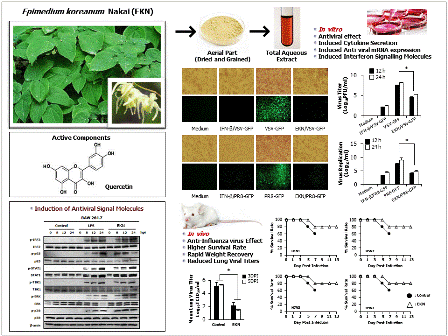
Epimedium koreanum Nakai Displays Broad Spectrum of Antiviral Activity in Vitro and in Vivo by Inducing Cellular Antiviral State
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and extract Preparation
2.2. Cells and Viruses
2.3. Determination of Effective Concentration (EC50) of Epimedium koreanum Nakai in Vitro
2.4. Determination of the Cytotoxic Concentration (CC50) of Epimedium koreanum Nakai in Vitro
2.6. NDV-GFP mRNA Expression and Virus Titration in RAW 264.7 Cells
2.7. Virus Titration of Treated Cell Supernatants and Infected Cells
2.8. Detection of IFN-β and Pro-Inflammatory Cytokines in Epimedium koreanum Nakai-Treated RAW264.7 and HEK293T Cells by Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Determination of the Level of mRNA Induction by Epimedium koreanum Nakai in Vitro by Real-Time PCR Analysis
2.10. Immunoblot Analysis to Determine the Effect of Epimedium koreanum Nakai on Type I IFN-Related Protein Phosphorylation in RAW264.7 Cells
2.11. Oral Inoculation of Epimedium koreanum Nakai and Viral Challenge in BALB/c Mice
2.12. Determination of Lung Viral Titer
2.13. Anti-Influenza Effect and Induction of Cytokines by Quercetin on RAW264.7 Cells
2.14. Statistical Analysis
2.15. Biodiversity Rights
3. Results and Discussion
3.1. Determination of the Effective Concentration (EC50) and Cytotoxic Concentration (CC50) of Epimedium koreanum Nakai in Vitro
| Cell line | EC50 ± S.D.a (µg/mL) | CC50 ± S.D.b (µg/mL) | |||
|---|---|---|---|---|---|
| PR8-GFP | VSV-GFP | NDV-GFP | HSV-GFP | ||
| Raw264.7 | 0.94±0.23 | 0.82±0.28 | 0.49±0.25 | 0.62±0.14 | 14.6±1.68 |
| HEK293T | - | 1.12±0.31 | - | 1.41±0.41 | 8.4±1.96 |
3.2. Inhibitory Effects of Epimedium koreanum Nakai on Viruses in RAW264.7 Cells
3.3. Inhibitory Effects of Epimedium koreanum Nakai on Viruses in HEK293T Cells


3.4. Detection of IFN-β and Pro-Inflammatory Cytokines by Epimedium koreanum Nakai in Vitro

3.5. Epimedium koreanum Nakai Induces the Activation of Signal Molecules in the Type I IFN Signaling Pathway
3.7. Protection Against Diverse Influenza A Virus Infection by Oral Administration of Epimedium koreanum Nakai in Balb/c Mice

3.8. Inhibitory Effect of Quercetin on Influenza Virus (PR8-GFP) and Induction of IFN-β or Pro-Inflammatory Cytokines in RAW264.7 Cells


3.9. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interests
References
- McCaughey, C. Influenza: A virus of our times. Ulst. Med. J. 2010, 79, 46–51. [Google Scholar]
- Morens, M.D.; Folkers, G.K.; Fauci, A.S. Perspective What Is a Pandemic; National Institute of Allergy and Infectious Diseases: National Institutes of Health, Bethesda, MD, USA, 2009; pp. 1533–1579. [Google Scholar]
- Brownlie, J.; Peckham, C.; Waage, J.; Woolhouse, M.; Lyall, C. Foresight. Infectious Diseases: Preparing for the future Threats; Office of Science and Innovation, Department of Trade and Industry: London, UK, 2006. Available online: https://www.gov.uk/government/uploads/system/uploads/attachmentdata/file/294243/06-760-infectious-diseases-report.pdf (accessed on 17 November 2014).
