Phages Preying on Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis: Past, Present and Future
Abstract
:1. Introduction
“A strong feeling of adventure is animating those who are working on bacterial viruses, a feeling that they have a small part in the great drive towards a fundamental problem in biology”.—Max Delbrück, 1946 [1].
2. The B. cereus Group and Its Taxonomic Issues
| Characteristics | B. anthracis | B. cereus | B. thuringiensis |
|---|---|---|---|
| Motility | No a | Yes | Yes |
| Crystal parasporal inclusion(s) | No | No | Yes |
| Lysis by Gamma phage | Yes | No b | No |
| Mucoid colony (capsule synthesis on bicarbonate medium) | Yes | No | No |
| Hemolytic activity on 5% blood agar (sheep or horse) | No a | Yes c | Yes c |
| Penicillin resistance (β-lactamase production) | No a | Yes | Yes |
| Phospholipase C activity | No | Yes c | Yes c |
| Chitinase activity | No | Yes | Yes |
| Tyrosine decomposition | No | Yes | Yes c |
| Mutation non-sense in plcR regulator | Yes | No a | No a |
| Four genomic prophages | Yes | No | No |
3. Pioneer Milestones

4. Phages of B. anthracis, B. cereus and B. thuringiensis
4.1. Classification of B. cereus Group Phages
| Order | Family | Morphology | Shape | Virion Size (nm) | Schematic Representation a |
|---|---|---|---|---|---|
| Caudovirales | Myoviridae | Isometric head, contractile tail and a small base plate. | Tailed | Icosahedral heads: 50–145 Elongated heads: 80 × 110 Tail: 16–20 × 80–455 |  |
| Siphoviridae | Isometric head, long non-contractile tail. Some have elongated heads. | Tailed | Head: 40–80 Tail: 5–10 × 100–210 |  | |
| Podoviridae | Isometric head, short non-contractile tail. Some have elongated heads. | Tailed | Head: 60–70 Tail: 10–20 |  | |
| Unassigned | Tectiviridae | Isometric virion with apical spikes. Capsid encloses an inner membrane vesicle. | Polyhedral | Virion: 66 Spikes: 20 |  |
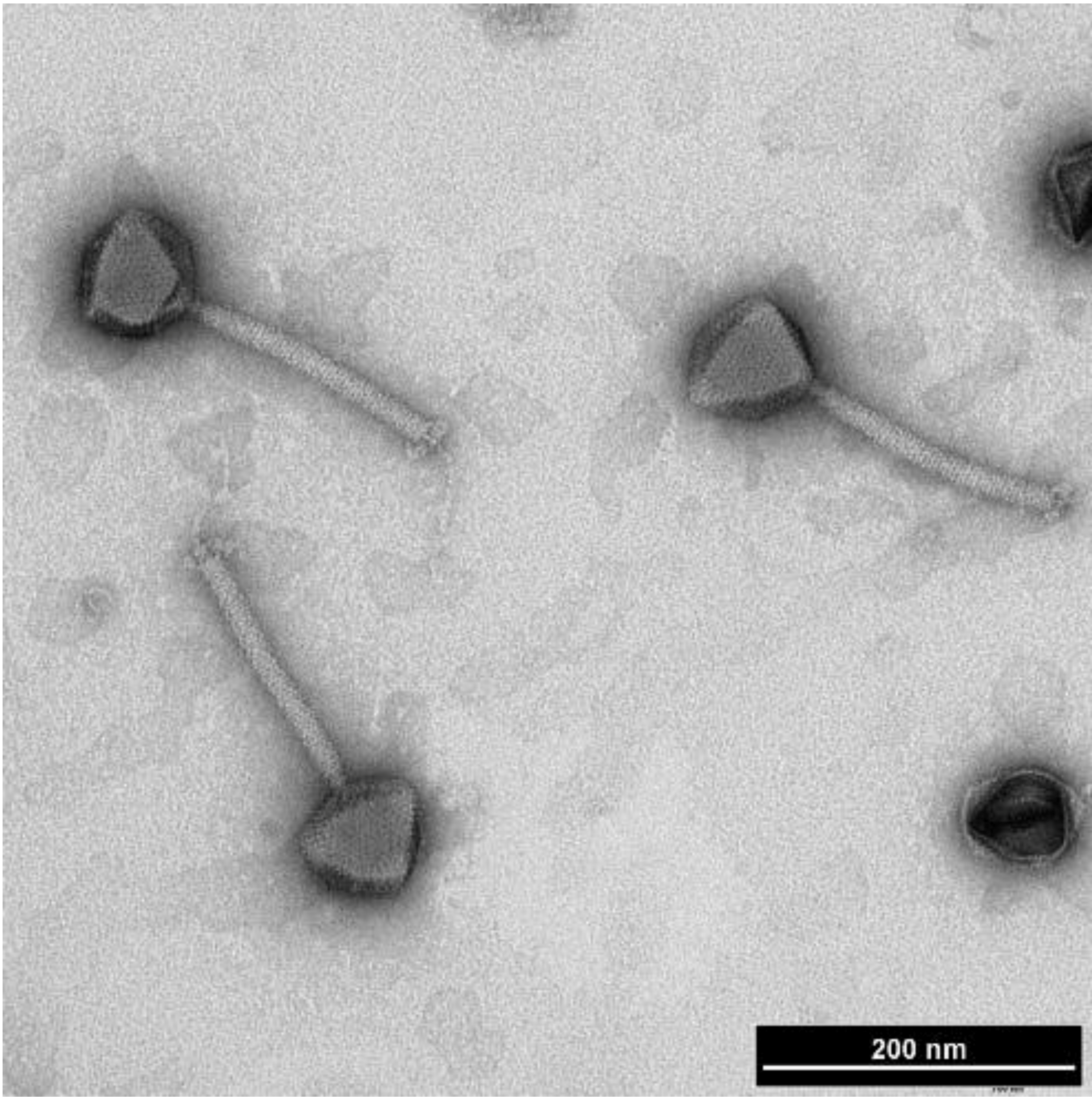
| Morphology | Phage | (Original) Host | Estimated Genome Size | Lifestyle | Particular Features | References |
|---|---|---|---|---|---|---|
| Myoviridae | Bace-11 | B. cereus | ND | ND | Virion morphology related to the 0305ϕ8-36 jumbo phage. | [69,70,71] |
| Myoviridae | BCP1-1 | B. cereus | ~150 kb | Virulent | Able to eradicate B. cereus from food. Divalent cations (Ca2+, Mg2+ or Mn2+) required for activity. | [72] |
| Myoviridae | BCP8-2 | B. cereus | ~150 kb | Virulent | Able to eradicate B. cereus from food Divalent cations (Ca2+, Mg2+ or Mn2+) required for activity. | [72] |
| Myoviridae (SPO1-like) | CP-51 | B. cereus NRRL 569 (ATCC 10876) | ~138 (88) kb a | ND | Mediates generalized transduction. Instability at low temperatures. Infects sporulating B. cereus cells. Related to B. subtilis phage SPO1. | [58,59,64,73,74] |
| Myoviridae | CP-54 | B. thuringiensis sv. alesti NRRL 4041 | 84–116 (339) kb a | ND | Mediates generalized transduction. | [59] |
| Myoviridae | CP-54Ber | B. thuringiensis sv. thuringiensis Berliner 1715 | 84–116 (339) kb a | ND | Mediates generalized transduction. | [75] |
| Myoviridae | FWLBc1 | B. cereus | >90 kb | Virulent | Biocontrols B. cereus. | [76] |
| Myoviridae | FWLBc2 | B. cereus | >90 kb | Virulent | Biocontrols B. cereus. | [76] |
| Myoviridae | JBP901 | B. cereus | ~150 kb | Virulent | Biocontrols B. cereus in liquid cultures and in fermented Korean food products. | [77] |
| Myoviridae | Tg13 | B. thuringiensis | 61 kb | ND | Mediates transduction. | [78] |
| Myoviridae | TP-13 | B. thuringiensis | Possibly 380 kb | ND | Mediates generalized transduction. Converting phage for sporulation and crystal formation. Related to B. subtilis phages SP15. | [79] |
| Myoviridae | TP-18 | B. thuringiensis | 55 kb | ND | Mediates generalized transduction. | [60] |
| Myoviridae | Tt91 | B. thuringiensis | ND | Virulent | Mediates specialized transduction. | [80] |
| Siphoviridae | ϕ20 | B. anthracis Sterne 34F2 (pXO1+ pXO2−) | 48.7 kb | ND | Has a circular plasmidial prophage state. | [81] |
| Siphoviridae | CP-53 | B. cereus ATCC 6464 | 25 kb | ND | Mediates generalized transduction. | [73,74] |
| Siphoviridae | J7W-1 | B. thuringiensis sv. sotto/dendrolimus AF101 | 48 kb | Temperate | Integrates into the 69 kb plasmid pAF101. Is induced by temperature or during mating. | [82,83,84] |
| Siphoviridae | MZTP01 | B. thuringiensis sv. kurstaki MZ1 | ND | Temperate | None. | [85] |
| Siphoviridae | Px1 | B. thuringiensis sv. galleriae 69/6 | ND | Temperate | Mediates transduction. Very sensitive to chloroform. | [86] |
| Siphoviridae | TP21-H | B. thuringiensis | ND | Temperate | None. | [87,88] |
| Siphoviridae | SU-11 | B. thuringiensis sv. israelensis | ND | ND | Has a circular plasmidial prophage state. | [89] |
| Tectiviridae | Emet | B. cereus 5975c | ~15 kb | Temperate | Isolated from an emetic B. cereus. Has a linear plasmidial prophage state. | [90] |
| Tectiviridae | Sand | B. cereus VD184 | ~15 kb | Temperate | Has a linear plasmidial prophage state. | [90] |
| Tectiviridae | Sato | B. cereus AND1284 | ~15 kb | Temperate | Isolated from an emetic B. cereus. Has a linear plasmidial prophage state. | [90] |
| Tectiviridae | Sole | B. cereus VD166 | ~15 kb | Temperate | Has a linear plasmidial prophage state. | [90] |
| ND | - | B. anthracis | ND | Virulent | First phage isolated for this bacterium. | [41] |
| ND | ϕ42 | B. thuringiensis sv. gelechiae Bt1134 | ND | ND | Mediates generalized transduction. | [91] |
| ND | ϕ63 | B. thuringiensis | ND | ND | Mediates generalized transduction. | [91] |
| ND | ϕ64 | B. thuringiensis sv. alesti | 79–85 kb | ND | Mediates generalized transduction. Possibly a mutant of ϕ63. | [92] |
| ND | ϕHD67 | B. thuringiensis sv. alesti | 45.7 kb | Temperate | Mediates generalized transduction. | [93,94,95] |
| ND | ϕHD130 | B. thuringiensis | 38.1 kb | Temperate | Mediates transduction. | [93,94,95] |
| ND | ϕHD228 | B. thuringiensis | 36 kb | Temperate | Mediates transduction. | [93,94,95] |
| ND | ϕHD248 | B. thuringiensis | 47.1 kb | Temperate | Mediates transduction. Used for fine-structure chromosomal mapping. | [93,94,95] |
| ND | TP-21 (TP21-T) | B. thuringiensis sv. kurstaki HD-1 (HD1-9) | ND | ND | Mediates specialized transduction. Plasmidial phage. | [96,97] |
| ND | 12826 | B. cereus WS2453 | ND | ND | Its endolysin (Ply12) lysis several Bacillus sp. | [98] |
| ND | BcpI | ND | ND | ND | Its endolysin (PlyB) has a potent lytic action against B. anthracis. | [99] |
| ND | TP-10 | B. thuringiensis | ND | ND | Mediates generalized transduction. | [79] |
| ND | W | B. cereus W (ATCC 11950) | ND | Temperate | Parental phage for Gamma phage, probably same as Wβ. | [100] |
