Abstract
Mosquito-borne viruses such as dengue, West Nile and chikungunya viruses cause significant morbidity and mortality in human populations. Since current methods are not sufficient to control disease occurrence, novel methods to control transmission of arboviruses would be beneficial. Recent studies have shown that virus infection and transmission in insects can be impeded by co-infection with the bacterium Wolbachia pipientis. Wolbachia is a maternally inherited endosymbiont that is commonly found in insects, including a number of mosquito vector species. In Drosophila, Wolbachia mediates antiviral protection against a broad range of RNA viruses. This discovery pointed to a potential strategy to interfere with mosquito transmission of arboviruses by artificially infecting mosquitoes with Wolbachia. This review outlines research on the prevalence of Wolbachia in mosquito vector species and the impact of antiviral effects in both naturally and artificially Wolbachia-infected mosquitoes.
Keywords:
Wolbachia; Drosophila; mosquito; arbovirus; insect virus; symbiosis; antiviral protection; antiviral effects 1. Introduction
Understanding the factors that contribute to the transmission of arboviruses may facilitate strategies to limit the spread of disease. Mosquito transmission of viruses is impacted by interactions between the virus, host and other microbes. Presence of the endosymbiotic bacterium Wolbachia pipientis can interfere with microbial and parasite infection in insects, including viruses in mosquitoes (reviewed in [1,2,3,4]). As a result of this characteristic, there is increased interest in exploiting Wolbachia as a means of biological control of arthropod transmitted infectious pathogens (reviewed in [1,5,6]). This review is focused on the impact of both natural and artificial Wolbachia infection on the outcome of virus infection in vector mosquitoes.
2. Wolbachia in Insects
Wolbachia is an alphaproteobacterium predicted to infect more than 40% of insect species [7,8]. Wolbachia infection can have a wide range of impacts on insects [9]. An obligate intracellular bacterium, Wolbachia lives in the cytoplasm of host cells and is dependent on host cell resources for replication. The primary transmission route of Wolbachia is vertical inheritance through the cytoplasm of the maternal line, although horizontal transmission between insect species also contributes to Wolbachia prevalence [10,11,12,13,14]. Invasion of invertebrate populations is generally achieved via Wolbachia induced modification of host reproductive systems. Invasion into insect populations can occur very rapidly; for example, Wolbachia swept through Californian Drosophila simulans (D. simulans) populations in three years [15]. The ability to invade populations together with the recent finding that Wolbachia can interfere with virus transmission has led to interest in utilising Wolbachia to control mosquito transmission of arboviruses [1,3,6].
Cytoplasmic incompatibility (CI) is a prevalent Wolbachia reproductive manipulation in insects [16], which increases the proportion of Wolbachia-infected individuals in the population. Wolbachia-infected females can successfully mate with an uninfected male or male infected with the same or a compatible Wolbachia type (see Figure 1). CI occurs when a Wolbachia-infected male mates with a female that is either not infected with Wolbachia (unidirectional CI) or infected with an incompatible type of Wolbachia (bidirectional CI) [17]. That is, “if the male is infected with an infection (type) that is not present in his mate, it is an incompatible cross” [18]. In mosquitoes, Wolbachia-induced CI skews the population toward Wolbachia-infected females. In contrast to the female gametes, Wolbachia is not present in the male sperm. The molecular events that lead to CI are not completely clear but involve changes in condensation of male chromatin in Wolbachia free zygotes and lack of mitotic synchrony between the parental chromosomes [19,20,21,22]. In diploid insects such as mosquitoes, viable progeny are not produced from these eggs. CI is rescued in Wolbachia-infected eggs, as there is a restoration of synchrony between the male and female chromosomes, therefore producing diploid Wolbachia-infected progeny [20,22]. For biological control approaches, CI can be harnessed to establish Wolbachia-infected populations in the field [23].
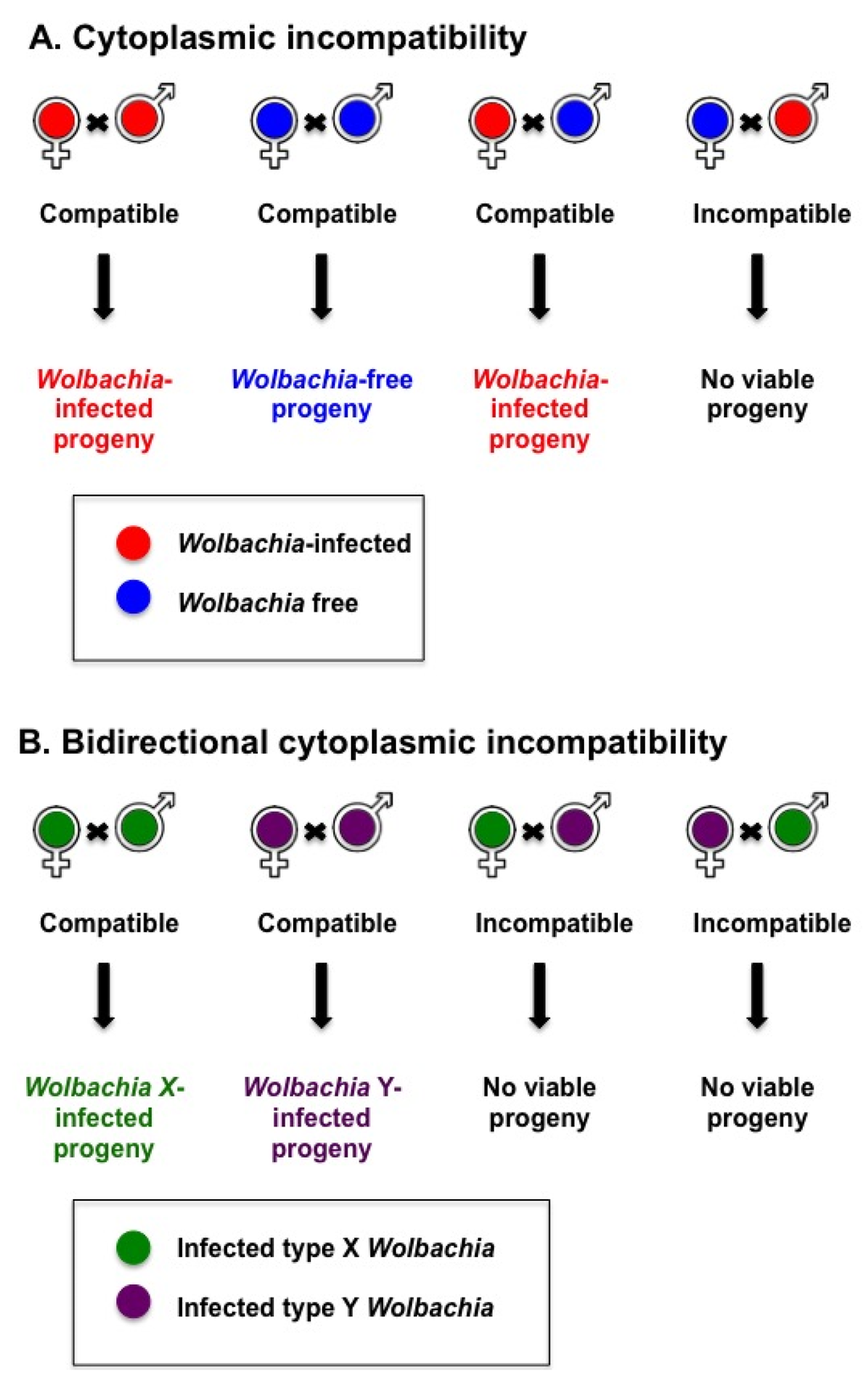
Figure 1.
