Abstract
Griffithsin (GRFT), an algae-derived lectin, is one of the most potent viral entry inhibitors discovered to date. It is currently being developed as a microbicide with broad-spectrum activity against several enveloped viruses. GRFT can inhibit human immunodeficiency virus (HIV) infection at picomolar concentrations, surpassing the ability of most anti-HIV agents. The potential to inhibit other viruses as well as parasites has also been demonstrated. Griffithsin’s antiviral activity stems from its ability to bind terminal mannoses present in high-mannose oligosaccharides and crosslink these glycans on the surface of the viral envelope glycoproteins. Here, we review structural and biochemical studies that established mode of action and facilitated construction of GRFT analogs, mechanisms that may lead to resistance, and in vitro and pre-clinical results that support the therapeutic potential of this lectin.
1. Introduction
A number of life-threatening human diseases are caused by viruses as diverse as human immunodeficiency virus (HIV), Ebola, yellow fever, Zika, influenza, and severe acute respiratory syndrome and Middle East respiratory syndrome corona viruses (SARS-CoV and MERS-CoV, respectively). Though these viruses are taxonomically and genetically distinct, they are all enveloped viruses and therefore possess a lipid bilayer that protects the viral capsid and genetic material that is inside the viral particle. Despite the efforts to eradicate these diseases, a number of them continue to affect us either as pandemic diseases or as outbreaks that appear from time to time. For example, according to the World Health Organization (WHO), by the end of 2015 there were nearly 37 million people infected with HIV, with two million new cases and about one million deaths occurring that year (http://www.who.int/). On the other hand, nearly 29,000 cases and over 11,000 deaths were reported during the most recent Ebola outbreak that started in 2014. SARS-CoV and MERS-CoV belong to the corona virus family that includes the common cold. Outbreaks of both viruses (SARS-CoV in Asia in 2003 and MERS-CoV in Saudi Arabia in 2012) led to several thousand cases of SARS and MERS, and hundreds of deaths. Efforts to create vaccines against these viral diseases have been successful in some cases such as yellow fever, but other cases like HIV have been more challenging.
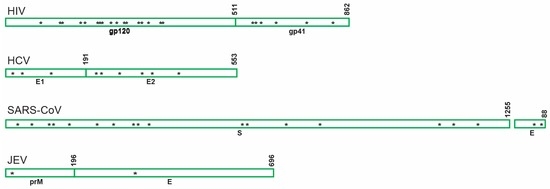
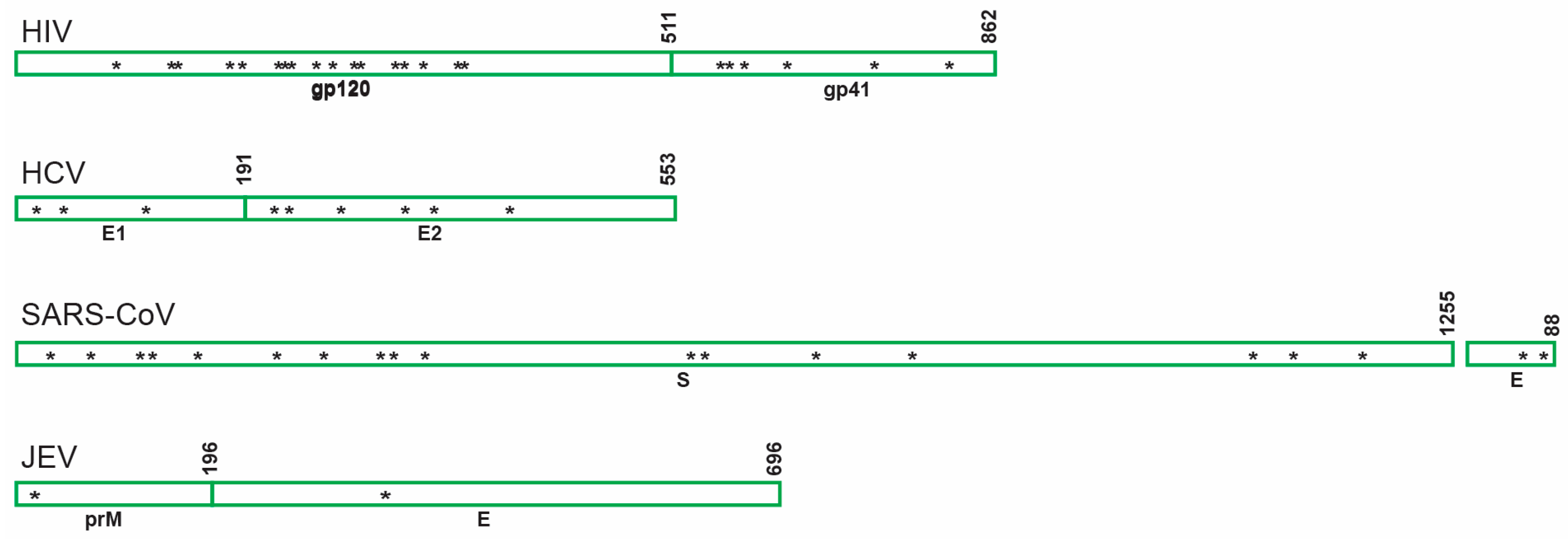
Enveloped viruses have surface glycoproteins that mediate attachment and fusion with the target cell membrane [1,2]. These proteins constitute the first encounter with the host and the most exposed target that the immune system can attack; hence viruses have evolved to hide the features that would make them more susceptible to antibody neutralization. These strategies include hiding fundamental structural motifs through oligomerization or conformational occlusion, rapid mutation rates that lead to high sequence variability in non-essential regions such as variable loops, and extensive posttranslational glycosylation. Vaccine development against enveloped viruses has mostly focused on targeting the envelope glycoproteins primarily because they are the sole targets of neutralizing antibodies. Some viruses, including hepatitis C virus (HCV) and HIV, have thus far eluded vaccine efforts, mostly due to the high variability among strains, high mutation rates within a given virus, and physical masking of neutralizing epitopes [3,4]. Development of vaccines against diseases that appear as outbreaks, such as Ebola and SARS-CoV, are also challenging because of the lack of infected patient populations necessary for testing efficacy of vaccines and therapeutics. Other diseases such as yellow fever can be prevented by vaccination.
For those diseases in which vaccine development has been challenging, other therapies have nevertheless been developed. HIV is arguably the most studied viral disease; highly-active antiretroviral therapy (HAART) consisting of a combination of small molecule antiretroviral drugs has progressively improved the lives of patients in some parts of the world, and effectively extends life expectancy. Antiretroviral drugs target different proteins and enzymes essential for the virus life cycle. On the other hand, antibody therapeutics are emerging as powerful alternative forms of therapy against HIV and other diseases. Such therapies depend on isolation of neutralizing monoclonal antibodies, primarily from patient sera, that will bind specifically to the envelope glycoprotein of the virus. Another way to treat viral diseases that is currently being studied is through the use of proteins capable of targeting the glycans present on the surface of the envelope glycoproteins, namely, lectins [5,6]. Lectins are sugar-binding proteins that are ubiquitous, present in microorganisms, plants and animals. They participate in many important cellular processes including cell–cell interactions and protein folding. Some lectins provide protection to the host from other organisms. Lectins have been developed widely as probes to investigate cell surface structure and functions; they have also found applications as antiviral drugs and in the delivery of chemotherapeutic agents. A number of lectins capable of binding the high-mannose glycans commonly found in the surface of the envelope glycoproteins are currently under study to be used as microbicides [7,8]. Some of the most promising antiviral lectins include griffithsin (GRFT), cyanovirin (CV-N) and banana lectin (BanLec). Their use has been mostly suggested as antiviral microbicides, acting as a barrier to prevent transmission of HIV through their incorporation into vaginal and rectal gels, creams or suppositories. In such systems, they can bind the viruses and prevent viral entry and fusion to target cells, thereby preventing infection. This review summarizes the activity, biochemical, biophysical and structural data available for GRFT, one of the most potent anti-HIV agents reported to date.