| ND | Wα | B. cereus W (ATCC 11950) | ND | Virulent | Rare virulent mutant of phage W. | [101] |
| ND | wx | B. cereus W (ATCC 11950) | ND | Temperate | Cryptic plasmid involved in lysogenic conversion to phospholipase A production. | [102,103] |
| ND | wxc | B. cereus W (ATCC 11950) | ND | Virulent | Virulent mutant of phage wx | [102] |
| Morphology b | Phage | Host | Genome Size (bp) | GC% | Predicted ORFs | No. tRNAs | Lifestyle | GenBank Accession No. | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Myoviridae | 0305ϕ8-36 | B. thuringiensis | 218,948 | 41.80 | 247 | 2 | Virulent | EF583821 | [104] |
| Myoviridae (Twort-like) | B4 | B. cereus | 162,596 | 37.71 | 277 | 0 | Virulent | JN790865 | [105] |
| Myoviridae (Twort-like) | B5S | B. cereus | 162,598 | 37.71 | 272 | 0 | Virulent | JN797796 | [67] |
| Myoviridae (Twort-like) | Bastille | B. cereus | 153,962 | 38.14 | 273 | 7 | Virulent | JF966203 | [64] |
| Myoviridae | BCD7 | B. cereus | 93,839 | 38.04 | 140 | 0 | Virulent | JN712910 | - |
| Myoviridae | BCP78 | B. cereus | 156,176 | 39.86 | 227 | 18 | Virulent | JN797797 | [106] |
| Myoviridae | BCU4 | B. cereus | 154,371 | 39.86 | 223 | 19 | Virulent | JN797798 | [67] |
| Myoviridae | BigBertha | B. thuringiensis | 162,661 | 37.80 | 287 | 0 | Virulent ? | KF669647 | [107] |
| Myoviridae | BPS10C | B. cereus | 159,590 | 38.74 | 271 | 0 | Virulent | KC430106 | [108,109] |
| Myoviridae | BPS13 | B. cereus | 158,305 | 38.75 | 268 | 0 | Virulent | JN654439 | [108,109] |
| Myoviridae | JL | B. cereus | 137,918 | 40.80 | 22 | 4 | ND | KC595512 | [110] |
| Myoviridae | Shanette | B. cereus | 138,877 | 40.80 | 223 | 3 | ND | KC595513 | [110] |
| Myoviridae | Spock | B. thuringiensis | 161,497 | 38.20 | 280 | 0 | Virulent ? | KF669662 | [111] |
| Myoviridae | Troll | B. thuringiensis | 163,019 | 37.80 | 289 | 0 | ND | KF208639 | [112] |
| Myoviridae (Twort-like) | vB_BceM_Bc431v3 | B. cereus | 158,621 | 39.98 | 239 | 20 | Virulent | JX094431 | [68] |
| Myoviridae | W.Ph. | B. cereus | 156,897 | 36.45 | 274 | 0 | Virulent | HM144387 | - |
| Siphoviridae | 11143 | B. cereus | 39,077 | 34.96 | 49 | 0 | Temperate | GU233956 | [113] |
| Siphoviridae | 250 | B. cereus | 56,505 | 36.45 | 54 | 0 | Temperate | GU229986 | [114] |
| Siphoviridae | Basilisk | B. cereus | 81,790 | 33.90 | 140 | 2 | ND | KC595511 | [110] |
| Siphoviridae | BceA1 | B. cereus | 42,932 | 35.66 | 63 | 0 | Temperate | HE614282 | [115] |
| Siphoviridae | BMBtp2 | B. thuringiensis | 36,932 | 37.79 | 53 | 0 | Temperate | JX887877 | [116] |
| Siphoviridae | BtCS33 | B. thuringiensis | 41,992 | 35.22 | 57 | 0 | Temperate | JN191664 | [117] |
| Siphoviridae | Cherry c | B. anthracis | 36,615 | 35.26 | 51 | 0 | Virulent | DQ222851 | [118] |
| Siphoviridae | Fah c | B. anthracis | 37,974 | 34.94 | 50 | 0 | Virulent | DQ150593 | [119] |
| Siphoviridae | Gamma USAMRIID c | B. anthracis | 37,253 | 35.22 | 53 | 0 | Virulent | DQ222853 | [118] |
| Siphoviridae | Gamma LSU c | B. anthracis | 38,067 | 35.63 | 50 | 0 | Virulent | DQ222855 | [118] |
| Siphoviridae | Gamma isolate d’Herelle c | B. anthracis | 37,373 | 35.12 | 53 | 0 | Virulent | DQ289556 | [120] |
| Siphoviridae | Gamma Porton c | B. anthracis | 36,083 | 35.10 | ND | 0 | Virulent | DQ221100 | - |
| Siphoviridae | MZTP02 | B. thuringiensis | 15,717 | 37.55 | 20 | 0 | Temperate | AY894696 | [121] |
| Siphoviridae | PBC1 | B. cereus | 41,164 | 41.68 | 50 | 0 | Virulent | JQ619704 | [122] |
| Siphoviridae | phIS3501 | B. thuringiensis | 44,401 | 34.86 | 53 | 1 | Temperate | JQ062992 | [123] |
| Siphoviridae | phiCM3 | B. thuringiensis | 38,772 | 35.46 | 56 | 0 | Virulent | KF296718 | [124] |
| Siphoviridae | TP21-L | B. cereus | 37,456 | 37.80 | 56 | 0 | Temperate | EU887664 | [88,98] |
| Siphoviridae | vB_BanS-Tsamsa | B. anthracis | 168,876 | 37.80 | 272 | 19 | Temperate | KC481682 | [125] |
| Siphoviridae | vB_BceS-IEBH | B. cereus | 53,104 | 36.42 | 86 | 0 | Temperate | EU874396 | [126] |
| Siphoviridae | Wβ | B. cereus | 40,867 | 35.26 | 53 | 0 | Temperate | DQ289555 | [120] |
| Podoviridae | MG-B1 | B. weihenstephanensis | 27,190 | 30.75 | 43 | 0 | Virulent | KC685370 | [127] |
| Tectiviridae | AP50 d | B. anthracis | 14,398 | 38.65 | 31 | 0 | Temperate | EU408779 | [128] |
| Tectiviridae | Bam35 d | B. thuringiensis | 14,935 | 39.72 | 32 | 0 | Temperate | AY257527 | [129] |
| Tectiviridae | GIL01 | B. thuringiensis | 14,931 | 39.73 | 30 | 0 | Temperate | AJ536073 | [130] |
| Tectiviridae | GIL16 d | B. thuringiensis | 14,844 | 40.07 | 31 | 0 | Temperate | AY701338 | [131] |
| Tectiviridae | Wip1 | B. anthracis | 14,319 | 36.84 | 27 | 0 | Temperate | KF188458 | [132] |
| ND | lambdaBa01 | B. anthracis | 50,482 | 35.3 | ND | 0 | Temperate | AE016879 | [16] |
| ND | lambdaBa02 | B. anthracis | 44,043 | 35.0 | ND | 0 | Temperate | AE016879 | [16] |
| ND | lambdaBa03 | B. anthracis | 16,759 | 35.0 | ND | 0 | Temperate | AE016879 | [16] |
| ND | lambdaBa04 | B. anthracis | 37,385 | 34.0 | ND | 0 | Temperate | AE016879 | [16] |
| ND | phBC6A51 | B. cereus | 61,395 | 37.69 | 75 | 0 | Temperate | NC_004820 | [17] |
| ND | phBC6A52 | B. cereus | 38,472 | 34.72 | 49 | 0 | Temperate | NC_004821 | [17] |
| ND | proCM3 | B. thuringiensis | 43,278 | 37.40 | 58 | 0 | Temperate | KF296717 | [124] |
| Characteristics | CP-51 | CP-53 | CP-54 | CP-54Ber |
|---|---|---|---|---|
| Family | Myoviridae | Siphoviridae | Myoviridae | Myoviridae |
| Head diameter (nm) | 90 | 66 | 120–122 | 120 |
| Tail length (nm) | 160–185 | 276 | 198–200 | 200 |
| Estimated genome size (kb) | 138 (88) a | 25 | 84–116 (339) b | 84–116 (339) b |
| GC% | 43.9 | 37 | 43 | 43 |
| Genome particular features | Fixed ends, HMU c | No unusual bases | HMU | HMU |
| Lifestyle | Virulent | Temperate | Virulent | Temperate |
| Transduction frequencies | 10−7–10−5 | 10−7–10−6 | 10−7–10−5 | 10−7–10−5 |
| Host range | Active on different B. thuringiensis, B.cereus and B. anthracis strains | ND | Broader than CP-51 | Narrower than CP-54, but active on B.thuringiensis sv. thuringiensis strain Berliner 1715 |
| Stability at 4 °C | No | Yes | No (more cold sensitive than CP-51) | No |
| Stability at 15 °C (plus divalent cations) | Yes | ND | Yes (less than CP-51) | Yes (less than CP-51) |
| Infection of sporulating cells | Yes | ND | Yes | Yes |
4.2. The Transducing Phages
4.2.1. Phages CP-51, CP-53, CP-54 and CP-54Ber
4.2.2. Phages TP-13 and TP-18
4.2.3. Phages ϕ63 and its Derivative Mutant ϕ64
4.2.4. Other Transducing Phages
4.3. Phages with a Chromosomal Prophage State

The γ-Like Phages

4.4. Phages with a Plasmidial Prophage State
4.4.1. Circular Plasmidial Prophages

4.4.2. Tectiviruses

4.5. The Jumbo Phages