- Morens, D.M.; Fauci, A.S. Emerging Infectious Diseases: Threats to Human Health and Global Stability. PLoS Pathog. 2013, 9, e1003467. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, D.; Palombo, E.A.; Chia, Y.T.; Lim, S.L.D.; Lee, T.C. Identification of Traditional Medicinal Plant Extracts with Novel Anti-Influenza Activity. PLoS One 2013, 8, e79293. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, W.; Zhao, A. Anti-influenza agents from plants and traditional Chinese medicine. Phytother. Res. 2006, 20, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Uchide, N.; Toyoda, H. Future target molecules for influenza treatment. Mini. Rev. Med. Chem. 2008, 8, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Grienke, U.; Schmidtke, M.; Kirchmair, J.; Pfarr, K.; Wutzler, P. Antiviral Potential and Molecular Insight into Neuraminidase Inhibiting Diaryl heptanoids from Alpiniakatsumadai. J. Med. Chem. 2009, 53, 778–786. [Google Scholar] [CrossRef]
- Ison, M.G. Antivirals and resistance: Influenza virus. Curr. Opin. Virol. 2011, 1, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, A.; Eswariah, M.C. Herbalism—A Review. Inter. J. Photother. 2012, 2, 96–105. [Google Scholar]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbil. Rev. 1999, 12, 564–582. [Google Scholar]
- Chen, Y.; Zhao, Y.Z.; Jia, X.B.; Hu, M. Intestinal Absorption Mechanisms of Prenylated Flavonoids Present in the Heat-Processed Epimedium koreanum Nakai. Pharm. Res. 2008, 25, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; He, X.; Yang, Y.; Li, M.; Hao, D. The genus Epimedium: An ethno pharmacological and photochemical review. J. Ethnopharmacol. 2011, 134, 519–541. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.K.; Kim, H.; Choi, Y.J.; Yim, N.H.; Ma, J.Y. Epimedium koreanum Nakai Water Extract Exhibits Antiviral Activity against Porcine Epidermic Diarrhea Virus In Vitro and In Vivo. Evid. Based Complement. Altern. Med. 2008. [Google Scholar] [CrossRef]
- Pachaly, P.; Weibarth, C.S.; Sin, K.S. New Prenyl flavonoid Glycosides from Epimedium koreanum. Planta Med. 1990, 56, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Zhao, X.L.; Liu, Z.Q.; Xing, J.P. Isolation and Extraction of Total Flavonoids from Epimedium Koreanum Nakai by Supercritical Fluid Extraction. Chem. Res. Chin. Univ. 2004, 20, 707–710. [Google Scholar]
- Wang, T.; Zhang, J.C.; Chen, Y.; Huang, F.; Yang, M.S. Comparison of anti-oxidative and antitumor activities of six flavonoids from Epimedium koreanum. J. Chin. Mater. Med. 2007, 32, 715–718. [Google Scholar]
- Zhang, W.; Chen, H.; Wang, Z.; Lan, G.; Zhang, L. Comparative studies on antioxidant activities of extracts and fractions from the leaves and stem of Epimedium koreanum Nakai. Int. J. Food Sci. Technol. 2011, 50, 1122–1129. [Google Scholar] [CrossRef]
- Ou, X.; Li, W. Effect on enhancing physical strength and anti-stress activity of flavonoids from the Chinese medicinal plant Epimedium koreanum Nakai. Sci. Res. Essays 2010, 5, 883–886. [Google Scholar]
- Tohda, C.; Nagata, A. Epimedium koreanum Extract and Its Constituent Icariin Improve Motor Dysfunction in Spinal Cord Injury. Evid. Based Complement. Altern. Med. 2012. [Google Scholar] [CrossRef]
- Strober, W. Trypan blue exclusion test for cell viability. Curr. Protoc. Cytom. 2001, 64, 9–2. [Google Scholar]
