Peters, C.J. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science 1993, 262, 914–917. [Google Scholar]
- Childs, J.E.; Ksiazek, T.G.; Spiropoulou, C.F.; Krebs, J.W.; Morzunov, S.; Maupin, G.O.; Gage, K.L.; Rollin, P.E.; Sarisky, J.; Enscore, R.E. Serologic and genetic identification of Peromyscus maniculatus as the primary rodent reservoir for a new hantavirus in the southwestern United States. J. Infect. Dis. 1994, 169, 1271–1280. [Google Scholar] [CrossRef]
- Schmaljohn, C.S.; Nichol, S.T. Bunyaviridae. In Field’s Virology; Knipe, D.M., Howley, P.M., Griffin, D.E., Lamb, R.A., Martin, M.A., Roizman, B., Straus, S.E., Eds.; Lippincott Wiliams & Wilkins: Philadelphia, PA, USA, 2007; pp. 1741–1789. [Google Scholar]
- Lee, H.W.; Lee, P.W.; Baek, L.J.; Song, C.K.; Seong, I.W. Intraspecific transmission of Hantaan virus, etiologic agent of Korean hemorrhagic fever, in the rodent Apodemus agrarius. Am. J. Trop. Med. Hyg. 1981, 30, 1106–1112. [Google Scholar]
- Bi, Z.; Formenty, P.B.H.; Roth, C.E. Hantavirus Infection: A review and global update. J. Infect. Dev. Ctries. 2008, 2, 3–23. [Google Scholar]
- Watson, D.C.; Sargianou, M.; Papa, A.; Chra, P.; Starakis, I.; Panos, G. Epidemiology of Hantavirus infections in humans: A comprehensive, global overview. Crit. Rev. Microbiol. 2013, 7828, 1–12. [Google Scholar]
- Plyusnin, A.; Elliot, R.M. Bunyaviridae: Molecular and Cellular Biology; Caister Academic Press: Norfolk, UK, 2011; p. 213. [Google Scholar]
- Plyusnin, A. Genetics of hantaviruses: Implications to taxonomy. Arch. Virol. 2002, 147, 665–682. [Google Scholar] [CrossRef]
- Elliot, R.M.; Bouloy, M.; Calisher, C.H.; Goldbach, R.; Moyer, J.T.; Nichol, S.T.; Pettersson, R.; Plyusnin, A.; Schmaljohn, C.S. Family Bunyaviridae. In Virus Taxonomy. Seventh Report of the International Committee on Taxonomy of Viruses; Van-Regenmortel, M.H.V., Fauquet, C.M., Bishop, D.H.L., Carsten, E.B., Estes, M.K., Lemon, S.M., Maniloff, J., Mayo, M.A., McGeoch, D.J., Pringle, C.R., et al., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2000; pp. 599–621. [Google Scholar]
- Fauquet, C.M.; Mayo, M.A.; Maniloff, J.; Desselberger, U.; Ball, L.A. Virus Taxonomy: Classification and Nomeclature of Viruses. In Eighth Report of the International Committee on the Taxonomy of Viruses; Fauquet, C.M., Mayo, M.A., Maniloff, J., Desselberger, U., Ball, L.A., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2005; pp. 695–723. [Google Scholar]
- Firth, C.; Tokarz, R.; Simith, D.B.; Nunes, M.R.T.; Bhat, M.; Rosa, E.S.T.; Medeiros, D.B.A.; Palacios, G.; Vasconcelos, P.F.C.; Lipkin, W.I. Diversity and distribution of hantaviruses in South America. J. Virol. 2012, 86, 13756–13766. [Google Scholar] [CrossRef]
- Plyusnin, A.; Morzunov, S.P. Virus evolution and genetic diversity of hantaviruses and their rodent hosts. Curr. Top. Microbiol. Immunol. 2001, 256, 47–75. [Google Scholar]
- Carey, D.E.; Reuben, R.; Panicker, K.N.; Shope, R.E.; Myers, R.M. Thottapalayam virus: A presumptive arbovirus isolated from a sherw in India. Indian J. Med. Res. 1971, 59, 1758–1760. [Google Scholar]
- Zeller, H.G.; Karabatsos, N.; Calisher, C.H.; Digoutte, J.P.; Cropp, C.B.; Murphy, F.A.; Shope, R.E. Electron microscopic and antigenic studies of uncharacterized viruses. III. Evidence suggesting the placement of viruses in the family Reoviridae. Arch. Virol. 1989, 109, 253–261. [Google Scholar] [CrossRef]
- Klempa, B.; Fichet-Calvet, E.; Lecompte, E.; Auste, B.; Aniskin, V.; Meisel, H.; Barrière, P.; Koivogui, L.; Ter Meulen, J.; Krüger, D.H. Novel hantavirus sequences in Shrew, Guinea. Emerg. Infect. Dis. 2007, 13, 520–522. [Google Scholar] [CrossRef]
- Song, J.-W.; Gu, S.H.; Bennett, S.N.; Arai, S.; Puorger, M.; Hilbe, M.; Yanagihara, R. Seewis virus, a genetically distinct hantavirus in the Eurasian common shrew (Sorex araneus). Virol. J. 2007, 4, 114. [Google Scholar] [CrossRef] [Green Version]
- Song, J.-W.; Kang, H.J.; Song, K.-J.; Truong, T.T.; Bennett, S.N.; Arai, S.; Truong, N.U.; Yanagihara, R. Newfound hantavirus in Chinese mole shrew, Vietnam. Emerg. Infect. Dis. 2007, 13, 1784–1787. [Google Scholar] [CrossRef]
- Arai, S.; Song, J.-W.; Sumibcay, L.; Bennett, S.N.; Nerurkar, V.R.; Parmenter, C.; Cook, J.A.; Yates, T.L.; Yanagihara, R. Hantavirus in northern short-tailed shrew, United States. Emerg. Infect. Dis. 2007, 13, 1420–1423. [Google Scholar]
- Arai, S.; Ohdachi, S.D.; Asakawa, M.; Kang, H.J.; Mocz, G.; Arikawa, J.; Okabe, N.; Yanagihara, R. Molecular phylogeny of a newfound hantavirus in the Japanese shrew mole (Urotrichus talpoides). Proc. Natl. Acad. Sci. USA 2008, 105, 16296–16301. [Google Scholar]
- Kang, H.J.; Bennett, S.N.; Dizney, L.; Sumibcay, L.; Arai, S.; Ruedas, L.A.; Song, J.-W.; Yanagihara, R. Host switch during evolution of a genetically distinct hantavirus in the American shrew mole (Neurotrichus gibbsii). Virology 2009, 388, 8–14. [Google Scholar] [CrossRef]
- Kang, H.J.; Arai, S.; Hope, A.G.; Cook, J.A.; Yanagihara, R. Novel hantavirus in the flat-skulled shrew (Sorex roboratus). Vector borne Zoonotic Dis. (Larchmt. N. Y.) 2010, 10, 593–597. [Google Scholar] [CrossRef]
- Kang, H.J.; Bennett, S.N.; Hope, A.G.; Cook, J.A.; Yanagihara, R. Shared ancestry between a newfound mole-borne hantavirus and hantaviruses harbored by cricetid rodents. J. Virol. 2011, 85, 7496–7503. [Google Scholar] [CrossRef]
- Sumibcay, L.; Kadjo, B.; Gu, S.H.; Kang, H.J.; Lim, B.K.; Cook, J.A.; Song, J.-W.; Yanagihara, R. Divergent lineage of a novel hantavirus in the banana pipistrelle (Neoromicia nanus) in Côte d’Ivoire. Virol. J. 2012, 9, 34. [Google Scholar] [CrossRef]
- Weiss, S.; Witkowski, P.T.; Auste, B.; Nowak, K.; Weber, N.; Fahr, J.; Mombouli, J.-V.; Wolfe, N.D.; Drexler, J.F.; Drosten, C.; et al. Hantavirus in bat, Sierra Leone. Emerg. Infect. Dis. 2012, 18, 159–161. [Google Scholar] [CrossRef]
- Goodin, D.G.; Koch, D.E.; Owen, R.D.; Chu, Y.K.; Hutchinson, J.M.S.; Jonsson, C.B. Land cover associated with hantavirus presence in Paraguay. Glob. Ecol. Biogeogr. 2006, 15, 519–527. [Google Scholar] [CrossRef]
- Mills, J.N. Biodiversity loss and emerging infectious disease: An example from the rodent-borne hemorrhagic fevers. Biodiversity 2006, 7, 9–17. [Google Scholar]
- Mills, J.N.; Amman, B.R.; Glass, G.E. Ecology of hantaviruses and their hosts in North America. Vector borne Zoonotic Dis. (Larchmt. N. Y.) 2010, 10, 563–574. [Google Scholar] [CrossRef]
- Mills, J.N.; Childs, J.E. Ecologic studies of rodent reservoirs: Their relevance for human health. Emerg. Infect. Dis. 1998, 4, 529–537. [Google Scholar] [CrossRef]
- Drebot, M.A.; Artsob, H.; Werker, D. Hantavirus pulmonary syndrome in Canada, 1989–1999. Can. Commun. Dis. Rep. 2000, 26, 65–69. [Google Scholar]
- Musser, G.G.; Carleton, M.D. Superfamily Muroidea. In Mammal Species of the World: A Taxonomic and Geographic Reference; Wilson, D.E., Reeder, D.M., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2005; pp. 849–1531. [Google Scholar]
- Raboni, S.M.; Hoffmann, F.G.; Oliveira, R.C.; Teixeira, B.R.; Bonvicino, C.R.; Stella, V.; Carstensen, S.; Bordignon, J.; D’Andrea, P.S.; Lemos, E.R.S.; et al. Phylogenetic characterization of hantaviruses from wild rodents and hantavirus pulmonary syndrome cases in the state of Paraná (southern Brazil). J. Gen. Virol. 2009, 90, 2166–2171. [Google Scholar] [CrossRef]
- McCormack, R.K.; Allen, L.J.S. Disease emergence in multi-host epidemic models. Math. Med. Biol. 2007, 24, 17–34. [Google Scholar] [CrossRef]
- Palma, R.E.; Polop, J.J.; Owen, R.D.; Mills, J.N. Ecology of rodent-associated hantaviruses in the Southern Cone of South America: Argentina, Chile, Paraguay, and Uruguay. J. Wildl. Dis. 2012, 48, 267–281. [Google Scholar] [CrossRef]
- Allen, L.J.S.; Wesley, C.L.; Owen, R.D.; Goodin, D.G.; Koch, D.; Jonsson, C.B.; Chu, Y.-K.; Shawn Hutchinson, J.M.; Paige, R.L. A habitat-based model for the spread of hantavirus between reservoir and spillover species. J. Theor. Biol. 2009, 260, 510–522. [Google Scholar]
- Morzunov, S.P.; Rowe, J.E.; Ksiazek, T.G.; Peters, C.J.; St Jeor, S.C.; Nichol, S.T. Genetic analysis of the diversity and origin of hantaviruses in Peromyscus leucopus mice in North America. J. Virol. 1998, 72, 57–64. [Google Scholar]
- Vapalahti, O.; Lundkvist, A.; Fedorov, V.; Conroy, C.J.; Hirvonen, S.; Plyusnina, A.; Nemirov, K.; Fredga, K.; Cook, J.A.; Niemimaa, J.; et al. Isolation and characterization of a hantavirus from Lemmus sibiricus: Evidence for host switch during hantavirus evolution. J. Virol. 1999, 73, 5586–5592. [Google Scholar]
- Nemirov, K.; Henttonen, H.; Vaheri, A.; Plyusnin, A. Phylogenetic evidence for host switching in the evolution of hantaviruses carried by Apodemus mice. Virus Res. 2002, 90, 207–215. [Google Scholar] [CrossRef]
- Weksler, M. Phylogenetic relationships of oryzomine rodents (Muroidea: Sigmodontinae): separate and combined analyses of morphological and molecular data. Bull. Am. Mus. Nat. Hist. 2006, 296, 1–149. [Google Scholar] [CrossRef]
- Bonvicino, C.; Seuánez, M.; Moreira, H. Análise Genética de Mamíferos Silvestres de Importância Sanitária e Econômica. In Dimensões Humanas da Biodiversidade; Gary, I., Becker, B., Eds.; Vozes: Petrópolis, Rio de Janeiro, Brazil, 2006; pp. 283–298. [Google Scholar]