4.6. Other Remarkable Phages
5. What about Phages in Other Members of the B. cereus Group?
6. Applications of B. cereus Group Phages
6.1. Phage Typing
6.2. Biocontrol
7. Concluding Remarks
Acknowledgments
Conflicts of Interest
References and Notes
- Delbrück, M.L.H. Experiments with bacterial viruses (bacteriophages). Harvey Lect. 1946, 41, 187. [Google Scholar]
- Hendrix, R.W.; Smith, M.C.; Burns, R.N.; Ford, M.E.; Hatfull, G.F. Evolutionary relationships among diverse bacteriophages and prophages: All the world’s a phage. Proc. Natl. Acad. Sci. USA 1999, 96, 2192–2197. [Google Scholar]
- Brüssow, H.; Canchaya, C.; Hardt, W.D. Phages and the evolution of bacterial pathogens: From genomic rearrangements to lysogenic conversion. Microbiol. Mol. Biol. Rev. 2004, 68, 560–602. [Google Scholar] [CrossRef]
- Twort, F.W. An investigation on the nature of ultra-microscopic viruses. Lancet 1915, 186, 1241–1243. [Google Scholar] [CrossRef]
- Duckworth, D.H. Who discovered bacteriophage? Bacteriol. Rev. 1976, 40, 793–802. [Google Scholar]
- D’Herelle, F. Sur un microbe invisible antogoniste des bacilles dysentériques. Comptes Rendus Acad. Sci. (Paris) 165, 373–375.
- Fruciano, D.E.; Bourne, S. Phage as an antimicrobial agent: D’Herelle’s heretical theories and their role in the decline of phage prophylaxis in the West. Can. J. Infect. Dis. Med. Microbiol. 2007, 18, 19–26. [Google Scholar]
- Sano, E.; Carlson, S.; Wegley, L.; Rohwer, F. Movement of viruses between biomes. Appl. Environ. Microbiol. 2004, 70, 5842–5846. [Google Scholar] [CrossRef]
- Dinsdale, E.A.; Edwards, R.A.; Hall, D.; Angly, F.; Breitbart, M.; Brulc, J.M.; Furlan, M.; Desnues, C.; Haynes, M.; Li, L.; et al. Functional metagenomic profiling of nine biomes. Nature 2008, 452, 629–632. [Google Scholar] [CrossRef]
- Farrar, W.E.; Reboli, A. The Genus Bacillus—Medical. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer US: New York, NY, USA, 2006; pp. 609–630. [Google Scholar]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Fouet, A.; Mock, M. Regulatory networks for virulence and persistence of Bacillus anthracis. Curr. Opin. Microbiol. 2006, 9, 160–166. [Google Scholar]
- Bottone, E.J. Bacillus cereus, a volatile human pathogen. Clin. Microbiol. Rev. 2010, 23, 382–398. [Google Scholar] [CrossRef]
- Bravo, A.; Likitvivatanavong, S.; Gill, S.S.; Soberon, M. Bacillus thuringiensis: A story of a successful bioinsecticide. Insect. Biochem. Mol. Biol. 2011, 41, 423–431. [Google Scholar]
- Sozhamannan, S.; Chute, M.; McAfee, F.; Fouts, D.; Akmal, A.; Galloway, D.; Mateczun, A.; Baillie, L.; Read, T. The Bacillus anthracis chromosome contains four conserved, excision-proficient, putative prophages. BMC Microbiol. 2006, 6, 34. [Google Scholar]
- Read, T.D.; Peterson, S.N.; Tourasse, N.; Baillie, L.W.; Paulsen, I.T.; Nelson, K.E.; Tettelin, H.; Fouts, D.E.; Eisen, J.A.; Gill, S.R.; et al. The genome sequence of Bacillus anthracis Ames and comparison to closely related bacteria. Nature 2003, 423, 81–86. [Google Scholar] [CrossRef]
- Ivanova, N.; Sorokin, A.; Anderson, I.; Galleron, N.; Candelon, B.; Kapatral, V.; Bhattacharyya, A.; Reznik, G.; Mikhailova, N.; Lapidus, A.; et al. Genome sequence of Bacillus cereus and comparative analysis with Bacillus anthracis. Nature 2003, 423, 87–91. [Google Scholar] [CrossRef]
- Anderson, I.; Sorokin, A.; Kapatral, V.; Reznik, G.; Bhattacharya, A.; Mikhailova, N.; Burd, H.; Joukov, V.; Kaznadzey, D.; Walunas, T.; et al. Comparative genome analysis of Bacillus cereus group genomes with Bacillus subtilis. FEMS Microbiol. Lett. 2005, 250, 175–184. [Google Scholar] [CrossRef]
- Jensen, G.B.; Hansen, B.M.; Eilenberg, J.; Mahillon, J. The hidden lifestyles of Bacillus cereus and relatives. Environ. Microbiol. 2003, 5, 631–640. [Google Scholar]
- Guinebretière, M.H.; Auger, S.; Galleron, N.; Contzen, M.; de Sarrau, B.; de Buyser, M.L.; Lamberet, G.; Fagerlund, A.; Granum, P.E.; Lereclus, D.; et al. Bacillus cytotoxicus sp. nov. is a novel thermotolerant species of the Bacillus cereus group occasionally associated with food poisoning. Int. J. Syst. Evol. Microbiol. 2013, 63, 31–40. [Google Scholar] [CrossRef]
- Nakamura, L.K. Bacillus pseudomycoides sp. nov. Int. J. Syst. Bacteriol. 1998, 48, 1031–1035. [Google Scholar]
- Priest, F.G. Biodiversity of the entomopathogenic, endospore-forming bacteria. In Entomopathogenic bacteria: From laboratory to field application; Charles, J.F., Delecluse, A., Nielsen-Leroux, C., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 1–22. [Google Scholar]
- Turnbull, P.C.B. Guidelines for the Surveillance and Control of Anthrax in Humans and Animals, 3rd ed.; World Health Organization (WHO): Switzerland, 1998; WHO/EMC/ZDI/98.6; pp. 1–97. [Google Scholar]
- Ash, C.; Collins, M.D. Comparative analysis of 23S ribosomal RNA gene sequences of Bacillus anthracis and emetic Bacillus cereus determined by PCR-direct sequencing. FEMS Microbiol. Lett. 1992, 94, 75–80. [Google Scholar] [CrossRef]
- Ash, C.; Farrow, J.A.E.; Dorsch, M.; Stackebrandt, E.; Collins, M.D. Comparative analysis of Bacillus anthracis, Bacillus cereus, and related species on the basis of reverse transcriptase sequencing of 16S rRNA. Int. J. Syst. Bacteriol. 1991, 41, 343–346. [Google Scholar] [CrossRef]
- Bavykin, S.G.; Lysov, Y.P.; Zakhariev, V.; Kelly, J.J.; Jackman, J.; Stahl, D.A.; Cherni, A. Use of 16S rRNA, 23S rRNA, and gyrB gene sequence analysis to determine phylogenetic relationships of Bacillus cereus group microorganisms. J. Clin. Microbiol. 2004, 42, 3711–3730. [Google Scholar] [CrossRef]
- Daffonchio, D.; Raddadi, N.; Merabishvili, M.; Cherif, A.; Carmagnola, L.; Brusetti, L.; Rizzi, A.; Chanishvili, N.; Visca, P.; Sharp, R.; et al. Strategy for identification of Bacillus cereus and Bacillus thuringiensis strains closely related to Bacillus anthracis. Appl. Environ. Microbiol. 2006, 72, 1295–1301. [Google Scholar] [CrossRef]
- Helgason, E.; Caugant, D.A.; Lecadet, M.M.; Chen, Y.; Mahillon, J.; Lovgren, A.; Hegna, I.; Kvaloy, K.; Kolsto, A.B. Genetic diversity of Bacillus cereus/B. thuringiensis isolates from natural sources. Curr. Microbiol. 1998, 37, 80–87. [Google Scholar] [CrossRef]
- Helgason, E.; Økstad, O.A.; Caugant, D.A.; Johansen, H.A.; Fouet, A.; Mock, M.; Hegna, I.; Kolstø, A.-B. Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis—One species on the basis of genetic evidence. Appl. Environ. Microbiol. 2000, 66, 2627–2630. [Google Scholar] [CrossRef]
- Helgason, E.; Tourasse, N.J.; Meisal, R.; Caugant, D.A.; Kolstø, A.-B. Multilocus sequence typing scheme for bacteria of the Bacillus cereus group. Appl. Environ. Microbiol. 2004, 70, 191–201. [Google Scholar] [CrossRef]
- Ticknor, L.O.; Kolstø, A.-B.; Hill, K.K.; Keim, P.; Laker, M.T.; Tonks, M.; Jackson, P.J. Fluorescent amplified fragment length polymorphism analysis of Norwegian Bacillus cereus and Bacillus thuringiensis soil isolates. Appl. Environ. Microbiol. 2001, 67, 4863–4873. [Google Scholar] [CrossRef]
- Guinebretière, M.H.; Velge, P.; Couvert, O.; Carlin, F.; Debuyser, M.L.; Nguyen-The, C. Ability of Bacillus cereus group strains to cause food poisoning varies according to phylogenetic affiliation (groups I to VII) rather than species affiliation. J. Clin. Microbiol. 2010, 48, 3388–3391. [Google Scholar] [CrossRef] [Green Version]
- Tourasse, N.J. HyperCAT: An extension of the SuperCAT database for global multi-scheme and multi-datatype phylogenetic analysis of the Bacillus cereus group population. Database 2010, 2010, baq017. [Google Scholar] [CrossRef]