- Moon, H.J.; Lee, J.S.; Choi, Y.K.; Park, J.Y.; Talactac, M.R. Induction of Type I interferon by high-molecular poly-gamma-glutamate protects B6. A2G-Mx1 mice against influenza A virus. Antivir. Res. 2012, 94, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.M.; Kwon, J.H.; Lee, S.; Kong, H.S.; Lee, S.J.; Lee, C.K.; Cho, K.H.; Ha, N.J.; Kim, K.J. Immunostimulatory effects of Cordyceps militaris on macrophages through enhanced production of cytokines via the activation of NF-KB. Immune Netw. 2010, 10, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Seal, B.S.; King, D.J.; Bennett, J.D. Characterization of Newcastle Disease Virus Isolates by Reverse Transcription PCR Coupled to Direct Nucleotide Sequencing and Development of Sequence Database for Pathotype Prediction and Molecular Epidemiological Analysis. J. Clin. Microbiol. 1995, 33, 2624–2630. [Google Scholar] [PubMed]
- Coil, D.A.; Miller, A.D. Phosphatidylserine is not the cell surface receptor for Vesicular Stomatitis Virus. J. Virol. 2004, 78, 10920–10926. [Google Scholar] [CrossRef] [PubMed]
- Wadsworth, T.L.; Koop, D.R. Effects of the wine polyphenol icsquercetin and resveratrol on pro-inflammatory cytokine expression in RAW 264.7 macrophages. Inflamm. Immunopharmacol. 1999, 8, 941–949. [Google Scholar]
- Quan, F.S.; Compans, R.W.; Huang, C.; Kang, S.M. Virus Like ParticleVaccine Induces Protective Immunity against Homologous and Heterologous Strains of Influenza Virus. J. Virol. 2007, 81, 3514–3524. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Lin, Y.; Du, L.; Guan, J.; Sun, S.; Sui, H.; Kou, Z.; Chan, C.C.; Guo, Y.; Jiang, S.; Zheng, B.J.; Zhou, Y. An M2e-based multiple antigenic peptide vaccine protects mice from lethal challenge with divergent H5N1 influenza viruses. Virol. J. 2010, 7, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Magadula, J.J.; Suleimania, H.O. Cytotoxic and anti-HIV activities of some Tanzanian Garcinia species. Tanzan. J. Health Res. 2010, 12, 24–28. [Google Scholar] [CrossRef]
- Lin, Xu.; Su, W.; Jin, J.; Chen, J.; Li, X.; Zhang, X.; Sun, M.; Sun, S.; Fan, P.; An, D.; et al. Identification of Luteolin as Enterovirus 71 and Coxsackievirus A16 Inhibitors through Reporter viruses and Cell Viability-Based Screening. Viruses 2014, 6, 2778–2795. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; Zhou, S.F.; Gao, S.H.; Yu, Z.L.; Ko, K.M. New Perspectives on How to Discover Drugs from Herbal Medicines: CAM’s Outstanding Contribution to Modern Therapeutics. Evid. Based Complement. Altern. Med. 2013. [Google Scholar] [CrossRef]
- Fong, H.H. Integration of Herbal medicines in to modern medical practices: Issues and prospects. Integr. Cancer Ther. 2002, 1, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Dattner, A.M. From medical herbalism to phytotherapy in dermatology: Back to the future. Dermatol. Ther. 2008, 16, 106–113. [Google Scholar] [CrossRef]
- Thatte, U.M.; Rege, N.N.; Phatak, S.D.; Dahanukar, S.A. The flip side of Ayurveda. J. Postgrad. Med. 1993, 39, 179–182. [Google Scholar] [PubMed]
- Salem, M.L.; Hossain, M.S. Protective effect of black seed oil from Nigella sativa against murine cytomegalovirus infection. Int. J. Immunopharmacol. 2000, 22, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Parab, S.; Kulkarni, R.; Thatte, U. Heavy metals in herbal T medicines. Indian J. Gastroenterol. 2003, 22, 111–112. [Google Scholar] [PubMed]
- Huffman, M.A. Animal self-medication and ethno-medicine: Exploration and exploitation of the medicinal properties of plants. Proc. Nutr. Soc. 2003, 62, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.L.; Liebowitz, R.S.; Newby, L.K. Complementary and alternative medicine in cardiovascular disease: A review of biologically based approaches. Am. Heart J. 2004, 147, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, T.; Kobayashi, M.; Pollard, R.B.; Suzuki, F. Glycyrrhizin an Active Component of Licorice Roots, Reduces Morbidity and Mortality of Mice Infected with Lethal Doses of Influenza Virus. Antimicrob. Agents Chemother. 1997, 41, 551–556. [Google Scholar] [PubMed]