- Bonvicino, C.R.; de Oliveira, J.A.; D’Andrea, P.S. Guia dos roedores do Brasil, com chaves para gêneros baseadas em caracteres externos; Centro Pan-Americano de Febre Adtosa—OPAS/OMS: Rio de Janeiro, Brazil, 2008; Volume 15, p. 120. [Google Scholar]
- Oliveira, J.; Bonvicino, C. Ordem Rodentia. In Mamíferos do Brasil; Reis, N., Peracchi, A., Pedro, W., Lima, I., Eds.; Suprema: Londrina, Paraná, Brazil, 2006; pp. 347–400. [Google Scholar]
- Agrellos, R.; Bonvicino, C.R.; Travassos da Rosa, E.S.; Marques, A.R.; D’Andrea, P.S.; Weksler, M. The taxonomic status of the Castelo dos Sonhos Hantavirus reservoir, Oligoryzomys utiaritensis Allen 1916 (Rodentia: Cricetidae: Sigmodontinae). Zootaxa 2012, 28, 1–28. [Google Scholar]
- Sholl, T.G.C.; de Moura, J.F.; Ott, P.H.; Bonvicino, C.R.; Reis, E.C.; Tavares, D.C.; Siciliano, S. Cytochrome b sequencing for the species identification of whale carcasses washed ashore in Brazil. Mar. Biodivers. Rec. 2013, 6, 1–4. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Ratnasingham, S.; Al, E. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. R. Soc. 2003, 270, 96–99. [Google Scholar] [CrossRef]
- Galewski, T.; Tilak, M.; Sanchez, S.; Chevret, P.; Paradis, E.; Douzery, E.J.P. The evolutionary radiation of Arvicolinae rodents (voles and lemmings): Relative contribution of nuclear and mitochondrial DNA phylogenies. BMC Evol. Biol. 2006, 6, 80. [Google Scholar] [CrossRef]
- Borisenko, A.V.; Lim, B.K.; Ivanova, N.V.; Hanner, R.H.; Hebert, P.D.N. DNA barcoding in surveys of small mammal communities: A field study in Suriname. Mol. Ecol. Resour. 2008, 8, 471–479. [Google Scholar] [CrossRef]
- Bryja, J.; Granjon, L.; Dobigny, G.; Patzenhauerová, H.; Konečný, A.; Duplantier, J.M.; Gauthier, P.; Colyn, M.; Durnez, L.; Lalis, A.; et al. Plio-Pleistocene history of West African Sudanian savanna and the phylogeography of the Praomys daltoni complex (Rodentia): The environment/geography/genetic interplay. Mol. Ecol. 2010, 19, 4783–4799. [Google Scholar] [CrossRef]
- Nicolas, V.; Olayemi, A.; Wendelen, W.; Colyn, M. Mitochondrial DNA and morphometrical identification of a new species of Hylomyscus (Rodentia: Muridae) from West Africa. Zootaxa 2010, 44, 30–44. [Google Scholar]
- Nicolas, V.; Schaeffer, B.; Missoup, A.D.; Kennis, J.; Colyn, M.; Denys, C.; Tatard, C.; Cruaud, C.; Laredo, C. Assessment of three mitochondrial genes (16S, Cytb, CO1) for identifying species in the Praomyini tribe (Rodentia: Muridae). PLoS One 2012, 7, e36586. [Google Scholar] [CrossRef] [Green Version]
- Bradley, R.D.; Baker, R.J. A test of the genetic species concept: Cytochrome-b sequences and mammals. J. Mammal. 2001, 82, 960–973. [Google Scholar] [CrossRef]
- Mouline, K.; Granjon, L.; Galan, M.; Tatard, C.; Abdoullaye, D.; Ag Atteyine, S.; Duplantier, J.-M.; Cosson, J.-F. Phylogeography of a Sahelian rodent species Mastomys huberti: A Plio-Pleistocene story of emergence and colonization of humid habitats. Mol. Ecol. 2008, 17, 1036–1053. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Saitoh, T.; Abe, S.; Yoshida, M.C. Sex-related spatial kin structure in a spring population of grey-sided voles Clethrionomys rufocanus as revealed by mitochondrial and microsatellite DNA analyses. Mol. Ecol. 1997, 6, 63–71. [Google Scholar] [CrossRef]
- Iwasa, M.A.; Kariwa, H.; Cui, B.-Z.; Lokugamage, K.; Lokugamage, N.; Hagiya, T.; Mizutani, T.; Takashima, I. Modes of hantavirus transmission in a population of Clethrionomys rufocanus bedfordiae inferred from mitochondrial and microsatellite DNA analyses. Arch. Virol. 2004, 149, 929–941. [Google Scholar] [CrossRef]
- Mills, J.N.; Ksiazek, T.G.; Ellis, B.A.; Rollin, P.E.; Nichol, S.T.; Yates, T.L.; Gannon, W.L.; Levy, C.E.; Engelthaler, D.M.; Davis, T.; et al. Patterns of association with host and habitat: Antibody reactive with Sin Nombre virus in small mammals in the major biotic communities of the southwestern United States. Am. J. Trop. Med. Hyg. 1997, 56, 273–284. [Google Scholar]
- Percequillo, A.R.; Hingst-Zaher, E.; Bonvicino, C.R. Systematic review of genus Cerradomys Weksler, Percequillo and Voss, 2006 (Rodentia: Cricetidae: Sigmodontinae: Oryzomyini), with description of two new species from eastern Brazil. Am. Mus. Novit. 2008, 3622, 1–46. [Google Scholar] [CrossRef]
- De, O.J.A.; Bonvicino, C.R. A new species of sigmodontine rodent from the Atlantic forest of eastern Brazil. Acta Theriol. (Warsz.) 2002, 47, 307–322. [Google Scholar]
- Gonçalves, P.; Almeida, F.; Bonvicino, C. A new species of Wiedomys (Rodentia: Sigmodontinae) from Brazilian Cerrado. Mamm. Biol. 2005, 70, 46–60. [Google Scholar]
- Percequillo, A.; Carmignotto, A.; Silva, M. A new species of Neusticomys (Ichthyomyini, Sigmodontinae) from Central Brazilian Amazonia. J. Mammal. 2005, 86, 873–880. [Google Scholar] [CrossRef]
- Percequillo, A.R.; Weksler, M.; Costa, L.P. A new genus and species of rodent from the Brazilian Atlantic Forest (Rodentia: Cricetidae: Sigmodontinae: Oryzomyini), with comments on oryzomyine biogeography. Zool. J. Linn. Soc. 2011, 161, 357–390. [Google Scholar] [CrossRef]
- Bonvicino, C.; Oliveira, J.; Gentile, R. A new species of Calomys (Rodentia: Sigmodontinae) from Eastern Brazil. Zootaxa 2010, 2336, 19–25. [Google Scholar]
- Rocha, R.G.; Ferreira, E.; Costa, B.M.A.; Martins, I.C.M.; Leite, Y.L.R.; Costa, L.P.; Fonseca, C. Small mammals of the mid-Araguaia River in central Brazil, with the description of a new species of climbing rat. Zootaxa 2011, 34, 1–34. [Google Scholar]
- Reig, O.A. A new fossil genus of the South American Cricetid rodents allied to Wiedomys, with an assessment of the Sigmodontinae. J. Zool. 1980, 192, 257–281. [Google Scholar] [CrossRef]
- Irwin, D.M.; Kocher, T.D.; Wilson, A.C. Evolution of the cytochrome b gene of mammals. J. Mol. Evol. 1991, 32, 128–144. [Google Scholar] [CrossRef]
- Steppan, S.J. Revision of the tribe Phyllotini (Rodentia: Sigmodontinae), with a phylogenetic hypothesis for the Sigmodontinae. Fieldiana Zool. 1994, 80, 1–112. [Google Scholar]
- Engel, S.R.; Hogan, K.M.; Taylor, J.F.; Davis, S.K. Molecular systematics and paleobiogeography of the South American sigmodontine rodents. Mol. Biol. Evol. 1998, 15, 35–49. [Google Scholar] [CrossRef]
- Smith, M.F.; Patton, J.L. Phylogenetic relationships and the radiation of sigmodontine rodents in South America: Evidence from cytochrome b. J. Mamm. Evol. 1999, 6, 89–128. [Google Scholar] [CrossRef]
- D’Elía, G. Phylogenetics of Sigmodontinae (Rodentia, Muroidea, Cricetidae), with special reference to the akodont group, and with additional comments on historical biogeography. Cladistics 2003, 19, 307–323. [Google Scholar] [CrossRef]
- Weksler, M. Phylogeny of Neotropical oryzomyine rodents (Muridae: Sigmodontinae) based on the nuclear IRBP exon. Mol. Phylogenet. Evol. 2003, 29, 331–349. [Google Scholar] [CrossRef]
- D’Elía, G.; Luna, L.; González, E.M.; Patterson, B.D. On the Sigmodontinae radiation (Rodentia, Cricetidae): An appraisal of the phylogenetic position of Rhagomys. Mol. Phylogenet. Evol. 2006, 38, 558–564. [Google Scholar] [CrossRef]
- Nowak, R.M. Walker’s Mammals of the World, 6th ed.; Johns Hopkins University Press: Baltimore, MD, USA, 1991; p. 1921. [Google Scholar]
- Musser, G.G.; Carleton, M.D. Order Rodentia. In Mammal Species of the World A Taxonomic and Geographic Reference; Johns Hopkins University Press: Baltimore, MD, USA, 2005; Volume 2, p. 2142. [Google Scholar]
- Bergallo, H.G.; Magnusson, W.E. Effects of climate and food availability on four rodent species in southeastern Brazil. J. Mammal. 1999, 80, 472–486. [Google Scholar] [CrossRef]
- Klempa, B. Hantaviruses and climate change. Clin. Microbiol. Infect. 2009, 15, 518–523. [Google Scholar] [CrossRef]
- Heyman, P.; Ceianu, C.S.; Christova, I.; Tordo, N.; Beersma, M.; João Alves, M.; Lundkvist, A.; Hukic, M.; Papa, A.; Tenorio, A.; et al. A five-year perspective on the situation of haemorrhagic fever with renal syndrome and status of the hantavirus reservoirs in Europe, 2005–2010. Euro Surveill. 2011, 16, 19961. [Google Scholar]
- Meyer, B.J.; Schmaljohn, C.S. Persistent hantavirus infections: Characteristics and mechanisms. Trends Microbiol. 2000, 8, 61–67. [Google Scholar] [CrossRef]
- Nuzum, E.O.; Rossi, C.A.; Stephenson, E.H.; LeDuc, J.W. Aerosol transmission of Hantaan and related viruses to laboratory rats. Am. J. Trop. Med. Hyg. 1988, 38, 636–640. [Google Scholar]
- Padula, P.; Figueroa, R.; Navarrete, M.; Pizarro, E.; Cadiz, R.; Bellomo, C.; Jofre, C.; Zaror, L.; Rodriguez, E.; Murúa, R. Transmission study of Andes hantavirus infection in wild sigmodontine rodents. J. Virol. 2004, 78, 11972–11979. [Google Scholar] [CrossRef]
- Mills, J.N.; Schmidt, K.; Ellis, B.A.; Calderón, G.; Enría, D.A.; Ksiazek, T.G. A longitudinal study of hantavirus infection in three sympatric reservoir species in agroecosystems on the Argentine Pampa. Vector Borne Zoonotic Dis. (Larchmt. N. Y.) 2007, 7, 229–240. [Google Scholar] [CrossRef]
- Glass, G.E.; Childs, J.E.; Korch, G.W.; LeDuc, J.W. Association of intraspecific wounding with hantaviral infection in wild rats (Rattus norvegicus). Epidemiol. Infect. 1988, 101, 459–472. [Google Scholar] [CrossRef]