Cytoplasmic incompatibility induced by Wolbachia can lead to an increased number of Wolbachia-infected progeny in the population. (A) An incompatible cross arises when a male infected with Wolbachia mates with a Wolbachia-free female; (B) Crosses between parents infected with different Wolbachia strains will be incompatible when their Wolbachia strains are incompatible.
4. Arboviruses in Mosquitoes
A subset of the over 3000 species of mosquitoes vector viruses that cause human disease (Table 1). The major mosquito-borne human pathogenic viruses come from three RNA virus families: Flaviviridae genus Flavivirus, Togaviridae genus Alphavirus and Bunyaviridae genera Orthobunyavirus and Phlebovirus (Table 1) [62]. These viruses are often found in both mosquitoes and animals, with virus replication occurring in both host types. Human disease occurs following transmission of the virus via a bite from an infected mosquito. For many of these viruses, humans are a dead-end host with viral population being necessarily maintained in other animal hosts. Viruses including dengue virus (DENV) and chikungunya virus (CHIKV) have adapted to a human-mosquito transmission cycle and no longer require amplification in other animals. While there are a complex range of factors that determine vector competence for transmission of arboviruses in mosquitoes [63], the presence of Wolbachia could influence vector competence by altering mosquito susceptibility to virus infection.

Table 1.
Mosquito vectored arboviruses and their common vectors *.
| Virus Family (Genome Nucleic Acid) | Genera | Examples of Arboviruses | Common Vectors |
|---|---|---|---|
| Flaviviridae (ss (+) RNA) | Flavivirus | Dengue virus | Aedes aegypti, Aedes albopictus |
| Japanese encephalitis virus | Culex spp. | ||
| St Louis encephalitis virus | Culex spp. | ||
| West Nile virus | Culex spp. | ||
| Yellow fever virus | Aedes spp. | ||
| Togaviridae (ss (+) RNA) | Alphavirus | Chikungunya virus | Aedes albopictus, Aedes aegypti |
| O’nyong nyong virus | Anopheles spp. | ||
| Semliki Forest virus | Aedes spp. | ||
| Venezuelan equine encephalitis virus | Aedes spp., Culex spp. | ||
| Bunyaviridae (ss (−) RNA) | Orthobunyavirus | La Crosse virus | Aedes triseriatus |
| Phlebovirus | Rift Valley fever virus | Aedes spp., Culex spp. |
* For references see [62,64] and references there in.
5. The Distribution of Wolbachia in Vector Mosquitoes
Wolbachia was first discovered in the mosquito Culex pipiens [65] and is present in populations of various wild mosquito species. Surveys focused on disease transmitting mosquito genera identified Wolbachia in 7%–42% of the Culex pecies analysed and 0%–30% of the Aedes species analysed; until recently, no Wolbachia was detected in any of the tested Anopheles species [66,67,68]. It is interesting to note that Wolbachia is frequently detected in several of the common arbovirus vectors including the Culex pipiens complex and Aedes species including Ae. albopictus but not Ae. aegypti. The establishment of Wolbachia in some mosquito species can be impeded by the native microbiome and this may in part explain the absence of Wolbachia in some mosquito species in nature [69]. Improved methods of detection will likely lead to detection of Wolbachia in a wider variety of species. For example Wolbachia was recently detected for the first time in a limited number of Anopheles gambiae mosquitoes using high throughput sequencing of the 16s rRNA gene amplified from field caught mosquitoes [70]. The presence of Wolbachia in various arbovirus mosquito vectors raises the question of the impact of Wolbachia infection on arbovirus transmission in natural vector populations.
The ability to experimentally transfer Wolbachia into naïve hosts can create new vector-Wolbachia associations that do not occur in nature. This is attractive as a way of introducing Wolbachia into Wolbachia-free mosquitoes such as Ae. aegypti. In the laboratory Wolbachia can be transferred between insects by a process called transinfection (see [71]). Wolbachia is extracted from infected donor insects and injected into naïve insects. A stable transinfected insect line is established if the Wolbachia productively infects the female gonads and is passed from one generation to the next. Stable transinfection of Wolbachia into mosquito species is challenging, but has been successfully achieved for several species of mosquitoes including: Ae. aegypti, Ae. albopictus, Ae. polynesiensis, Cx. pipiens, and An. stephensi [41,50,72,73,74,75,76,77,78,79,80,81,82,83]. In addition, mosquitoes can be transiently transinfected by injection of Wolbachia into adult mosquitoes. In both cases Wolbachia invades various tissues of the mosquito and can be recognised as foreign, therefore stimulating the host immune responses [54,56,84].