- Pleschka, S.; Stein, M.; Schoop, R.; Hudson, J.B. Anti-viral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian Influenza virus (H5N1, H7N7) and swine-origin H1N1 (S-OIV). Virol. J. 2009, 6, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Ge, U.; Wang, Y.F.; Xu, J.; Gu, Q.; Liu, H.B.; Xiao, P.G.; Zhou, J.; Liu, Y.; Yang, Z.; Su, H. Anti-influenza agents from Traditional Chinese Medicine. Nat. Prod. Rep. 2010, 27, 1758–1780. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Han, H.; Wang, W.; Gao, B. Anti-influenza virus effect of aqueous extracts from dandelion. Virol. J. 2011, 8, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Haruyama, T.; Nagata, K. Anti-influenza virus activity of Ginkgo biloba leaf extracts. J. Nat. Med. 2013, 67, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Makau, J.N.; Watanabe, K.; Kobayashi, N. Anti-influenza activity of Alchemilla mollis extract: Possible virucidal activity against influenza virus particles. Drug Discov. Ther. 2013, 7, 189–195. [Google Scholar] [PubMed]
- Makarovaa, M.N.; Pozharitskayaa, O.N.; Shikova, A.N.; Tesakovaa, S.V.; Tikhonov, V.P. Effect of lipid-based suspension of Epimedium koreanum Nakai extract on sexual behavior in rats. J. Ethnopharmacol. 2007, 114, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Manicassamy, B.; Medina, R.A.; Hai, R.; Tsibane, T.; Stertz, S. Protection of Mice against Lethal Challenge with 2009 H1N1 Influenza A Virus by 1918-Like and Classical Swine H1N1 Based Vaccines. PLoS Pathog. 2010, 6, e1000745. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Recognition of viruses by innate immunity. Immunol. Rev. 2007, 220, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Konopka, J.L.; Thompson, J.M.; Whitmore, A.C.; Webb, D.L.; Johnston, R.E. Acute Infection with Venezuelan Equine Encephalitis Virus Replicon Particles Catalyzes a Systemic Antiviral State and Protects from Lethal Virus Challenge. J. Virol. 2009, 83, 12432–12442. [Google Scholar] [CrossRef] [PubMed]
- Teijaro, J.R.; Walsh, K.B.; Rice, S.; Rosen, H.; Oldstone, M.B. Mapping the innate signaling cascade essential for cytokine storm during influenza virus infection. Proc. Natl. Acad. Sci. USA 2014, 111, 3799–3804. [Google Scholar] [CrossRef] [PubMed]
- Goodbourn, S.; Didcock, L.; Randall, R.E. Interferons: Cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 2000, 81, 2341–2364. [Google Scholar] [PubMed]
- Perry, A.K.; Chow, E.K.; Cheng, G. Differential Requirement for TANK-binding Kinase-1 in Type I Interferon Responses to Toll-like Receptor Activation and Viral Infection. J. Exp. Med. 2004, 199, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Tenoever, B.R.; Ng, S.L.; Chua, M.A.; McWhirter, S.M.; Garcia-Sastre, A.; Maniatis, T. Multiple functions of the IKK-related kinase IKK-epsilon in interferon-mediated antiviral immunity. Science 2007, 315, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Boasso, A. Type I Interferon at the Interface of Antiviral Immunity and Immune Regulation: The Curious Case of HIV-1. Scientifica 2013. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, P.O.; Lopes, A.M.; Mazzola, P.G.; Rangel-Yagui, C.; Penna, T.C. Methods of endotoxin removal from biological preparations: A review. J. Pharm. Sci. 2007, 10, 388–404. [Google Scholar]
- Maurya, M.R.; Gupta, S.; Li, X.; Fahy, E.; Dinasarapu, A.R.; Sud, M.; Brown, H.A.; Glass, C.K.; Murphy, R.C.; Russell, D.W.; et al. Analysis of inflammatory and lipid metabolic networks across RAW264.7 and thioglycolate-elicited macrophages. J. Lipid Res. 2013, 54, 2525–2542. [Google Scholar] [CrossRef] [PubMed]