- Bagamian, K.H.; Towner, J.S.; Kuenzi, A.J.; Douglass, R.J.; Rollin, P.E.; Waller, L.A.; Mills, J.N. Transmission ecology of Sin Nombre hantavirus in naturally infected North American deermouse populations in outdoor enclosures. PLoS One 2012, 7, e47731. [Google Scholar]
- Pearce-Duvet, J.M.C.; St Jeor, S.C.; Boone, J.D.; Dearing, M.D. Changes in sin nombre virus antibody prevalence in deer mice across seasons: The interaction between habitat, sex, and infection in deer mice. J. Wildl. Dis. 2006, 42, 819–824. [Google Scholar] [CrossRef]
- Yanagihara, R.; Amyx, H.L.; Gajdusek, D.C. Experimental infection with Puumala virus, the etiologic agent of nephropathia epidemica, in bank voles (Clethrionomys glareolus). J. Virol. 1985, 55, 34–38. [Google Scholar]
- Kariwa, H.; Fujiki, M.; Yoshimatsu, K.; Arikawa, J.; Takashima, I.; Hashimoto, N. Urine-associated horizontal transmission of Seoul virus among rats. Arch. Virol. 1998, 143, 365–374. [Google Scholar] [CrossRef]
- Bernshtein, A.D.; Apekina, N.S.; Mikhailova, T.V.; Myasnikov, Y.A.; Khlyap, L.A.; Korotkov, Y.S.; Gavrilovskaya, I.N. Dynamics of Puumala hantavirus infection in naturally infected bank voles (Clethrinomys glareolus). Arch. Virol. 1999, 144, 2415–2428. [Google Scholar] [CrossRef]
- Dohmae, K.; Koshimizu, U.; Nishimune, Y. In utero and mammary transfer of hantavirus antibody from dams to infant rats. Lab. Anim. Sci. 1993, 43, 557–561. [Google Scholar]
- Kallio, E.R.; Klingström, J.; Gustafsson, E.; Manni, T.; Vaheri, A.; Henttonen, H.; Vapalahti, O.; Lundkvist, A. Prolonged survival of Puumala hantavirus outside the host: Evidence for indirect transmission via the environment. J. Gen. Virol. 2006, 87, 2127–2134. [Google Scholar] [CrossRef]
- Hasselquist, D.; Nilsson, J.-A. Maternal transfer of antibodies in vertebrates: Trans-generational effects on offspring immunity. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2009, 364, 51–60. [Google Scholar] [CrossRef]
- Innes, D.G.L.; Millar, J.S. Life histories of Clethrionomys and Microtus (Microtinae). Mamm. Rev. 1994, 24, 179–207. [Google Scholar]
- Kallio, E.R.; Begon, M.; Henttonen, H.; Koskela, E.; Mappes, T.; Vaheri, A.; Vapalahti, O. Hantavirus infections in fluctuating host populations: The role of maternal antibodies. Proc. R. Soc. B Biol. Sci. 2010, 277, 3783–3791. [Google Scholar] [CrossRef]
- Olsson, G.E.; Leirs, H.; Henttonen, H. Hantaviruses and their hosts in Europe: Reservoirs here and there, but not everywhere? Vector borne Zoonotic Dis. (Larchmt. N. Y.) 2010, 10, 549–561. [Google Scholar] [CrossRef]
- Piudo, L.; Monteverde, M.J.; Walker, R.S.; Douglass, R.J. Oligoryzomys longicaudatus characteristics’ associated with the presence of Andes virus (Hantavirus). Rev. Chil. Infectol. 2012, 29, 200–206. [Google Scholar] [CrossRef]
- Yahnke, C.J.; Meserve, P.L.; Ksiazek, T.G.; Mills, J.N. Patterns of infection with Laguna Negra virus in wild populations of Calomys laucha in the central Paraguayan chaco. Am. J. Trop. Med. Hyg. 2001, 65, 768–776. [Google Scholar]
- Lyubsky, S.; Gavrilovskaya, I.; Luft, B.; Mackow, E. Histopathology of Peromyscus leucopus naturally infected with pathogenic NY-1 hantaviruses: Pathologic markers of HPS viral infection in mice. Lab. Investig. 1996, 74, 627–633. [Google Scholar]
- Peters, C.J.; Simpson, G.L.; Levy, H. Spectrum of hantavirus infection: Hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. Annu. Rev. Med. 1999, 50, 531–545. [Google Scholar] [CrossRef]
- Enria, D.A.; Pinheiro, F. Rodent-borne emerging viral zoonosis. Hemorrhagic fevers and hantavirus infections in South America. Infect. Dis. Clin. N. Am. 2000, 14, 167–184. [Google Scholar]
- Korva, M.; Duh, D.; Puterle, A.; Trilar, T.; Zupanc, T.A. First molecular evidence of Tula hantavirus in Microtus voles in Slovenia. Virus Res. 2009, 144, 318–322. [Google Scholar] [CrossRef]
- Hutchinson, K.L.; Rollin, P.E.; Peters, C.J. Pathogenesis of a North American hantavirus, Black Creek Canal virus, in experimentally infected Sigmodon hispidus. Am. J. Trop. Med. Hyg. 1998, 59, 58–65. [Google Scholar]
- Hardestam, J.; Karlsson, M.; Falk, K.I.; Olsson, G.; Klingström, J.; Lundkvist, A. Puumala hantavirus excretion kinetics in bank voles (Myodes glareolus). Emerg. Infect. Dis. 2008, 14, 1209–1215. [Google Scholar]
- Hart, C.A.; Bennett, M. Hantavirus infections: Epidemiology and pathogenesis. Microbes Infect. 1999, 1, 1229–1237. [Google Scholar] [CrossRef]
- Escutenaire, S.; Chalon, P.; de Jaegere, F.; Karelle-Bui, L.; Mees, G.; Brochier, B.; Rozenfeld, F.; Pastoret, P.-P. Behavioral, physiologic, and habitat influences on the dynamics of Puumala virus infection in bank voles (Clethrionomys glareolus). Emerg. Infect. Dis. 2002, 8, 930–936. [Google Scholar] [CrossRef]
- Klein, S.L.; Bird, B.H.; Glass, G.E. Sex differences in immune responses and viral shedding following Seoul virus infection in Norway rats. Am. J. Trop. Med. Hyg. 2001, 65, 57–63. [Google Scholar]
- Gavrilovskaya, I.N.; Chumakov, M.P.; Apekina, N.S.; Ryltseva, E.V; Martiyanova, L.I.; Gorbachkova, E.A.; Bernshtein, A.D.; Zakharova, M.A.; Boiko, V.A. Adaptation to laboratory and wild animals of the haemorrhagic fever with renal syndrome virus present in the foci of European U.S.S.R. Brief report. Arch. Virol. 1983, 77, 87–90. [Google Scholar] [CrossRef]
- Netski, D.; Thran, B.H.; St Jeor, S.C. Sin Nombre virus pathogenesis in Peromyscus maniculatus. J. Virol. 1999, 73, 585–591. [Google Scholar]
- Botten, J.; Mirowsky, K.; Kusewitt, D.; Bharadwaj, M.; Yee, J.; Ricci, R.; Feddersen, R.M.; Hjelle, B. Experimental infection model for Sin Nombre hantavirus in the deer mouse (Peromyscus maniculatus). Proc. Natl. Acad. Sci. USA 2000, 97, 10578–10583. [Google Scholar] [CrossRef]
- Botten, J.; Mirowsky, K.; Kusewitt, D.; Ye, C.; Gottlieb, K.; Prescott, J.; Hjelle, B. Persistent Sin Nombre virus infection in the deer mouse (Peromyscus maniculatus) model: Sites of replication and strand-specific expression. J. Virol. 2003, 77, 1540–1550. [Google Scholar] [CrossRef]
- Schountz, T.; Prescott, J.; Cogswell, A.C.; Oko, L.; Mirowsky-Garcia, K.; Galvez, A.P.; Hjelle, B. Regulatory T cell-like responses in deer mice persistently infected with Sin Nombre virus. Proc. Natl. Acad. Sci. USA 2007, 104, 15496–15501. [Google Scholar]
- Schountz, T.; Shaw, T.I.; Glenn, T.C.; Feldmann, H.; Prescott, J. Expression profiling of lymph node cells from deer mice infected with Andes virus. BMC Immunol. 2013, 14, 18. [Google Scholar]
- Klein, S.L.; Calisher, C.H. Emergence and persistence of hantaviruses. Curr. Top. Microbiol. Immunol. 2007, 315, 217–252. [Google Scholar]
- Kallio, E.R.; Voutilainen, L.; Vapalahti, O.; Vaheri, A.; Henttonen, H.; Koskela, E.; Mappes, T. Endemic hantavirus infection impairs the winter survival of its rodent host. Ecology 2007, 88, 1911–1916. [Google Scholar] [CrossRef]
- Luis, A.D.; Douglass, R.J.; Hudson, P.J.; Mills, J.N.; Bjørnstad, O.N. Sin Nombre hantavirus decreases survival of male deer mice. Oecologia 2012, 169, 431–439. [Google Scholar] [CrossRef]
- Hjelle, B.; Chavez-Giles, F.; Torrez-Martinez, N.; Yamada, T.; Sarisky, J.; Ascher, M.; Jenison, S. Dominant glycoprotein epitope of four corners hantavirus is conserved across a wide geographical area. J. Gen. Virol. 1994, 75, 2881–2888. [Google Scholar] [CrossRef]
- Ravkov, E.V.; Rollin, P.E.; Ksiazek, T.G.; Peters, C.J.; Nichol, S.T. Genetic and serologic analysis of black-creek-canal-virus and its association with human-disease and Sigmodon hispidus infection. Virology 1995, 210, 482–489. [Google Scholar]
- Sestaro, C.; Castanheira Fernandes, S.R.; Vilela, R.S.; Henriques, W.N. Hantavirus Pulmonary Syndrome: An Alert to Latin American Countries. Braz. J. Infect. Dis. 1999, 3, 203–214. [Google Scholar]
- Easterbrook, J.D.; Klein, S.L. Immunological mechanisms mediating hantavirus persistence in rodent reservoirs. PLoS Pathog. 2008, 4, e1000172. [Google Scholar] [CrossRef]
- Easterbrook, J.D.; Kaplan, J.B.; Glass, G.E.; Pletnikov, M.V.; Klein, S.L. Elevated testosterone and reduced 5-HIAA concentrations are associated with wounding and hantavirus infection in male Norway rats. Horm. Behav. 2007, 52, 474–481. [Google Scholar] [CrossRef]
- Schountz, T.; Acuna-Retamar, M.; Feinstein, S.; Prescott, J.; Torres-Perez, F.; Podell, B.; Peters, S.; Ye, C.; Black, W.C.; Hjelle, B. Kinetics of immune responses in deer mice experimentally infected with Sin Nombre virus. J. Virol. 2012, 86, 10015–10027. [Google Scholar] [CrossRef]
- Guivier, E.; Galan, M.; Malé, P.-J.G.; Kallio, E.R.; Voutilainen, L.; Henttonen, H.; Olsson, G.E.; Lundkvist, A.; Tersago, K.; Augot, D.; et al. Associations between MHC genes and Puumala virus infection in Myodes glareolus are detected in wild populations, but not from experimental infection data. J. Gen. Virol. 2010, 91, 2507–2512. [Google Scholar] [CrossRef]
- Vaheri, A.; Strandin, T.; Hepojoki, J.; Sironen, T.; Henttonen, H.; Mäkelä, S.; Mustonen, J. Uncovering the mysteries of hantavirus infections. Nat. Rev. Microbiol. 2013, 11, 539–550. [Google Scholar] [CrossRef]
- Levine, J.R.; Prescott, J.; Brown, K.S.; Best, S.M.; Ebihara, H.; Feldmann, H. Antagonism of type I interferon responses by new world hantaviruses. J. Virol. 2010, 84, 11790–11801. [Google Scholar] [CrossRef]