- Maughan, H.; van der Auwera, G. Bacillus taxonomy in the genomic era finds phenotypes to be essential though often misleading. Infect. Genet. Evol. 2011, 11, 789–797. [Google Scholar]
- Blevins, S.M.; Bronze, M.S. Robert Koch and the “golden age” of bacteriology. Int. J. Infect. Dis. 2010, 14, e744–e751. [Google Scholar] [CrossRef]
- Bardell, D. An 1898 Report by Gamaleya for a lytic agent specific for Bacillus anthracis. J. Hist. Med. Allied Sci. 1982, 37, 222–225. [Google Scholar] [CrossRef]
- Lemos Monteiro, J. Sobre o phenomeno de Twort-d’Hérelle. Presenca do principio lytico nas culturas, em meio solido do Bacillo. anthrac. c. pestio et c. dys. Shiga-Kruse. Bol. Soc. Med. c. cir. de. S. Paolo 1922, Nr.4. [Google Scholar]
- Lemos Monteiro, J. Sobre o phenomeno de d’Hérelle; presenca do principio lytico nas culturas, em meio solido, do Bacillo anthracis, B. pestis e B. dys. Shiga Kruse. Brazil-Méd. 1922, 36, 297. [Google Scholar]
- Brown, J.H.; Basaca, M. Pseudobacteriophage of Bacillus anthracis. Proc. Soc. Exp. Biol. Med. 1926, 23, 625. [Google Scholar] [CrossRef]
- Raiga, A. Traitement des furoncles et ses anthrax par le bactériophage de d’Hérelle. Presse méd. 1929, Nr. 12, 187. [Google Scholar]
- Cowles, P.B. A bacteriophage for B. anthracis. J. Bacteriol. 1931, 21, 161–166. [Google Scholar]
- Klee, S.R.; Brzuszkiewicz, E.B.; Nattermann, H.; Bruggemann, H.; Dupke, S.; Wollherr, A.; Franz, T.; Pauli, G.; Appel, B.; Liebl, W.; et al. The genome of a Bacillus isolate causing anthrax in chimpanzees combines chromosomal properties of B. cereus with B. anthracis virulence plasmids. PLoS One 2010, 5, e10986. [Google Scholar] [CrossRef]
- Brown, E.R.; Moody, M.D.; Treece, E.L.; Smith, C.W. Differential diagnosis of Bacillus cereus, Bacillus anthracis, and Bacillus cereus var. mycoides. J. Bacteriol. 1958, 75, 499–509. [Google Scholar]
- Buck, C.A.; Anacker, R.L.; Newman, F.S.; Eisenstark, A. Phage isolated from lysogenic Bacillus anthracis. J. Bacteriol. 1963, 85, 1423–1430. [Google Scholar]
- Sansinenea, E. Discovery and description of Bacillus thuringiensis. In Bacillus Thuringiensis Biotechnology; Sansinenea, E., Ed.; Springer Netherlands: Berlin, The Netherlands, 2012; pp. 3–18. [Google Scholar]
- Kellenberger, G.; Kellenberger, E. La lysogénie d’une de souche Bacillus cereus. Mise en évidence par le microscope electronique. Schweiz. Z. Allgem. Pathol. Bakteriol. 1952, 15, 225–233. [Google Scholar]
- Afrikian, E. Causal agents of bacterial diseases of the silkworm and the use of antibiotics in their control. J. Insect. Path. 1960, 2, 299–304. [Google Scholar]
- Norris, J.R. Bacteriophages of Bacillus cereus and of crystal-forming insect pathogens related to B. cereus. J. Gen. Microbiol. 1961, 26, 167–173. [Google Scholar] [CrossRef]
- Khachatrian, L.S.; Rautenshtein, I. Comparative study of various bacteriophages of cultures of the Bac. cereus-thuringiensis group. Mikrobiologiia 1963, 32, 813–818. [Google Scholar]
- Ciuca, M.; Stamatin, N.; Zilisteanu, E.; Nafta, I.; Anghelesco, S. Research on the “cereus-anthracis-mycoides” phages. Arch. Roum. Pathol. Exp. Microbiol. 1962, 21, 400–405. [Google Scholar]
- Stiube, P.; Dimitriu, C. Electron microscopic studies of mesophilic and psychrophilic cereus-anthracis-mycoides (CAM) bacteriophages. Arch. Roum. Pathol. Exp. Microbiol. 1969, 28, 809–821. [Google Scholar]
- Dawson, I.M.; Smillie, E.; Norris, J.R. The morphology of Bacillus cereus bacteriophages. J. Gen. Microbiol. 1962, 28, 517–519. [Google Scholar] [CrossRef]
- Chapman, H.M.; Norris, J.R. Four new bacteriophages of Bacillus thuringiensis. J. Appl. Bacteriol. 1966, 29, 529–535. [Google Scholar] [CrossRef]
- Colasito, D.J.; Rogoff, M.H. Characterization of lytic bacteriophages of Bacillus thuringiensis. J. Gen. Virol. 1969, 5, 267–274. [Google Scholar] [CrossRef]
- Colasito, D.J.; Rogoff, M.H. Characterization of temperate bacteriophages of Bacillus thuringiensis. J. Gen. Virol. 1969, 5, 275–281. [Google Scholar] [CrossRef]
- King, A.M.Q.; Lefkowitz, E.; Adams, M.J.; Carstens, E.B.E. Virus Taxonomy. Ninth Report of the International Committee on Taxonomy of Viruses; Academic Press: London, UK, 2012; pp. 1–1327. [Google Scholar]
- Tikhonenko, A.S. Ultrastructure of Bacterial Viruses; Plenum Press: New York, NY, USA, 1970. [Google Scholar]
- Thorne, C.B. Transducing bacteriophage for Bacillus cereus. J. Virol. 1968, 2, 657–662. [Google Scholar]
- Thorne, C.B. Transduction in Bacillus thuringiensis. Appl. Environ. Microbiol. 1978, 35, 1109–1115. [Google Scholar]
- Barsomian, G.D.; Robillard, N.J.; Thorne, C.B. Chromosomal mapping of Bacillus thuringiensis by transduction. J. Bacteriol. 1984, 157, 746–750. [Google Scholar]
- Ackermann, H.W. Classification of Bacteriophages. In The Bacteriophages, 2nd ed.; Calendar, R., Ed.; Oxford University Press, Inc.: New York, NY, USA, 2006; pp. 8–16. [Google Scholar]
- Hulo, C.; de Castro, E.; Masson, P.; Bougueleret, L.; Bairoch, A.; Xenarios, I.; Le Mercier, P. ViralZone: A knowledge resource to understand virus diversity. Nucleic Acids Res. 2011, 39, D576–D582. [Google Scholar]
- Lavigne, R.; Darius, P.; Summer, E.; Seto, D.; Mahadevan, P.; Nilsson, A.; Ackermann, H.; Kropinski, A. Classification of Myoviridae bacteriophages using protein sequence similarity. BMC Microbiol. 2009, 9, 224. [Google Scholar] [CrossRef]
- Klumpp, J.; Lavigne, R.; Loessner, M.J.; Ackermann, H.W. The SPO1-related bacteriophages. Arch. Virol. 2010, 155, 1547–1561. [Google Scholar] [CrossRef]
- Barylski, J.; Nowicki, G.; Goździcka-Józefiak, A. The discovery of phiAGATE, a novel phage infecting Bacillus pumilus, leads to new insights into the phylogeny of the subfamily Spounavirinae. PLoS One 2014, 9, e86632. [Google Scholar]
- Ackermann, H.W.; DuBow, M.S. Viruses of Prokaryotes, Vol II. Natural groups of Bacteriophages; CRC Press: Boca de Raton, FL, USA, 1987; pp. 72–81. [Google Scholar]
- Lee, J.H.; Shin, H.; Ryu, S. Characterization and comparative genomic analysis of bacteriophages infecting members of the Bacillus cereus group. Arch. Virol. 2014, 159, 871–884. [Google Scholar] [CrossRef]
- El-Arabi, T.; Griffiths, M.; She, Y.-M.; Villegas, A.; Lingohr, E.; Kropinski, A. Genome sequence and analysis of a broad-host range lytic bacteriophage that infects the Bacillus cereus group. Virol. J. 2013, 10, 48. [Google Scholar]
- Ahmed, R.; Sankar-Mistry, P.; Jackson, S.; Ackermann, H.W.; Kasatiya, S.S. Bacillus cereus phage typing as an epidemiological tool in outbreaks of food poisoning. J. Clin. Microbiol. 1995, 33, 636–640. [Google Scholar]
- Ackermann, H.-W.; Yoshino, S.; Ogata, S. A Bacillus phage that is a living fossil. Can. J. Microbiol. 1995, 41, 294–297. [Google Scholar] [CrossRef]
- Lavigne, R.; Ceyssens, P.-J. Family Myoviridae. In Virus Taxonomy. Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M.Q., Lefkowitz, E., Adams, M.J., Carstens, E.B., Eds.; Academic Press: London, UK, 2012; pp. 46–62. [Google Scholar]
- Bandara, N.; Jo, J.; Ryu, S.; Kim, K.-P. Bacteriophages BCP1–1 and BCP8–2 require divalent cations for efficient control of Bacillus cereus in fermented foods. Food Microbiol. 2012, 31, 9–16. [Google Scholar] [CrossRef]
- Yelton, D.B.; Thorne, C.B. Comparison of Bacillus cereus bacteriophages CP-51 and CP-53. J. Virol. 1971, 8, 242–253. [Google Scholar]
- Yelton, D.B.; Thorne, C.B. Transduction in Bacillus cereus by each of two bacteriophages. J. Bacteriol. 1970, 102, 573–579. [Google Scholar]
- Lecadet, M.M.; Blondel, M.O.; Ribier, J. Generalized transduction in Bacillus thuringiensis var. berliner 1715 using bacteriophage CP-54Ber. J. Gen. Microbiol. 1980, 121, 203–212. [Google Scholar]