2. Discovery and Recombinant Expression of Griffithsin
Griffithsin was discovered as an anti-HIV lead by researchers at the National Cancer Institute (NCI). GRFT was isolated from a marine red alga Griffithsia sp. present in the NCI Natural Products Repository. Mass spectroscopic and nuclear magnetic resonance (NMR) data indicated the active compound was a protein rather than a small molecule natural product. Its sequence was determined through a combination of N-terminal Edman degradation of the intact protein and N-terminal sequencing of peptide fragments obtained from endopeptidase and cyanogen bromide treatments [9]. The wild-type protein from the alga contained an uncommon amino acid of 151.05 Da at position 31 that was replaced by alanine (Ala) in recombinant protein preparations without affecting anti-HIV activity. GRFT has no homology to any other proteins previously reported. It has been shown to have anti-HIV activity against T cell tropic and macrophage-tropic viruses. It is capable of inhibiting cell–cell fusion between chronically infected and uninfected cells and its efficacy as an antiviral agent against other enveloped viruses has also been shown (see Section 6).
A large-scale expression system is essential for the development of GRFT as an affordable drug. To that end GRFT has been expressed recombinantly in different organisms (Table 1). It was first expressed recombinantly in Escherichia coli (E. coli) with a polyhistidine tag on the N-terminus to facilitate purification by metal affinity chromatography [9,10]. Expression of GRFT in Nicotiana benthamiana using an infectious tobacco mosaic virus (TMV)-based vector has yielded gram amounts of the protein [11,12]. Expression in rice seeds through the stable transformation of plants has also been reported [13]. Purification of GRFT has been also optimized, including the use of ceramic filtration followed by two-stage chromatography [11], and a combination of heat, magnesium chloride and bentonite, followed by a single chromatographic step [14]. Importantly, GRFT expression and purification has been proven robust, an essential feature in making a drug available for clinical testing.

Table 1.
Recombinant expression of griffithsin.
3. Three-Dimensional Structure
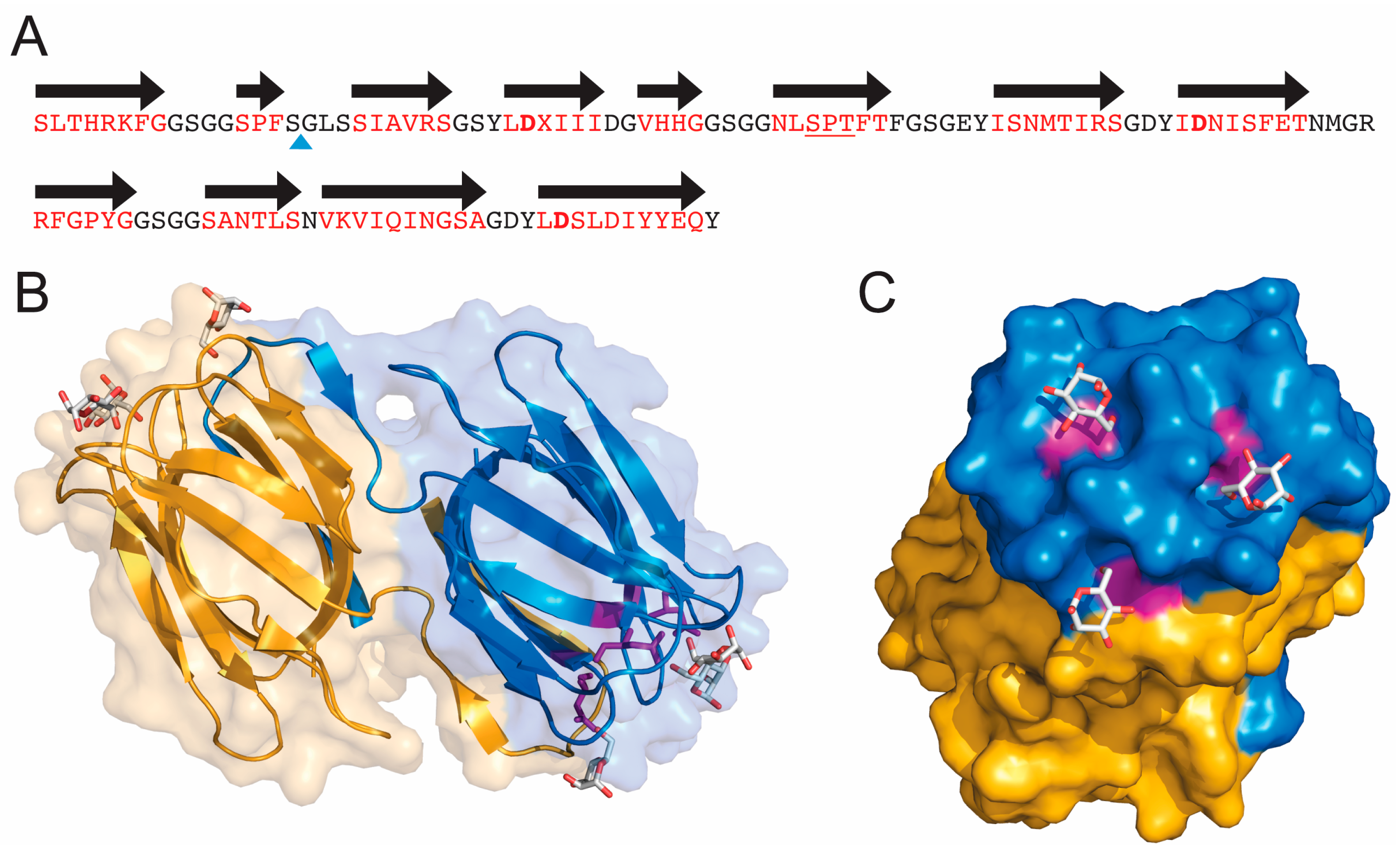
Griffithsin exists as a stable homodimer where each subunit contains 121 amino acids (Figure 1A). It has no cysteines in its sequence and no homology to any other proteins. Structures of GRFT in the absence of any ligand as well as in the presence of different monosaccharides and disaccharides have been solved by X-ray crystallography [15,16,17]. GRFT folds into a domain-swapped dimer (Figure 1B), where each subunit presents nearly perfect internal three-fold symmetry. The structure is composed by three repeats of an antiparallel four-stranded β-sheet [15] that superficially resembles a β-prism-I motif found in other lectins of the jacalin family. Two out of 12 β-strands (16 amino acids) swap from one monomer to the other to form a β-prism of three four-stranded sheets. Each subunit of the homodimer griffithsin is capable of binding three monosaccharides. Each binding site is located in an equilateral triangle, with each site separated by approximately 15 Å (Figure 1C). Crystal structures with the monosaccharides mannose, glucose and N-acetylglycosamine, and the disaccharides 1-6α-mannobiose and maltose have been reported [15,16,17]. When interacting with oligosaccharides, it has been shown that GRFT preferentially interacts with the terminal sugar [16]. Crystals with mannose have shown that all binding sites are almost identical and each one contains an aspartic acid (Asp) residue that makes extensive contacts with the sugar. These include Asp30, Asp70 and Asp112 that make hydrogen bonding interactions with O5 and O6 of mannose [15] (Figure 1C). Mutations of these residues to Ala do not affect the folding of the protein but weaken the binding to a mannose column [18].