- Karavitis, J.; Kovacs, E.J. Macrophage phagocytosis: Effects of environmental pollutants, alcohol, cigarette smoke, and other external factors. J. Leukoc. Biol. 2011, 90, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Woods, J.A.; Lu, Q.; Lowder, T. Exercise-induced modulation of macrophage function. Immunol. Cell Biol. 2000, 78, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yuan, Y.; Yue, T. Immunostimulatory activities of -d-glucan from Ganoderma lucidum. Carbohydr. Polym. 2014, 102, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, S.J.; Rim, H.K.; Shin, J.S.; Jung, J.Y.; Heo, J.S.; Kim, J.B.; Lee, M.S.; Lee, K.T. In vitro and in vivo immunostimulatory effects of hot water extracts from the leaves of Artemisia princeps Pampanini cv. Sajabal. J. Ethnopharmacol. 2013, 149, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Vázquez, M.C.; Alonso-Castro, A.J.; García-Carrancá, A. Kaempferitrin induces immunostimulatory effects in vitro. J. Ethnopharmacol. 2013, 148, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Wilden, H.; Schirrmacher, V.; Fournier, P. Important role of interferon regulatory factor (IRF)-3 in the interferon response of mouse macrophages upon infection by Newcastle disease virus. Int. J. Oncol. 2011, 39, 493–504. [Google Scholar] [PubMed]
- Wilden, H.; Fournier, P.; Zawatzky, L.; Schirrmacher, V. Expression of RIG-I, IRF-3, IFN-B and IRF-7 determines resistance or susceptibility of cells to infection by Newcatle Disease Virus. Int. J. Oncol. 2009, 34, 971–982. [Google Scholar] [PubMed]
- Graham, K.L.; Lee, L.Y.; Higgins, J.P.; Steinman, L.; Utz, P.J.; Ho, P.P. Treatment with a Toll-like receptor inhibitory GpG oligonucleotide delays and attenuates lupus nephritis in NZB/W mice. Autoimmunity 2014, 43, 140–155. [Google Scholar] [CrossRef]
- Wu, C.; Sheng, Y.; Zhang, Y.; Zhang, J.; Guo, B. Identification and characterization of active compoundsand their metabolites by high-performance liquidchromatography/Fourier transform ion cyclotronresonance mass spectrometry after oral administration of a herbal extract of Epimedium koreanum Nakai to rats. Rapid. Commun. Mass. Spectrom. 2008, 22, 2813–2824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.W.; Cheng, Y.; Wang, N.L.; Zhang, J.C.; Yang, M.S. Effects of total flavonoids and flavonol glycosides from Epimedium koreanum Nakai on the proliferation and differentiation of primary osteoblasts. Phytomedicine 2008, 15, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Kima, J.H.; Lee, C.H.; Ahn, Y.J.; Song, J.H.; Baek, S.H.; Kwona, D.H. Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antivir. Res. 2009, 81, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Shim, J.K.; Choi, H.J. Quercetin 7-rhamnoside reduces porcine epidemic species. Virol. J. 2011, 4, 460–465. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, W.-K.; Weeratunga, P.; Lee, B.-H.; Park, J.-S.; Kim, C.-J.; Ma, J.Y.; Lee, J.-S. Epimedium koreanum Nakai Displays Broad Spectrum of Antiviral Activity in Vitro and in Vivo by Inducing Cellular Antiviral State. Viruses 2015, 7, 352-377. https://doi.org/10.3390/v7010352
Cho W-K, Weeratunga P, Lee B-H, Park J-S, Kim C-J, Ma JY, Lee J-S. Epimedium koreanum Nakai Displays Broad Spectrum of Antiviral Activity in Vitro and in Vivo by Inducing Cellular Antiviral State. Viruses. 2015; 7(1):352-377. https://doi.org/10.3390/v7010352
Chicago/Turabian StyleCho, Won-Kyung, Prasanna Weeratunga, Byeong-Hoon Lee, Jun-Seol Park, Chul-Joong Kim, Jin Yeul Ma, and Jong-Soo Lee. 2015. "Epimedium koreanum Nakai Displays Broad Spectrum of Antiviral Activity in Vitro and in Vivo by Inducing Cellular Antiviral State" Viruses 7, no. 1: 352-377. https://doi.org/10.3390/v7010352
APA StyleCho, W.-K., Weeratunga, P., Lee, B.-H., Park, J.-S., Kim, C.-J., Ma, J. Y., & Lee, J.-S. (2015). Epimedium koreanum Nakai Displays Broad Spectrum of Antiviral Activity in Vitro and in Vivo by Inducing Cellular Antiviral State. Viruses, 7(1), 352-377. https://doi.org/10.3390/v7010352