- Lee, W.J.; Billington, C.; Hudson, J.A.; Heinemann, J.A. Isolation and characterization of phages infecting Bacillus cereus. Lett. Appl. Microbiol. 2011, 52, 456–464. [Google Scholar] [CrossRef]
- Shin, H.; Bandara, N.; Shin, E.; Ryu, S.; Kim, K.-P. Prevalence of Bacillus cereus bacteriophages in fermented foods and characterization of phage JBP901. Res. Microbiol. 2011, 162, 791–797. [Google Scholar] [CrossRef]
- Pogosbekova, M.R.; Azizbekian, R.R. Intervariant transduction in Bacillus thuringiensis. Genetika 1984, 20, 187–189. [Google Scholar]
- Perlak, F.J.; Mendelsohn, C.L.; Thorne, C.B. Converting bacteriophage for sporulation and crystal formation in Bacillus thuringiensis. J. Bacteriol. 1979, 140, 699–706. [Google Scholar]
- Koroleva, I.V.; Minenkova, I.B.; Shamshina, T.N.; Azizbekian, R.R. Structural and molecular-biological characteristics of a new transducing phage from Bacillus thuringiensis Tt 91. Mol. Gen. Mikrobiol. Virusol. 1993, 1, 35–38. [Google Scholar]
- Inal, J.M.; Karunakaran, K.V. phi 20, a temperate bacteriophage isolated from Bacillus anthracis exists as a plasmidial prophage. Curr. Microbiol. 1996, 32, 171–175. [Google Scholar] [CrossRef]
- Kanda, K.; Tan, Y.; Aizawa, K. A novel phage genome integrated into a plasmid in Bacillus thuringiensis strain AF101. J. Gen. Microbiol. 1989, 135, 3035–3041. [Google Scholar]
- Kanda, K.; Kayashima, T.; Kato, F.; Murata, A. Temperature influences induction of a J7W-1-related phage in Bacillus thuringiensis serovar indiana. Acta Virol. (Praha) 2000, 44, 183–187. [Google Scholar]
- Kanda, K.; Takada, Y.; Kawasaki, F.; Kato, F.; Murata, A. Mating in Bacillus thuringiensis can induce plasmid integrative prophage J7W-1. Acta Virol. (Praha) 2000, 44, 189–192. [Google Scholar]
- Liao, W.; Sun, F.; Song, S.Y.; Shi, W.; Pang, Y. Biology of two lysogenic phages from Bacillus thuringiensis MZ1. Acta Microbiologica Sinica 2007, 47, 92–97. [Google Scholar]
- Koretskaia, N.G.; Kuzin, A.I.; Svetoch, O.E.; Dobritsa, A.P. Structure and biological features of the temperate phage of Bacillus thuringiensis var. galleriae 69/9, sensitive to chloroform. Mol. Gen. Mikrobiol. Virusol. 1989, 8, 27–34. [Google Scholar]
- He, N.B.; Chen, J.Z.; Lin, C.C. Six distinct types of bacteriophage attacking Bacillus thuringiensis. Acta Microbiologica Sinica 1978, 18, 220–224. [Google Scholar]
- Klumpp, J.; Calendar, R.; Loessner, M.J. Complete nucleotide sequence and molecular characterization of Bacillus phage TP21 and its relatedness to other phages with the same name. Viruses 2010, 2, 961–971. [Google Scholar] [CrossRef]
- Kanda, K.; Ohderaotoshi, T.; Shimojyo, A.; Kato, F.; Murata, A. An extrachromosomal prophage naturally associated with Bacillus thuringiensis serovar israelensis. Lett. Appl. Microbiol. 1999, 28, 305–308. [Google Scholar] [CrossRef]
- Jalasvuori, M.; Palmu, S.; Gillis, A.; Kokko, H.; Mahillon, J.; Bamford, J.K.H.; Fornelos, N. Identification of five novel tectiviruses in Bacillus strains: Analysis of a highly variable region generating genetic diversity. Res. Microbiol. 2013, 164, 118–126. [Google Scholar] [CrossRef]
- Landén, R.; Heierson, A.; Boman, H.G. A phage for generalized transduction in Bacillus thuringiensis and mapping of four genes for antibiotic resistance. J. Gen. Microbiol. 1981, 123, 49–59. [Google Scholar]
- Heierson, A.; Landén, R.; Boman, H. Transductional mapping of nine linked chromosomal genes in Bacillus thuringiensis. Mol. Gen. Genet. 1983, 192, 118–123. [Google Scholar]
- Jones, D.R.; Karunakaran, V.; Burges, H.D. Phages naturally associated with the aizawai variety of insect pathogen Bacillus thuringiensis and their relevance to strain identification. J. Appl. Microbiol. 1983, 54, 373–377. [Google Scholar]
- Inal, J.R.M.; Karunakaran, V.; Burges, H.D. Isolation and propagation of phages naturally associated with the aizawai variety of Bacillus thuringiensis. J. Appl. Microbiol. 1990, 68, 17–21. [Google Scholar]
- Inal, J.R.M.; Karunakaran, V.; Burges, H.D. Generalized transduction in Bacillus thuringiensis var. aizawai. J. Appl. Microbiol. 1992, 72, 87–90. [Google Scholar]
- Ruhfel, R.E.; Robillard, N.J.; Thorne, C.B. Interspecies transduction of plasmids among Bacillus anthracis, B. cereus, and B. thuringiensis. J. Bacteriol. 1984, 157, 708–711. [Google Scholar]
- Walter, T.M.; Aronson, A.I. Transduction of certain genes by an autonomously replicating Bacillus thuringiensis phage. Appl. Environ. Microbiol. 1991, 57, 1000–1005. [Google Scholar]
- Loessner, M.J.; Maier, S.K.; Daubek-Puza, H.; Wendlinger, G.; Scherer, S. Three Bacillus cereus bacteriophage endolysins are unrelated but reveal high homology to cell wall hydrolases from different bacilli. J. Bacteriol. 1997, 179, 2845–2851. [Google Scholar]
- Porter, C.J.; Schuch, R.; Pelzek, A.J.; Buckle, A.M.; McGowan, S.; Wilce, M.C.J.; Rossjohn, J.; Russell, R.; Nelson, D.; Fischetti, V.A.; et al. The 1.6 Å crystal structure of the catalytic domain of PlyB, a bacteriophage lysin active against Bacillus anthracis. J. Mol. Biol. 2007, 366, 540–550. [Google Scholar] [CrossRef]
- McCloy, E.W. Studies on a lysogenic Bacillus strain. I. A bacteriophage specific for Bacillus anthracis. J. Hyg. (Lond.) 1951, 49, 114–125. [Google Scholar] [CrossRef]
- Mccloy, E.W. Lysogenicity and Immunity to Bacillus Phage W. J. Gen. Microbiol. 1958, 18, 198–220. [Google Scholar]
- Gaal, V.; Ivanovics, G. A cryptic prophage carried by Bacillus cereus. Acta Microbiol. Acad. Sci. Hung. 1973, 20, 209–219. [Google Scholar]
- Ivánovics, G.; Gaál, V.; Prágai, B. Lysogenic conversion to phospholipase a production in Bacillus cereus. J. Gen. Virol. 1974, 24, 349–358. [Google Scholar] [CrossRef]
- Thomas, J.A.; Hardies, S.C.; Rolando, M.; Hayes, S.J.; Lieman, K.; Carroll, C.A.; Weintraub, S.T.; Serwer, P. Complete genomic sequence and mass spectrometric analysis of highly diverse, atypical Bacillus thuringiensis phage 0305ϕ8–36. Virology 2007, 368, 405–421. [Google Scholar] [CrossRef]
- Lee, J.-H.; Shin, H.; Son, B.; Heu, S.; Ryu, S. Characterization and complete genome sequence of a virulent bacteriophage B4 infecting food-borne pathogenic Bacillus cereus. Arch. Virol. 2013, 158, 2101–2108. [Google Scholar] [CrossRef]
- Lee, J.-H.; Shin, H.; Son, B.; Ryu, S. Complete genome sequence of Bacillus cereus bacteriophage BCP78. J. Virol. 2012, 86, 637–638. [Google Scholar]
- Ting, J.H.; Smyth, T.B.; Chamakura, K.R.; Kuty Everett, G.F. Complete genome of Bacillus thuringiensis myophage BigBertha. Genome Announc. 2013, 1, e00853–e00813. [Google Scholar]
- Park, J.; Yun, J.; Lim, J.-A.; Kang, D.-H.; Ryu, S. Characterization of an endolysin, LysBPS13, from a Bacillus cereus bacteriophage. FEMS Microbiol. Lett. 2012, 332, 76–83. [Google Scholar] [CrossRef]
- Shin, H.; Lee, J.-H.; Park, J.; Heu, S.; Ryu, S. Characterization and genome analysis of the Bacillus cereus-infecting bacteriophages BPS10C and BPS13. Arch. Virol. 2014, in press. [Google Scholar]
- Grose, J.H.; Jensen, J.D.; Merrill, B.D.; Fisher, J.N.B.; Burnett, S.H.; Breakwell, D.P. Genome sequences of three novel Bacillus cereus bacteriophages. Genome Announc. 2014, 2, e01118–e01113. [Google Scholar]
- Maroun, J.W.; Whitcher, K.J.; Chamakura, K.R.; Kuty Everett, G.F. Complete genome of Bacillus thuringiensis myophage Spock. Genome Announc. 2013, 1, e00863–e00813. [Google Scholar]
- The Bacillus phage database. Available online: http://bacillus.phagesdb.org/ (accessed on 11 February 2014).