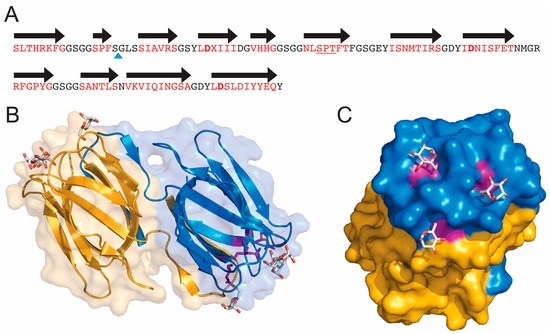
Figure 1.
Sequence and three-dimensional structure of griffithsin (GRFT). (A) Sequence of wild-type GRFT. Amino acids located within beta strands are colored red and the black arrows correspond to secondary structure. X represents an unknown amino acid of mass 151.05 present in the natural alga-derived material. The blue triangle shows the site of the Gly-Ser insertion used to produce the monomeric version of GRFT (mGRFT). The three Asp residues located in the three mannose-binding sites are shown in bold; (B) Ribbon drawing showing the three-dimensional structure of GRFT. Each monomer of the domain swapped dimer is colored yellow or blue. Mannose residues bound to the carbohydrate binding sites are shown in stick representation. Aspartic acids present in the binding sites are colored purple; (C) Rotated view of GRFT showing the carbohydrate binding face and mannose binding sites. (B,C) were generated using the program Pymol (Delano Scientific LLC, Palo Alto, CA, USA) and protein data bank accession number 2GUD.
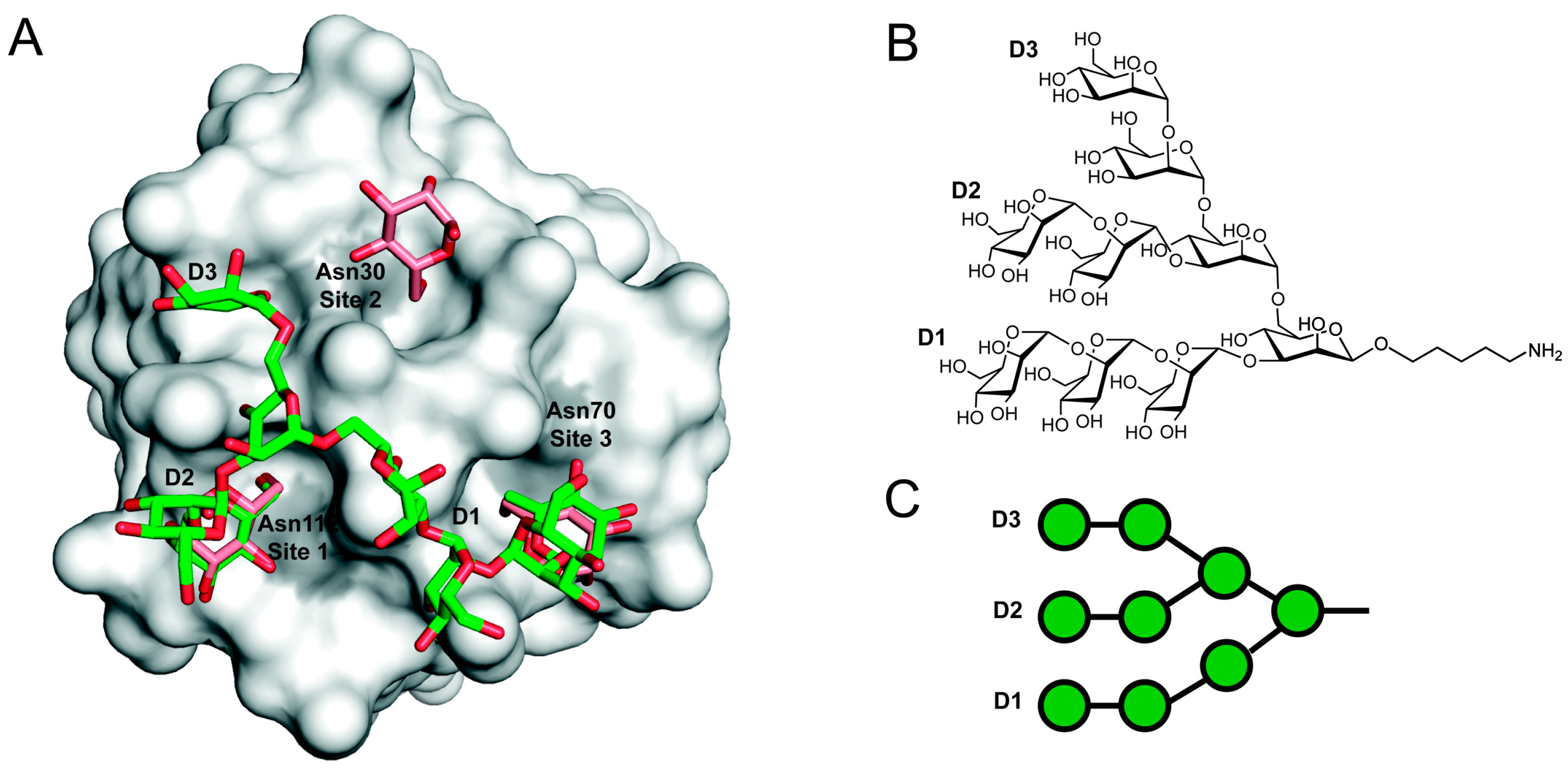
Attempts to crystalize GRFT with oligosaccharides have been mostly unsuccessful because of extensive precipitation of the protein in the presence of the oligosaccharides [16]. This problem was solved by Moulaei et al. who were able to create a monomeric version of GRFT (mGRFT) by insertion of a short linker, Gly-Ser, that separates the β-strands that undergo intermolecular domain swapping [19]. A second mutation, leucine in position 2 to serine, was introduced to minimize the hydrophobic patch opposite to the swap region. A complex of mGRFT bound to a synthetic nonamannoside (Man9) that contains the nine mannose residues present in full-length mannonanose-di-(N-acetyl-D-glucosamine) (Man9GlcNAc2) but lacks the core. In that structure, they found two of the three arms of nonamannoside bound to two of the three mannose-binding sites (Figure 2). In particular, the terminal mannose units on the D2 and D1 arms were bound to sites 1 and 3 (as defined in ref [15]), respectively [19]. The third mannose-binding site, site 2, is occupied by the D2 arm of another nonamannoside in what likely is a more transient interaction [19]. This interaction was inconsistent with the model proposed by Ziókowska et al. [16] where it was proposed that all three arms of Man9GlcNAc2 could interact with GRFT with a one-to-one stoichiometry. In addition to the crystal structures, NMR assignments for the backbone atoms of GRFT in the absence of ligand and in the presence of mannose have been reported [18].