The creation of a new stable association between a host and Wolbachia strain and can lead to phenotypic and genetic changes. In naturally infected insects, maternal inheritance of Wolbachia across many generations maintains a close association between the host and symbiont, leading to co-evolution and stable interactions. In comparison, theory predicts that new host-Wolbachia associations are likely to be maladapted [85], and artificial transfer of Wolbachia to a new host is known to induce novel host phenotypes [86,87,88], and can also result in a burst of changes in the Wolbachia genome [89]. However, adaptation in the new host can occur relatively rapidly [86,88].
6. The Intrinsic Effects of Wolbachia on Virus Infection in Mosquitoes
The presence of Wolbachia in mosquitoes has varied impacts on arbovirus infection. This is a complex tripartite system with contributions from the host, Wolbachia and virus on the outcome of virus infection. In addition, Wolbachia infections in the mosquitoes analysed in the laboratory can either be naturally occurring or introduced by transinfection. Thus studies of antiviral effects in mosquitoes may be confounded by the Wolbachia infection mode [2].
Wolbachia-mediated antiviral effects have been well documented in stably transinfected mosquitoes (Table 2). The mosquito species Ae. albopictus, Ae. polynesiensis, and Ae. Aegypti have been stably transfected with one or more of three Wolbachia strains (wMel, wMelPop and wAlbB). These studies have included viruses from the families Flaviviridae (WNV, DENV and YFV) and Togaviridae (CHIKV) (Table 2). Arboviruses from the Bunyaviridae family are yet to be analysed in mosquitoes. A range of parameters can be examined for Wolbachia antiviral effects, these include: measuring the number of virus infected individuals, measuring virus load in whole or parts of mosquitoes, measuring dissemination and measuring virus in saliva as a proxy for transmission. In contrast to Wolbachia-mediated protection in Drosophila, since arboviruses have little impact on the survival of mosquitoes, protection against virus-induced mortality is not documented in mosquitoes. The impact of the presence of stably transinfected Wolbachia on the outcome of virus infection can range from a modest reduction in rate of infection amongst individuals or virus accumulation within infected individuals, to near complete interference with virus replication and transmission. Studies differ in the parameters tested and methods used, so it is difficult to make comprehensive comparisons across study systems. For example, in several cases antiviral effects were more prominent when virus was delivered orally rather than by injection [33,46]. However, in all cases where mosquito lines stably transinfected with Wolbachia have been analysed the presence of Wolbachia has decreased virus infection in at least one of the evaluated parameters [33,36,41,46,49,50,53,90].
Transient transinfection of Wolbachia into adult mosquitoes can lead to enhancement of virus infection. WNV infection rate was enhanced following transient transinfection of wAlbB into Cx. tarsalis mosquitoes [54]. Interestingly once the mosquitoes were infected with the virus, there was no impact of Wolbachia on virus accumulation, dissemination or transmission. This is the only enhancement of virus infection in mosquitoes that is linked to the presence of Wolbachia. It should be noted that transient transinfection is very different to natural stable infections where the host and Wolbachia have co-adapted to each other over many generations. It will be interesting to see if the enhancement in infection rate is limited to transiently transinfected mosquitoes. Cx. tarsalis mosquitoes are naturally Wolbachia-free [68] and stable transinfections of this mosquito have not been achieved so to date this comparison cannot be made. The possibility of arbovirus enhancement is an important consideration given there is one example of a natural Wolbachia infection stimulating increased susceptibility to a DNA virus in the African armyworm [43] and other examples of Wolbachia-induced enhancement of plasmodium infection in mosquitoes [84,91,92,93].
In contrast to stably transinfected mosquitoes, those naturally infected with Wolbachia do not ubiquitously exhibit antiviral effects (Table 2). Ae. albopictus mosquitoes naturally co-infected with wAlbA and wAlbB have similar total CHIKV or DENV loads to Wolbachia-free mosquitoes [34,51,52] and a small impact of Wolbachia on dissemination to the salivary glands was noted in one study for DENV [52]. Wolbachia-mediated antiviral effects for CHIKV were not stimulated by wAlbA and wAlbB introgressed into a new Ae. albopictus host background. The results of two studies on Wolbachia antiviral effects in naturally infected Culex mosquitoes were contrasting. While wPip was shown to mediate reduced WNV loads and transmission in Cx. quinquefasciatus mosquitoes [94], no effects of natural Wolbachia infection were detected for Cx. pipiens mosquitoes infected with WNV [95]. Interestingly, the two studies were performed by the same research group and they identified that the laboratory population of Cx. quinquefasciatus used in the original study had much higher somatic density of Wolbachia than the recently caught Cx. pipiens mosquitoes used in the second study [95]. Further analysis of somatic Wolbachia density in recently caught Cx. quinquefasciatus mosquitoes was even lower than that of the Cx. pipiens. This suggests that while presence of Wolbachia in Cx. quinquefasciatus can lead to reduced vector competence, the Wolbachia density in natural Culex populations may not be high enough to support these antiviral effects. As a consequence, Wolbachia may not impact vector competence in the field. Taken together, these studies suggest that Wolbachia may not have a major impact on competence of mosquitoes with a naturally occurring Wolbachia infection to transmit arboviruses; however, a limitation is that there are few studies on recently caught populations of mosquitoes and further research in this area is required before conclusions can be made.
Comparison of transinfected and naturally infected mosquitoes may give insight into factors important for Wolbachia-mediated antiviral effects. Robust antiviral effects induced by Wolbachia strain wMel transinfected into Ae. albopictus shows that this host can support Wolbachia antiviral effects [50,90]. However, natural wAlbA and wAlbB infection in this same host has either no or little impact on virus infection [34,51,52]. It is interesting that wAlbB transinfected into a different host, Ae. aegypti, is able to induce antiviral effects [49]. Thus, while both Ae. albopictus and wAlbB are individually competent partners for Wolbachia-mediated antiviral effects, no antiviral effects are demonstrated with this host-Wolbachia combination. This indicates that it is not a feature of either host or the Wolbachia strain per se which determines antiviral effects, but involves the interaction between the two. Transinfection of the Wolbachia into a new host in both these cases has stimulated antiviral effects. In mosquitoes there are two common effects of transinfection combinations that have antiviral effects: increased Wolbachia density and increased immune stimulation.