- Lee, Y.D.; Park, J.H. Genome organization of temperate phage 11143 from emetic Bacillus cereus NCTC11143. J. Microbiol. Biotechnol. 2012, 22, 649–653. [Google Scholar] [CrossRef]
- Lee, Y.-D.; Park, J.-H. Genomic sequence of temperate phage 250 isolated from emetic B. cereus and cloning of putative endolysin. Food Sci. Biotechnol. 2010, 19, 1643–1648. [Google Scholar] [CrossRef]
- Swanson, M.M.; Reavy, B.; Makarova, K.S.; Cock, P.J.; Hopkins, D.W.; Torrance, L.; Koonin, E.V.; Taliansky, M. Novel bacteriophages containing a genome of another bacteriophage within their genomes. PLoS One 2012, 7, e40683. [Google Scholar]
- Dong, Z.; Peng, D.; Wang, Y.; Zhu, L.; Ruan, L.; Sun, M. Complete genome sequence of Bacillus thuringiensis bacteriophage BMBtp2. Genome Announc. 2013, 1, e00011–e00012. [Google Scholar]
- Yuan, Y.; Gao, M.; Wu, D.; Liu, P.; Wu, Y. Genome characteristics of a novel phage from Bacillus thuringiensis showing high similarity with phage from Bacillus cereus. PLoS One 2012, 7, e37557. [Google Scholar] [CrossRef]
- Fouts, D.E.; Rasko, D.A.; Cer, R.Z.; Jiang, L.; Fedorova, N.B.; Shvartsbeyn, A.; Vamathevan, J.J.; Tallon, L.; Althoff, R.; Arbogast, T.S.; et al. Sequencing Bacillus anthracis typing phages Gamma and Cherry reveals a common ancestry. J. Bacteriol. 2006, 188, 3402–3408. [Google Scholar] [CrossRef]
- Minakhin, L.; Semenova, E.; Liu, J.; Vasilov, A.; Severinova, E.; Gabisonia, T.; Inman, R.; Mushegian, A.; Severinov, K. Genome sequence and gene expression of Bacillus anthracis bacteriophage Fah. J. Mol. Biol. 2005, 354, 1–15. [Google Scholar] [CrossRef]
- Schuch, R.; Fischetti, V.A. Detailed genomic analysis of the Wβ and γ phages infecting Bacillus anthracis: Implications for evolution of environmental fitness and antibiotic resistance. J. Bacteriol. 2006, 188, 3037–3051. [Google Scholar] [CrossRef]
- Liao, W.; Song, S.; Sun, F.; Jia, Y.; Zeng, W.; Pang, Y. Isolation, characterization and genome sequencing of phage MZTP02 from Bacillus thuringiensis MZ1. Arch. Virol. 2008, 153, 1855–1865. [Google Scholar] [CrossRef]
- Kong, M.; Kim, M.; Ryu, S. Complete genome sequence of Bacillus cereus bacteriophage PBC1. J. Virol. 2012, 86, 6379–6380. [Google Scholar] [CrossRef]
- Moumen, B.; Nguen-The, C.; Sorokin, A. Sequence analysis of inducible prophage phIS3501 integrated into the haemolysin II gene of Bacillus thuringiensis var israelensis ATCC35646. Genet. Res. Int. 2012, 2012, 543286. [Google Scholar]
- Yuan, Y.; Gao, M.; Peng, Q.; Wu, D.; Liu, P.; Wu, Y. Genomic analysis of a phage and prophage from a Bacillus thuringiensis strain. J. Gen. Virol. 2014, 95, 751–761. [Google Scholar] [CrossRef]
- Ganz, H.H.; Law, C.; Schmuki, M.; Eichenseher, F.; Calendar, R.; Loessner, M.J.; Getz, W.M.; Korlach, J.; Beyer, W.; Klumpp, J. Novel giant siphovirus from Bacillus anthracis features unusual genome characteristics. PLoS One 2014, 9, e85972. [Google Scholar] [CrossRef]
- Smeesters, P.R.; Drèze, P.A.; Bousbata, S.; Parikka, K.J.; Timmery, S.; Hu, X.; Perez-Morga, D.; Deghorain, M.; Toussaint, A.; Mahillon, J.; et al. Characterization of a novel temperate phage originating from a cereulide-producing Bacillus cereus strain. Res. Microbiol. 2011, 162, 446–459. [Google Scholar] [CrossRef]
- Redondo, R.A.F.; Kupczok, A.; Stift, G.; Bollback, J.P. Complete genome sequence of the novel phage MG-B1 Infecting Bacillus weihenstephanensis. Genome Announc. 2013, 1, e00216–e00213. [Google Scholar]
- Sozhamannan, S.; McKinstry, M.; Lentz, S.M.; Jalasvuori, M.; McAfee, F.; Smith, A.; Dabbs, J.; Ackermann, H.-W.; Bamford, J.K.H.; Mateczun, A.; et al. Molecular characterization of a variant of Bacillus anthracis-specific phage AP50 with improved bacteriolytic activity. Appl. Environ. Microbiol. 2008, 74, 6792–6796. [Google Scholar] [CrossRef]
- Strömsten, N.J.; Benson, S.D.; Burnett, R.M.; Bamford, D.H.; Bamford, J.K. The Bacillus thuringiensis linear double-stranded DNA phage Bam35, which is highly similar to the Bacillus cereus linear plasmid pBClin15, has a prophage state. J. Bacteriol. 2003, 185, 6985–6989. [Google Scholar] [CrossRef]
- Verheust, C.; Jensen, G.; Mahillon, J. pGIL01, a linear tectiviral plasmid prophage originating from Bacillus thuringiensis serovar israelensis. Microbiology 2003, 149, 2083–2092. [Google Scholar] [CrossRef]
- Verheust, C.; Fornelos, N.; Mahillon, J. GIL16, a new Gram-positive tectiviral phage related to the Bacillus thuringiensis GIL01 and the Bacillus cereus pBClin15 elements. J. Bacteriol. 2005, 187, 1966–1973. [Google Scholar] [CrossRef]
- Kan, S.; Fornelos, N.; Schuch, R.; Fischetti, V.A. Identification of a ligand on the Wip1 bacteriophage highly specific for a receptor on Bacillus anthracis. J. Bacteriol. 2013, 195, 4355–4364. [Google Scholar] [CrossRef]
- Ackermann, H.W.; Azizbekyan, R.R.; Emadi Konjin, H.P.; Lecadet, M.M.; Seldin, L.; Yu, M.X. New Bacillus bacteriophage species. Arch. Virol. 1994, 135, 333–344. [Google Scholar] [CrossRef]
- Thorne, C.B. Transduction in Bacillus cereus and Bacillus anthracis. Bacteriol. Rev. 1968, 32, 358–361. [Google Scholar]
- Griffiths, A.J.F.; Milller, J.H.; Suzuki, D.T.; Lewontin, R.C.; Gelbart, W.M. An Introduction to Genetic Analysis, 7th ed.; W.H. Freeman: New York, NY, USA, 2000; pp. 1–860. [Google Scholar]
- Altenbern, R.A.; Stull, H.B. Inducible lytic systems in the genus Bacillus. J. Gen. Microbiol. 1965, 39, 53–62. [Google Scholar] [CrossRef]
- Altenbern, R.A.; Stull, H.B. Edema factor and phospholipase release by a strain of Bacillus cereus. Can. J. Microbiol. 1964, 10, 717–725. [Google Scholar] [CrossRef]
- Thorne, C.B.; Holt, S.C. Cold lability of Bacillus cereus bacteriophage CP-51. J. Virol. 1974, 14, 1008–1012. [Google Scholar]
- Green, B.D.; Battisti, L.; Koehler, T.M.; Thorne, C.B.; Ivins, B.E. Demonstration of a capsule plasmid in Bacillus anthracis. Infect. Immun. 1985, 49, 291–297. [Google Scholar]
- Cohen, A.; Ben-Ze'Ev, H.; Yashouv, J. Outgrowth of Bacillus cereus spores harboring bacteriophage CP-51 DNA I. Initiation of bacteriophage development. J. Virol. 1973, 11, 648–654. [Google Scholar]
- Van Tassell, R.L.; Yousten, A.A. Response of Bacillus thuringiensis to bacteriophage CP-51. Can. J. Microbiol. 1976, 22, 583–586. [Google Scholar] [CrossRef]
- Clements, M.O.; Moir, A. Role of the gerI operon of Bacillus cereus 569 in the response of spores to germinants. J. Bacteriol. 1998, 180, 6729–6735. [Google Scholar]
- Lecadet, M.M.; Chaufaux, J.; Ribier, J.; Lereclus, D. Construction of novel Bacillus thuringiensis strains with different insecticidal activities by transduction and transformation. Appl. Environ. Microbiol. 1992, 58, 840–849. [Google Scholar]
- Koretskaia, N.G.; Svetoch, O.; Khachaturian, S.V.; Dobrina, A.P. Transduction ability of mutants of phage CP51, virulent for bacteria of the Bacillus cereus group. Genetika 1989, 25, 1013–1020. [Google Scholar]
- Stepanov, A.S.; Gavrilov, S.V.; Puzanova, O.B.; Grigor’eva, T.M.; Azizbekian, R.R. Plasmid transduction by Bacillus anthracis bacteriophage CP54. Mol. Gen. Mikrobiol. Virusol. 1989, 14–19. [Google Scholar]
- Klumpp, J.; Fouts, D.E.; Sozhamannan, S. Next generation sequencing technologies and the changing landscape of phage genomics. Bacteriophage 2012, 2, 190–199. [Google Scholar] [CrossRef]
- Tyeryar, F.J., Jr.; Taylor, M.J.; Lawton, W.D.; Goldberg, I.D. Cotransduction and cotransformation of genetic markers in Bacillus subtilis and Bacillus licheniformis. J. Bacteriol. 1969, 100, 1027–1036. [Google Scholar]
- Inal, J.M.; Karunakaran, V.; Jones, D.R. Bacillus thuringiensis subsp. aizawai generalized transducing phage ϕHD248: Restriction site map and potential for fine-structure chromosomal mapping. Microbiology 1996, 142, 1409–1416. [Google Scholar] [CrossRef]
- Ruhfel, R.; Thorne, C. Physical and Genetic Characterisation of the Bacillus thuringiensis subsp. kurstaki HD-1 extrachromosomal temperate phage TP-21. In Proceedings of the 88th Annual Meeting of the American Social Microbiology Abstracts, Washington, DC, USA, 8–13 May 1988; American Society for Microbiology: Washington, DC, USA.