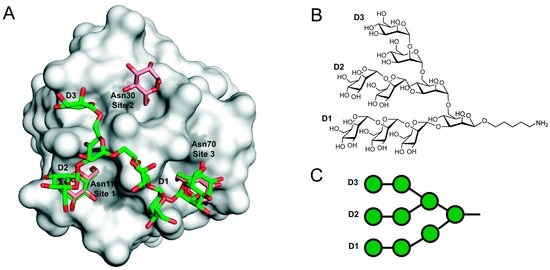
Figure 2.
Model of a GRFT-Man9 complex. (A) Representation of the structure of monomeric GRFT (gray surface) bound to nonamannoside (green sticks), Protein Data Bank accession 3LL2. Aspartic acid residues from each of the three glycan-binding sites are highlighted in grey. The structures of a GRFT:synthetic nonamannoside complex and a 1:3 mGRFT:mannose complex have been superimposed to compare the mannose binding sites. Only the D1 and D2 terminal mannoses are bound to a single GRFT monomer. Figure was rendered using PyMol. (B) Chemical structure of nonamannoside showing the D1, D2 and D3 arms; (C) Symbol representation of nonamannoside.
The crystal structures of GRFT:mannoside complexes suggested the importance of Asp residues present in each of the three mannose binding sites. Single mutations of any one of the Asp residues to Ala slightly weaken the affinity of binding to HIV-1 envelope glycoprotein gp120. However, the single mutations significantly decrease the potency in inhibition in single round HIV infection and cytopathicity assays as well as cell-to-cell transmission assays (cocultivation assays) [18,20]. To further study the importance of having three binding sites on each monomer, Xue et al. [21] designed a series of mutants where two monomers connected by an amino acid linker were expressed to form an obligate dimer. They compared a construct having all six binding sites (three from each monomer), with a mutant where three of the binding sites in one of the subunits were mutated to abolish binding to mannose (one-arm obligate dimer) and another mutant in which all six mannose binding sites were mutated. Binding studies showed that the obligate dimer as well as the construct containing mannose binding sites on only one subunit bound to gp120 with comparable affinities, whereas the mutant lacking all mannose binding sites did not bind gp120. In contrast, neutralization assays showed that the anti-HIV activity of the one-arm obligate dimer was reduced by several orders of magnitude compared to the wild-type obligate dimer [21].
Isothermal titration calorimetry (ITC) showed that binding to mono- and disaccharides is exothermic and entropically favored with micromolar KD values. Enthalpies among different saccharides indicated favorable electrostatic contacts. Large changes in entropy were reasoned by large displacement of water molecules from the binding sites [16,17,19]. Interestingly, nonamannoside binding was largely enthalpically driven and entropically unfavorable, indicating the formation of more favorable contacts between the sugar and the protein. The unfavorable entropy of binding indicated that the multivalent interactions between GRFT and nonamannoside diminished the degrees of freedom of glycan and protein [19].
4. GRFT Fragments, Conjugates and Stability
4.1. Peptides Derived from GRFT
Using the structure and carbohydrate binding arrangement of GRFT, Micewicz et al. constructed a peptide, grifonin-1 (GRFN-1), that contained three covalently linked beta sheets and displayed three-fold symmetry [22]. Grifonin-1 inhibited laboratory strains of HIV and bound to viral glycoproteins. Though antiviral potency was reduced by many hundred-fold compared to GRFT, grifonin-1 nevertheless exhibited submicromolar half maximal inhibitory concentrations (IC50s) in single-round HIV infectivity assays.
4.2. GRFT-C37
To enhance the ability of GRFT to inhibit HIV infection, Kagiampkis et al. created a covalently linked fusion protein composed of GRFT and the known HIV entry inhibitor C37 [23]. C37 is a 37-amino acid peptide containing sequences from the C-terminal region of the HIV transmembrane protein gp41. C37 blocks HIV fusion by binding to the N-terminal helices of gp41 and preventing the formation of the six-helix bundle that leads to membrane fusion. The fusion constructs demonstrated 5–8-fold improvements in potency compared to GRFT alone in C-C chemokine receptor type 5 (CCR5)-tropic or C-X-C chemokine receptor type 5 (CXCR5)-tropic cell-cell fusion assays and single-round infection assays [23].
4.3. Tandemers
Following on their work with monomeric GRFT, Moulaei and co-workers created several GRFT tandemers [24]. The tandemer proteins were constructed by linking GRFT monomers to one another through covalent linkages formed by insertion of Gly-Thr-Gly linkers. The tandemers were analyzed by electron microscopy that showed the double and triple repeats to exist in a linear arrangement while the tandemer containing four mGRFT repeats formed a more globular structure. In HIV neutralization assays, improvements in potency were observed for the double and triple tandemers but potency plateaued there, with no increase in potency observed for the 4× mGRFT construct.
4.4. GRFT Stability
GRFT is an unusually stable protein, withstanding extraction procedures used in natural products’ isolation and purification. These may include exposure to organic solvents and repeated lyophilization. In addition to this physicochemical stability, its resistance to protease degradation was established in a study examining the stability of antimicrobial peptides. There, the authors showed that GRFT was resistant to digestion by eight out of nine different proteases and was degraded only by the metalloprotease elastase [25]. In addition, GRFT was not affected under bacterial degradation conditions.
5. Carbohydrate-Mediated Crosslinking
The importance of intermolecular crosslinking by GRFT has been suggested in several publications [18,19,20,21,26]. Evidence has been obtained from the protein perspective as well as from the glycan perspective. It has been shown that monomeric GRFT binds with comparable affinity to gp120, yet the IC50 for neutralization is several orders of magnitude weaker than wild-type GRFT [19]. Crystal structures with a synthetic nonamannoside have been obtained only for the monomeric GRFT construct. The mode of binding seen in the crystals was in disagreement with a model proposing all three terminal mannose units of Man9 interacting with each of the mannose-binding sites in GRFT [16,19]. The inability of Man9 to engage all three binding sites on GRFT was further supported by studies that showed that glycopeptides that mimic Man9 required distances greater than those separating the arms of natural Man9GlcNAc2 in order to engage all three binding sites simultaneously [26]. Most recently, cryo-electron microscopy and surface plasmon resonance studies of viral particles treated with GRFT or GRFT mutants demonstrated that the unique combination of multivalency, strict recognition of Man9 and homodimeric structure of GRFT led to bridging of viral particles and aggregation [27].