Table 2.
Antiviral protection in mosquitoes naturally or artificially infected with Wolbachia.
| Host Species | Mode of Wolbachia Infection | Wolbachia Strain | Virus * | Antiviral Effect ** | Reference |
|---|---|---|---|---|---|
| Culex quinquefasciatus | Natural | wPip | WNV | Reduced virus load and transmission | [94] |
| Culex pipiens | Natural | Not typed | WNV | No effect | [95] |
| Culex tarsalis | Transient transinfection | wAlbB | WNV | Enhanced infection rate | [54] |
| Aedes albopictus | Natural | wAlbA and wAlbB | DENV | No effect | [34] |
| Natural | wAlbA and wAlbB | DENV | No effect on virus load, reduced dissemination | [52] | |
| CHIKV | No effect | [51] | |||
| Introgressed | wAlbA and wAlbB | CHIKV | No effect | [90] | |
| Stable transinfection | wMel | DENV | Reduced transmission | [50] | |
| CHIKV | Reduced transmission | [90] | |||
| Aedes polynesiensis | Stable transinfection | wAlbB | DENV | Decreased virus load, reduced transmission (compared to line naturally infected with wPolA) | [33] |
| Aedes aegypti | Stable transinfection | wMelPop | DENV | Reduced infection rate, virus load and transmission | [36] |
| CHIKV | Reduced infection rate and virus load | [36] | |||
| WNV | Reduced infection rate, viral load and transmission | [53] | |||
| YFV | Reduced infection rate and virus load | [46] | |||
| wMel | DENV | Reduced virus load, dissemination and transmission | [41] | ||
| CHIKV | Reduced virus load and transmission | [46] | |||
| WNV | Delayed virus accumulation, reduced transmission | [53] | |||
| YFV | Reduced virus load | [46] | |||
| wAlbB | DENV | Reduced infection rate, virus load and transmission | [49] |
* WNV, West Nile virus; DENV, dengue virus; CHIKV, Chikungunya virus; YFV, yellow fever virus. ** reduced transmission is measured by a reduction of virus load in the mosquito saliva; reduced infection rate indicates a decrease in number of individuals infected with virus, reduced virus load indicates that there is reduction in either viral genome copies or virus titre.
Immune stimulation has been noted following stable transinfection of mosquitoes, and has been proposed as a potential mechanism for antiviral effects [36,49,56,57,58]. Wolbachia infection also induces reactive oxygen species in transinfected Ae. aegypti [58] and naturally infected Drosophila [59]. An increase in reactive oxygen species corresponds with Toll pathway restriction of DENV infection leading to the suggestion that Wolbachia mediates anti-DENV effects through stimulation of the Toll pathway [58]. Contrasting this, broad immune stimulation is not observed in Wolbachia-mediated antiviral protection in Drosophila, either in naturally infected or heterologous transinfected flies [44,96,97]. In addition, no factors that influence Wolbachia-mediated protection have been identified by experimental disruption of Drosophila immune pathways including the Toll pathway [98,99,100]. Involvement of the Toll pathway in Wolbachia-mediated antiviral effects in transinfected mosquitoes could be directly tested through analysis of virus infection in Toll impaired mosquitoes.
Density is key to antiviral effects both in Drosophila and mosquitoes. The implication from several studies is that antiviral effects in transinfected mosquitoes are linked to an increase in Wolbachia density [39,90]. The principle of density being important for Wolbachia-antiviral effects is also well supported from both direct and indirect studies in Drosophila and cell culture systems [26,27,31,32,34,35]. It is not currently clear whether stable transinfection itself leads to an increase in density, or whether other factors such as a new host-Wolbachia association is involved.
Whether Wolbachia-mediated antiviral effects in mosquitoes will attenuate over time following transinfection remains to be determined [101]. Reduction in antiviral effects may eventuate if adaptation of Wolbachia to the transinfected host leads to a decrease in Wolbachia density or an attenuation of features that lead to the antiviral effects. Alternatively, the virus could evolve to “escape” the antiviral mechanisms mediated by Wolbachia. There is strong evolutionary pressure for the virus to overcome the Wolbachia antiviral effect and lack of evidence of strong effects in natural mosquito populations may suggest that evolutionary adaptation will lead to reduction of antiviral effects [101].
7. Conclusions
Currently, there is a contrast of antiviral effects reported in naturally versus artificially infected mosquitoes. The current literature suggests that natural infection of vector species with Wolbachia may not have widespread impact of arbovirus transmission. However, there are few studies on natural populations so it is not appropriate to draw strong conclusions at this point. In contrast, stable transinfection of Wolbachia into heterologous mosquito hosts clearly produces antiviral effects against arboviruses including DENV, WNV, YFV and CHIKV. These antiviral effects are likely related to increased Wolbachia density and possibly immune stimulation in the new host, although direct evidence for this is lacking. If antiviral effects are stimulated by the new Wolbachia-host association, it is possible that as adaption occurs these effects may decrease, which will be an important consideration for release of artificially infected mosquitoes as biocontrol for arbovirus transmission.
Acknowledgments
The author’s research is supported by an Australian Research Council Discovery Grant DP1092492. I thank the anonymous reviewers for suggestions that helped improve the review.
Conflicts of Interest
The author declares no conflict of interest.