- Spengler, J.R.; Haddock, E.; Gardner, D.; Hjelle, B.; Feldmann, H.; Prescott, J. Experimental Andes virus infection in deer mice: Characteristics of infection and clearance in a heterologous rodent host. PLoS One 2013, 8, e55310. [Google Scholar]
- Madhav, N.K.; Wagoner, K.D.; Douglass, R.J.; Mills, J.N. Delayed density-dependent prevalence of Sin Nombre virus antibody in Montana deer mice (Peromyscus maniculatus) and implications for human disease risk. Vector Borne Zoonotic Dis. (Larchmt. N. Y.) 2007, 7, 353–364. [Google Scholar] [CrossRef]
- Xiao, S.Y.; Diglisic, G.; Avsic-Zupanc, T.; LeDuc, J.W. Dobrava virus as a new Hantavirus: Evidenced by comparative sequence analysis. J. Med. Virol. 1993, 39, 152–155. [Google Scholar] [CrossRef]
- Lokugamage, K.; Kariwa, H.; Hayasaka, D.; Cui, B.Z.; Iwasaki, T.; Lokugamage, N.; Ivanov, L.I.; Volkov, V.I.; Demenev, V.A.; Slonova, R.; et al. Genetic characterization of hantaviruses transmitted by the Korean field mouse (Apodemus peninsulae), Far East Russia. Emerg. Infect. Dis. 2002, 8, 768–776. [Google Scholar] [CrossRef]
- Jiang, J.-F.; Zhang, W.-Y.; Wu, X.-M.; Zhang, P.-H.; Cao, W.-C. Soochong virus and Amur virus might be the same entities of hantavirus. J. Med. Virol. 2007, 79, 1792–1795. [Google Scholar] [CrossRef]
- Klempa, B.; Tkachenko, E.A.; Dzagurova, T.K.; Yunicheva, Y.V.; Morozov, V.G.; Okulova, N.M.; Slyusareva, G.P.; Smirnov, A.; Kruger, D.H. Hemorrhagic fever with renal syndrome caused by 2 lineages of Dobrava hantavirus, Russia1. Emerg. Infect. Dis. 2008, 14, 617–625. [Google Scholar] [CrossRef]
- Chan, Y.C.; Wong, T.W.; Yap, E.H.; Tan, H.C.; Lee, H.W.; Chu, Y.K.; Lee, P.W. Haemorrhagic fever with renal syndrome involving the liver. Med. J. Aust. 1987, 147, 248–249. [Google Scholar]
- Plyusnina, A.; Ibrahim, I.-N.; Plyusnin, A. A newly recognized hantavirus in the Asian house rat (Rattus tanezumi) in Indonesia. J. Gen. Virol. 2009, 90, 205–209. [Google Scholar] [CrossRef]
- Hugot, J.-P.; Plyusnina, A.; Herbreteau, V.; Nemirov, K.; Laakkonen, J.; Lundkvist, A.; Supputamongkol, Y.; Henttonen, H.; Plyusnin, A. Genetic analysis of Thailand hantavirus in Bandicota indica trapped in Thailand. Virol. J. 2006, 3, 72. [Google Scholar]
- Pattamadilok, S.; Lee, B.-H.; Kumperasart, S.; Yoshimatsu, K.; Okumura, M.; Nakamura, I.; Araki, K.; Khoprasert, Y.; Dangsupa, P.; Panlar, P.; et al. Geographical distribution of hantaviruses in Thailand and potential human health significance of Thailand virus. Am. J. Trop. Med. Hyg. 2006, 75, 994–1002. [Google Scholar]
- Vapalahti, O.; Mustonen, J.; Lundkvist, Å.; Henttonen, H.; Plyusnin, A.; Vaheri, A. Review Hantavirus infections in Europe. Lancet Infect. Dis. 2003, 3, 653–661. [Google Scholar] [CrossRef]
- Plyusnin, A.; Vapalahti, O.; Lankinen, H.; Lehväslaiho, H.; Apekina, N.; Myasnikov, Y.; Kallio-Kokko, H.; Henttonen, H.; Lundkvist, A.; Brummer-Korvenkontio, M. Tula virus: A newly detected hantavirus carried by European common voles. J. Virol. 1994, 68, 7833–7839. [Google Scholar]
- Plyusnina, A.; Laakkonen, J.; Niemimaa, J.; Nemirov, K.; Muruyeva, G.; Pohodiev, B.; Lundkvist, A.; Vaheri, A.; Henttonen, H.; Vapalahti, O.; et al. Genetic analysis of hantaviruses carried by Myodes and Microtus rodents in Buryatia. Virol. J. 2008, 5, 4. [Google Scholar] [CrossRef]
- Schmidt-Chanasit, J.; Essbauer, S.; Petraityte, R.; Yoshimatsu, K.; Tackmann, K.; Conraths, F.J.; Sasnauskas, K.; Arikawa, J.; Thomas, A.; Pfeffer, M.; et al. Extensive host sharing of central European Tula virus. J. Virol. 2010, 84, 459–474. [Google Scholar] [CrossRef]
- Plyusnina, A.; Deter, J.; Charbonnel, N.; Cosson, J.-F.; Plyusnin, A. Puumala and Tula hantaviruses in France. Virus Res. 2007, 129, 58–63. [Google Scholar] [CrossRef]
- Childs, J.E.; McLafferty, S.L.; Sadek, R.; Miller, G.L.; Khan, A.S.; DuPree, E.R.; Advani, R.; Mills, J.N.; Glass, G.E. Epidemiology of rodent bites and prediction of rat infestation in New York City. Am. J. Epidemiol. 1998, 148, 78–87. [Google Scholar] [CrossRef]
- Glass, G.E.; Gardner-Santana, L.C.; Holt, R.D.; Chen, J.; Shields, T.M.; Roy, M.; Schachterle, S.; Klein, S.L. Trophic garnishes: Cat-rat interactions in an urban environment. PLoS One 2009, 4, e5794. [Google Scholar]
- Heyman, P.; Baert, K.; Plyusnina, A.; Cochez, C.; Lundkvist, A.; van Esbroeck, M.; Goossens, E.; Vandenvelde, C.; Plyusnin, A.; Stuyck, J. Serological and genetic evidence for the presence of Seoul hantavirus in Rattus norvegicus in Flanders, Belgium. Scand. J. Infect. Dis. 2009, 41, 51–56. [Google Scholar] [CrossRef]
- Sanfeliu, I.; Nogueras, M.M.; Gegúndez, M.I.; Segura, F.; Lledó, L.; Font, B.; Saz, J.V. Seroepidemiological survey of hantavirus infection in healthy people in Vallès Occidental, Barcelona. Vector borne Zoonotic Dis. (Larchmt. N. Y.) 2011, 11, 697–700. [Google Scholar] [CrossRef]
- Klempa, B.; Radosa, L.; Kruger, D.H. The broad spectrum of hantaviruses and their hosts in Central Europe. Acta Virol. 2013, 57, 130–137. [Google Scholar] [CrossRef]
- Kim, B.-N.; Choi, B.-D. Hemorrhagic fever with renal syndrome complicated with pregnancy: A case report. Korean J. Intern. Med. 2006, 21, 150–153. [Google Scholar] [CrossRef]
- Amori, G.; Hutterer, R.; Kryštufek, B.; Yigit, N.; Mitsain, G.; Palomo, L.; Henttonen, H.; Vohralík, V.; Zagorodnyuk, I.; Juškaitis, R.; et al. IUCN Red List of Threatened Species. Available online: http://www.iucnredlist.org/ (accessed on 30 September 2013).
- Kotlík, P.; Deffontaine, V.; Mascheretti, S.; Zima, J.; Michaux, J.R.; Searle, J.B. A northern glacial refugium for bank voles (Clethrionomys glareolus). Proc. Natl. Acad. Sci. USA 2006, 103, 14860–14864. [Google Scholar] [CrossRef]
- Plyusnin, A.; Vapalahti, O.; Ulfves, K.; Lehväslaiho, H.; Apekina, N.; Gavrilovskaya, I.; Blinov, V.; Vaheri, A. Sequences of wild Puumala virus genes show a correlation of genetic variation with geographic origin of the strains. J. Gen. Virol. 1994, 75, 405–409. [Google Scholar] [CrossRef]
- Hörling, J.; Lundkvist, A.; Jaarola, M.; Plyusnin, A.; Tegelström, H.; Persson, K.; Lehväslaiho, H.; Hörnfeldt, B.; Vaheri, A.; Niklasson, B. Distribution and genetic heterogeneity of Puumala virus in Sweden. J. Gen. Virol. 1996, 77, 2555–2562. [Google Scholar] [CrossRef]
- Heiske, A.; Anheier, B.; Pilaski, J.; Klenk, H.D.; Gröne, H.J.; Feldmann, H. Polymerase chain reaction detection of Puumala virus RNA in formaldehyde-fixed biopsy material. Kidney Int. 1999, 55, 2062–2069. [Google Scholar] [CrossRef]
- Asikainen, K.; Hänninen, T.; Henttonen, H.; Niemimaa, J.; Laakkonen, J.; Andersen, H.K.; Bille, N.; Leirs, H.; Vaheri, A.; Plyusnin, A. Molecular evolution of Puumala hantavirus in Fennoscandia: Phylogenetic analysis of strains from two recolonization routes, Karelia and Denmark. J. Gen. Virol. 2000, 81, 2833–2841. [Google Scholar]
- Escutenaire, S.; Chalon, P.; Heyman, P.; van der Auwera, G.; van der Groen, G.; Verhagen, R.; Thomas, I.; Karelle-Bui, L.; Vaheri, A.; Pastoret, P.P.; et al. Genetic characterization of Puumala hantavirus strains from Belgium: Evidence for a distinct phylogenetic lineage. Virus Res. 2001, 74, 1–15. [Google Scholar] [CrossRef]
- Sironen, T.; Vaheri, A.; Plyusnin, A. Molecular evolution of Puumala hantavirus. J. Virol. 2001, 75, 11803–11810. [Google Scholar] [CrossRef]
- Johansson, P.; Olsson, G.E.; Low, H.-T.; Bucht, G.; Ahlm, C.; Juto, P.; Elgh, F. Puumala hantavirus genetic variability in an endemic region (Northern Sweden). Infect. Genet. Evol. 2008, 8, 286–296. [Google Scholar] [CrossRef]
- Razzauti, M.; Plyusnina, A.; Sironen, T.; Henttonen, H.; Plyusnin, A. Analysis of Puumala hantavirus in a bank vole population in northern Finland: Evidence for co-circulation of two genetic lineages and frequent reassortment between strains. J. Gen. Virol. 2009, 90, 1923–1931. [Google Scholar] [CrossRef]
- Nemirov, K.; Leirs, H.; Lundkvist, A.; Olsson, G.E. Puumala hantavirus and Myodes glareolus in northern Europe: No evidence of co-divergence between genetic lineages of virus and host. J. Gen. Virol. 2010, 91, 1262–1274. [Google Scholar] [CrossRef]
- Razzauti, M.; Plyusnina, A.; Niemimaa, J.; Henttonen, H.; Plyusnin, A. Co-circulation of two Puumala hantavirus lineages in Latvia: A Russian lineage described previously and a novel Latvian lineage. J. Med. Virol. 2012, 84, 314–318. [Google Scholar] [CrossRef]
- Schlegel, M.; Kindler, E.; Essbauer, S.S.; Wolf, R.; Thiel, J.; Wolf, R.; Groschup, M.H.; Heckel, G.; Oehme, R.M.; Ulrich, R.G. Tula virus infections in the Eurasian water vole in Central Europe. Vector borne Zoonotic Dis. (Larchmt. N. Y.) 2012, 12, 503–513. [Google Scholar] [CrossRef]
- Song, J.-W.; Gligic, A.; Yanagihara, R. Identification of Tula hantavirus in Pitymys subterraneus captured in the Cacak region of Serbia-Yugoslavia. Int. J. Infect. Dis. 2002, 6, 31–36. [Google Scholar] [CrossRef]
- Hjelle, B.; Torres-Pérez, F. Hantaviruses in the americas and their role as emerging pathogens. Viruses 2010, 2, 2559–2586. [Google Scholar] [CrossRef]
- Jonsson, C.B.; Figueiredo, L.T.M.; Vapalahti, O. A global perspective on hantavirus ecology, epidemiology, and disease. Clin. Microbiol. Rev. 2010, 23, 412–441. [Google Scholar] [CrossRef]
- International Committee on the Taxonomy of Viruses (ICTV). Available online: http://www.ictvonline.org/virusTaxonomy.asp/ (accessed on 20 January 2014).