The ability of monomeric GRFT tandemers, linked together by Gly-Thr-Gly amino acid linkers, showed that two individual monomeric units were needed to recover (and improve by up to 5-fold) the potency of wild-type GRFT [24]. Three units of mGRFT improved the neutralization potential even further (5-fold more). However, no further improvements in potency were observed for tandemers containing a greater number of mGRFT domains [24]. Interestingly, dynamic light scattering showed that wild-type GRFT causes virus aggregation, however, monomeric GRFT as well as the mGRFT tandemers did not cause virus aggregation; in addition, cryo-electron microscopy showed that viruses exposed to wild-type GRFT had protein aggregates on their surface but immunostaining was not performed [24].
7. Activity against Protists
Trichomoniasis is a sexually transmitted parasitic disease, caused by Trichomonas vaginalis. Development of microbicide agents against HIV can benefit also the prevention against Trichomonas. GRFT binds to the surface of Trichomonas and Tritrichomonas and causes flagellated trichomonads to undergo self-aggregation and precipitation [52]. Such interaction is likely due to the binding of GRFT to unprocessed glycans on the surface of the parasite. Topical application of GRFT during infection with Tritichomonas in mice reduced the recovery of parasites in mice, but it did not eliminate it [52].
8. Toxicity and Immunogenicity of GRFT
The cytotoxicity profile of GRFT has been studied extensively. While GRFT is by now well known to prevent infection by a number of diverse viruses, there has been no sign of cellular toxicity against a variety of cells even at concentrations as high as 500 nM [28]. If used as an antiviral therapeutic, the mode of GRFT administration may be determined by the mechanism through which the virus infects new hosts. For example, in the case of HIV, its most promising application is as a topical microbicide. Microbicides do not carry substantial risk of systemic side effects because they are unlikely to be absorbed efficiently. Alternatively, in respiratory infections via SARS-CoV, GRFT could be administered as an intranasal spray, or intravenously in the case of HCV.
Studies have shown that GRFT is stable and maintains similar anti-HIV activity after incubation for long periods of time in a variety of environments, including in the acidic (pH 4–6) cervical/vaginal lavage fluids from pig-tailed macaques [28]. GRFT binds the outermost layer of the squamous epithelium (human cervical epithelium) [53]. However, no loss in cell viability of endocervical and ectocervical cell lines was observed even at doses of 1 mg/mL GRFT. In addition, GRFT does not induce cell proliferation in cervicovaginal cell lines in contrast with other lectins that show high cytotoxicity and significant mitogenic activity [53]. Treatment of human cervical explants as well as cultured human endocervical, ectocervical and vaginal cell lines with GRFT, or intravaginal GRFT treatment of rabbits, have shown no significant perturbations on the levels of cytokines and chemokines [11,53]. RNA microarray analysis of cultured ectocervical cells line showed that GRFT treatment has minimal alteration in the gene expression profile [53]. An in vivo rabbit vaginal irritation (RVI) assay showed good safety profiles for GRFT, with no inflammatory responses or damage to epithelia. RVI testing is required by the Food and Drug Administration (FDA) to proceed for clinical testing [11].
HIV can infect PBMCs, which are often used in evaluating HIV infectivity. GRFT binds the surface of PMBCs and prevents viral replication even after washing the cells [53]. However, exposure of human PBMCs for up to three days showed no mitogenic activity, that is, it does not stimulate lymphocyte proliferation [11,53]. GRFT does not stimulate the expression of PMBC activation markers of immune activation (namely, markers for T cell activation) and it has minimal effect on cytokine and chemokine release on PMBC [53].
GRFT has also been proposed to treat respiratory infections such as SARS. In such cases, the protein would be administered intranasally. Preliminary studies where GRFT was administered intranasally against SARS-CoV infected mice showed that the levels of cytokines decreased compared those infected with SARS-CoV alone [30]. Perivascular infiltrates were observed on GRFT-treated mice, however, no further analysis was performed [30].
Subcutaneous injection has been tested to show the potential of GRFT to treat HCV. Subcutaneous injection of GRFT in immunodeficient transgenic mice indicated that the lectin remained bioavailable even after 18 days, with only mild transient alterations in health parameters such as body weight and morbidity scores. However, prolonged dosing had deleterious effects on the overall health of these mice [32]. The authors of this study suggest that this could be a consequence of the inherent fragility of the mouse model and not toxicity to implanted human hepatocytes [32]. Treatment of HCV-challenged transgenic mice showed a 2.5 log reduction in the HCV viral titer for the GRFT-treated mice [32]. In another HCV animal model, subcutaneous injections into chimeric mice harboring human hepatocytes in their livers resulted in two out of six animal deaths; similar to the previous study, the authors suggest that this is likely due to the fragile nature of the mice used in this model rather than lectin-induced toxicity [31]. Lastly, intraperitoneal administration of GRFT to mice before a lethal dose of JEV resulted in 100% survival whereas none of the mice in the control group survived [33].
Pharmacokinetic properties of GRFT were further tested in two rodent models. Subcutaneous injections of high amounts of GRFT (50 mg/kg) showed that GRFT persists in plasma for at least two weeks, and sera tested in HIV infectivity assays demonstrated that the GRFT in sera retains antiviral activity during this time. All animals survived the high dose GRFT treatment, and some accumulation in spleen, kidney and liver was observed [54]. Although minimal signs of toxicity were seen, juvenile animals showed weight loss compared to control animals. Importantly, the authors were unable to detect anti-GRFT antibodies in sera even in the high dose animals [54].
Another study assessed the immunogenicity of HIV-1 gp120 and Gag, in the presence and absence of GRFT [40,54]. Immunization of mice with a gp120-GRFT complex enhanced the anti-gp120 response compared to mice immunized with gp120 alone, but immunization with a non-glycosylated Gag-GRFT complex had no effect on the levels of anti-Gag antibodies compared to immunization with Gag alone. Interestingly, an increased anti-GRFT immune response was observed in mice immunized with gp120-GRFT but not the Gag-GRFT complex, leading the authors to conclude that immunization with GRFT-bound gp120 may improve the humoral immune response to gp120 [40].
9. Conclusions
GRFT belongs to a group of lectins capable of inhibiting HIV infection as well as infection by other enveloped viruses. GRFT binds the terminal mannose units present on high mannose oligosaccharides present on the surface of various viral envelope glycoproteins. Structural studies by X-ray crystallography and NMR have shown that the unique structure and distribution of mannose binding sites engender GRFT with an unprecedented ability to block HIV at much lower concentrations than other anti-HIV drugs. Even though GRFT has three mannose binding sites on each subunit, it has been shown that only two of them can be simultaneously engaged by the Man9 oligosaccharide, leaving the third binding site available for binding a second Man9 molecule. This precise distribution of carbohydrate binding sites together with the multivalent character of GRFT arising from its homo dimeric structure leads to the formation of a complex crosslinked network, likely one of the determining factors for its picomolar activity.
Given that the primary binding site of GRFT comprises the carbohydrates present on the surface of HIV-1 gp120, it was reasonable to evaluate the activity against other enveloped viruses. All the viruses that have been shown to be sensitive to GRFT inhibition contain moderate- to heavily-glycosylated envelopes, with a high density of high-mannose oligosaccharides present on many. The therapeutic potential of GRFT against these viruses will depend on the mode of entry of each virus. In closing, a growing body of literature has now established the remarkable potency, broad-spectrum anti-viral activity and safety profile of GRFT supporting its development as a microbicide, especially for diseases where preventative measures are unavailable.