References
- Bourtzis, K.; Dobson, S.L.; Xi, Z.; Rasgon, J.L.; Calvitti, M.; Moreira, L.A.; Bossin, H.C.; Moretti, R.; Baton, L.A.; Hughes, G.L.; et al. Harnessing mosquito-Wolbachia symbiosis for vector and disease control. Acta Trop. 2014, 132, S150–S163. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.N. Bacteria and antiviral immunity in insects. Curr. Opin. Insect Sci. 2015, 8, 97–103. [Google Scholar] [CrossRef]
- Rainey, S.M.; Shah, P.; Kohl, A.; Dietrich, I. Understanding the Wolbachia-mediated inhibition of arboviruses in mosquitoes: Progress and challenges. J. Gen. Virol. 2014, 95, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Sinkins, S.P. Wolbachia and arbovirus inhibition in mosquitoes. Future Microbiol. 2013, 8, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.; Moreira, L.A. Can Wolbachia be used to control malaria? Mem. Inst. Oswaldo Cruz 2011, 106, S212–S217. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Montgomery, B.L.; Popovici, J.; Iturbe-Ormaetxe, I.; Johnson, P.H.; Muzzi, F.; Greenfield, M.; Durkan, M.; Leong, Y.S.; Dong, Y.; et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 2011, 476, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Zug, R.; Hammerstein, P. Still a host of hosts for Wolbachia: Analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS One 2012, 7, e38544. [Google Scholar] [CrossRef] [PubMed]
- Hilgenboecker, K.; Hammerstein, P.; Schlattmann, P.; Telschow, A.; Werren, J.H. How many species are infected with Wolbachia?—A statistical analysis of current data. FEMS Microbiol. Lett. 2008, 281, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.Z.; Li, S.J.; Xue, X.; Yin, X.J.; Ren, S.X.; Jiggins, F.M.; Greeff, J.M.; Qiu, B.L. The intracellular bacterium Wolbachia uses parasitoid wasps as phoretic vectors for efficient horizontal transmission. PLoS Pathog. 2015, 10, e1004672. [Google Scholar] [CrossRef] [PubMed]
- Jiggins, F.M.; Hurst, G.D. Microbiology. Rapid insect evolution by symbiont transfer. Science 2011, 332, 185–186. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.L.; Giordano, R.; Colbert, A.M.; Karr, T.L.; Robertson, H.M. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc. Natl. Acad. Sci. USA 1992, 89, 2699–2702. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Zhang, W.; Guo, L.R. Evolution and phylogeny of Wolbachia: Reproductive parasites of arthropods. Proc. R. Soc. London B Biol. Sci. 1995, 261, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Vavre, F.; Fleury, F.; Lepetit, D.; Fouillet, P.; Bouletreau, M. Phylogenetic evidence for horizontal transmission of Wolbachia in host-parasitoid associations. Mol. Biol. Evol. 1999, 16, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Turelli, M.; Hoffmann, A.A. Rapid spread of an inherited incompatibility factor in California Drosophila. Nature 1991, 353, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.J.; Vavre, F.; Beukeboom, L.W. Manipulation of arthropod sex determination by endosymbionts: Diversity and molecular mechanisms. Sex. Dev. Genet. Mol. Biol. Evol. Endocrinol. Embryol. Pathol. Sex Determ. Differ. 2014, 8, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Stouthamer, R.; Breeuwer, J.A.; Hurst, G.D. Wolbachia pipientis: Microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 1999, 53, 71–102. [Google Scholar] [CrossRef] [PubMed]
- Cytoplasmic Incompatibility. Available online: http://dobsonserv.ca.uky.edu/DobsonSite/CI1.html (accessed on 25 July 2015).
- Tram, U.; Fredrick, K.; Werren, J.H.; Sullivan, W. Paternal chromosome segregation during the first mitotic division determines Wolbachia-induced cytoplasmic incompatibility phenotype. J. Cell Sci. 2006, 119, 3655–3663. [Google Scholar] [CrossRef] [PubMed]
- Tram, U.; Sullivan, W. Role of delayed nuclear envelope breakdown and mitosis in Wolbachia-induced cytoplasmic incompatibility. Science 2002, 296, 1124–1126. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.B.; Stainton, K.; Harris, S.; Kambris, Z.; Sutton, E.R.; Bonsall, M.B.; Parkhill, J.; Sinkins, S.P. Transcriptional regulation of Culex pipiens mosquitoes by Wolbachia influences cytoplasmic incompatibility. PLoS Pathog. 2013, 9, e1003647. [Google Scholar] [CrossRef] [PubMed]
- Landmann, F.; Orsi, G.A.; Loppin, B.; Sullivan, W. Wolbachia-mediated cytoplasmic incompatibility is associated with impaired histone deposition in the male pronucleus. PLoS Pathog. 2009, 5, e1000343. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Iturbe-Ormaetxe, I.; Callahan, A.G.; Phillips, B.L.; Billington, K.; Axford, J.K.; Montgomery, B.; Turley, A.P.; O’Neill, S.L. Stability of the wMel Wolbachia infection following invasion into Aedes aegypti populations. PLoS Negl. Trop. Dis. 2014, 8, e3115. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.; Ferreira, A.; Ashburner, M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 2008, 6, e1000002. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.M.; Brownlie, J.C.; O’Neill, S.L.; Johnson, K.N. Wolbachia and virus protection in insects. Science 2008, 322. [Google Scholar] [CrossRef] [PubMed]
- Osborne, S.E.; Leong, Y.S.; O’Neill, S.L.; Johnson, K.N. Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans. PLoS Pathog. 2009, 5, e1000656. [Google Scholar] [CrossRef] [PubMed]
- Osborne, S.E.; Iturbe-Ormaetxe, I.; Brownlie, J.C.; O’Neill, S.L.; Johnson, K.N. Antiviral protection and the importance of Wolbachia density and tissue tropism in Drosophila simulans. Appl. Environ. Microbiol. 2012, 78, 6922–6929. [Google Scholar] [CrossRef] [PubMed]