- Hjelle, B.; Lee, S.W.; Song, W.; Torrez-Martinez, N.; Song, J.W.; Yanagihara, R.; Gavrilovskaya, I.; Mackow, E.R. Molecular linkage of hantavirus pulmonary syndrome to the white-footed mouse, Peromyscus leucopus: Genetic characterization of the M genome of New York virus. J. Virol. 1995, 69, 8137–8141. [Google Scholar]
- Song, J.W.; Baek, L.J.; Nagle, J.W.; Schlitter, D.; Yanagihara, R. Genetic and phylogenetic analyses of hantaviral sequences amplified from archival tissues of deer mice (Peromyscus maniculatus nubiterrae) captured in the eastern United States. Arch. Virol. 1996, 141, 959–967. [Google Scholar] [CrossRef]
- Kariwa, H.; Takashima, I.; Ramos, C.; Romero-Almaraz Mde, L.; Seto, T.; Sánchez-Hernández, C.; Totani, M.; Takano, A.; Yoshida, H.; Yoshimatsu, K.; et al. Genetic diversity of hantaviruses in Mexico: Identification of three novel hantaviruses from Neotominae rodents. Virus Res. 2012, 163, 486–494. [Google Scholar] [CrossRef]
- López, N.; Padula, P.; Rossi, C.; Miguel, S.; Edelstein, A.; Ramírez, E.; Franze-Fernández, M.T. Genetic characterization and phylogeny of Andes virus and variants from Argentina and Chile. Virus Res. 1997, 50, 77–84. [Google Scholar] [CrossRef]
- Levis, S.; Morzunov, S.P.; Rowe, J.E.; Enria, D.; Pini, N.; Calderon, G.; Sabattini, M.; St Jeor, S.C. Genetic diversity and epidemiology of hantaviruses in Argentina. J. Infect. Dis. 1998, 177, 529–538. [Google Scholar]
- Johnson, A.M.; de Souza, L.T.M.; Ferreira, I.B.; Pereira, L.E.; Ksiazek, T.G.; Rollin, P.E.; Peters, C.J.; Nichol, S.T. Genetic investigation of novel hantaviruses causing fatal HPS in Brazil. J. Med. Virol. 1999, 535, 527–535. [Google Scholar]
- Rosa, E.S.T.; Mills, J.N.; Padula, P.J.; Elkhoury, M.R.; Ksiazek, T.G.; Mendes, W.S.; Santos, E.D.; Araújo, G.C.B.; Martinez, V.P.; Rosa, J.F.S.T.; et al. Newly recognized hantaviruses associated with hantavirus pulmonary syndrome in northern Brazil: Partial genetic characterization of viruses and serologic implication of likely reservoirs. Vector Borne Zoonotic Dis. (Larchmt. N. Y.) 2005, 5, 11–19. [Google Scholar]
- Travassos da Rosa, E.S.; Sampaio de Lemos, E.R.; de Almeida Medeiros, D.B.; Simith, D.B.; de Souza Pereira, A.; Elkhoury, M.R.; Mendes, W.S.; Vidigal, J.R.B.; de Oliveira, R.C.; D’Andrea, P.S.; et al. Hantaviruses and hantavirus pulmonary syndrome, Maranhao, Brazil. Emerg. Infect. Dis. 2010, 16, 1952–1955. [Google Scholar] [CrossRef]
- Johnson, A.M.; Bowen, M.D.; Ksiazek, T.G.; Williams, R.J.; Bryan, R.T.; Mills, J.N.; Peters, C.J.; Nichol, S.T. Laguna Negra virus associated with HPS in western Paraguay and Bolivia. Virology 1997, 238, 115–127. [Google Scholar] [CrossRef]
- Travassos da Rosa, E.S.; Medeiros, D.B.A.; Nunes, M.R.T.; Simith, D.B.; Pereira, A.D.S.; Elkhoury, M.R.; Santos, E.D.; Lavocat, M.; Marques, A.A.; Via, A.V.G.; et al. Molecular epidemiology of Laguna Negra virus, Mato Grosso State, Brazil. Emerg. Infect. Dis. 2012, 18, 982–985. [Google Scholar] [CrossRef]
- Chu, Y.K.; Milligan, B.; Owen, R.D.; Goodin, D.G.; Jonsson, C.B. Phylogenetic and geographical relationships of hantavirus strains in eastern and western Paraguay. Am. J. Trop. Med. Hyg. 2006, 75, 1127–1134. [Google Scholar]
- Chu, Y.K.; Goodin, D.; Owen, R.D.; Koch, D.; Jonsson, C.B. Sympatry of 2 hantavirus strains, paraguay, 2003–2007. Emerg. Infect. Dis. 2009, 15, 1977–1980. [Google Scholar] [CrossRef]
- De Oliveira, R.C.; Padula, P.J.; Gomes, R.; Martinez, V.P.; Bellomo, C.; Bonvicino, C.R.; Freire e Lima, D.I.; Bragagnolo, C.; Caldas, A.C.S.; D’Andrea, P.S.; et al. Genetic characterization of hantaviruses associated with sigmodontine rodents in an endemic area for hantavirus pulmonary syndrome in southern Brazil. Vector borne Zoonotic Dis. (Larchmt. N. Y.) 2011, 11, 301–314. (accessed on 20 January 2014). [Google Scholar] [CrossRef]
- Williams, R.J.; Bryan, R.T.; Mills, J.N.; Palma, R.E.; Vera, I.; de Velasquez, F.; Baez, E.; Schmidt, W.E.; Figueroa, R.E.; Peters, C.J.; et al. An outbreak of hantavirus pulmonary syndrome in western Paraguay. Am. J. Trop. Med. Hyg. 1997, 57, 274–282. [Google Scholar]
- Levis, S.; Garcia, J.; Pini, N.; Calderón, G.; Ramírez, J.; Bravo, D.; St Jeor, S.; Ripoll, C.; Bego, M.; Lozano, E.; et al. Hantavirus pulmonary syndrome in northwestern Argentina: Circulation of Laguna Negra virus associated with Calomys callosus. Am. J. Trop. Med. Hyg. 2004, 71, 658–663. [Google Scholar]
- Carroll, D.S.; Mills, J.N.; Montgomery, J.M.; Bausch, D.G.; Blair, P.J.; Burans, J.P.; Felices, V.; Gianella, A.; Iihoshi, N.; Nichol, S.T.; et al. Hantavirus pulmonary syndrome in Central Bolivia: Relationships between reservoir hosts, habitats, and viral genotypes. Am. J. Trop. Med. Hyg. 2005, 72, 42–46. [Google Scholar]
- Sanchez, A.J.; Abbott, K.D.; Nichol, S.T. Genetic identification and characterization of limestone canyon virus, a unique Peromyscus-borne hantavirus. Virology 2001, 286, 345–353. [Google Scholar] [CrossRef]
- Milazzo, M.L.; Cajimat, M.N.B.; Romo, H.E.; Estrada-Franco, J.G.; Iñiguez-Dávalos, L.I.; Bradley, R.D.; Fulhorst, C.F. Geographic distribution of hantaviruses associated with neotomine and sigmodontine rodents, Mexico. Emerg. Infect. Dis. 2012, 18, 571–576. [Google Scholar]
- Peters, C.J.; Khan, A.S. Hantavirus pulmonary syndrome: The new American hemorrhagic fever. Clin. Infect. Dis. 2002, 34, 1224–1231. [Google Scholar] [CrossRef]
- Bohlman, M.C.; Morzunov, S.P.; Meissner, J.; Taylor, M.B.; Ishibashi, K.; Rowe, J.; Levis, S.; Enria, D.; St Jeor, S.C. Analysis of hantavirus genetic diversity in Argentina: S segment-derived phylogeny. J. Virol. 2002, 76, 3765–3773. [Google Scholar] [CrossRef]
- Ramsden, C.; Holmes, E.C.; Charleston, M.A. Hantavirus evolution in relation to its rodent and insectivore hosts: No evidence for codivergence. Mol. Biol. Evol. 2009, 26, 143–153. [Google Scholar]
- Chu, Y.-K.; Owen, R.D.; Jonsson, C.B. Phylogenetic exploration of hantaviruses in Paraguay reveals reassortment and host switching in South America. Virol. J. 2011, 8, 399. [Google Scholar] [CrossRef]
- Goodin, D.G.; Paige, R.; Owen, R.D.; Ghimire, K.; Koch, D.E.; Chu, Y.-K.; Jonsson, C.B. Microhabitat characteristics of Akodon montensis, a reservoir for hantavirus, and hantaviral seroprevalence in an Atlantic forest site in eastern Paraguay. J. Vector Ecol. 2009, 34, 104–113. [Google Scholar] [CrossRef]
- Rivera, P.C.; Ittig, R.E.G.; Fraire, H.J.R.; Levis, S.; Gardenal, C.N. Molecular identification and phylogenetic relationships among the species of the genus Oligoryzomys (Rodentia, Cricetidae) present in Argentina, putative reservoirs of hantaviruses. Zool. Scr. 2007, 36, 231–239. [Google Scholar] [CrossRef]
- González-Ittig, R.E.; Salazar-Bravo, J.; Barquez, R.M.; Gardenal, C.N. Phylogenetic relationships among species of the genus Oligoryzomys (Rodentia, Cricetidae) from Central and South America. Zool. Scr. 2010, 39, 511–526. [Google Scholar] [CrossRef]
- Palma, R.E.; Rodríguez-Serrano, E.; Rivera-Milla, E.; Hernandez, C.E.; Salazar-Bravo, J.; Carma, M.I.; Belmar-Lucero, S.; Gutierrez-Tapia, P.; Zeballos, H.; Yates, T.L. Phylogenetic relationships of the pygmy rice rats of the genus Oligoryzomys Bangs, 1900 (Rodentia, Sigmodontinae). Zool. J. Linn. Soc. 2010, 160, 551–566. [Google Scholar] [CrossRef]
- Ksiazek, T.G.; Nichol, S.T.; Mills, J.N.; Groves, M.G.; Wozniak, A.; McAdams, S.; Monroe, M.C.; Johnson, A.M.; Martin, M.L.; Peters, C.J.; et al. Isolation, genetic diversity, and geographic distribution of Bayou virus (Bunyaviridae: Hantavirus). Am. J. Trop. Med. Hyg. 1997, 57, 445–448. [Google Scholar]
- Delfraro, A.; Clara, M.; Tomé, L.; Achaval, F.; Levis, S.; Calderón, G.; Enria, D.; Lozano, M.; Russi, J.; Arbiza, J. Yellow pigmy rice rat (Oligoryzomys flavescens) and hantavirus pulmonary syndrome in Uruguay. Emerg. Infect. Dis. 2003, 9, 846–852. [Google Scholar] [CrossRef]
- Armien, B.; Pascale, J.M.; Muñoz, C.; Mariñas, J.; Núñez, H.; Herrera, M.; Trujillo, J.; Sánchez, D.; Mendoza, Y.; Hjelle, B.; et al. Hantavirus fever without pulmonary syndrome in Panama. Am. J. Trop. Med. Hyg. 2013, 89, 489–494. [Google Scholar] [CrossRef]
- Reis, N.L.; Perachi, A.L.; Pedro, W.A.; Lima, I.P. Mamíferos do Brasil; Universidade Estadual de Londrina: Londrina, Brazil, 2006. [Google Scholar]
- Morris, D.W. Coexistence of specialist and generalist rodents via habitat selection. Ecology 1996, 77, 2352–2364. [Google Scholar] [CrossRef]
- Yahnke, C.J. Habitat use and natural history of small mammals in the central Paraguayan Chaco. Mastozool. Neotrop. 2006, 13, 103–116. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Fonseca, G.A.B.; Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Metzger, J.P.; Martensen, A.C.; Ponzoni, F.J.; Hirota, M.M. The Brazilian Atlantic Forest: How much is left, and how is the remaining\nforest distributed? Implications for conservation. Biol. Conserv. 2009, 142, 1141–1153. [Google Scholar] [CrossRef]
- Umetsu, F. Pequenos mamíferos em um mosaico de habitats remanescentes e antropogênicos: Qualidade da matriz e conectividade em uma paisagem fragmentada da Mata Atlântica; Universidade de São Paulo: São Paulo, Brazil, 2005; p. 125. [Google Scholar]
- Suzuki, A.; Bisordi, I.; Levis, S.; Garcia, J.; Pereira, L.E.; Souza, R.P.; Sugahara, T.K.N.; Pini, N.; Enria, D.; Souza, L.T.M. Identifying rodent hantavirus reservoirs, Brazil. Emerg. Infect. Dis. 2004, 10, 2127–2134. [Google Scholar] [CrossRef]
- Travassos da Rosa, E.S.; Medeiros, D.B.A.; Nunes, M.R.T.; Simith, D.B.; de Souza Pereira, A.; Elkhoury, M.R.; Lavocat, M.; Marques, A.A.R.; Via, A.V.; D’Andrea, P.; et al. Pygmy rice rat as potential host of Castelo dos Sonhos Hantavirus. Emerg. Infect. Dis. 2011, 17, 1527–1530. [Google Scholar] [CrossRef]
- Püttker, T.; Pardini, R.; Meyer-Lucht, Y.; Sommer, S. Responses of five small mammal species to micro-scale variations in vegetation structure in secondary Atlantic Forest remnants, Brazil. BMC Ecol. 2008, 8, 9. [Google Scholar] [CrossRef]