- Sorokin, A. Bacillus thuringiensis genetics and phages—From transduction and sequencing to recombineering. In Bacillus Thuringiensis Biotechnology; Sansinenea, E., Ed.; Springer The Netherlands: Dordrecht, The Netherlands, 2012; pp. 131–157. [Google Scholar]
- Stenfors Arnesen, L.P.; Fagerlund, A.; Granum, P.E. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 2008, 32, 579–606. [Google Scholar] [CrossRef]
- Canchaya, C.; Proux, C.; Fournous, G.; Bruttin, A.; Brüssow, H. Prophage genomics. Microbiol. Mol. Biol. Rev. 2003, 67, 238–276. [Google Scholar] [CrossRef]
- Dwyer, K.; Lamonica, J.; Schumacher, J.; Williams, L.; Bishara, J.; Lewandowski, A.; Redkar, R.; Patra, G.; DelVecchio, V. Identification of Bacillus anthracis specific chromosomal sequences by suppressive subtractive hybridization. BMC Genomics 2004, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Klee, S.R.; Nattermann, H.; Becker, S.; Urban-Schriefer, M.; Franz, T.; Jacob, D.; Appel, B. Evaluation of different methods to discriminate Bacillus anthracis from other bacteria of the Bacillus cereus group. J. Appl. Microbiol. 2006, 100, 673–681. [Google Scholar] [CrossRef]
- Rasko, D.A.; Altherr, M.R.; Han, C.S.; Ravel, J. Genomics of the Bacillus cereus group of organisms. FEMS Microbiol. Rev. 2005, 29, 303–329. [Google Scholar]
- Tourasse, N.J.; Kolstø, A.-B. Survey of group I and group II introns in 29 sequenced genomes of the Bacillus cereus group: Insights into their spread and evolution. Nucleic Acids Res. 2008, 36, 4529–4548. [Google Scholar] [CrossRef]
- Salvetti, S.; Faegri, K.; Ghelardi, E.; Kolsto, A.B.; Senesi, S. Global gene expression profile for swarming Bacillus cereus bacteria. Appl. Environ. Microbiol. 2011, 77, 5149–5156. [Google Scholar] [CrossRef]
- Gillis, A.; Dupres, V.; Delestrait, G.; Mahillon, J.; Dufrene, Y.F. Nanoscale imaging of Bacillus thuringiensis flagella using atomic force microscopy. Nanoscale 2012, 4, 1585–1591. [Google Scholar] [CrossRef]
- Gillis, A.; Dupres, V.; Mahillon, J.; Dufrene, Y.F. Atomic force microscopy: A powerful tool for studying bacterial swarming motility. Micron 2012, 43, 1304–1311. [Google Scholar] [CrossRef]
- Wang, J.; Steggles, J.R.; Ellar, D.J. Molecular characterization of virulence defects in Bacillus thuringiensis mutants. FEMS Microbiol. Lett. 2008, 280, 127–134. [Google Scholar] [CrossRef]
- Mencia, M.; Gella, P.; Camacho, A.; de Vega, M.; Salas, M. Terminal protein-primed amplification of heterologous DNA with a minimal replication system based on phage Phi29. Proc. Natl. Acad. Sci. USA 2011, 108, 18655–18660. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Svensson, B.; Guinebretiere, M.H.; Lindback, T.; Andersson, M.; Schulz, A.; Fricker, M.; Christiansson, A.; Granum, P.E.; Martlbauer, E.; et al. Emetic toxin formation of Bacillus cereus is restricted to a single evolutionary lineage of closely related strains. Microbiology 2005, 151, 183–197. [Google Scholar] [CrossRef]
- Reynolds, R.B.; Reddy, A.; Thorne, C.B. Five unique temperate phages from a polylysogenic strain of Bacillus thuringiensis subsp. aizawai. J. Gen. Microbiol. 1988, 134, 1577–1585. [Google Scholar]
- Azizbekian, K.R.; Shamshina, T.N.; Dobrzhanskaia, E.O. Morphologic characterization and protein analysis of phages isolated from type strains of Bacillus thuringiensis. Mikrobiologiya 1997, 66, 242–246. [Google Scholar]
- Schuch, R.; Nelson, D.; Fischetti, V.A. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature 2002, 418, 884–889. [Google Scholar] [CrossRef]
- Davison, S.; Couture-Tosi, E.; Candela, T.; Mock, M.; Fouet, A. Identification of the Bacillus anthracis Gamma phage receptor. J. Bacteriol. 2005, 187, 6742–6749. [Google Scholar] [CrossRef]
- Brown, E.R.; Cherry, W.B. Specific identification of Bacillus anthracis by means of a variant bacteriophage. J. Infect. Dis. 1955, 96, 34–39. [Google Scholar] [CrossRef]
- Meynell, E.W. Reverting and non-reverting rough variants of Bacillus anthracis. J. Gen. Microbiol. 1963, 32, 55–60. [Google Scholar] [CrossRef]
- Watanabe, T.; Morimoto, A.; Shiomi, T. The fine structure and the protein composition of gamma phage of Bacillus anthracis. Can. J. Microbiol. 1975, 21, 1889–1892. [Google Scholar]
- Fulmer, P.A. Susceptibility of Bacillus Anthracis to Gamma and Cherry Bacteriophage; Louisiana State University: Baton Rouge, LA, USA, 2003. [Google Scholar]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A genome comparison visualizer. Bioinformatics 2011, 27, 1009–1010. [Google Scholar]
- Casjens, S. Prophages and bacterial genomics: What have we learned so far? Mol. Microbiol. 2003, 49, 277–300. [Google Scholar]
- Aronson, A.I.; Beckman, W. Transfer of chromosomal genes and plasmids in Bacillus thuringiensis. Appl. Environ. Microbiol. 1987, 53, 1525–1530. [Google Scholar]
- Mahillon, J.; Seurinck, J.; van Rompuy, L.; Delcour, J.; Zabeau, M. Nucleotide sequence and structural organization of an insertion sequence element (IS231) from Bacillus thuringiensis strain berliner 1715. EMBO J. 1985, 4, 3895–3899. [Google Scholar]
- Casjens, S.R.; Gilcrease, E.B.; Winn-Stapley, D.A.; Schicklmaier, P.; Schmieger, H.; Pedulla, M.L.; Ford, M.E.; Houtz, J.M.; Hatfull, G.F.; Hendrix, R.W. The generalized transducing Salmonella bacteriophage ES18: Complete genome sequence and DNA packaging strategy. J. Bacteriol. 2005, 187, 1091–1104. [Google Scholar]
- Kanda, K.; Kitajima, Y.; Moriyama, Y.; Kato, F.; Murata, A. Association of plasmid integrative J7W-1 prophage with Bacillus thuringiensis strains. Acta Virol. (Praha) 1998, 42, 315–318. [Google Scholar]
- Tam, A.; Fitz-James, P. Plasmids associated with a phagelike particle and with a satellite inclusion in Bacillus thuringiensis ssp. israelensis. Can. J. Microbiol. 1986, 32, 382–388. [Google Scholar] [CrossRef]
- Oksanen, H.M.; Bamford, D.H. Family Tectiviridae. In Virus Taxonomy. Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Academic Press: London, UK, 2012; pp. 317–321. [Google Scholar]
- Caldentey, J.; Blanco, L.; Bamford, D.H.; Salas, M. In vitro replication of bacteriophage PRD1 DNA. Characterization of the protein-primed initiation site. Nucleic Acids Res. 1993, 21, 3725–3730. [Google Scholar] [CrossRef]
- Schuch, R.; Fischetti, V.A. The secret life of the anthrax agent Bacillus anthracis: Bacteriophage-mediated ecological adaptations. PLoS One 2009, 4, e6532. [Google Scholar] [CrossRef]
- Nagy, E.; Pragai, B.; Ivanovics, G. Characteristics of phage AP50, an RNA phage containing phospholipids. J. Gen. Virol. 1976, 32, 129–132. [Google Scholar] [CrossRef]
- Saren, A.-M.; Ravantti, J.J.; Benson, S.D.; Burnett, R.M.; Paulin, L.; Bamford, D.H.; Bamford, J.K.H. A snapshot of viral evolution from genome analysis of the Tectiviridae family. J. Mol. Biol. 2005, 350, 427–440. [Google Scholar] [CrossRef]
- Schuch, R.; Pelzek, A.J.; Kan, S.; Fischetti, V.A. Prevalence of Bacillus anthracis-like organisms and bacteriophages in the intestinal tract of the earthworm Eisenia fetida. Appl. Environ. Microbiol. 2010, 76, 2286–2294. [Google Scholar] [CrossRef]
- Yu, M.X.; Slater, M.R.; Ackermann, H.W. Isolation and characterization of Thermus bacteriophages. Arch. Virol. 2006, 151, 663–679. [Google Scholar] [CrossRef]
- Nagy, E. A highly specific phage attacking Bacillus anthracis strain Sterne. Acta Microbiol. Acad. Sci. Hung. 1974, 21, 257–263. [Google Scholar]
- Ackermann, H.W.; Roy, R.; Martin, M.; Murthy, M.R.; Smirnoff, W.A. Partial characterization of a cubic Bacillus phage. Can. J. Microbiol. 1978, 24, 986–993. [Google Scholar] [CrossRef]
- Gillis, A.; Mahillon, J. Prevalence, genetic diversity and host range of tectiviruses among members of the Bacillus cereus group. Appl. Environ. Microbiol. 2014, 80, 4138–4152. [Google Scholar] [CrossRef]
- Hendrix, R.W. Jumbo bacteriophages. Curr. Top. Microbiol. Immunol. 2009, 328, 229–240. [Google Scholar]