Acknowledgments
We thank the NIH Intramural Research Program (National Institute of Diabetes and Digestive and Kidney Diseases, NIDDK) for financial support, and Cinque Soto and Peter Kwong, for coordinates for the fully glycosylated (with Man9GlcNAc2) model of gp120.
Author Contributions
S.L. and C.A.B wrote this review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Doms, R.W.; Moore, J.P. HIV-1 membrane fusion: Targets of opportunity. J. Cell Biol. 2000, 151, F9–F14. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.C. Viral membrane fusion. Virology 2015, 479–480, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.R.; Poignard, P.; Stanfield, R.L.; Wilson, I.A. Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. Science 2012, 337, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.J. Current progress in development of hepatitis C virus vaccines. Nat. Med. 2013, 19, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Acharya, P.; Lusvarghi, S.; Bewley, C.A.; Kwong, P.D. HIV-1 gp120 as a therapeutic target: Navigating a moving labyrinth. Expert Opin. Ther. Targets 2015, 19, 765–783. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, J. Targeting the glycans of glycoproteins: A novel paradigm for antiviral therapy. Nat. Rev. Microbiol. 2007, 5, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Akkouh, O.; Ng, T.B.; Singh, S.S.; Yin, C.; Dan, X.; Chan, Y.S.; Pan, W.; Cheung, R.C. Lectins with anti-HIV activity: A review. Molecules 2015, 20, 648–668. [Google Scholar] [CrossRef] [PubMed]
- Francois, K.O.; Balzarini, J. Potential of carbohydrate-binding agents as therapeutics against enveloped viruses. Med. Res. Rev. 2012, 32, 349–387. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; O’Keefe, B.R.; Sowder, R.C., 2nd; Bringans, S.; Gardella, R.; Berg, S.; Cochran, P.; Turpin, J.A.; Buckheit, R.W., Jr.; McMahon, J.B.; et al. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J. Biol. Chem. 2005, 280, 9345–9353. [Google Scholar] [CrossRef] [PubMed]
- Giomarelli, B.; Schumacher, K.M.; Taylor, T.E.; Sowder, R.C., 2nd; Hartley, J.L.; McMahon, J.B.; Mori, T. Recombinant production of anti-HIV protein, griffithsin, by auto-induction in a fermentor culture. Protein Expr. Purif. 2006, 47, 194–202. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, B.R.; Vojdani, F.; Buffa, V.; Shattock, R.J.; Montefiori, D.C.; Bakke, J.; Mirsalis, J.; d’Andrea, A.L.; Hume, S.D.; Bratcher, B.; et al. Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. Proc. Natl. Acad. Sci. USA 2009, 106, 6099–6104. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.; Giritch, A.; Bartels, D.; Bortesi, L.; Gleba, Y. A novel and fully scalable Agrobacterium spray-based process for manufacturing cellulases and other cost-sensitive proteins in plants. Plant Biotechnol. J. 2015, 13, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Vamvaka, E.; Arcalis, E.; Ramessar, K.; Evans, A.; O’Keefe, B.R.; Shattock, R.J.; Medina, V.; Stoger, E.; Christou, P.; Capell, T. Rice endosperm is cost-effective for the production of recombinant griffithsin with potent activity against HIV. Plant Biotechnol. J. 2016, 14, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Fuqua, J.L.; Wanga, V.; Palmer, K.E. Improving the large scale purification of the HIV microbicide, griffithsin. BMC Biotechnol. 2015, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowska, N.E.; O’Keefe, B.R.; Mori, T.; Zhu, C.; Giomarelli, B.; Vojdani, F.; Palmer, K.E.; McMahon, J.B.; Wlodawer, A. Domain-swapped structure of the potent antiviral protein griffithsin and its mode of carbohydrate binding. Structure 2006, 14, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Ziolkowska, N.E.; Shenoy, S.R.; O’Keefe, B.R.; McMahon, J.B.; Palmer, K.E.; Dwek, R.A.; Wormald, M.R.; Wlodawer, A. Crystallographic, thermodynamic, and molecular modeling studies of the mode of binding of oligosaccharides to the potent antiviral protein griffithsin. Proteins 2007, 67, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Ziolkowska, N.E.; Shenoy, S.R.; O’Keefe, B.R.; Wlodawer, A. Crystallographic studies of the complexes of antiviral protein griffithsin with glucose and N-acetylglucosamine. Protein Sci. 2007, 16, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Gao, Y.; Hoorelbeke, B.; Kagiampakis, I.; Zhao, B.; Demeler, B.; Balzarini, J.; LiWang, P.J. The role of individual carbohydrate-binding sites in the function of the potent anti-HIV lectin griffithsin. Mol. Pharm. 2012, 9, 2613–2625. [Google Scholar] [CrossRef] [PubMed]
- Moulaei, T.; Shenoy, S.R.; Giomarelli, B.; Thomas, C.; McMahon, J.B.; Dauter, Z.; O’Keefe, B.R.; Wlodawer, A. Monomerization of viral entry inhibitor griffithsin elucidates the relationship between mulitivalent binding to carbohydrates and anti-HIV activity. Structure 2010, 18, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Hoorelbeke, B.; Xue, J.; LiWang, P.J.; Balzarini, J. Role of the carbohydrate-binding sites of griffithsin in the prevention of DC-SIGN-mediated capture and transmission of HIV-1. PLoS ONE 2013, 8, e64132. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Hoorelbeke, B.; Kagiampakis, I.; Demeler, B.; Balzarini, J.; Liwang, P.J. The griffithsin dimer is required for high-potency inhibition of HIV-1: Evidence for manipulation of the structure of gp120 as part of the griffithsin dimer mechanism. Antimicrob. Agents Chemother. 2013, 57, 3976–3989. [Google Scholar] [CrossRef] [PubMed]