- Dalmagro, A.D.; Vieira, E.M. Patterns of habitat utilization of small rodents in an area of Araucaria forest in Southern Brazil. Austral Ecol. 2005, 30, 353–362. [Google Scholar] [CrossRef]
- Trott, A.; Callegari-Jacques, S.M.; Oliveira, L.F.B.; Langguth, A.; Mattevi, M.S. Genetic diversity and relatedness within and between species of the genus Oligoryzomys (Rodentia; Sigmodontinae). Braz. J. Biol. 2007, 67, 153–160. [Google Scholar] [CrossRef]
- Weksler, M.; Bonvicino, C.R. Taxonomy of pigmy rice rats genus Oligoryzomys bangs, 1900 (rodentia, sigmodontinae) of the brazilian cerrado. Arq. do Mus. Nac. 2005, 63, 113–130. [Google Scholar]
- Bonvicino, C.R.; Langguth, A.; Lindbergh, S.M.; Paula, A.C. An elevational gradient study of small mammals at Caparaó National Park, South eastern Brazil. Mammalia 1997, 61, 547–560. [Google Scholar]
- Eisenberg, J.; Redford, K. Mammals of the Neotropics, 3rd ed.; University of Chicago Press: Chicago, IL, USA, 1999; p. 1999. [Google Scholar]
- Streilein, K.E. The ecology of small mammals in the semiarid Brazilian Caatinga. IV. Habitat selection. Ann. Carnegie Mus. 1982, 51, 331–343. [Google Scholar]
- Graipel, M.; Cherem, J.; Miller, P.; Glock, L. Trapping small mammals in the forest understory: A comparison of three methods. Mammalia 2003, 67, 551–558. [Google Scholar]
- Pardinas, U.; D’Elia, G.; Fagundes, V.; Christoff, A.; Geise, L. IUCN Red List of Threatened Species. Available online: http://www.iucnredlist.org/ (accessed on 30 September 2013).
- Jordão, J.C.; Ramos, F.N.; da Silva, V.X. Demographic parameters of Akodon montensis (Mammalia: Rodentia) in an Atlantic Forest remnant of Southeastern Brazil. Mammalia 2010, 74, 395–400. [Google Scholar]
- Kang, H.J.; Bennett, S.N.; Sumibcay, L.; Arai, S.; Hope, A.G.; Mocz, G.; Song, J.-W.; Cook, J.A.; Yanagihara, R. Evolutionary insights from a genetically divergent hantavirus harbored by the European common mole (Talpa europaea). PLoS One 2009, 4, e6149. [Google Scholar] [CrossRef]
- Okumura, M.; Yoshimatsu, K.; Kumperasart, S.; Nakamura, I.; Ogino, M.; Taruishi, M.; Sungdee, A.; Pattamadilok, S.; Ibrahim, I.N.; Erlina, S.; et al. Development of serological assays for Thottapalayam virus, an insectivore-borne Hantavirus. Clin. Vaccine Immunol. 2007, 14, 173–181. [Google Scholar] [CrossRef]
- Gliwicz, J.; Taylor, R.E. Comparing life histories of shrews and rodents. Acta Theriol. (Warsz.) 2002, 47, 185–208. [Google Scholar] [CrossRef]
- Hutterer, R. Mammal Species of the World. In Mammal Species of the World; Wilson, D.E., Reeder, D.M., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2005; pp. 220–311. [Google Scholar]
- William, J.; Platt, W.J. Metabolic rates of short-tailed shrews. Physiol. Zool. 1974, 47, 75–90. [Google Scholar]
- Barnard, C.J. Shrews. In The Encyclopedia of Mammals; Macdonald, D., Ed.; Facts on File: New York, NY, USA, 1984; pp. 758–763. [Google Scholar]
- Churchfield, S. The Natural History of Shrews; Comstock Pub. Associates: Colorado Springs, CO, USA, 1990; p. 178. [Google Scholar]
- Campbell, K.; Hochachka, P. Thermal biology and metabolism of the American shrew-mole, Neurotrichus gibbsii. J. Mammal. 2000, 81, 578–585. [Google Scholar] [CrossRef]
- Dalquest, W.W.; Orcutt, D.R. The biology of the least shrew-mole, Neurotrichus gibbsii minor. Am. Midl. Nat. 1942, 27, 387–401. [Google Scholar] [CrossRef]
- Song, J.-W.; Kang, H.J.; Gu, S.H.; Moon, S.S.; Bennett, S.N.; Song, K.-J.; Baek, L.J.; Kim, H.-C.; O’Guinn, M.L.; Chong, S.-T.; et al. Characterization of Imjin virus, a newly isolated hantavirus from the Ussuri white-toothed shrew (Crocidura lasiura). J. Virol. 2009, 83, 6184–6191. [Google Scholar] [CrossRef]
- Arai, S.; Bennett, S.N.; Sumibcay, L.; Cook, J.A.; Song, J.-W.; Hope, A.; Parmenter, C.; Nerurkar, V.R.; Yates, T.L.; Yanagihara, R. Phylogenetically distinct hantaviruses in the masked shrew (Sorex cinereus) and dusky shrew (Sorex monticolus) in the United States. Am. J. Trop. Med. Hyg. 2008, 78, 348–351. [Google Scholar]
- Yan, D.Y.; Xie, Y.J.; Zhang, C.A.; McCormick, J.B.; Sanchez, A.; Engelman, H.M.; Chen, S.Z.; Gu, X.S.; Tang, W.T.; Zhang, J. New isolates of HFRS virus in Sichuan, China and characterisation of antigenic differences by monoclonal antibodies. Lancet 1986, 7, 1328. [Google Scholar]
- Tang, Y.W.; Ruo, S.L.; Xu, X.; Sanchez, A.; Fisher-Hoch, S.P.; McCormick, J.B.; Xu, Z.Y. Hantavirus strains isolated from rodentia and insectivora in rural China differentiated by polymerase chain reaction assay. Arch. Virol. 1990, 115, 37–46. [Google Scholar] [CrossRef]
- Arai, S.; Gu, S.H.; Baek, L.J.; Tabara, K.; Bennett, S.N.; Oh, H.-S.; Takada, N.; Kang, H.J.; Tanaka-Taya, K.; Morikawa, S.; et al. Divergent ancestral lineages of newfound hantaviruses harbored by phylogenetically related crocidurine shrew species in Korea. Virology 2012, 424, 99–105. [Google Scholar] [CrossRef]
- Guo, W.-P.; Lin, X.-D.; Wang, W.; Tian, J.-H.; Cong, M.-L.; Zhang, H.-L.; Wang, M.-R.; Zhou, R.-H.; Wang, J.-B.; Li, M.-H.; et al. Phylogeny and origins of hantaviruses harbored by bats, insectivores, and rodents. PLoS Pathog. 2013, 9, e1003159. [Google Scholar] [CrossRef]
- Arai, S.; Nguyen, S.T.; Boldgiv, B.; Fukui, D.; Araki, K.; Dang, C.N.; Ohdachi, S.D.; Nguyen, N.X.; Pham, T.D.; Boldbaatar, B.; et al. Novel bat-borne hantavirus, Vietnam. Emerg. Infect. Dis. 2013, 19, 1159–1161. [Google Scholar] [CrossRef]
- Calisher, C.H.; Childs, J.E.; Field, H.E.; Holmes, K.V.; Schountz, T. Bats: Important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006, 19, 531–545. [Google Scholar] [CrossRef]
- Simmons, N.B. Evolution. An Eocene big bang for bats. Science 2005, 307, 527–528. [Google Scholar] [CrossRef]
- Peracchi, A.L.; Lima, I.P.; Reis, N.R.; Nogueira, M.R.; Ortêncio Filho, H. Ordem Chiroptera. In Mamíferos do Brasil; Reis, N.R., Peracchi, A.L., Pedro, W.A., Lima, I.P., Eds.; Universidade Estadual de Londrina: Londrina, Paraná, Brazil, 2006; pp. 153–230. [Google Scholar]
- Munshi-South, J.; Wilkinson, G.S. Bats and birds: Exceptional longevity despite high metabolic rates. Ageing Res. Rev. 2010, 9, 12–19. [Google Scholar] [CrossRef]
- Luis, A.D.; Hayman, D.T.S.; O’Shea, T.J.; Cryan, P.M.; Gilbert, A.T.; Pulliam, J.R.C.; Mills, J.N.; Timonin, M.E.; Willis, C.K.R.; Cunningham, A.A.; et al. A comparison of bats and rodents as reservoirs of zoonotic viruses: Are bats special? Proc. Biol. Sci. 2013, 280, 20122753. [Google Scholar] [CrossRef]
- O’Shea, T.J.; Neubaum, D.J.; Neubaum, M.A.; Cryan, P.M.; Ellison, L.E.; Stanley, T.R.; Rupprecht, C.E.; Pape, W.J.; Bowen, R.A. Bat ecology and public health surveillance for rabies in an urbanizing region of Colorado. Urban Ecosyst. 2011, 14, 665–697. [Google Scholar] [CrossRef]
- Plowright, R.K.; Foley, P.; Field, H.E.; Dobson, A.P.; Foley, J.E.; Eby, P.; Daszak, P. Urban habituation, ecological connectivity and epidemic dampening:The emergence of Hendra virus from flying foxes (Pteropus spp.). Proc. R. Soc. 2011, 278, 3703–3712. [Google Scholar] [CrossRef]
- Mickleburgh, S.; Waylen, K.; Racey, P. Bats as bushmeat: A global review. Oryx 2009, 43, 217–234. [Google Scholar] [CrossRef]
- Kamins, A.O.; Restif, O.; Ntiamoa-Baidu, Y.; Suu-Ire, R.; Hayman, D.T.S.; Cunningham, A.A.; Wood, J.N.L.; Rowcliffe, J. Uncovering the fruit bat bushmeat commodity chain and the true extent of fruit bat hunting in Ghana, West Africa. Biol. Conserv. 2011, 144, 3000–3008. [Google Scholar] [CrossRef]
- Pulliam, J.R.; Epstein, J.H.; Dushoff, J.; Rahman, S.A.; Bunning, M.; Jamaluddin, A.A.; Hyatt, A.D.; Field, H.E.; Dobson, A.P.; Daszak, P. Agricultural intensification, priming for persistence and the emergence of Nipah virus: A lethal bat-borne zoonosis. J. R. Soc. Interface 2012, 9, 89–101. [Google Scholar] [CrossRef]
- De Araujo, J.; Thomazelli, L.M.; Henriques, D.A.; Lautenschalager, D.; Ometto, T.; Dutra, L.M.; Aires, C.C.; Favorito, S.; Durigon, E.L. Detection of hantavirus in bats from remaining rain forest in São Paulo, Brazil. BMC Res. Notes 2012, 5, 690. [Google Scholar] [Green Version]
- Jung, Y.T.; Kim, G.R. Genomic characterization of M and S RNA segments of hantaviruses isolated from bats. Acta Virol. 1995, 39, 231–233. [Google Scholar]
- Araujo, J.; Pereira, A.; Nardi, M.S.; Henriques, D.A.; Lautenschalager, D.A.; Dutra, L.M.; Ometto, T.L.; Hurtado, R.F.; Maués, F.; Nava, A.; et al. Detection of hantaviruses in Brazilian rodents by SYBR-Green-based real-time RT-PCR. Arch. Virol. 2011, 156, 1269–1274. [Google Scholar] [CrossRef]
- Paglia, A.P.; Rylands, A.B.; Herrmann, G.; Aguiar, L.M.S.; Chiarello, A.G.; Leite, Y.L.R.; Costa, L.P.; Siciliano, S. Annotated Checklist of Brazilian Mammals; Occasional Papers in Conservation Biology Conservation International: Arlington, VA, USA, 2012; Volume 76. [Google Scholar]
- Torres-Pérez, F.; Navarrete-Droguett, J.; Aldunate, R.; Yates, T.L.; Mertz, G.J.; Vial, P.A.; Ferrés, M.; Marquet, P.A.; Palma, R.E. Peridomestic small mammals associated with confirmed cases of human hantavirus disease in southcentral Chile. Am. J. Trop. Med. Hyg. 2004, 70, 305–309. [Google Scholar]
- Martinez, V.P.; Bellomo, C.M.; Cacace, M.L.; Suarez, P.; Bogni, L.; Padula, P.J. Hantavirus pulmonary syndrome in Argentina, 1995–2008. Emerg. Infect. Dis. 2010, 16, 1853–1860. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Xiao, D.-L.; Wang, Y.; Wang, H.-X.; Sun, L.; Tao, X.-X.; Qu, Y.-G. The epidemic characteristics and preventive measures of hemorrhagic fever with syndromes in China. Zhonghua Liu Xing Bing Xue Za Zhi 2004, 25, 466–469. [Google Scholar]
- LeDuc, J.W. Epidemiology of Hantaan and related viruses. Lab. Anim. Sci. 1987, 37, 413–418. [Google Scholar]
- CDC Update: Outbreak of Hantavirus Infection—Southwestern United States, 1993. Morb. Mortal. Wkly. Rep. 1993, 42, 441–443.