- Dobson, S.L.; Bourtzis, K.; Braig, H.R.; Jones, B.F.; Zhou, W.; Rousset, F.; O’Neill, S.L. Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem. Mol. Biol. 1999, 29, 153–160. [Google Scholar] [CrossRef]
- Dutton, T.J.; Sinkins, S.P. Strain-specific quantification of Wolbachia density in Aedes albopictus and effects of larval rearing conditions. Insect Mol. Biol. 2004, 13, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.J.; Riegler, M. Evolutionary dynamics of wAu-like Wolbachia variants in neotropical Drosophila spp. Appl. Environ. Microbiol. 2006, 72, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Longdon, B.; Bauer, S.; Chan, Y.S.; Miller, W.J.; Bourtzis, K.; Teixeira, L.; Jiggins, F.M. Symbionts commonly provide broad spectrum resistance to viruses in insects: A comparative analysis of Wolbachia strains. PLoS Pathog. 2014, 10, e1004369. [Google Scholar] [CrossRef] [PubMed]
- Chrostek, E.; Marialva, M.S.; Esteves, S.S.; Weinert, L.A.; Martinez, J.; Jiggins, F.M.; Teixeira, L. Wolbachia variants induce differential protection to viruses in Drosophila melanogaster: A phenotypic and phylogenomic analysis. PLoS Genet. 2013, 9, e1003896. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.W.; Zhou, G.L.; Lu, P.; Xi, Z.Y. Replacing a native Wolbachia with a novel strain results in an increase in endosymbiont load and resistance to Dengue virus in a mosquito vector. PLoS Negl. Trop. Dis. 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Bian, G.W.; Pan, X.L.; Xi, Z.Y. Wolbachia induces density-dependent inhibition to Dengue virus in mosquito cells. PLoS Negl. Trop. Dis. 2012, 6. [Google Scholar] [CrossRef] [PubMed]
- Frentiu, F.D.; Robinson, J.; Young, P.R.; McGraw, E.A.; O’Neill, S.L. Wolbachia-mediated resistance to dengue virus infection and death at the cellular level. PLoS ONE 2010, 5, e13398. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.J.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia symbiont in Aedes aegypti limits infection with Dengue, Chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Aksoy, S. Tissue tropism, transmission and expression of foreign genes in vivo in midgut symbionts of tsetse flies. Insect Mol. Biol. 1999, 8, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Ruel, T.D.; Zhou, W.; Moloo, S.K.; Majiwa, P.; O’Neill, S.L.; Aksoy, S. Tissue distribution and prevalence of Wolbachia infections in tsetse flies, Glossina spp. Med. Vet. Entomol. 2000, 14, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Frydman, H.M.; Li, J.M.; Robson, D.N.; Wieschaus, E. Somatic stem cell niche tropism in Wolbachia. Nature 2006, 441, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Min, K.T.; Benzer, S. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc. Natl. Acad. Sci. USA 1997, 94, 10792–10796. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.; Johnson, P.H.; Moreira, L.A.; Iturbe-Ormaetxe, I.; Frentiu, F.D.; McMeniman, C.J.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P.; et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011, 476, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Jaenike, J. Coupled population dynamics of endosymbionts within and between hosts. Oikos 2009, 118, 353–362. [Google Scholar] [CrossRef]
- Graham, R.I.; Grzywacz, D.; Mushobozi, W.L.; Wilson, K. Wolbachia in a major African crop pest increases susceptibility to viral disease rather than protects. Ecol. Lett. 2012, 15, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Rances, E.; Ye, Y.H.; Woolfit, M.; McGraw, E.A.; O’Neill, S.L. The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathog. 2012, 8, e1002548. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.E.; Veronesi, E.; Maurin, G.; Ftaich, N.; Guiguen, F.; Rixon, F.; Ratinier, M.; Mertens, P.; Carpenter, S.; Palmarini, M.; et al. Drosophila melanogaster as a model organism for Bluetongue virus replication and tropism. J. Virol. 2012, 86, 9015–9024. [Google Scholar] [CrossRef] [PubMed]
- Van den Hurk, A.F.; Hall-Mendelin, S.; Pyke, A.T.; Frentiu, F.D.; McElroy, K.; Day, A.; Higgs, S.; O’Neill, S.L. Impact of Wolbachia on infection with Chikungunya and Yellow Fever viruses in the mosquito vector Aedes aegypti. PLoS Negl. Trop. Dis. 2012, 6. [Google Scholar] [CrossRef] [PubMed]
- Longdon, B.; Fabian, D.K.; Hurst, G.D.D.; Jiggins, F.M. Male-killing Wolbachia do not protect Drosophila bifasciata against viral infection. BMC Microbiology 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Unckless, R.L.; Jaenike, J. Maintenance of a male-killing Wolbachia in Drosophila innubila by male-killing dependent and male-killing independent mechanisms. Evolution 2012, 66, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.W.; Xu, Y.; Lu, P.; Xie, Y.; Xi, Z.Y. The endosymbiotic bacterium Wolbachia induces resistance to Dengue virus in Aedes aegypti. PLoS Pathog. 2010, 6, e1000833. [Google Scholar] [CrossRef] [PubMed]
- Blagrove, M.S.; Arias-Goeta, C.; Failloux, A.B.; Sinkins, S.P. Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proc. Natl. Acad. Sci. USA 2012, 109, 255–260. [Google Scholar] [CrossRef]
- Mousson, L.; Martin, E.; Zouache, K.; Madec, Y.; Mavingui, P.; Failloux, A.B. Wolbachia modulates Chikungunya replication in Aedes albopictus. Mol. Ecol. 2010, 19, 1953–1964. [Google Scholar] [CrossRef] [PubMed]