- Micewicz, E.D.; Cole, A.L.; Jung, C.L.; Luong, H.; Phillips, M.L.; Pratikhya, P.; Sharma, S.; Waring, A.J.; Cole, A.M.; Ruchala, P. Grifonin-1: A small HIV-1 entry inhibitor derived from the algal lectin, Griffithsin. PLoS ONE 2010, 5, e14360. [Google Scholar] [CrossRef] [PubMed]
- Kagiampakis, I.; Gharibi, A.; Mankowski, M.K.; Snyder, B.A.; Ptak, R.G.; Alatas, K.; LiWang, P.J. Potent strategy to inhibit HIV-1 by binding both gp120 and gp41. Antimicrob. Agents Chemother. 2011, 55, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Moulaei, T.; Alexandre, K.B.; Shenoy, S.R.; Meyerson, J.R.; Krumpe, L.R.; Constantine, B.; Wilson, J.; Buckheit, R.W., Jr.; McMahon, J.B.; Subramaniam, S.; et al. Griffithsin tandemers: Flexible and potent lectin inhibitors of the human immunodeficiency virus. Retrovirology 2015, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Moncla, B.J.; Pryke, K.; Rohan, L.C.; Graebing, P.W. Degradation of naturally occurring and engineered antimicrobial peptides by proteases. Adv. Biosci. Biotechnol. 2011, 2, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Lusvarghi, S.; Ghirlando, R.; Wong, C.H.; Bewley, C.A. Glycopeptide mimetics recapitulate high-mannose-type oligosaccharide binding and function. Angew. Chem. Int. Ed. 2015, 54, 5603–5608. [Google Scholar] [CrossRef] [PubMed]
- Lusvarghi, S.; Lohith, K.; Morin-Leisk, J.; Ghirlando, R.; Hinshaw, J.E.; Bewley, C.A. Binding Site Geometry and Subdomain Valency Control Effects of Neutralizing Lectins on HIV-1 Viral Particles. ACS Infect. Dis. 2016. [Google Scholar] [CrossRef] [PubMed]
- Emau, P.; Tian, B.; O’Keefe, B.R.; Mori, T.; McMahon, J.B.; Palmer, K.E.; Jiang, Y.; Bekele, G.; Tsai, C.C. Griffithsin, a potent HIV entry inhibitor, is an excellent candidate for anti-HIV microbicide. J. Med. Primatol. 2007, 36, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Férir, G.; Huskens, D.; Palmer, K.E.; Boudreaux, D.M.; Swanson, M.D.; Markovitz, D.M.; Balzarini, J.; Schols, D. Combinations of griffithsin with other carbohydrate-binding agents demonstrate superior activity against HIV type 1, HIV type 2, and selected carbohydrate-binding agent-resistant HIV type 1 strains. AIDS Res. Hum. Retrovir. 2012, 28, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, B.R.; Giomarelli, B.; Barnard, D.L.; Shenoy, S.R.; Chan, P.K.; McMahon, J.B.; Palmer, K.E.; Barnett, B.W.; Meyerholz, D.K.; Wohlford-Lenane, C.L.; et al. Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae. J. Virol. 2010, 84, 2511–2521. [Google Scholar] [CrossRef] [PubMed]
- Meuleman, P.; Albecka, A.; Belouzard, S.; Vercauteren, K.; Verhoye, L.; Wychowski, C.; Leroux-Roels, G.; Palmer, K.E.; Dubuisson, J. Griffithsin has antiviral activity against hepatitis C virus. Antimicrob. Agents Chemother. 2011, 55, 5159–5167. [Google Scholar] [CrossRef] [PubMed]
- Takebe, Y.; Saucedo, C.J.; Lund, G.; Uenishi, R.; Hase, S.; Tsuchiura, T.; Kneteman, N.; Ramessar, K.; Tyrrell, D.L.; Shirakura, M.; et al. Antiviral lectins from red and blue-green algae show potent in vitro and in vivo activity against hepatitis C virus. PLoS ONE 2013, 8, e64449. [Google Scholar] [CrossRef] [PubMed]
- Ishag, H.Z.; Li, C.; Huang, L.; Sun, M.X.; Wang, F.; Ni, B.; Malik, T.; Chen, P.Y.; Mao, X. Griffithsin inhibits Japanese encephalitis virus infection in vitro and in vivo. Arch. Virol. 2013, 158, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Levendosky, K.; Mizenina, O.; Martinelli, E.; Jean-Pierre, N.; Kizima, L.; Rodriguez, A.; Kleinbeck, K.; Bonnaire, T.; Robbiani, M.; Zydowsky, T.M.; et al. Griffithsin and carrageenan combination to target Herpes Simplex Virus 2 and Human Papillomavirus. Antimicrob. Agents Chemother. 2015, 59, 7290–7298. [Google Scholar] [CrossRef] [PubMed]
- Turville, S.G.; Aravantinou, M.; Stossel, H.; Romani, N.; Robbiani, M. Resolution of de novo HIV production and trafficking in immature dendritic cells. Nat. Methods 2008, 5, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, K.B.; Gray, E.S.; Lambson, B.E.; Moore, P.L.; Choge, I.A.; Mlisana, K.; Karim, S.S.A.; McMahon, J.; O’Keefe, B.; Chikwamba, R.; et al. Mannose-rich glycosylation patterns on HIV-1 subtype C gp120 and sensitivity to the lectins, Griffithsin, Cyanovirin-N and Scytovirin. Virology 2010, 402, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Ferir, G. Griffithsin, alone and combined with all classes of antiretroviral drugs, potently inhibits HIV cell-cell transmission and destruction of CD4+ T cells. J. Antivir. Antiretrovir. 2012, 4, 103–112. [Google Scholar] [CrossRef]