- Vitek, C.R.; Breiman, R.F.; Ksiazek, T.G.; Rollin, P.E.; McLaughlin, J.C.; Umland, E.T.; Nolte, K.B.; Loera, A.; Sewell, C.M.; Peters, C.J. Evidence against person-to-person transmission of hantavirus to health care workers. Clin. Infect. Dis. 1996, 22, 824–826. [Google Scholar] [CrossRef]
- Ferreira, M.S. Hantaviruses. Rev. Soc. Bras. Med. Trop. 2003, 36, 81–96. [Google Scholar] [CrossRef]
- Schmaljohn, C.; Hjelle, B. Hantaviruses: A global disease problem. Emerg. Infect. Dis. 1997, 3, 95–104. [Google Scholar] [CrossRef]
- Mills, J.N. Regulation of rodent-borne viruses in the natural host: Implications for human disease. Arch. Virol. Suppl. 2005, 19, 45–57. [Google Scholar]
- Engelthaler, D.M.; Mosley, D.G.; Cheek, J.E.; Levy, C.E.; Komatsu, K.K.; Ettestad, P.; Davis, T.; Tanda, D.T.; Miller, L.; Frampton, J.W.; et al. Climatic and environmental patterns associated with hantavirus pulmonary syndrome, Four Corners region, United States. Emerg. Infect. Dis. 1999, 5, 87–94. [Google Scholar] [CrossRef]
- Gubler, D.J.; Reiter, P.; Ebi, K.L.; Yap, W.; Nasci, R.; Patz, J.A. Climate variability and change in the United States: Potential impacts on vector- and rodent-borne diseases. Environ. Health Perspect. 2001, 109, 223–233. [Google Scholar] [CrossRef]
- Tersago, K.; Schreurs, A.; Linard, C.; Verhagen, R.; van Dongen, S.; Leirs, H. Population, environmental, and community effects on local bank vole (Myodes glareolus) Puumala virus infection in an area with low human incidence. Vector Borne Zoonotic Dis. (Larchmt. N. Y.) 2008, 8, 235–244. [Google Scholar] [CrossRef]
- Clement, J.; Vercauteren, J.; Verstraeten, W.W.; Ducoffre, G.; Barrios, J.M.; Vandamme, A.-M.; Maes, P.; van Ranst, M. Relating increasing hantavirus incidences to the changing climate: The mast connection. Int. J. Health Geogr. 2009, 8, 1. [Google Scholar] [CrossRef]
- Heyman, P.; Cochez, C.; Ducoffre, G.; Mailles, A.; Zeller, H.; Abu Sin, M.; Koch, J.; van Doornum, G.; Koopmans, M.; Mossong, J.; et al. Haemorrhagic Fever with Renal Syndrome: An analysis of the outbreaks in Belgium, France, Germany, the Netherlands and Luxembourg in 2005. Euro Surveill. 2007, 12, E15–E16. [Google Scholar]
- Hörnfeldt, B.; Hipkiss, T.; Eklund, U. Fading out of vole and predator cycles? Proc. Biol. Sci. 2005, 272, 2045–2049. [Google Scholar] [CrossRef]
- Kausrud, K.L.; Mysterud, A.; Steen, H.; Vik, J.O.; Østbye, E.; Cazelles, B.; Framstad, E.; Eikeset, A.M.; Mysterud, I.; Solhøy, T.; et al. Linking climate change to lemming cycles. Nature 2008, 456, 93–97. [Google Scholar] [CrossRef]
- Slonova, R.A.; Tkachenko, E.A.; Astakhova, T.I.; Dzagurova, T.K. Hantaan virus serotypes circulating in foci of the Far Eastern region of the USSR. Vopr. Virusol. 1990, 35, 391–393. [Google Scholar]
- Bi, P.; Wu, X.; Zhang, F.; Parton, K.A.; Tong, S. Seasonal rainfall variability, the incidence of hemorrhagic fever with renal syndrome, and prediction of the disease in low-lying areas of China. Am. J. Epidemiol. 1998, 148, 276–281. [Google Scholar] [CrossRef]
- Hu, W.; Mengersen, K.; Bi, P.; Tong, S. Time-series analysis of the risk factors for haemorrhagic fever with renal syndrome: Comparison of statistical models. Epidemiol. Infect. 2007, 135, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.X.; Qiu, F.X.; Dong, B.J.; Ji, S.Z.; Li, Y.T.; Wang, Y.; Wang, H.M.; Zuo, G.F.; Tao, X.X.; Gao, S.Y. Epidemiological studies on hemorrhagic fever with renal syndrome in China. J. Infect. Dis. 1986, 154, 394–398. [Google Scholar] [CrossRef]
- Chen, H.X.; Qiu, F.X. Epidemiologic surveillance on the hemorrhagic fever with renal syndrome in China. Chin. Med. J. (Engl.) 1993, 106, 857–863. [Google Scholar]
- Mackelprang, R.; Dearing, M.D.; St Jeor, S.C. High prevalence of Sin Nombre virus in rodent populations, central Utah: A consequence of human disturbance? Emerg. Infect. Dis. 2001, 7, 480–482. [Google Scholar] [CrossRef]
- Pinto, V.L.; DE Sousa, A.I.; DE Lemos, E.R.S. Regional variations and time trends of hantavirus pulmonary syndrome in Brazil. Epidemiol. Infect. 2014, 1–6. [Google Scholar]
- Santos, J.P.; Steinke, E.T.; García-Zapata, A. Land use and occupation and hantavirosis dissemination in the São Sebastião region, Federal District: 2004–2008. Rev. Soc. Bras. Med. Trop. 2011, 44, 53–57. [Google Scholar] [CrossRef]
- Jaksic, F.M.; Lima, M. Myths and facts on ratadas: Bamboo blooms, rainfall peaks and rodent outbreaks in South America. Austral Ecol. 2003, 28, 237–251. [Google Scholar] [CrossRef]
- Caldas, A.C.S. Santa Catarina Departament of Health, Florianópolis, SC, Brazil. Personal communication, 2005. [Google Scholar]
- Pereira, C. Sobre as “ratadas” no sul do Brasil e o ciclo vegetativo das taquaras. Arq. do Inst. Biol. São Paulo 1941, 12, 175–200. [Google Scholar]
- Dearing, M.D.; Dizney, L. Ecology of hantavirus in a changing world. Ann. N. Y. Acad. Sci. 2010, 1195, 99–112. [Google Scholar]
- Oliveira, R.C.; Gentile, R.; Guterres, A.; Fernandes, J.; Teixeira, B.R.; Vaz, V.; Valdez, F.P.; Vicente, L.H.B.; da Costa-Neto, S.F.; Bonvicino, C.; et al. Ecological study of hantavirus infection in wild rodents in an endemic area in Brazil. Acta Trop. 2014, 131C, 1–10. [Google Scholar]
- Wójcik-Fatla, A.; Zając, V.; Knap, J.P.; Dutkiewicz, J. Hantavirus RNA not detected in Ixodes ricinus ticks. Ann. Agric. Environ. Med. 2011, 18, 446–447. [Google Scholar]
- Wójcik-Fatla, A.; Zając, V.; Knap, J.P.; Dutkiewicz, J. Hantavirus RNA was not detected in Dermacentor reticulatus ticks. Ann. Agric. Environ. Med. 2013, 20, 452–454. [Google Scholar]
- Costa, L.P.; Leite, Y.L.R.; Mendes, S.L.; Ditchfield, A.D. Conservação de mamíferos no Brasil. Megadiversidade 2005, 1, 103–112. [Google Scholar]
- Xiao, S.Y.; Leduc, J.W.; Chu, Y.K.; Schmaljohn, C.S. Phylogenetic analyses of virus isolates in the genus Hantavirus, family Bunyaviridae. Virology 1994, 198, 205–217. [Google Scholar] [CrossRef]
- Elwell, M.R.; Ward, G.S.; Tingpalapong, M.; LeDuc, J.W. Serologic evidence of Hantaan-like virus in rodents and man in Thailand. Southeast Asian J. Trop. Med. Public Health 1985, 16, 349–354. [Google Scholar]
- Wang, H.; Yoshimatsu, K.; Ebihara, H.; Ogino, M.; Araki, K.; Kariwa, H.; Wang, Z.; Luo, Z.; Li, D.; Hang, C.; et al. Genetic diversity of hantaviruses isolated in China and characterization of novel hantaviruses isolated from Niviventer confucianus and Rattus rattus. Virology 2000, 278, 332–345. [Google Scholar] [CrossRef]
- Plyusnin, A.; Hörling, J.; Kanerva, M.; Mustonen, J.; Cheng, Y.; Partanen, J.; Vapalahti, O.; Kukkonen, S.K.; Niemimaa, J.; Henttonen, H.; et al. Puumala hantavirus genome in patients with nephropathia epidemica: Correlation of PCR positivity with HLA haplotype and link to viral sequences in local rodents. J. Clin. Microbiol. 1997, 35, 1090–1096. [Google Scholar]
- Németh, V.; Oldal, M.; Madai, M.; Horváth, G.; Kemenesi, G.; Dallos, B.; Bányai, K.; Jakab, F. Molecular characterization of Dobrava and Kurkino genotypes of Dobrava-Belgrade hantavirus detected in Hungary and Northern Croatia. Virus Genes 2013, 47, 546–549. [Google Scholar] [CrossRef]
- Dzagurova, T.K.; Witkowski, P.T.; Tkachenko, E.A.; Klempa, B.; Morozov, V.G.; Auste, B.; Zavora, D.L.; Iunicheva, I.V; Mutnih, E.S.; Kruger, D.H. Isolation of sochi virus from a fatal case of hantavirus disease with fulminant clinical course. Clin. Infect. Dis. 2012, 54, e1–e4. [Google Scholar] [CrossRef]
- Liang, M.; Li, D.; Xiao, S.Y.; Hang, C.; Rossi, C.A.; Schmaljohn, C.S. Antigenic and molecular characterization of hantavirus isolates from China. Virus Res. 1994, 31, 219–233. [Google Scholar] [CrossRef]