- Mousson, L.; Zouache, K.; Arias-Goeta, C.; Raquin, V.; Mavingui, P.; Failloux, A.B. The native Wolbachia symbionts limit transmission of dengue virus in Aedes albopictus. PLoS Negl. Trop. Dis. 2012, 6, e1989. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Lu, G.J.; Torres, S.; Edmonds, J.H.; Kay, B.H.; Khromykh, A.A.; Asgari, S. Effect of Wolbachia on replication of West Nile Virus in a mosquito cell line and adult mosquitoes. J. Virol. 2013, 87, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Dodson, B.L.; Hughes, G.L.; Paul, O.; Matacchiero, A.C.; Kramer, L.D.; Rasgon, J.L. Wolbachia enhances West Nile virus (WNV) infection in the mosquito Culex tarsalis. PLoS Negl. Trop. Dis. 2014, 8, e2965. [Google Scholar] [CrossRef] [PubMed]
- Caragata, E.P.; Rances, E.; Hedges, L.M.; Gofton, A.W.; Johnson, K.N.; O’Neill, S.L.; McGraw, E.A. Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog. 2013, 9, e1003459. [Google Scholar] [CrossRef] [PubMed]
- Kambris, Z.; Blagborough, A.M.; Pinto, S.B.; Blagrove, M.S.C.; Godfray, H.C.J.; Sinden, R.E.; Sinkins, S.P. Wolbachia stimulates immune gene expression and inhibits Plasmodium development in Anopheles gambiae. PLoS Pathog. 2010, 6, e1001143. [Google Scholar] [CrossRef] [PubMed]
- Kambris, Z.; Cook, P.E.; Phuc, H.K.; Sinkins, S.P. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science 2009, 326, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.L.; Zhou, G.L.; Wu, J.H.; Bian, G.W.; Lu, P.; Raikhel, A.S.; Xi, Z.Y. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2012, 109, E23–E31. [Google Scholar] [CrossRef] [PubMed]
- Wong, Z.S.; Brownlie, J.C.; Johnson, K.N. Oxidative stress correlates with Wolbachia-mediated antiviral protection in Wolbachia-Drosophila associations. Appl. Environ. Microbiol. 2015, 81, 3001–3005. [Google Scholar] [CrossRef] [PubMed]
- Asgari, S. Role of microRNAs in arbovirus/vector interactions. Viruses 2014, 6, 3514–3534. [Google Scholar] [CrossRef] [PubMed]
- Asgari, S. Regulatory role of cellular and viral microRNAs in insect-virus interactions. Curr. Opin. Insect Sci. 2015, 8, 104–110. [Google Scholar] [CrossRef]
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antivir. Res. 2010, 85, 328–345. [Google Scholar] [CrossRef] [PubMed]
- Tabachnick, W.J. Nature, nurture and evolution of intra-species variation in mosquito arbovirus transmission competence. Int. J. Environ. Res. Public Health 2013, 10, 249–277. [Google Scholar] [CrossRef] [PubMed]
- Ruckert, C.; Bell-Sakyi, L.; Fazakerley, J.K.; Fragkoudis, R. Antiviral responses of arthropod vectors: An update on recent advances. Virusdisease 2014, 25, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Hertig, M.; Wolbach, S.B. Studies on Rickettsia-like micro-organisms in insects. J. Med. Res. 1924, 44, 329–374.7. [Google Scholar] [PubMed]
- Kittayapong, P.; Baisley, K.J.; Baimai, V.; O’Neill, S.L. Distribution and diversity of Wolbachia infections in Southeast Asian mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2000, 37, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Ricci, I.; Cancrini, G.; Gabrielli, S.; D’Amelio, S.; Favi, G. Searching for Wolbachia (Rickettsiales: Rickettsiaceae) in mosquitoes (Diptera: Culicidae): Large polymerase chain reaction survey and new identifications. J. Med. Entomol. 2002, 39, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Rasgon, J.L.; Scott, T.W. An initial survey for Wolbachia (Rickettsiales: Rickettsiaceae) infections in selected California mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2004, 41, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.L.; Dodson, B.L.; Johnson, R.M.; Murdock, C.C.; Tsujimoto, H.; Suzuki, Y.; Patt, A.A.; Cui, L.; Nossa, C.W.; Barry, R.M.; et al. Native microbiome impedes vertical transmission of Wolbachia in Anopheles mosquitoes. Proc. Natl. Acad. Sci. USA 2014, 111, 12498–12503. [Google Scholar] [CrossRef] [PubMed]
- Baldini, F.; Segata, N.; Pompon, J.; Marcenac, P.; Robert Shaw, W.; Dabire, R.K.; Diabate, A.; Levashina, E.A.; Catteruccia, F. Evidence of natural Wolbachia infections in field populations of Anopheles gambiae. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.L.; Rasgon, J.L. Transinfection: A method to investigate Wolbachia-host interactions and control arthropod-borne disease. Insect Mol. Biol. 2014, 23, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Dean, J.L.; Khoo, C.; Dobson, S.L. Generation of a novel Wolbachia infection in Aedes albopictus (Asian tiger mosquito) via embryonic microinjection. Insect Biochem. Mol. Biol. 2005, 35, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Khoo, C.C.; Dobson, S.L. Interspecific transfer of Wolbachia into the mosquito disease vector Aedes albopictus. Proc. Biol. Sci. 2006, 273, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Khoo, C.C.; Dobson, S.L. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 2005, 310, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.; Joshi, D.; Dong, Y.; Lu, P.; Zhou, G.; Pan, X.; Xu, Y.; Dimopoulos, G.; Xi, Z. Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science 2013, 340, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Ruang-Areerate, T.; Kittayapong, P. Wolbachia transinfection in Aedes aegypti: A potential gene driver of dengue vectors. Proc. Natl. Acad. Sci. USA 2006, 103, 12534–12539. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.; Song, S.; Sinkins, S.P. Wolbachia in the Culex pipiens group mosquitoes: Introgression and superinfection. J. Hered. 2009, 100, 192–196. [Google Scholar] [CrossRef] [PubMed]