- Hu, B.; Du, T.; Li, C.; Luo, S.; Liu, Y.; Huang, X.; Hu, Q. Sensitivity of transmitted and founder human immunodeficiency virus type 1 envelopes to carbohydrate-binding agents griffithsin, cyanovirin-N and Galanthus nivalis agglutinin. J. Gen. Virol. 2015, 96, 3660–3666. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, K.B.; Gray, E.S.; Pantophlet, R.; Moore, P.L.; McMahon, J.B.; Chakauya, E.; O’Keefe, B.R.; Chikwamba, R.; Morris, L. Binding of the mannose-specific lectin, griffithsin, to HIV-1 gp120 exposes the CD4-binding site. J. Virol. 2011, 85, 9039–9050. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Michael, E.; Eggink, D.; van Montfort, T.; Lasnik, A.B.; Palmer, K.E.; Sanders, R.W.; Moore, J.P.; Klasse, P.J. Occluding the mannose moieties on human immunodeficiency virus type 1 gp120 with griffithsin improves the antibody responses to both proteins in mice. AIDS Res. Hum. Retrovir. 2012, 28, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, K.B.; Gray, E.S.; Mufhandu, H.; McMahon, J.B.; Chakauya, E.; O’Keefe, B.R.; Chikwamba, R.; Morris, L. The lectins griffithsin, cyanovirin-N and scytovirin inhibit HIV-1 binding to the DC-SIGN receptor and transfer to CD4+ cells. Virology 2012, 423, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Nixon, B.; Stefanidou, M.; Mesquita, P.M.; Fakioglu, E.; Segarra, T.; Rohan, L.; Halford, W.; Palmer, K.E.; Herold, B.C. Griffithsin protects mice from genital herpes by preventing cell-to-cell spread. J. Virol. 2013, 87, 6257–6269. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jin, W.; Griffin, G.E.; Shattock, R.J.; Hu, Q. Removal of two high-mannose N-linked glycans on gp120 renders human immunodeficiency virus 1 largely resistant to the carbohydrate-binding agent griffithsin. J. Gen. Virol. 2011, 92, 2367–2373. [Google Scholar] [CrossRef] [PubMed]
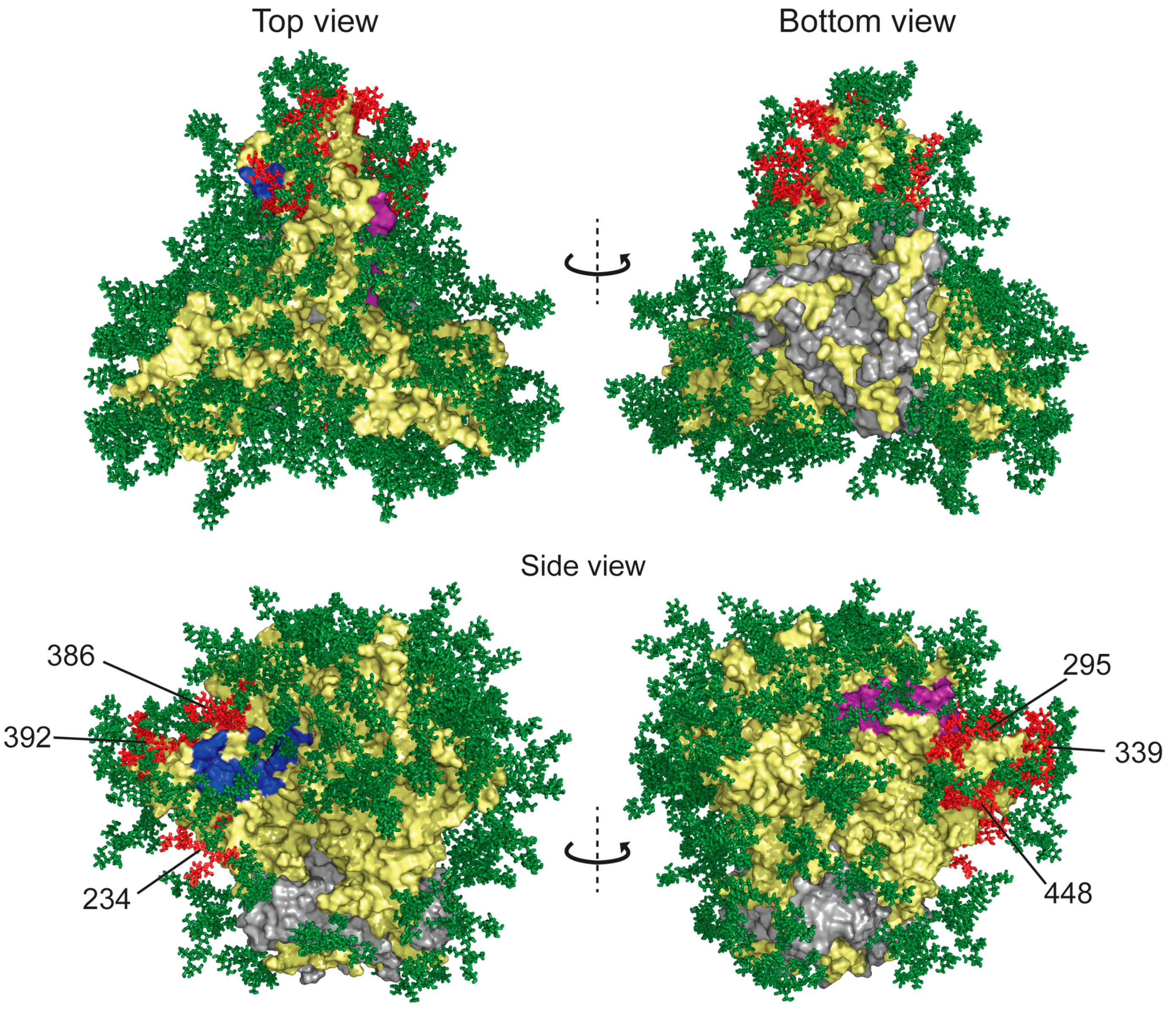
- Stewart-Jones, G.B.E.; Soto, C.; Lemmin, T.; Chuang, G.Y.; Druz, A.; Kong, R.; Thomas, P.V.; Wagh, K.; Zhou, T.Q.; Behrens, A.J.; et al. Trimeric HIV-1-Env structures define glycan shields from clades A, B, and G. Cell 2016, 165, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, K.B.; Moore, P.L.; Nonyane, M.; Gray, E.S.; Ranchobe, N.; Chakauya, E.; McMahon, J.B.; O’Keefe, B.R.; Chikwamba, R.; Morris, L. Mechanisms of HIV-1 subtype C resistance to GRFT, CV-N and SVN. Virology 2013, 446, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Ferir, G.; Huskens, D.; Noppen, S.; Koharudin, L.M.; Gronenborn, A.M.; Schols, D. Broad anti-HIV activity of the Oscillatoria agardhii agglutinin homologue lectin family. J. Antimicrob. Chemother. 2014, 69, 2746–2758. [Google Scholar] [CrossRef] [PubMed]
- Hamorsky, K.T.; Grooms-Williams, T.W.; Husk, A.S.; Bennett, L.J.; Palmer, K.E.; Matoba, N. Efficient single tobamoviral vector-based bioproduction of broadly neutralizing anti-HIV-1 monoclonal antibody VRC01 in Nicotiana benthamiana plants and utility of VRC01 in combination microbicides. Antimicrob. Agents Chemother. 2013, 57, 2076–2086. [Google Scholar] [CrossRef] [PubMed]
- Ferir, G.; Palmer, K.E.; Schols, D. Synergistic activity profile of griffithsin in combination with tenofovir, maraviroc and enfuvirtide against HIV-1 clade C. Virology 2011, 417, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, L.; Oliveira, C.; Fournier, C.; Descamps, V.; Morel, V.; Dubuisson, J.; Brochot, E.; Francois, C.; Castelain, S.; Duverlie, G.; et al. Hepatitis C Virus resistance to carbohydrate-binding agents. PLoS ONE 2016, 11, e0149064. [Google Scholar] [CrossRef] [PubMed]
- Ishag, H.Z.; Li, C.; Wang, F.; Mao, X. Griffithsin binds to the glycosylated proteins (E and prM) of Japanese encephalitis virus and inhibit its infection. Virus Res. 2016, 215, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.M.; Ruscetti, F.W.; Rein, A.; Bertolette, D.C.; Saucedo, C.J.; O’Keefe, B.R.; Jones, K.S. Differential inhibitory effects of cyanovirin-N, griffithsin, and scytovirin on entry mediated by envelopes of gammaretroviruses and deltaretroviruses. J. Virol. 2014, 88, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Ratner, D.M.; Ryan, C.M.; Johnson, P.J.; O’Keefe, B.R.; Secor, W.E.; Anderson, D.J.; Robbins, P.W.; Samuelson, J. Anti-retroviral lectins have modest effects on adherence of trichomonas vaginalis to epithelial cells in vitro and on recovery of Tritrichomonas foetus in a mouse vaginal model. PLoS ONE 2015, 10, e0135340. [Google Scholar] [CrossRef] [PubMed]
- Kouokam, J.C.; Huskens, D.; Schols, D.; Johannemann, A.; Riedell, S.K.; Walter, W.; Walker, J.M.; Matoba, N.; O’Keefe, B.R.; Palmer, K.E. Investigation of griffithsin’s interactions with human cells confirms its outstanding safety and efficacy profile as a microbicide candidate. PLoS ONE 2011, 6, e22635. [Google Scholar] [CrossRef] [PubMed]
- Barton, C.; Kouokam, J.C.; Lasnik, A.B.; Foreman, O.; Cambon, A.; Brock, G.; Montefiori, D.C.; Vojdani, F.; McCormick, A.A.; O’Keefe, B.R.; et al. Activity of and effect of subcutaneous treatment with the broad-spectrum antiviral lectin griffithsin in two laboratory rodent models. Antimicrob. Agents Chemother. 2014, 58, 120–127. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).