- Van Etten, J.L.; Lane, L.C.; Dunigan, D.D. DNA viruses: The really big ones (giruses). Annu. Rev. Microbiol. 2010, 64, 83–99. [Google Scholar] [CrossRef]
- Pathria, S.; Rolando, M.; Lieman, K.; Hayes, S.; Hardies, S.; Serwer, P. Islands of non-essential genes, including a DNA translocation operon, in the genome of bacteriophage 0305ϕ8–36. Bacteriophage 2012, 2, 25–35. [Google Scholar] [CrossRef]
- Serwer, P.; Hayes, S.J.; Lieman, K. Aggregates of bacteriophage 0305phi8–36 seed future growth. Virol. J. 2007, 4, 131. [Google Scholar] [CrossRef]
- Serwer, P.; Hayes, S.J.; Thomas, J.A.; Hardies, S.C. Propagating the missing bacteriophages: A large bacteriophage in a new class. Virol. J. 2007, 4, 21. [Google Scholar] [CrossRef]
- Hardies, S.C.; Thomas, J.A.; Serwer, P. Comparative genomics of Bacillus thuringiensis phage 0305phi8–36: Defining patterns of descent in a novel ancient phage lineage. Virol. J. 2007, 4, 97. [Google Scholar] [CrossRef]
- Serwer, P.; Hayes, S.; Thomas, J.; Demeler, B.; Hardies, S. Isolation of novel large and aggregating bacteriophages. In Bacteriophages. Vol. 1: Isolation, characterization, and interactions; Clokie, M.J., Kropinski, A., Eds.; Humana Press: Totowa, NJ, USA, 2009; Volume 501, pp. 55–66. [Google Scholar]
- Serwer, P.; Hayes, S.J.; Lieman, K.; Griess, G.A. In situ fluorescence microscopy of bacteriophage aggregates. J. Microsc. 2007, 228, 309–321. [Google Scholar] [CrossRef]
- Bailly-Bechet, M.; Vergassola, M.; Rocha, E. Causes for the intriguing presence of tRNAs in phages. Genome Res. 2007, 17, 1486–1495. [Google Scholar] [CrossRef]
- Son, B.; Yun, J.; Lim, J.A.; Shin, H.; Heu, S.; Ryu, S. Characterization of LysB4, an endolysin from the Bacillus cereus-infecting bacteriophage B4. BMC Microbiol. 2012, 12, 33. [Google Scholar] [CrossRef]
- Lewis, I.M.; Worley, G. Bacteriophagy of Bacillus mycoides with reference to effect on dissociation and transmission by spores. J. Bacteriol. 1936, 32, 195–198. [Google Scholar]
- Baer, B.S.; Krueger, A.P. The B. mycoides N host-virus system. I. Differences in appearance and “rate of growth” of the lysogenic and parent indicator strains. J. Gen. Physiol. 1952, 35, 857–864. [Google Scholar] [CrossRef]
- Baer, B.S.; Krueger, A.P. The B. mycoides N host-virus system. III. Release of phage from the lysogenic strain. J. Gen. Physiol 1952, 36, 127–138. [Google Scholar] [CrossRef]
- Baer, B.S.; Krueger, A.P. The B. mycoides N host-virus system. II. Interrelation of phage growth, bacterial multiplication, and lysis in infections of the indicator strain of B. mycoides N with phage N in nutrient broth. J. Gen. Physiol. 1952, 36, 111–125. [Google Scholar] [CrossRef]
- Tikhonenko, A.S.; Belyaeva, N.N.; Kretova, A.F. Bacillus mycoides phages and their satellites. Can. J. Microbiol. 1984, 30, 691–698. [Google Scholar] [CrossRef]
- Stiube, P.; Dimitriu, C. Electron microscope studies on cereus-specific bacteriophages. Arch. Roum. Pathol. Exp. Microbiol. 1969, 28, 794–801. [Google Scholar]
- Edintsov, I.M.; Ivanitskii, G.R.; Kuniskii, A.S. 3-dimensional reconstruction of the extended tail of bacteriophage H17 of Bacillus mycoides. Dokl. Akad. Nauk SSSR 1975, 224, 704–706. [Google Scholar]
- Stamatin, N.; Mintzer-Morgenstern, L. Action of streptomycin on various phages CAM (cereus-anthracis-mycoides). Arch. Roum. Pathol. Exp. Microbiol. 1966, 25, 378–383. [Google Scholar]
- Stamatin, N.; Hadarag, E.; Mintzer-Morgenstern, L. Particular sensitivity to heat of some cereus-anthracis-mycoides phage trains. Arch. Roum. Pathol. Exp. Microbiol. 1963, 23, 637–642. [Google Scholar]
- Stamatin, N. The temperatures of inactivation and multiplication of cereus-anthracis-mycoides phages. Ann. Inst. Pasteur (Paris) 1963, 105, 515–523. [Google Scholar]
- Tikhonenko, A.S.; Beliaeva, N.N. Changes in protein structures of Bacillus mycoides No. 1 and H 19 phages in the process of heating. Mikrobiologiia 1967, 36, 475–481. [Google Scholar]
- Toschkov, A.; Toschkoff, A.; Valerianov, Z.; Abraschev, I. Effect of various antibiotics on the replication of bacteriophages of group CAM (cereus anthracis mycoides). Arch. Exp. Veterinarmed. 1970, 24, 213–217. [Google Scholar]
- Tikhonenko, A.S. Defective morphogenesis of the Bacillus mycoides phage No. 1 head. Mikrobiologiia 1966, 35, 118–121. [Google Scholar]
- Coman, I.; Stiube, P.; Dimitriu, C. Defective bacteriophages in Bacillus mycoides. Arch. Roum. Pathol. Exp. Microbiol. 1969, 28, 857–866. [Google Scholar]
- Tikhonenko, A.S.; Bespalova, I.A. Phage maturation in Bacillus mycoides cells. Virology 1964, 23, 259–267. [Google Scholar] [CrossRef]
- Lechner, S.; Mayr, R.; Francis, K.P.; Pruss, B.M.; Kaplan, T.; Wiessner-Gunkel, E.; Stewart, G.S.; Scherer, S. Bacillus weihenstephanensis sp. nov. is a new psychrotolerant species of the Bacillus cereus group. Int. J. Syst. Bacteriol. 1998, 48, 1373–1382. [Google Scholar] [CrossRef]
- Lapidus, A.; Goltsman, E.; Auger, S.; Galleron, N.; Segurens, B.; Dossat, C.; Land, M.L.; Broussolle, V.; Brillard, J.; Guinebretiere, M.H.; et al. Extending the Bacillus cereus group genomics to putative food-borne pathogens of different toxicity. Chem. Biol. Interact. 2008, 171, 236–249. [Google Scholar] [CrossRef]
- Auger, S.; Galleron, N.; Bidnenko, E.; Ehrlich, S.D.; Lapidus, A.; Sorokin, A. The genetically remote pathogenic strain NVH391-98 of the Bacillus cereus group is representative of a cluster of thermophilic strains. Appl. Environ. Microbiol. 2008, 74, 1276–1280. [Google Scholar] [CrossRef]
- Abshire, T.G.; Brown, J.E.; Ezzell, J.W. Production and validation of the use of Gamma phage for identification of Bacillus anthracis. J. Clin. Microbiol. 2005, 43, 4780–4788. [Google Scholar] [CrossRef]
- Endersen, L.; O’Mahony, J.; Hill, C.; Ross, R.P.; McAuliffe, O.; Coffey, A. Phage therapy in the food industry. Annu. Rev. Food Sci. Technol. 2014, 5, 327–349. [Google Scholar] [CrossRef]
- Garcia, P.; Martinez, B.; Obeso, J.M.; Rodriguez, A. Bacteriophages and their application in food safety. Lett. Appl. Microbiol. 2008, 47, 479–485. [Google Scholar] [CrossRef]
- Fischetti, V.A. Bacteriophage lysins as effective antibacterials. Curr. Opin. Microbiol. 2008, 11, 393–400. [Google Scholar] [CrossRef]
- Verheust, C.; Fornelos, N.; Mahillon, J. The Bacillus thuringiensis phage GIL01 encodes two enzymes with peptidoglycan hydrolase activity. FEMS Microbiol. Lett. 2004, 237, 289–295. [Google Scholar]
- Verheust, C.; Pauwels, K.; Mahillon, J.; Helinski, D.; Herman, P. Contained use of bacteriophages: Risk assessment and biosafety recommendations. App. Biosaf. 2010, 15, 32–44. [Google Scholar]
- Klumpp, J.; Loessner, M.J. Listeria phages: Genomes, evolution, and application. Bacteriophage 2013, 3, e26861. [Google Scholar] [CrossRef]
- Huys, I.; Pirnay, J.-P.; Lavigne, R.; Jennes, S.; de Vos, D.; Casteels, M.; Verbeken, G. Paving a regulatory pathway for phage therapy. EMBO Rep. 2013, 14, 951–954. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gillis, A.; Mahillon, J. Phages Preying on Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis: Past, Present and Future. Viruses 2014, 6, 2623-2672. https://doi.org/10.3390/v6072623
Gillis A, Mahillon J. Phages Preying on Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis: Past, Present and Future. Viruses. 2014; 6(7):2623-2672. https://doi.org/10.3390/v6072623
Chicago/Turabian StyleGillis, Annika, and Jacques Mahillon. 2014. "Phages Preying on Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis: Past, Present and Future" Viruses 6, no. 7: 2623-2672. https://doi.org/10.3390/v6072623
APA StyleGillis, A., & Mahillon, J. (2014). Phages Preying on Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis: Past, Present and Future. Viruses, 6(7), 2623-2672. https://doi.org/10.3390/v6072623