- McMeniman, C.J.; Lane, R.V.; Cass, B.N.; Fong, A.W.C.; Sidhu, M.; Wang, Y.F.; O’Neill, S.L. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 2009, 323, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Suh, E.; Mercer, D.R.; Fu, Y.; Dobson, S.L. Pathogenicity of life-shortening Wolbachia in Aedes albopictus after transfer from Drosophila melanogaster. Appl. Environ. Microbiol. 2009, 75, 7783–7788. [Google Scholar] [CrossRef] [PubMed]
- Calvitti, M.; Moretti, R.; Lampazzi, E.; Bellini, R.; Dobson, S.L. Characterization of a new Aedes albopictus (Diptera: Culicidae)-Wolbachia pipientis (Rickettsiales: Rickettsiaceae) symbiotic association generated by artificial transfer of the wPip strain from Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 2010, 47, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Gavotte, L.; Mercer, D.R.; Dobson, S.L. Artificial triple Wolbachia infection in Aedes albopictus yields a new pattern of unidirectional cytoplasmic incompatibility. Appl. Environ. Microbiol. 2010, 76, 5887–5891. [Google Scholar] [CrossRef] [PubMed]
- Andrews, E.S.; Crain, P.R.; Fu, Y.; Howe, D.K.; Dobson, S.L. Reactive oxygen species production and Brugia pahangi survivorship in Aedes polynesiensis with artificial Wolbachia infection types. PLoS Pathog. 2012, 8, e1003075. [Google Scholar] [CrossRef] [PubMed]
- Calvitti, M.; Moretti, R.; Skidmore, A.R.; Dobson, S.L. Wolbachia strain wPip yields a pattern of cytoplasmic incompatibility enhancing a Wolbachia-based suppression strategy against the disease vector Aedes albopictus. Parasit. Vectors 2012, 5. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.L.; Vega-Rodriguez, J.; Xue, P.; Rasgon, J.L. Wolbachia strain wAlbB enhances infection by the rodent malaria parasite Plasmodium berghei in Anopheles gambiae mosquitoes. Appl. Environ. Microbiol. 2012, 78, 1491–1495. [Google Scholar] [CrossRef] [PubMed]
- Turelli, M. Evolution of incompatibility-inducing microbes and their hosts. Evolution 1994, 48, 1500–1513. [Google Scholar] [CrossRef]
- McGraw, E.A.; Merritt, D.J.; Droller, J.N.; O’Neill, S.L. Wolbachia density and virulence attenuation after transfer into a novel host. Proc. Natl. Acad. Sci. USA 2002, 99, 2918–2923. [Google Scholar] [CrossRef] [PubMed]
- Carrington, L.B.; Hoffmann, A.A.; Weeks, A.R. Monitoring long-term evolutionary changes following Wolbachia introduction into a novel host: The Wolbachia popcorn infection in Drosophila simulans. Proc. Biol. Sci. 2010, 277, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Weeks, A.R.; Turelli, M.; Harcombe, W.R.; Reynolds, K.T.; Hoffmann, A.A. From parasite to mutualist: Rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biol. 2007, 5, e114. [Google Scholar] [CrossRef] [PubMed]
- Woolfit, M.; Iturbe-Ormaetxe, I.; Brownlie, J.C.; Walker, T.; Riegler, M.; Seleznev, A.; Popovici, J.; Rances, E.; Wee, B.A.; Pavlides, J.; et al. Genomic evolution of the pathogenic Wolbachia strain, wMelPop. Genome Biol. Evol. 2013, 5, 2189–2204. [Google Scholar] [CrossRef] [PubMed]
- Blagrove, M.S.; Arias-Goeta, C.; di Genua, C.; Failloux, A.B.; Sinkins, S.P. A Wolbachia wMel transinfection in Aedes albopictus is not detrimental to host fitness and inhibits Chikungunya virus. PLoS Negl. Trop. Dis. 2013, 7, e2152. [Google Scholar] [CrossRef] [PubMed]
- Baton, L.A.; Pacidonio, E.C.; Goncalves, D.S.; Moreira, L.A. wFlu: Characterization and evaluation of a native Wolbachia from the mosquito Aedes fluviatilis as a potential vector control agent. PLoS ONE 2013, 8, e59619. [Google Scholar] [CrossRef] [PubMed]
- Zele, F.; Nicot, A.; Berthomieu, A.; Weill, M.; Duron, O.; Rivero, A. Wolbachia increases susceptibility to Plasmodium infection in a natural system. Proc. Biol. Sci. 2014, 281. [Google Scholar] [CrossRef] [PubMed]
- Murdock, C.C.; Blanford, S.; Hughes, G.L.; Rasgon, J.L.; Thomas, M.B. Temperature alters Plasmodium blocking by Wolbachia. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Glaser, R.L.; Meola, M.A. The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS ONE 2010, 5, e11977. [Google Scholar] [CrossRef] [PubMed]
- Micieli, M.V.; Glaser, R.L. Somatic Wolbachia (Rickettsiales: Rickettsiaceae) levels in Culex quinquefasciatus and Culex pipiens (Diptera: Culicidae) and resistance to West Nile virus infection. J. Med. Entomol. 2014, 51, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Wong, Z.S.; Hedges, L.M.; Brownlie, J.C.; Johnson, K.N. Wolbachia-mediated antibacterial protection and immune gene regulation in Drosophila. PLoS ONE 2011, 6, e25430. [Google Scholar] [CrossRef] [PubMed]
- Chrostek, E.; Marialva, M.S.; Yamada, R.; O’Neill, S.L.; Teixeira, L. High anti-viral protection without immune upregulation after interspecies Wolbachia transfer. PLoS One 2014, 9, e99025. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.M.; Yamada, R.; O’Neill, S.L.; Johnson, K.N. The small interfering RNA pathway is not essential for Wolbachia-mediated antiviral protection in Drosophila melanogaster. Appl. Environ. Microbiol. 2012, 78, 6773–6776. [Google Scholar] [CrossRef] [PubMed]
- Rances, E.; Johnson, T.K.; Popovici, J.; Iturbe-Ormaetxe, I.; Zakir, T.; Warr, C.G.; O’Neill, S.L. The Toll and Imd pathways are not required for Wolbachia-mediated dengue virus interference. J. Virol. 2013, 87, 11945–11949. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.G.; Naylor, H.; Esteves, S.S.; Pais, I.S.; Martins, N.E.; Teixeira, L. The Toll-dorsal pathway is required for resistance to viral oral infection in Drosophila. PLoS Pathog. 2014, 10, e1004507. [Google Scholar] [CrossRef] [PubMed]
- Bull, J.J.; Turelli, M. Wolbachia versus dengue: Evolutionary forecasts. Evol. Med. Public Health 2013, 2013, 197–207. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).