- Klempa, B.; Fichet-Calvet, E.; Lecompte, E.; Auste, B.; Aniskin, V.; Meisel, H.; Denys, C.; Koivogui, L.; ter Meulen, J.; Krüger, D.H. Hantavirus in African wood mouse, Guinea. Emerg. Infect. Dis. 2006, 12, 838–840. [Google Scholar] [CrossRef]
- Meheretu, Y.; Čížková, D.; Těšíková, J.; Welegerima, K.; Tomas, Z.; Kidane, D.; Girmay, K.; Bryja, J.; Schmidt-Chanasit, J.; Bryjová, A.; et al. High diversity of RNA viruses in rodents, Ethiopia. Emerg. Infect. Dis. 2012, 18, 2047–2050. [Google Scholar] [CrossRef]
- Kariwa, H.; Yoshizumi, S.; Arikawa, J.; Yoshimatsu, K.; Takahashi, K.; Takashima, I.; Hashimoto, N. Evidence for the existence of Puumula-related virus among Clethrionomys rufocanus in Hokkaido, Japan. Am. J. Trop. Med. Hyg. 1995, 53, 222–227. [Google Scholar]
- Plyusnin, A.; Vapalahti, O.; Lundkvist, A.; Henttonen, H.; Vaheri, A. Newly recognised hantavirus in Siberian lemmings. Lancet 1996, 347, 1835. [Google Scholar]
- Kariwa, H.; Yoshimatsu, K.; Sawabe, J.; Yokota, E.; Arikawa, J.; Takashima, I.; Fukushima, H.; Lundkvist, A.; Shubin, F.N.; Isachkova, L.M.; et al. Genetic diversities of hantaviruses among rodents in Hokkaido, Japan and Far East Russia. Virus Res. 1999, 59, 219–228. [Google Scholar] [CrossRef]
- Zou, Y.; Xiao, Q.-Y.; Dong, X.; Lv, W.; Zhang, S.-P.; Li, M.-H.; Plyusnin, A.; Zhang, Y.-Z. Genetic analysis of hantaviruses carried by reed voles Microtus fortis in China. Virus Res. 2008, 137, 122–128. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, J.; Yang, X.; Zhou, J.; Yang, W.; Peng, C.; Zhang, H.-L.; Shi, Z. A novel hantavirus detected in Yunnan red-backed vole (Eothenomys miletus) in China. J. Gen. Virol. 2011, 92, 1454–1457. [Google Scholar] [CrossRef]
- Pounder, K.C.; Begon, M.; Sironen, T.; Henttonen, H.; Watts, P.C.; Voutilainen, L.; Vapalahti, O.; Klempa, B.; Fooks, A.R.; McElhinney, L.M. Novel hantavirus in field vole, United Kingdom. Emerg. Infect. Dis. 2013, 19, 673–675. [Google Scholar] [CrossRef]
- Torrez-Martinez, N.; Hjelle, B. Enzootic of Bayou hantavirus in rice rats (Oryzomys palustris) in 1983. Lancet 1995, 346, 780–781. [Google Scholar] [CrossRef]
- Rollin, P.E.; Ksiazek, T.G.; Elliott, L.H.; Ravkov, E.V.; Martin, M.L.; Morzunov, S.; Livingstone, W.; Monroe, M.; Glass, G.; Ruo, S. Isolation of black creek canal virus, a new hantavirus from Sigmodon hispidus in Florida. J. Med. Virol. 1995, 46, 35–39. [Google Scholar] [CrossRef]
- Vincent, M.J.; Quiroz, E.; Gracia, F.; Sanchez, A.J.; Ksiazek, T.G.; Kitsutani, P.T.; Ruedas, L.A.; Tinnin, D.S.; Caceres, L.; Garcia, A.; et al. Hantavirus pulmonary syndrome in Panama: Identification of novel hantaviruses and their likely reservoirs. Virology 2000, 277, 14–19. [Google Scholar] [CrossRef]
- Fulhorst, C.F.; Monroe, M.C.; Salas, R.A.; Duno, G.; Utrera, A.; Ksiazek, T.G.; Nichol, S.T.; de Manzione, N.M.; Tovar, D.; Tesh, R.B. Isolation, characterization and geographic distribution of Caño Delgadito virus, a newly discovered South American hantavirus (family Bunyaviridae). Virus Res. 1997, 51, 159–171. [Google Scholar] [CrossRef]
- Milazzo, M.L.; Cajimat, M.N.B.; Hanson, J.D.; Bradley, R.D.; Quintana, M.; Sherman, C.; Velásquez, R.T.; Fulhorst, C.F. Catacamas virus, a hantaviral species naturally associated with Oryzomys couesi (Coues’ oryzomys) in Honduras. Am. J. Trop. Med. Hyg. 2006, 75, 1003–1010. [Google Scholar]
- Levis, S.; Rowe, J.E.; Morzunov, S.; Enria, D.A.; St Jeor, S. New hantaviruses causing hantavirus pulmonary syndrome in central Argentina. Lancet 1997, 349, 998–999. [Google Scholar] [CrossRef]
- Hanson, J.D.; Utrera, A.; Fulhorst, C.F. The delicate pygmy rice rat (Oligoryzomys delicatus) is the principal host of Maporal virus (family Bunyaviridae, genus Hantavirus). Vector Borne Zoonotic Dis. (Larchmt. N. Y.) 2011, 11, 691–696. [Google Scholar] [CrossRef]
- Rawlings, J.A.; Torrez-Martinez, N.; Neill, S.U.; Moore, G.M.; Hicks, B.N.; Pichuantes, S.; Nguyen, A.; Bharadwaj, M.; Hjelle, B. Cocirculation of multiple hantaviruses in Texas, with characterization of the small (S) genome of a previously undescribed virus of cotton rats (Sigmodon hispidus). Am. J. Trop. Med. Hyg. 1996, 55, 672–679. [Google Scholar]
- Chu, Y.-K.; Owen, R.D.; Sánchez-Hernández, C.; Romero-Almaraz Mde, L.; Jonsson, C.B. Genetic characterization and phylogeny of a hantavirus from Western Mexico. Virus Res. 2008, 131, 180–188. [Google Scholar] [CrossRef]
- Hjelle, B.; Torrez-Martinez, N.; Koster, F.T. Hantavirus pulmonary syndrome-related virus from Bolivia. Lancet 1996, 6, 347–357. [Google Scholar]
- Hjelle, B.; Chavez-Giles, F.; Torrez-Martinez, N.; Yates, T.; Sarisky, J.; Webb, J.; Ascher, M. Genetic identification of a novel hantavirus of the harvest mouse Reithrodontomys megalotis. J. Virol. 1994, 68, 6751–6754. [Google Scholar]
- Hjelle, B.; Anderson, B.; Torrez-Martinez, N.; Song, W.; Gannon, W.L.; Yates, T.L. Prevalence and geographic genetic variation of hantaviruses of New World harvest mice (Reithrodontomys): Identification of a divergent genotype from a Costa Rican Reithrodontomys mexicanus. Virology 1995, 207, 452–459. [Google Scholar] [CrossRef]
- Hjelle, B.; Jenison, S.A.; Goade, D.E.; Green, W.B.; Feddersen, R.M.; Scott, A.A. Hantaviruses: Clinical, microbiologic, and epidemiologic aspects. Crit. Rev. Clin. Lab. Sci. 1995, 32, 469–508. [Google Scholar] [CrossRef]
- Song, W.; Torrez-Martinez, N.; Irwin, W.; Harrison, F.J.; Davis, R.; Ascher, M.; Jay, M.; Hjelle, B. Isla Vista virus: A genetically novel hantavirus of the California vole Microtus californicus. J. Gen. Virol. 1995, 76, 3195–3199. [Google Scholar] [CrossRef]
- Teta, P.; Jayat, J.P.; Ortiz, P.E.; Elía, G.D. The taxonomic status of Oligoryzomys brendae Massoia, 1998 (Rodentia, Cricetidae), with comments on the availability of this name. Zootaxa 2013, 3641, 433–447. [Google Scholar] [CrossRef]
- Radosa, L.; Schlegel, M.; Gebauer, P.; Ansorge, H.; Heroldová, M.; Jánová, E.; Stanko, M.; Mošanský, L.; Fričová, J.; Pejčoch, M.; et al. Detection of shrew-borne hantavirus in Eurasian pygmy shrew (Sorex minutus) in Central Europe. Infect. Genet. Evol. 2013, 19, 403–410. [Google Scholar] [CrossRef]
- Kang, H.J.; Kadjo, B.; Dubey, S.; Jacquet, F.; Yanagihara, R. Molecular evolution of Azagny virus, a newfound hantavirus harbored by the West African pygmy shrew (Crocidura obscurior) in Côte d’Ivoire. Virol. J. 2011, 8, 373. [Google Scholar] [CrossRef]
- Gu, S.H.; Markowski, J.; Kang, H.J.; Hejduk, J.; Sikorska, B.; Liberski, P.P.; Yanagihara, R. Boginia virus, a newfound hantavirus harbored by the Eurasian water shrew (Neomys fodiens) in Poland. Virol. J. 2013, 10, 160. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Oliveira, R.C.; Guterres, A.; Fernandes, J.; D'Andrea, P.S.; Bonvicino, C.R.; De Lemos, E.R.S. Hantavirus Reservoirs: Current Status with an Emphasis on Data from Brazil. Viruses 2014, 6, 1929-1973. https://doi.org/10.3390/v6051929
De Oliveira RC, Guterres A, Fernandes J, D'Andrea PS, Bonvicino CR, De Lemos ERS. Hantavirus Reservoirs: Current Status with an Emphasis on Data from Brazil. Viruses. 2014; 6(5):1929-1973. https://doi.org/10.3390/v6051929
Chicago/Turabian StyleDe Oliveira, Renata Carvalho, Alexandro Guterres, Jorlan Fernandes, Paulo Sérgio D'Andrea, Cibele Rodrigues Bonvicino, and Elba Regina Sampaio De Lemos. 2014. "Hantavirus Reservoirs: Current Status with an Emphasis on Data from Brazil" Viruses 6, no. 5: 1929-1973. https://doi.org/10.3390/v6051929
APA StyleDe Oliveira, R. C., Guterres, A., Fernandes, J., D'Andrea, P. S., Bonvicino, C. R., & De Lemos, E. R. S. (2014). Hantavirus Reservoirs: Current Status with an Emphasis on Data from Brazil. Viruses, 6(5), 1929-1973. https://doi.org/10.3390/v6051929




